Abstract
Neural crest cells (NCCs) are indispensable for the development of the cardiac outflow tract (OFT). Here, we show that mice lacking Smad4 in NCCs have persistent truncus arteriosus (PTA), severe OFT cushion hypoplasia, defective OFT elongation, and mispositioning of the OFT. Cardiac NCCs lacking Smad4 have increased apoptosis, apparently due to decreased Msx1/2 expression. This contributes to the reduction of NCCs in the OFT. Unexpectedly, mutants have MF20-expressing cardiomyocytes in the splanchnic mesoderm within the second heart field (SHF). This may result from abnormal differentiation or defective recruitment of differentiating SHF cells into OFT. Alterations in Bmp4, Sema3C and PlexinA2 signals in the mutant OFT, SHF, and NCCs, disrupt the communications among different cell populations. Such disruptions can further affect the recruitment of NCCs into the OFT mesenchyme, causing severe OFT cushion hypoplasia and OFT septation failure. Furthermore, these NCCs have drastically reduced levels of Ids and MT1-MMP, affecting the positioning and remodeling of the OFT. Thus, Smad-signaling in cardiac NCCs has cell autonomous effects on their survival and non-cell autonomous effects on coordinating the movement of multiple cell lineages in the positioning and the remodeling of the OFT.
Keywords: Smad, Neural Crest, Outflow Tract, Secondary Heart Field, Congenital Heart Diseases
Introduction
Nearly one percent of live births in humans have congenital heart diseases (CHDs) with about a third of these CHDs affecting the outflow tract (OFT) and its derivatives (Thom et al., 2006). Many of these defects result from abnormalities in the incorporation of distinct cellular lineages into the cardiac outflow tract, which requires precise timing and cell-cell communications. Cardiac development initiates with progenitor cells in the two bilaterally regions of the lateral mesoderm, namely the primary or first heart field (FHF) (Srivastava, 2006). Recent studies have provided convincing evidence that a second heart field (SHF), comprised of cells in pharyngeal and splanchnic mesoderm anterior and medial to the FHF, contributes myocardium to the OFT and right ventricle (Abu-Issa et al., 2004; Black, 2007; Eisenberg and Markwald, 2004; Kelly, 2005; Kelly et al., 2001; Mjaatvedt et al., 2001; Waldo et al., 2001). The recruitment of the SHF mesoderm into the OFT and right ventricle requires precise control of gene expression and interactions among different cell lineages (Black, 2007; Kelly, 2005).
The involvement of cardiac neural crest (CNC), a subgroup of neural crest cells (NCCs), in OFT development has been well documented by CNC ablation studies in chick as well as by genetic studies in mice and other model organisms (Brown and Baldwin, 2006; Hutson and Kirby, 2007; Stoller and Epstein, 2005). After populating pharyngeal arch (PA) 3, 4, and 6, NCCs migrate into the OFT as early as E9.5 in mice (Jiang et al., 2000), and mediate the remodeling of the OFT into the pulmonary trunk and the ascending aorta. NCC ablation or the deletion of genes in NCCs results in a spectrum of defects, including pharyngeal arch patterning defects, double outlet of right ventricle, tetralogy of Fallot and persistent truncus arteriosus (PTA) in which the aorticopulmonary septum fails to form (Brown and Baldwin, 2006; Hutson and Kirby, 2007; Stoller and Epstein, 2005). In addition, cell ablation studies in chick have shown that CNC is necessary for the addition of the myocardium from the SHF to the OFT and the caudal movement of the OFT (Waldo et al., 2005a; Waldo et al., 2001; Yelbuz et al., 2002).
Smad transcription factors are at the core of the transcriptional responses in the transforming growth factor β (Tgfβ) signaling pathway. Tgfβ superfamily members are structurally related secreted cytokines that include Tgfβ isoforms, activins, bone morphogenetic proteins (BMPs), and others. The binding of ligands to their receptors leads to the phosphorylation of the receptor-regulated Smads (R-Smads). The phosphorylated R-Smads complex with the common Smad, Smad4, before translocating into the nucleus to regulate the transcription of the target genes (Massague et al., 2005). Signals from different Tgfβ ligands and receptors diverge and converge on different sets of R-Smads, producing distinct and sometimes opposing outcomes. The Tgfβ pathway is one of the most versatile cytokine signaling pathways in metazoans, regulating biological processes from cell division to the patterning of the organism (Massague et al., 2005). Previous studies have shown that a spectrum of OFT and pharyngeal arch artery (PAA) defects can result from germline deletions and NCC-specific inactivation of a number of Tgfβ superfamily ligands and receptors (Choudhary et al., 2006; Gaussin et al., 2005; Kaartinen et al., 2004; Kim et al., 2001; Ma et al., 2005; Molin et al., 2004; Sanford et al., 1997; Stottmann et al., 2004; van Wijk et al., 2007; Wang et al., 2006; Wurdak et al., 2005). Recent advances have revealed that besides the kinase activities of the Tgfβ type I receptors, other kinases, such as MAPK, CDK, CamK II, and GRK2, can also phosphorylate Smads (Massague et al., 2005; Xu, 2006). In addition, Smad-independent Tgfβ responses have been reported in Smad-deficient cell lines and animal models (Massague et al., 2005; Xu, 2006). Signal transduction from Tgfβ ligands and receptors to Smads is complicated and nonlinear. Thus, to better understand the mechanism by which NCCs regulate cardiac development, it is necessary to investigate the precise role of Smad signaling in this context in addition to the studies of the Tgfβ ligands and receptors.
In this study, we have found that the absence of Smad4 in NCCs causes a wide spectrum of OFT defects, including OFT cushion hypoplasia, OFT septation defect, OFT elongation defect, and OFT alignment defect. We have observed increased apoptosis in the mutant cardiac NCCs, suggesting an indispensable cell autonomous role of Smad signaling in NCC survival. Furthermore, mice with NCCs lacking Smad4 have alterations in the expression of Bmp4, Sema3C, and PlexinA2 and other molecules in the OFT myocardium, SHF mesoderm, or NCCs, reflecting disrupted communications among these cell lineages. These defects lead to disruptions in NCC recruitment to OFT cushion, contributing to the observed OFT cushion hypoplasia. We have also observed abnormal presence of MF20-expressing cardiomyocytes in the splanchnic mesoderm within the SHF and a concurrent failure in the OFT caudal movement. The ectopic presence of MF20-expressing cells in the SHF may be a result of defective recruitment of mesodermal cells from the SHF to OFT myocardium, or abnormal differentiation due to the altered signaling between the Smad4-deficient NCCs and the SHF mesodermal cells. Our data also show that cardiac NCCs lacking Smad4 have greatly reduced expression of Inhibitor of differentiation (Id) genes and Membrane type-1 matrix metalloproteinase (MT1-MMP), both of which are critical for tissue remodeling. Thus the reduction of Ids and MT1-MMP may provide the basis for the failure of OFT caudal movement in the mutants that involves extensive tissue remodeling. This study reveals both a direct role of Smad signaling on NCC survival and indirect effects, through communications with other cell lineages, in orchestrating gene expression and the integration of multiple cell lineages for the remodeling of the OFT.
