Abstract
Background
Clinical islet transplantation is associated with loss of transplanted islets necessitating tissue from more than one donor to obtain insulin independence. The instant blood-mediated inflammatory reaction (IBMIR) is one explanation to the tissue loss. Complement activation is an important cytotoxic component of the IBMIR, and in the present study, we have investigated this component in detail.
Methods
Isolated human islets were analyzed by large particle flow cytometry and confocal microscopy after incubation in human ABO-compatible hirudinplasma.
Results
After incubation in plasma, the islets bound IgG and IgM, CIq, C4, C3 and C9. The binding of C3b/iC3b was evident already after 5 min. The binding of C3b/iC3b and the generation of C3a and sC5b-9 were inhibited by the complement inhibitor Compstatin. Lysis as reflected by propidium iodide (PI) staining and release of C-peptide was also inhibited by Compstatin. There were significant correlations between IgM/IgG versus C3b/iC3b and between sC5b-9 and C-peptide.
Conclusion
The conclusion is that complement is activated by natural IgG and IgM antibodies already after 5 min. The complement activation leads to lysis of cells of the pancreatic islets. This very rapid reaction may be an essential entity of the damage induced by the IBMIR in clinical islet transplantation.
Keywords: Human islets, Natural antibodies, Complement activation, Compstatin
Clinical islet transplantation is rapidly becoming an established procedure for the treatment of diabetics with uncontrolled hypoglycemia, but the procedure still requires the transplantation of islets from more than one donor to produce insulin independence (1). In recent studies using positron-emission tomography/computed tomography (PET/CT) technology in both humans and in pigs, we have demonstrated that less than 50% of the transplanted islets engraft after 2 hr and that the majority of the tissue loss has already occurred during infusion of the islets (2, 3). This rapid tissue loss is consistent with the Edmonton group’s demonstration that the functional capacity of the transplanted islets from up to four donors corresponds to only about 20% to 30% of that found in a nondiabetic person (4). Together, these reports indicate that only a small fraction of the transplanted islets successfully engraft. The long-term consequences of this marginal engraftment and drastically reduced β-cell mass may explain the observation that most of the patients receiving transplants according to the Edmonton protocol become insulin-dependent again within 2 to 3 years of transplantation (5).
The reason for this poor engraftment is complex. A major contributor to the poor outcome of clinical islet transplantation is likely to be the occurrence of the destructive instant blood-mediated inflammatory reaction (IBMIR), which leads to loss of transplanted tissue when the islets encounter the blood in the portal vein (6–8). This reaction is triggered by transcription factor expression by the endocrine cells of the islets, combined with an array of other proinflammatory events, such as the expression of monocyte chemotactic protein (MCP)-1 (9), interleukin-8, and macrophage migration inhibitory factor (MIF) (10, 11).
The very rapid destruction of the islets that we observed in our recent PET/CT studies points to the existence of other damaging reactions. The only component of innate immunity that could cause such rapid destruction is the complement system. Complement activation is an important component of the IB-MIR, and it occurs secondary to the thrombotic reaction (12). However, the involvement of a direct complement attack in allogeneic islet transplantation has been postulated by other investigators (13).
Until recently, no firm conclusions about such involvement have been possible to draw, however, because the available methods either required single-cell preparations of pancreatic islets, in which many of the cells become damaged, or used less-sensitive immunohistochemical techniques. We have now investigated complement activation and complement-mediated attack in hirudinanticoagulated (thrombin-inhibited) ABO-compatible plasma to avoid interference with the complement reaction by anticoagulants such as citrate, heparin, or EDTA. We have also used techniques that allowed us to examine whole islets, and we have verified the involvement of complement activation by using the complement inhibitor Compstatin. Our studies demonstrate that complement triggered by antibodies and the classical pathway is an important player in damaging the islet cells and further suggest that inhibition of complement may be crucial for success in allogeneic islet transplantation.
MATERIALS AND METHODS
Islets Isolation
Islets of Langerhans were isolated using a modification of the previously described semiautomated digestion-filtration method (8, 14, 15), followed by purification on a continuous density gradient in a refrigerated COBE 2991 centrifuge (COBE Blood Component Technology, Lakewood, CO). Islet volume and purity were determined by microscopic sizing on a grid after staining with diphenylthiocarbazone. The purity of the islets ranged from 40% to 80%. The protocol for isolation of human islets from cadaver donors was approved by the regional research ethics committee.
The islet preparations were cultured in CMRL 1066 culture medium (Mediatech, Inc. Herndon, VA) supplemented with 10 mM nicotinamide (Sigma Aldrich, Schnelldorf, Germany), 10 mM HEPES buffer (Invitrogen, Paisley, Scotland), 0.25 μg/mL Fungizone (Invitrogen), 50 μg/mL gentamycin (Invitrogen), 2 mM L-glutamine (Invitrogen), 10 μg/mL Ciprofloxacin (Bayer AG, Leverkusen, Germany), and 10% (v/v) heat-inactivated ABO-compatible human serum. They were kept at 37°C in humidified air containing 5% CO2. Human islets, cultured for 2 to 7 days, were collected and washed twice in cold phosphate-buffered saline (PBS).