Materials and methods
Mouse (Mus musculus) strains and sample collection
The generation of the floxed-Smad4 allele was described previously (Yang et al., 2002). Mice carrying this floxed-Smad4 allele were crossed with the Wnt1Cre transgenic mice to produce Wnt1Cre;Smad4floxed/floxed embryos that would have homozygous deletion of Smad4 in NCCs. Wnt1Cre;Smad4floxed/floxed embryos are designated as mutants in this study. Their littermates with no homozygous deletion of Smad4 in any cells are considered controls. To fate map the NCCs, the Rosa26RLacZ transgene was introduced into the Wnt1Cre;floxed-Smad4 mice. Direct comparison was made between littermates. All experiments were repeated at least three times.
Histological Analysis
For histological analyses, embryos were fixed with 4% paraformaldehyde and embedded in paraffin. Sections of 7 μm were collected and stained following standard protocol. For immunohistochemistry, sections were stained with a rabbit polyclonal anti-beta galactosidase antibody (MP Biomedical, 7A6, 1:1000) and a mouse monoclonal anti-MF20 (Developmental study hybridoma bank, 1:50). Appropriate AlexaFlour488 or 555-conjugated secondary antibodies (Molecular Probe, 1:1000) were used to detect the corresponding primary antibodies. Whole-mount immunostaining was carried out with an antibody for Pecam-1 (BD Pharmingen, CD31, 1:50) as described (Graef et al., 2001). 5-Bromo-4-chloro-3-indolyl-D-galactoside (Xgal) whole-mount staining of embryos were performed as described (Chang et al., 2004).
Proliferation and Apoptosis
BrdU was injected (i.p.) into pregnant mice 1.5 hours before embryo harvest and was detected by a mouse monoclonal anti-BrdU antibody (Developmental study hybridoma bank, 1:200). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analysis was performed on paraffin-embedded sections by using the ApopTag plus peroxidase in situ apoptosis detection kit (Roche, Nutley, NJ). Proliferation index is presented as the average number of BrdU positive cells per 100 cells counted. NCC proliferation index was determined by counting about 200 NCCs in the PA-OFT region for each sample (n=6 for each group). About 30–60 cells were counted in distal region of OFT myocardium in each mouse for OFT myocardium proliferation index (n=9 for each group). Exactly 30 cells in splanchnic mesoderm caudal to the OFT attachment point to the ventral pharynx in each mouse (n=9 for each group) were counted for the calculation of the proliferation index in this particular SHF region. The proliferation index was calculated individually for each mouse and Student t-test was employed to detect the statistical difference between the control and mutant groups.
RNA In situ hybridization
Whole-mount RNA in situ hybridization was performed as previously described (Chen and Capecchi, 1999). In situ probes for Nkx2.5, Gata4, Sema3C, Bmp4 and Vegf were synthesized from plasmids kindly provided by various laboratories (Acknowledgements). In situ probes for Has2, Sox9; Id1-3, Slug, PlexinA2, Msx1/2, Tbx1 and MT1-MMP were synthesized from T-easy vector (Qiagen, Valencia, CA) with cloned PCR inserts for different genes prepared by one step RT-PCR (Invitrogen, Carlsbad, CA). After RNA whole-mount in situ hybridization and microphotography, the embryos were embedded in paraffin and sectioned. For RNA in situ hybridization on paraffin sections, a DAKO Tyramide amplification kit was used following manufacturer’s protocol (Dako, Carpinteria, CA).
Ink injections and corrosive casting
To analyze vessel patterning, India ink was injected into the left ventricle with glass needles controlled by a mouth pipette and cleared in 1:2 benzyl alcohol/benzyl benzoate. Corrosive casting was done with Batson’s plastic replica and corrosion kit (Polysciences, Warrington, PA). The casting polymers were prepared immediately before the procedure and injected into the left ventricle using gauge 30 needles and 0.3 ml tuberculosis-injection syringes. Gentle pressure was applied to the syringe until the blue casting polymers fill the vasculature. The tissues were left at 4°C overnight before being placed in maceration solution at 50 °C to corrode for 6–8 hours.
Results
Smad4 deficiency in NCCs results in OFT septation defects and cushion hypoplasia
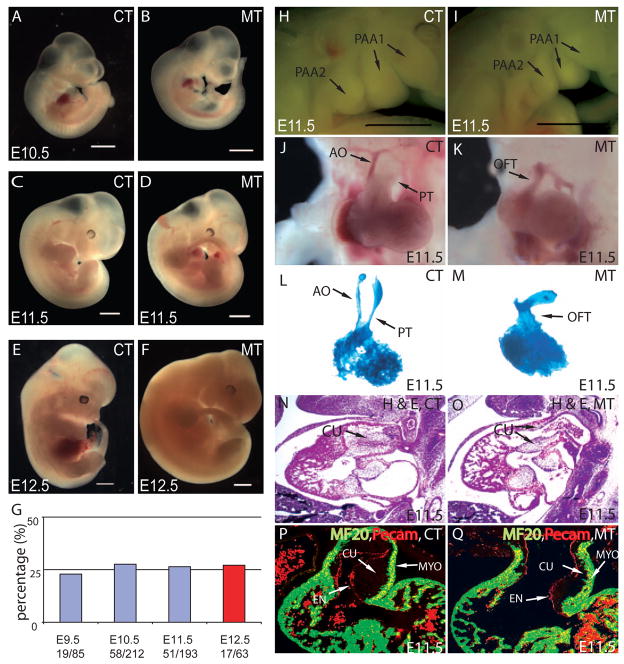
To study the role of Smad signaling in NCCs during cardiac development and to avoid the early gastrulation arrest found in Smad4 null animals, we use the Wnt1Cre transgene (Stottmann et al., 2004) to mediate the recombination of a loxP-flanked Smad4 allele (Yang et al., 2002) specifically in NCCs. Embryos with homozygous deletion of Smad4 in NCCs (Wnt1Cre/+;Smad4loxP/loxP) are described as mutants in this study. Littermates without homozygous deletion of Smad4 in NCCs have no phenotype and are described as controls. Except for the heart and the pharyngeal arch (PA) area, the overt morphology of the mutants appeared relatively normal up to E11.5 (Fig. 1A–D). Importantly, retrograde blood flow was observed in the mutant OFT at E11.5, indicating that the deletion of Smad4 in NCCs leads to severe cardiac OFT defects (data not shown) (Conway et al., 2003). While all E11.5 mutants recovered were alive, all mutant embryos died at E12.5 without exception (Fig. 1E,F). Mutant embryos were recovered at Mendelian ratio (25% from compound heterozygous intercross) at different developmental stages up to E12.5 (Fig. 1G). At E11.5, the mutant embryos exhibited hypoplasia of the pharyngeal arches (Fig. 1H,I). At this time, the single OFT was normally in the process of septating into two distinct channels: the ascending aorta and the pulmonary trunk (Fig. 1J). The mutant OFT, however, failed to septate and was connected to the aortic sac as a single tube, resembling PTA in humans (Fig. 1K). The failure of OFT septation is also evident in the cardiac casting (Fig. 1L,M). In addition, the OFT cushion was hypoplastic in the mutants at E11.5, particularly in its distal portion, as shown by the H&E stained sections (Fig. 1N,O) and sections immunostained with antibodies against Pecam-1 (labeling endocardium) and MF20 (labeling myocardium) (Fig. 1P,Q). The OFT cushion hypoplasia may be a primary defect due to the Smad4 deficiency in the NCCs and the reduction of NCC contribution to the OFT (discussed in later sections). However, hemodynamic changes caused by other cardiac structural defects could cause or contribute to the OFT cushion defects (Bartman and Hove, 2005; Butcher and Markwald, 2007). Nevertheless, severe OFT cushion hypoplasia is likely the major cause of the observed regurgitation at E11.5 with subsequent embryonic death at E12.5. The thickness and trabeculation of the ventricular myocardium were similar between control and mutant samples at E10.5 (control N=22, mutant N=22). The thickness, but not the level of trabeculation, of the right ventricular wall was slightly reduced in all E11.5 mutants examined (Control N=4; Mutant N=4) (Fig. 1P,Q and data not shown). The subtle reduction of right ventricular wall thickness in older mutant embryos may be an accumulative effect of defective myocardium accruement or as a result of hemodynamic changes due to defects in other cardiac structures, most noticeably the OFT.
Figure 1. Smad4 deficiency in NCCs results in abnormal OFT septation and severe OFT cushion hypoplasia.
(A–D) From E9.5 to E11.5, except for OFT defects and PA hypoplasia at E11.5, no other morphological difference was overtly apparent between Wnt1Cre;Smad4loxP/loxP mice (MT) and their littermate controls (CT) that do not have homozygous deletion of Smad4 in NCCs. (E,F) Mutant mice died at E12.5. (G) Mutant embryos were recovered at Mendelian ratio (25% from compound heterozygotes intercross) at different developmental stages up to E12.5. All mutants died at E12.5 as evidenced by the absence of heart beat (Red). Scar bar: 1mm. (H,I) PA hypoplasia was observed in mutants at E11.5. Scar bar: 1mm. (J,K) At E11.5, the OFT has developed into pulmonary trunk (PT) and ascending aorta (AO) in the control, while the OFT of the mutant was still not divided. (L,M) Casting experiments revealed two separated outlet channels in the OFT from the control but only one common outlet in the mutant. (N,O) Haematoxylin and Eosin (H&E) staining of sagittal sections at E11.5 showed severe OFT cushion (CU) hypoplasia and persistent truncus arteriosus (PTA) in mutant compared to the control. (P,Q) MF20 staining (green) and Pecam staining (red) of frontal section at E11.5 revealed a thinner layer of cushion in mutant OFT compared to the control.
NCC-specific inactivation of Smad4 leads to reduced NCC contribution to the OFT and disruption in the formation of the OFT cushion mesenchyme
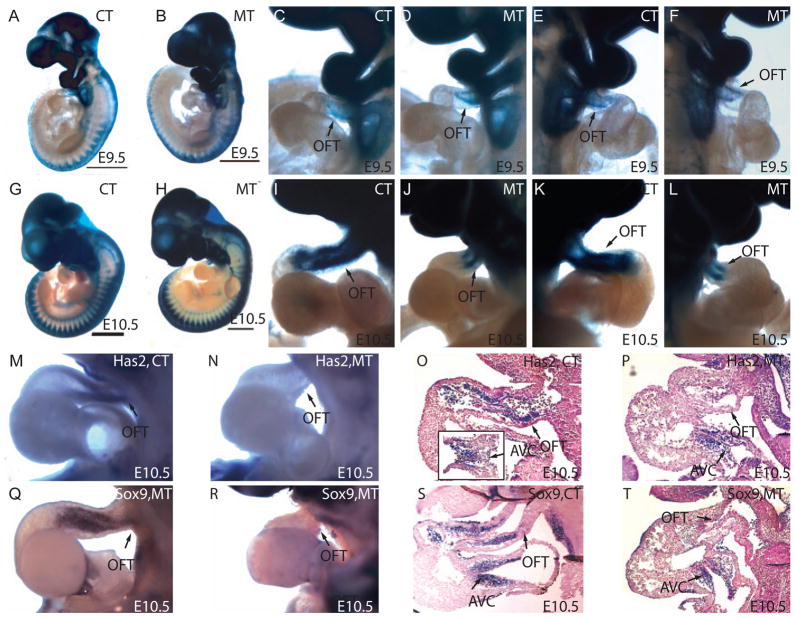
To study the migration of NCCs and OFT cushion development, we analyzed the migration of NCCs in Wnt1Cre-Rosa26RLacZ mice by whole-mount β-galactosidase staining and examined the expression of genes involved in cushion development. At E9.5, a significant number of NCCs had arrived in the distal OFT (away from the right ventricle) in both the control and mutant (Fig. 2A–F). At E10.5, when NCCs had normally reached the proximal OFT (close to the right ventricle), the NCCs in the mutant embryos only populated the distal part of the OFT without penetrating the proximal OFT. The decreased penetration and the shorter length of the mutant OFTs led to a significant reduction of the total number of NCCs in the OFT of the mutant embryos (Fig. 2G–L). This reduction of NCC contribution to the OFT was not due to a gross developmental delay or a cell autonomous migration defect in NCCs given that the control and mutant were morphologically comparable at this stage and the migration of NCCs to the dorsal root ganglia was not affected in mutants (Fig. 2A,B,G,H and data on Neurofilament staining not shown). The seemingly unaffected formation of the dorsal root ganglia and the severe OFT malformations suggest that the development of the OFT is more sensitive to the absence of Smad signaling in NCCs. This sensitivity may arise from the requirement of precise communications among diverse cell lineages during OFT development that would entail both cell autonomous and non-cell autonomous effects of Smad signaling in NCCs.
Figure 2. Smad4 deficiency in NCCs caused a reduction of NCC contribution to the OFT cushion mesenchyme.