Coating of Test Tubes With Heparin
Two-milliliters polystyrene test tubes were coated with the Corline heparin surface (Corline, Uppsala, Sweden) according to the manufacturer’s recommendation. The surface concentration of heparin was 0.5 μg/cm2, corresponding to approximately 0.1 IU/cm2, or an antithrombin binding capacity of 2 to 4 pmol/cm2. The heparinized surface has previously been shown by Andersson et al. (16) to be stable, with no leakage of heparin detected over a period of 1 hr.
Preparation of Human Hirudin-Treated Plasma
Blood was drawn from healthy blood donors into 7-mL tubes containing 500 μg of hirudin, a specific inhibitor of thrombin (Refludan; Pharmion Ltd., Cambridge, UK), to allow us to investigate complement activation in the absence of anticoagulants and activation of the coagulation system. For each experiment, blood was drawn from an individual with blood group compatibility to the islet donor. The plasma was harvested after centrifugation at 3300g for 15 min and thereafter stored at −70°C.
Treatment of Human Islets With ABO-Compatible (Allogeneic) Plasma
Approximately, 1000 human islets/40 μL (typically 5000 islets in 200 μL) of plasma were incubated in human ABO-compatible hirudin-treated plasma in heparinized test tubes. Eight donors and 14 different ABO-compatible plasma preparations were used in the experiments. In some experiments, ABO-compatible hirudin-treated plasma was preincubated in the presence or absence of 10 μM of the potent Compstatin analog, Ac-ICV(1MeW)QDWGAHRCT-NH2 (17), for 15 min at 37°C before the islets were added. The mixture of islets and plasma was incubated, with gentle shaking, at 37°C for up to 30 min. After centrifugation, the islets were immediately prepared for Complex object parametric analyzer and sorter (COPAS) analysis and confocal microscopy. After addition of EDTA (10 mM final concentration), the supernatants were stored at −70°C before analyses of C3a, sC5b-9, and C-peptide.
Preparation of Islets for Complex Object Parametric Analyzer and Sorter Analysis and Confocal Microscopy
Bound Immunoglobulins and Complement Components
Ten microliters of fluorescein isothiocyanate (FITC)-labeled antibodies recognizing the following proteins were added to 5000 islets (corresponding to approximately 10×106 cells) in 100 μL of PBS according to the manufacturer’s recommendations for single cells: C1q (1.0 g/L; AbCam, Cambridge, UK), C3c (3.2 g/L; for detection of C3b and iC3b; DakoCytomation, Glostrup, Denmark), C4 (1.3 g/L; Dako), C9 (2.6 g/L; Dako), mannose-binding lectin (MBL; 0.7 g/L; Dako), IgG (2.6 g/L; Dako), and IgM (4.0 g/L; Dako). Irrelevant mouse IgG1 (0.1 g/L; Dako) was used as a negative control.
Membrane-Bound Complement Regulators
Ten microliters of antibodies against CD46, CD55, and CD59 (BD Bioscience, Franklin Lakes, NJ) were used according the manufacture’s recommendations. All of these antibodies were FITC-conjugated except for CD59, which was conjugated with R-phycoerythrin.
Apoptotic and Necrotic Cells
Propidium iodide and Annexin V, respectively, were used: (1) Ten microliters of PI (0.5 μg PI/mL; BD Bioscience Pharmingen) was added to 5000 islets in 100 μL PBS for 5 min. (2) Ten microliters of Annexin V-FITC (BD Bioscience Pharmingen) was added to 5000 islets in 100 μL of PBS according to the manufacture’s recommendation for single cells. Islets that had not been incubated in plasma were used as controls.
For All the Immunostainings
The islets were incubated, gently rotating on ice, in the presence of each antibody for 30 min. After washing in PBS, the islets were treated with 1% formaldehyde (Apoteket, Gothenburg, Sweden) and held on ice until analysis. The antibody-, Annexin V-, and PI-stained islets were analyzed by COPAS and confocal microscopy.
Complex Object Parametric Analyzer and Sorter Analysis
The fluorescence-stained islets were analyzed using a COPAS (Union Biometrica, Somerville, MA), which is a large-particle-based flow cytometry instrument previously used for islet analysis (18). For each experiments 1000 islets were collected using a 488/514 multiline laser, and positive cells were sorted out for further analysis by confocal microscopy. The COPAS flow cytometry data were analyzed using CellQuest Pro software (BD Biosciences Immunocytometry Systems). Data were reported as mean fluorescent intensity.