(A–K) Whole-mount β-galactosidase assay. (A and B) At E9.5, overall neural crest development is similar between control and mutant, except for the OFT area. (C–F) There is a small reduction of NCCs at proximal OFT (close to the right ventricle) in the mutant compared to the control at E9.5. (C and D) left side; (E and F) right side. (G,H) At E10.5, NCC population in the PA area is reduced in the mutant when compared to the control. (I,L) The number of NCCs in OFT in the proximal OFT is significantly reduced. The length of the OFT in the mutants is shorter compared to the control. (M,N) Has2 whole mount in situ hybridization revealed the lack of Has2 in OFT. (O,P) Paraffin section after whole-mount RNA in situ showed reduced Has2 expression in OFT cushion. An insert in O showed Has2 expression in cushion of atria ventricular canal (AVC). (Q,R) Whole-mount RNA in situ hybridization showed that Sox9 was ablated in OFT. (S,T) Paraffin section after whole-mount RNA in situ hybridization indicated Sox9 was down-regulated both in outflow cushion and in NCCs in apposition to SHF in mutant compared to the controls.
To further study the OFT cushion hypoplasia (Fig. 1N–Q) in the mutants, we examined the expression of Has2 and Sox9, two important molecules expressed in cushion mesenchymal cells (Person et al., 2005). Has2 is essential for the biosynthesis of hyaluronan in the cardiac jelly within the cushions and Has2 null mice fail to undergo epithelial-mesenchymal transformation (EMT) (Camenisch et al., 2000). On the other hand, Sox9 is activated when endocardial cells transform into mesenchymal cells. Mice deficient for Sox9 die from heart failure with hyperplasia of both the OFT and AV cushions (Akiyama et al., 2004). By whole-mount RNA in situ hybridization, we found that expression of Has2 was abolished in the mutant OFT cushion (Fig. 2M–P). In contrast, Has2 expression in the atrioventricular (AV) cushion had no significant change (Fig. 2O,P). In addition, the expression of Sox9 was greatly reduced in the OFT cushion and in the NCCs in close apposition to the SHF, but not in the AV cushion in mutant mice (Fig. 2Q–T). These findings are consistent with previous observations that NCC defects specifically affect OFT cushion but not the AV cushion (de Lange et al., 2004; Stoller and Epstein, 2005). The drastic reduction of Has2 and Sox9 in the OFT cushion may further affect the OFT cushion development.
Smad4-deficient NCCs have reduced Msx1/2 expression and a higher apoptotic rate that may contribute to the reduction of NCCs in the developing cardiac OFT
To study whether potential changes in proliferation and cell death of NCCs lacking Smad4 contributed to the reduction of NCCs in the developing OFT, we used BrdU labeling and TUNEL assays to examine the proliferation and apoptosis of the NCCs (Fig. 3A–G). NCCs were visualized by an anti-β-galactosidase antibody in the presence of the Rosa26RLacZ transgene in these mice. We found no significant difference in the proliferation index of NCCs between control and mutant samples (Fig. 3A,B,G) at E10.5. However, TUNEL assay revealed a marked increase in the number of apoptotic NCCs in the pharyngeal arches and OFT area in mutant embryos compared with their controls (Fig. 3C,D). Immunostaining for β-galactosidase on adjacent sections revealed that most, but not all, of the apoptotic cells were NCCs (Fig. 3E,F). At E10.5, the intensity and the domain of expression of both Msx1 and Msx2 in the PA area were significantly reduced (Fig. 3H–O). This is consistent with previous finding that Msx1/2 are targets of Tgfβ superfamily signaling and are required for NCC survival (Ishii et al., 2005; Tribulo et al., 2003). Thus, our data suggest that the loss of Smad4 function in NCCs results in drastically reduced Msx1/2 expression in these cells, leading to increased apoptosis. The higher apoptotic rate contributes to the reduction of total NCCs in the pharynx and the OFT area. This reduction of NCCs will also inevitably disrupt the precise cellular interactions and signaling required for the correct morphogenetic remodeling of the OFT.
Figure 3. Smad4-deficient NCCs have reduced Msx1/2 expression and a higher apoptotic rate.
(A,B) BrdU labeling showed similar proliferation index between control and mutant. (C,D) TUNEL assay showed an increased apoptotic rate in PA mesenchyme in mutant compared to the control. (E,F) Immunofluoresence staining revealed the β-galactosidase positive cells (NCCs) in PA mesenchyme. (G) No difference (p=0.94) was detected in the proliferation rate of NCCs in the PA-OFT region in control (23±11%) and in mutant (23±7%); Values are means ± SEM, n = 6. (H–O) RNA whole-mount in situ hybridization indicated the expression of both Msx1 and Msx2 were downregulated in the PA-OFT area in the mutant. J, K, N and O are higher magnification views of the PA-OFT area in H, I, L and M respectively.
Mice with NCC-specific inactivation of Smad4 have abnormal presence of MF20 positive cardiomyocytes within the SHF
To assess how NCCs may influence the SHF cells and OFT remodeling, we examined the differentiation of SHF cells and the accruement of the cardiomyocytes from SHF to the elongating OFT. The NCCs were identified by an anti-β-galactosidase antibody in mice carrying both the Wnt1Cre and the Rosa26RLacZ transgenes. NCCs were located in direct apposition to SHF cells and in close proximity to myocardial cells in the OFT (Fig. 4A–D). At E10.5, cells in the SHF do not express MF20, a cardiomyocyte marker, in the controls (Fig. 4A,C). However, cells in the SHF of the mutant surprisingly expressed MF20 at E10.5 before they reached the OFT (Fig. 4B,D). A previous study suggested that there is myosin expression and some degree of differentiation in SHF at E8.5 (Prall et al., 2007). To ensure that the difference in MF20 expression in the splanchnic mesoderm caudal to OFT is truly associated with Smad4 deficiency in NCCs but not due to experimental variations, additional pairs of control and mutant samples were examined. We sectioned a total of 13 mutants and 13 littermate controls at E10.5. The MF20-expressing cells were present in the splanchnic mesoderm caudal to the OFT in every mutant examined and were absent in all the controls at E10.5. The abnormal presence of MF20 positive cells in this area suggests defective recruitment of these cells into the OFT (resulting in the shorter OFT) or abnormal differentiation due to the defective communication between these mesodermal cells and adjacent NCCs lacking Smad4. We further studied the proliferation of the cells in the splanchnic mesoderm caudal to OFT and the cardiomyocytes well inside OFT by BrdU labeling (Fig. 4E–F). We found no difference in the proliferation rate of cardiomyocytes in OFT myocardium between control and mutant samples (Fig. 4E–G). However, the proliferation rate of the SHF mesodermal cells caudal to the OFT in the mutant was significantly lower than those in the controls (Fig. 4E,F,H). The lower proliferation rate may reflect that these cells are differentiating into cardiomyocytes.
Figure 4. Abnormal presence of MF20 positive cardiomyocytes within the SHF.
(A, C) Double immunostaining with anti-MF20 (green) and anti-β-galactosidase (red) antibodies revealed that MF20 was expressed in OFT myocardium but not in the SHF in the control. (B, D) However, MF20 was expressed both in the OFT myocardium and in the SHF mesoderm in the mutant (arrows). (E, F) BrdU labeling revealed no difference (p=0.38) in the proliferation rate of the OFT myocardium between control (48±7%) and mutant (45±7%), Values are means ± SEM, n=9 (G); and a significant reduction (p=2.47E-07) in the proliferation rate in the splanchnic mesoderm caudal to the OFT between mutant (17±7%) and control (60±8%); * stands for statistical significant difference compared to the control. Values are means ± SEM, n = 9 (H).
Inactivation of Smad4 in NCCs impairs the caudal movement and the elongation of the OFT
To understand the mechanism by which inactivation of Smad4 in NCCs caused the observed cardiac anomalies, we set out to investigate the morphogenetic processes during OFT remodeling in the mutants. Whole-mount Pecam staining revealed that the connection of the OFT to the aortic sac was similar between the mutant and control at E9.5. The junction was slightly caudal to the second PA artery (PAA2) (Fig. 5A,B). At E10.5, PAA2 disappeared and the more caudal PAAs emerged. The connection of the OFT had shifted caudally to near the level of PAA4 at E10.5 in the controls, as revealed by Indian ink injection outlining the OFT and PAAs (Fig. 5C,E). The mutant OFT, however, failed to move caudally and the OFT junction remained at a level rostral to PAA3 near the level where the disappeared PAA2 would have been (Fig. 5D,F). Along with the failed OFT caudal movement, there was a significant decrease of OFT length in the mutants (Fig. 5D). As a result of the failure in caudal movement and elongation of the OFT, the mutant hearts were located in a more cranial position than that of the controls (Fig. 5C–F). The level of the OFT along the anterior-posterior axis did not change significantly from E10.5 to E11.5 in either the controls or the mutants. Further OFT remodeling, most noticeably septation, occurred only in the controls but not in the mutants at E11.5 (Fig. 5G–H). A summary of the level of OFT relative to the PAAs is provided in Fig. 5I.
Figure 5. Inactivation of Smad4 in NCCs impaired the caudal movement and the elongation of the OFT.
(A,B) Pecam whole-mount immunostaining revealed a shorter OFT (black arrows) at E9.5. The OFT attachment site to the ventral pharynx was at the level of PAA2 in both control and mutant (C, D) At E10.5, India ink injection also outlined a shortened OFT in the mutant compared to the control. The OFT attachment site to the ventral pharynx was located at the level caudal to PAA3 (and near PAA4) in the control, but at a position rostral to PAA3 in the mutant (near where the disappeared PAA2 should have been). (E,F) Ventral view of PAAs showed the rostral OFT attachment site in the mutant at E10.5. Triangle points to OFT attachment site to ventral pharynx. (G,H) At E11.5, the wild type OFT was separated into ascending aorta (AO) and pulmonary trunk (PT) which connected pharyngeal arch 4 and 6 respectively while the mutant OFT still connected the heart to the aortic sac as a single outlet. (I) A summary of the level of OFT attachment relative to PAAs at E9.5 and E10.5.
Absence of Smad4 in NCCs disrupts the communications among myocardium, NCCs, SHF mesoderm, and other cell types during OFT development
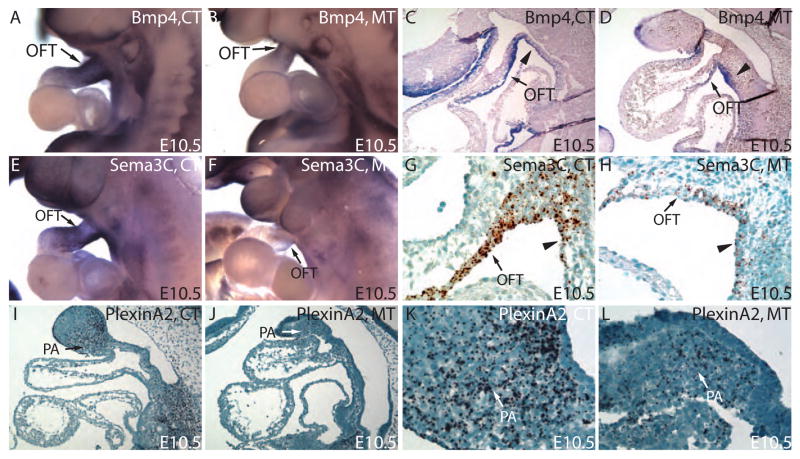
Multiple signal molecules, including BMPs, Sema3 isoforms and PlexinA2, are involved in the reciprocal signaling between NCCs and myocardial cells in the OFT. These interactions are essential for the migration of NCCs into the OFT and the normal development of the OFT cushion (Brown and Baldwin, 2006; Hutson and Kirby, 2007; Karafiat et al., 2005; Lepore et al., 2006; Stoller and Epstein, 2005). To understand the mechanisms of OFT remodeling defects in the mutants, we examined the expression of these factors by RNA in situ hybridization in the control and mutants at E10.5. We have observed alterations in the expression of Bmp4 in the OFT myocardium and the splanchnic mesoderm within the SHF in the mutants (Fig. 6A–D). Furthermore, we examined the transcript levels of Sema3A, F and C and PlexinA2 at E10.5 by whole-mount and section RNA in situ hybridization. We found that, among the Sema3 isoforms, only the expression of Sema3C was significantly decreased in the OFT myocardium and in the SHF mesodermal cells of the mutants (Fig. 6E–H, and data not shown). PlexinA2 mRNA, coding for the receptor for Sema3C, was significantly reduced in NCCs in areas from the PA region to the OFT (Fig. 6I–L). The expression changes in Bmp4, Sema3C, and PlexinA2 reflect the altered cell-cell signaling caused by the absence of Smad4 in the NCCs. These expression changes, in turn, may further affect NCC recruitment to OFT cushion and SHF mesoderm contribution to the OFT myocardium.
Figure 6. Defective OFT myocardial accruement of SHF mesodermal cells disrupts the normal distribution of signaling molecules essential for NCCs migration.
(A,B) Whole-mount RNA in situ hybridization revealed a reduction in Bmp4 expression in OFT. (C,D) Close examination of the subsequent sections further revealed that the reduction of Bmp4 expression is in OFT myocardium. (E,F) Whole-mount in situ hybridization reveals a marked reduction in Sema3C in OFT cuff in mutant compared to the control. (G,H) RNA In situ hybridization on paraffin section showed that the Sema3C was mainly expressed in outflow tract myocardium and was significantly down-regulated in mutants compared to the controls. (I,J) RNA In situ hybridization on paraffin section reveals a lower level of PlexinA2 transcripts in PA mesenchyme cells. K and L are higher magnification views of PA region in I and J. Triangle points to the splanchnic mesoderm within the SHF.
Smad4 deficiency in NCCs causes reduced expression of the Id genes and MT1-MMP that are important for extracellular matrix remodeling
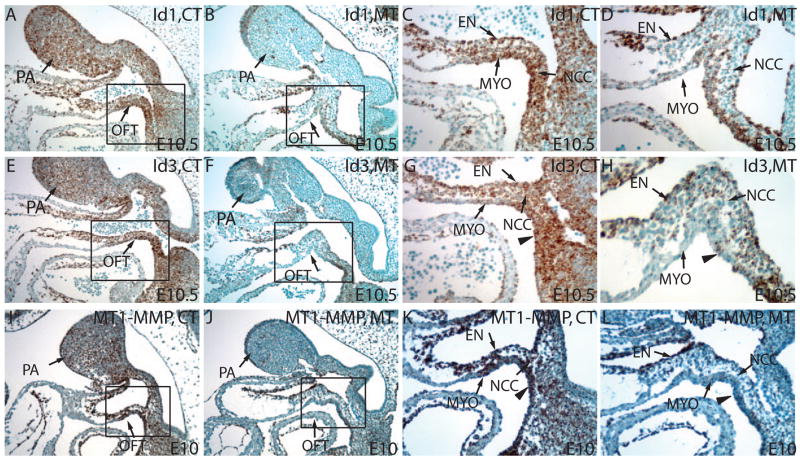
The remodeling of the cardiac OFT involves the restructuring of multiple tissues, including the vasculature. As in the remodeling of many other structures, degradation and reorganization of extracellular matrix (ECM) precedes the actual cell movement. To study if there was any misregulation of molecules in the remodeling of the cardiac OFT in mutants, we examined the expression of several genes known to regulate vascular or ECM remodeling. We found that the expression of Vegf, essential for vascular remodeling, had no significant difference between the control and mutant (data not shown). We also examined the expression of Ids that have been indicated as targets of Tgfβ signaling (Sakurai et al., 2004; ten Dijke et al., 2003) and as important factors in tissue remodeling (Benezra, 2001). By RNA In situ hybridization, we found that Id1 mRNA had wide spread expression in endocardium, myocardium, OFT cushion mesenchyme, and the PA areas (Fig. 7A,C). In the mutants, the expression of Id1 was abolished in the PA area and in the OFT cushion mesenchyme where normally a large number of NCCs reside (Fig. 7B,D). The expression of Id1 persisted in the endocardium and myocardium in the mutant (Fig. 7B,D). The expression domain of Id3 in this region was similar to that of Id1 except for the myocardium where Id3 expression was considerably less prominent (Fig. 7E,G). Similar to Id1, expression of Id3 in the mutant was also selectively decreased in NCCs within PAs and within the OFT cushion, but not in the adjacent tissues (Fig. 7F,H). Id2 expression level was also down regulated in PAs in mutants, though its normal expression level was much lower compared to Id1/3 (data not shown). The reduced level of the Ids may impair the expression of surface proteinases that are critical for the remodeling of the ECM during the collective migration of vascular endothelium and the associated tissues (Fong et al., 2003; Nieborowska-Skorska et al., 2006; Sakurai et al., 2004). To test this possibility, we examined the mRNA level of MT1-MMP (membrane type1 matrix metalloproteinase), one of the most important surface proteinases for ECM remodeling. Indeed, MT1-MMP mRNA was significantly reduced in NCCs populating the pharyngeal area and the OFT compared to the control at E10 (Fig. 7I–L). Thus, the reduction of Ids and MT1-MMP in NCCs may affect the normal extracellular matrix remodeling and OFT caudal movement.
Figure 7. Smad4 deficiency in NCCs causes reduced expression of Id genes and MT1-MMP important for extracellular matrix remodeling.
(A–D) RNA In situ hybridization on paraffin section reviewed a significant reduction of Id1 expression both in PA mesenchyme cells and in OFT mesenchyme cells at E10.5. C and D are higher magnification views of the rectangular regions in A and B. (E–H) RNA In situ hybridization on paraffin section reviewed a significant reduction of Id3 expression both in PA mesenchyme and OFT mesenchyme at E10.5. G and H are higher magnification views of the rectangular regions in E and F. (I–L) RNA In situ hybridization on paraffin section showed that MT1-MMP was down-regulated in PA mesenchyme and in OFT at E10.5 in the mutants compared to the controls. K and L are higher magnification views of the rectangular regions in I and J. EN: endothelia; MYO: myocardium. Triangles point to the splanchnic mesoderm within the SHF.
Discussion
In this study, we have shown that Smad4 deficiency in NCCs causes a range of severe morphogenetic defects in the cardiac OFT, resulting in valvular regurgitation and subsequent uniform embryonic lethality at E12.5. While the valvular regurgitation is most likely caused by the severe OFT cushion hypoplasia, OFT septation and elongation defects are also observed in all mutants. The spectrum of cardiac defects caused by Smad4 deficiency in NCCs resembles those associated with cardiac NCC (CNCC) ablation in avian embryos (Hutson and Kirby, 2007), indicating a central role of Smad signaling within NCCs for cardiac development. Our data suggest that both the reduction of the number of CNCCs and the inability of the remaining CNCCs to express key regulatory genes lead to the defective remodeling of the cardiac OFT.
Smad4 is in a key position in the Tgfβ superfamily signaling network in NCCs for the normal development of the cardiac OFT
Although NCC-specific inactivation of Tgfβ superfamily receptors commonly lead to OFT septum defect and PTA, only the Wnt1Cre; Bmpr1aflox/null (Stottmann et al., 2004) model have embryonic lethality in mid gestation similar to the Wnt1Cre; Smad4flox/flox mutants in this study. The migration of NCCs to OFT is impaired in mice with NCC-specific inactivation of Alk2 (Kaartinen et al., 2004). 40% of these mice died between E14-E18, while the rest survive till birth. However, similar reduction of NCCs in the OFT was not observed in mutants with NCC-specific inactivation of TgfβR1 or Tgfβr2 (Choudhary et al., 2006; Kaartinen et al., 2004; Wang et al., 2006; Wurdak et al., 2005), all of which can survive to birth. On one hand, these observations appear to confirm that the severity of the defects correlates with the degree of reduction in the number of CNCCs. On the other hand, the presence of defects in the OFT in mice lacking obvious changes in the number of CNCCs may suggest that qualitative changes in the CNCCs are equally important for the correct patterning of the OFT and pharyngeal structures. The OFT cushion defect in mice lacking Smad4 in NCCs is among the most severe in the models mentioned above. Since Smad4 is at the converging point of transcriptional responses from different types of Tgfβ superfamily ligands and receptors, the phenotypes we observed in mice with NCCs lacking Smad4 would, to some degree, reflect the combined effects of the loss of transcriptional responses from both Tgfβ signaling and Bmp signaling. Since, as described above, the inactivation of receptors specific for Bmp signaling in NCCs appears to lead to more severe phenotypes in the development of the OFT than those specifically associated with the inactivation of Tgfβ signaling, it is likely that Bmp signaling may have a more important role in these processes. We have shown that inactivating Smad4 in NCCs inhibits the expression of the Id genes (Fig. 7). This further supports a more important role for Bmp signaling in the observed phenotypes since Bmp signals have been shown to induce the expression of the Id genes while Tgfβ signaling has the opposite effect (Kowanetz et al., 2004; ten Dijke et al., 2003).
Absence of Smad4 in NCCs results in abnormal presence of cardiomyocytes in SHF
The observation of the ectopic presence of MF20-expressing cells in the splanchnic mesoderm within the SHF caudal to the OFT is very interesting and may have a number of possible explanations. First, a signal from the NCCs may normally suppress the differentiation of splanchnic mesodermal cells into cardiomyocytes until they migrate into the OFT. Such a signal may be missing in the Smad4-deficient NCCs, leading to the premature expression of myocardium marker in the splanchnic mesoderm. Second, the Smad4-deficient NCCs may send an abnormal signal to the SHF mesodermal cells that promotes premature myocardium differentiation. In fact, from E9.5 onward, NCCs are in close contact with the splanchnic mesodermal cells within the SHF. This close contact is the basis of the intimate signaling communication proposed in the first two possibilities. The third possibility is that these MF20-expressing cells may be normally differentiating cells programmed to enter the OFT from the SHF, but are “stranded” due to a failure in the accruement of these cells into the OFT myocardium. This accruement problem can be a result of defective migrating ability in SHF cells, alteration in cell migration cues along the migratory path, or the failure of OFT caudal movement. The latter possibility arises from the fact that MF20 positive cells within the SHF are located in an area that would normally be added to the OFT myocardium when the OFT migrates caudally across the splanchnic mesoderm within the SHF (Waldo et al., 2005b; Waldo et al., 2001). It is possible that during normal development, the caudally migrating OFT recruits the SHF mesodermal cells as they are differentiating into MF20-expressing cardiomyocytes. The well orchestrated temporal sequence of the OFT caudal movement/elongation and the differentiation of the SHF mesodermal cells ensures that no MF20-expressing cells are present in the SHF. In this model, Smad4 deficiency in NCCs leads to a cascade of events, causing a failure in OFT caudal movement/elongation and, possibly, the subsequent retention of MF20-expressing cells in the SHF. Lastly, accumulating evidence suggests that mechanical forces regulate many aspects of cardiac development, including gene expression, cellular differentiation, cell movement, and structural remodeling (Bartman and Hove, 2005; Butcher and Markwald, 2007). Thus, it is also possible that the observed ectopic presence of MF20-expressing cells is secondary to hemodynamic changes caused by primary defects in other cardiac structures. More studies are needed to further distinguish these possibilities.
Smad signaling in NCCs may regulate OFT development through its effects on genes important for tissue remodeling
OFT development involves the remodeling of the vasculature and the migration of the endothelial/endocardial cells along with other cells. Our studies suggest that Smad signaling in NCCs is essential for the caudal movement of the OFT through the regulation of the expression of genes required for the remodeling of vasculature and the reorganization of the ECM. These genes include the Id gene family and the MMPs. The Id family of helix-loop-helix transcription factors have indispensable in vivo roles in angiogenesis and tissue remodeling (Abe, 2006). We have detected a drastic reduction of the expression of Id1 and Id3 in the NCCs in the pharyngeal area along the OFT movement route at as early as E9.5 (Fig. 7 and data not shown). This reduction of Id expression is caused by Smad4 inactivation in NCCs since Ids are recognized targets of Bmp signaling (Miyazono and Miyazawa, 2002). Previous studies have shown that mice lacking both Id1/Id3 die before E13.5 with vascular malformation in multiple organs, undetectable levels of αVβ3-intergrin and some MMPs, as well as obvious ECM deposition (Lyden et al., 1999). MT1-MMP, a surface proteinase induced by Ids (Fong et al., 2003; Nieborowska-Skorska et al., 2006; Sakurai et al., 2004), is also critical in preparing the migratory path within extracellular matrix during cell migration (van Hinsbergh et al., 2006). Consistent with the Id1/Id3 inactivation studies, we also detected a significant reduction of MT1-MMP in NCCs (Fig. 7) and considerable deposition of ECM along the path of OFT caudal movement in the mutant (data not shown). The reduced expression of both Ids and MMPs in NCCs along the route of OFT caudal movement would impede OFT caudal migration by increasing ECM deposition. While only the reduction of MT1-MMP is presented here, based on previous studies by other laboratories, the reduction of other MMPs in the NCCs deficient for Smad4 is likely. This may explain the more severe embryonic defects, especially in OFT remodeling, in mice lacking Smad4 in NCCs than defects found in mice lacking MT1-MMP alone (Maxwell et al., 1996), which die only after birth.
Both increased apoptosis and disrupted communications between NCCs and myocardium may be responsible for the reduced NCC contribution to the OFT cushion mesenchyme
It has been suggested before that the number of available CNCCs is critical for the normal cardiac development (van den Hoff and Moorman, 2000). The requirement of sufficient number of NCCs may derive from the need for building materials, for providing appropriate signals, or for shielding off certain interfering signals (Brown and Baldwin, 2006; Hutson and Kirby, 2007; Stoller and Epstein, 2005; van den Hoff and Moorman, 2000). We have observed a significant reduction of the total number of NCCs in the OFT cushion mesenchyme from E10.5 onward in the mutants. This reduction contributes to the observed OFT cushion hypoplasia, OFT septation defect, and the valvular regurgitation. Our data suggest that this reduction of NCCs in the OFT may be due to both a cell autonomous effect of the absence of Smad4 in NCCs for their own survival and an indirect effect through the defective communications between NCCs and other cell types, especially the OFT myocardium. The direct effect of the absence of Smad4 on NCC survival is likely mediated by the reduced expression of both Msx1 and Msx2 (Fig. 3) and slug (data not shown), all of which are known to be essential for cell survival (Inukai et al., 1999; Ishii et al., 2005). On the other hand, the defective cell-cell communication is more complicated and is likely the result of defects in a number of interconnected morphogenetic events. The expression changes in Bmp4, Sema3C, and PlexinA2 reflect the altered cell-cell signaling caused by the absence of Smad4 in the NCCs. The altered signaling among OFT myocardium, SHF, and NCCs, in turn, could cause further disruptions in NCC recruitment to OFT cushion, resulting in the observed OFT cushion hypoplasia.
In summary, this study shows that Smad signaling in NCCs coordinates the interactions of various signaling molecules and pathways in multiple cell populations during OFT development. Smad-signaling in cardiac NCCs has cell autonomous effects for their survival and non-cell autonomous effects on the proliferation/differentiation/migration of other cell lineages, most noticeably the SHF mesodermal cells, for the correct positioning and remodeling of the OFT.
Acknowledgments
We thank Dr. J. A. Epstein for advice and comments on this manuscript; Dr. A. P. McMahon and Dr. Philippe Soriano for the Wnt1Cre and the Rosa26RLacZ mice, Drs. B. Bruneau, E. N. Olson, J. A Raper, M. Inoue, R. J. Miller for various in situ probes, and B. Black for advice on β-Galactosidase immunostaining. C.P.C. is supported by grants from American Heart Association (0535239N), NIH (5R01HL085345), and the Children’s Heart Foundation. F.C. is supported in part by institutional funds from the Department of Internal Medicine/Renal Division at Washington University School of Medicine, a March of Dimes Award (FY06-343), and a NIH grant (RO1DK067386).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe J. Bone morphogenetic protein (BMP) family, SMAD signaling and Id helix-loop-helix proteins in the vasculature: the continuous mystery of BMPs pleotropic effects. J Mol Cell Cardiol. 2006;41:4–7. doi: 10.1016/j.yjmcc.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Abu-Issa R, Waldo K, Kirby ML. Heart fields: one, two or more? Dev Biol. 2004;272:281–5. doi: 10.1016/j.ydbio.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. 2004;101:6502–7. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman T, Hove J. Mechanics and function in heart morphogenesis. Dev Dyn. 2005;233:373–81. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- Benezra R. Role of Id proteins in embryonic and tumor angiogenesis. Trends Cardiovasc Med. 2001;11:237–41. doi: 10.1016/s1050-1738(01)00117-7. [DOI] [PubMed] [Google Scholar]
- Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007 doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CB, Baldwin HS. Neural crest contribution to the cardiovascular system. Adv Exp Med Biol. 2006;589:134–54. doi: 10.1007/978-0-387-46954-6_8. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007 doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–8. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci U S A. 1999;96:541–6. doi: 10.1073/pnas.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, Sucov HM. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev Biol. 2006;289:420–9. doi: 10.1016/j.ydbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. doi: 10.1002/gene.10152. [DOI] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–54. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Cellular recruitment and the development of the myocardium. Dev Biol. 2004;274:225–32. doi: 10.1016/j.ydbio.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Fong S, Itahana Y, Sumida T, Singh J, Coppe JP, Liu Y, Richards PC, Bennington JL, Lee NM, Debs RJ, Desprez PY. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci U S A. 2003;100:13543–8. doi: 10.1073/pnas.2230238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JB, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97:219–26. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–75. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–10. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai T, Inoue A, Kurosawa H, Goi K, Shinjyo T, Ozawa K, Mao M, Inaba T, Look AT. SLUG, a ces-1-related zinc finger transcription factor gene with antiapoptotic activity, is a downstream target of the E2A-HLF oncoprotein. Mol Cell. 1999;4:343–52. doi: 10.1016/s1097-2765(00)80336-6. [DOI] [PubMed] [Google Scholar]
- Ishii M, Han J, Yen HY, Sucov HM, Chai Y, Maxson RE., Jr Combined deficiencies of Msx1 and Msx2 cause impaired patterning and survival of the cranial neural crest. Development. 2005;132:4937–50. doi: 10.1242/dev.02072. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–16. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–90. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Karafiat V, Dvorakova M, Krejci E, Kralova J, Pajer P, Snajdr P, Mandikova S, Bartunek P, Grim M, Dvorak M. Transcription factor c-Myb is involved in the regulation of the epithelial-mesenchymal transition in the avian neural crest. Cell Mol Life Sci. 2005;62:2516–25. doi: 10.1007/s00018-005-5297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RG. Molecular inroads into the anterior heart field. Trends Cardiovasc Med. 2005;15:51–6. doi: 10.1016/j.tcm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–66. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Valcourt U, Bergstrom R, Heldin CH, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol. 2004;24:4241–54. doi: 10.1128/MCB.24.10.4241-4254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore JJ, Mericko PA, Cheng L, Lu MM, Morrisey EE, Parmacek MS. GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J Clin Invest. 2006;116:929–39. doi: 10.1172/JCI27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–7. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–11. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Maxwell GD, Reid K, Elefanty A, Bartlett PF, Murphy M. Glial cell line-derived neurotrophic factor promotes the development of adrenergic neurons in mouse neural crest cultures. Proc Natl Acad Sci U S A. 1996;93:13274–9. doi: 10.1073/pnas.93.23.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002;2002:PE40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Molin DG, Poelmann RE, DeRuiter MC, Azhar M, Doetschman T, Gittenberger-de Groot AC. Transforming growth factor beta-SMAD2 signaling regulates aortic arch innervation and development. Circ Res. 2004;95:1109–17. doi: 10.1161/01.RES.0000150047.16909.ab. [DOI] [PubMed] [Google Scholar]
- Nieborowska-Skorska M, Hoser G, Rink L, Malecki M, Kossev P, Wasik MA, Skorski T. Id1 transcription inhibitor-matrix metalloproteinase 9 axis enhances invasiveness of the breakpoint cluster region/abelson tyrosine kinase-transformed leukemia cells. Cancer Res. 2006;66:4108–16. doi: 10.1158/0008-5472.CAN-05-1584. [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–59. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai D, Tsuchiya N, Yamaguchi A, Okaji Y, Tsuno NH, Kobata T, Takahashi K, Tokunaga K. Crucial role of inhibitor of DNA binding/differentiation in the vascular endothelial growth factor-induced activation and angiogenic processes of human endothelial cells. J Immunol. 2004;173:5801–9. doi: 10.4049/jimmunol.173.9.5801. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–70. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–48. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, Epstein JA. Cardiac neural crest. Semin Cell Dev Biol. 2005 doi: 10.1016/j.semcdb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–18. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–13. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–52. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- van den Hoff MJ, Moorman AF. Cardiac neural crest: the holy grail of cardiac abnormalities? Cardiovasc Res. 2000;47:212–6. doi: 10.1016/s0008-6363(00)00127-9. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–28. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–55. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML. Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Dev Biol. 2005a;281:66–77. doi: 10.1016/j.ydbio.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005b;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–88. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wang J, Nagy A, Larsson J, Dudas M, Sucov HM, Kaartinen V. Defective ALK5 signaling in the neural crest leads to increased postmigratory neural crest cell apoptosis and severe outflow tract defects. BMC Dev Biol. 2006;6:51. doi: 10.1186/1471-213X-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev. 2005;19:530–5. doi: 10.1101/gad.317405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. Regulation of Smad activities. Biochim Biophys Acta. 2006;1759:503–13. doi: 10.1016/j.bbaexp.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–1. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- Yelbuz TM, Waldo KL, Kumiski DH, Stadt HA, Wolfe RR, Leatherbury L, Kirby ML. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–10. doi: 10.1161/01.cir.0000023044.44974.8a. [DOI] [PubMed] [Google Scholar]