Confocal Microscopy
One to two hundred hand-picked stained islets were contained in a drop of PBS in a small Petri dish and protected from light before examination in the confocal microscope (Zeiss 510 Meta confocal, Carl Zeiss, Jena, Germany). Examination of the stained islets was performed using the 488-nm laser at ×10 magnification. Counterstaining with 4′,6-diamidino-2-phenylindole was used to visualize the nuclei of living islet cells.
Insulin Secretion in Response to Glucose Stimulation Using a Dynamic Perfusion System
Islet function was tested in a dynamic-perfusion system. Before glucose stimulation, islets were prepared in two ways: (1) Thousand human islets (from six different donors) were incubated in culture medium with or without addition of 10 μM Compstatin for 24 hr; (2) Islets from the same donors as in (1) were exposed to ABO-compatible plasma without Compstatin for 30 min (as described above). As control islets, cultured islets not treated with Compstatin or plasma, were used.
For each preparation, 10 islets were perfused with 1.67 mmol/L glucose, then with 16.7 mmol/L, and again with 1.67 mmol/L. During the 120-min perfusion, fractions were collected at 6-min intervals. The concentration of insulin was analyzed using a commercial enzyme-linked immunosorbent assay (EIA) kit (Mercodia, Uppsala, Sweden).
Enzyme-Linked Immunosorbent Assays
C3a and sC5b-9 were analyzed using EIAs previously described (19). C-peptide was assessed using an EIA kit from Mercodia (Uppsala, Sweden).
Statistics
Repeated measures ANOVA followed by Bonferroni post hoc test was used for comparison between groups. For correlation test, Spearman’s rank test was used. P values less than 0.05 were considered significant.
RESULTS
Binding of Complement Components and Expression of Complement Regulators
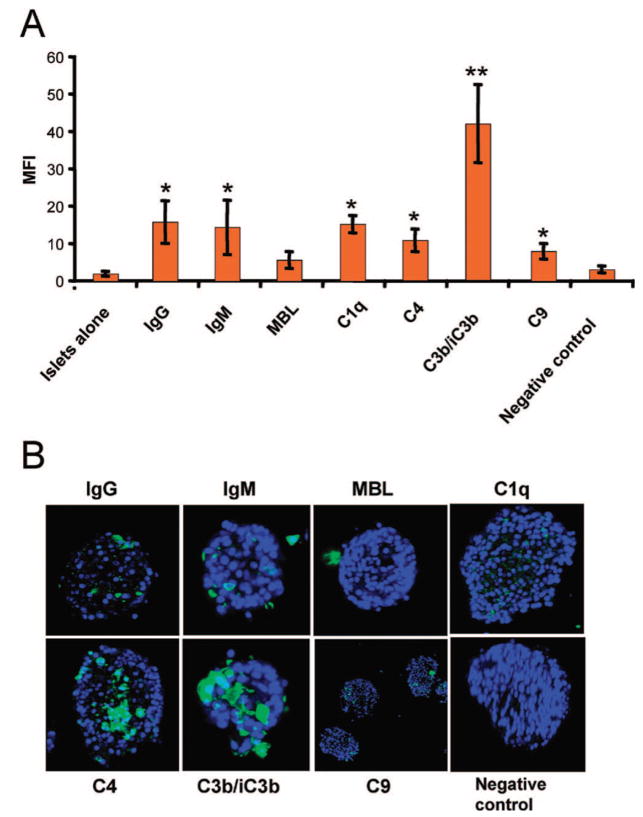
To determine whether human islets bind complement proteins after incubation in ABO-compatible hirudin-treated plasma, we stained the islets with FITC-conjugated antibodies recognizing IgG, IgM, C1q, C3b/iC3b fragments, C4, C9, CRP, and MBL. Antibodies against IgG, IgM, C1q, C4, and C3b/iC3b fragments bound strongly to the islets, but the binding of MBL and C9 was less prominent (Fig. 1A).
FIGURE 1.
Human islets (n=5) incubated in ABO-compatible hirudin-treated plasma (n=5) for 30 min. The islets were stained for IgG, IgM, MBL, C1q, C4, C3b/iC3b, C9, and negative control. Controls consisting of islets incubated without (islets alone) and with mouse isotype IgG1 (negative control) were included. The islets were analyzed by (A) COPAS and (B) confocal microscopy. All values were compared with values obtained from the negative control are given as mean±SEM (*P<0.05, **P<0.01).
Analysis by confocal microscopy revealed that the most extensive binding was seen for antibodies against C4 and C3, with C4 and C3b/iC3b fragments being found over large areas of the islets, and IgG, IgM, C1q, and C9 being detected in small, discrete spots all over the islet surface (Fig. 1B). No binding of MBL was detected by confocal microscopy.
No expression of the complement regulators MCP (CD46), DAF (CD55), and CD59 was seen on the surface of the islets, and no binding of IgG or IgM was detected on islets that had not been incubated in human plasma (not shown).
Complement Activation in the Presence and Absence of Compstatin
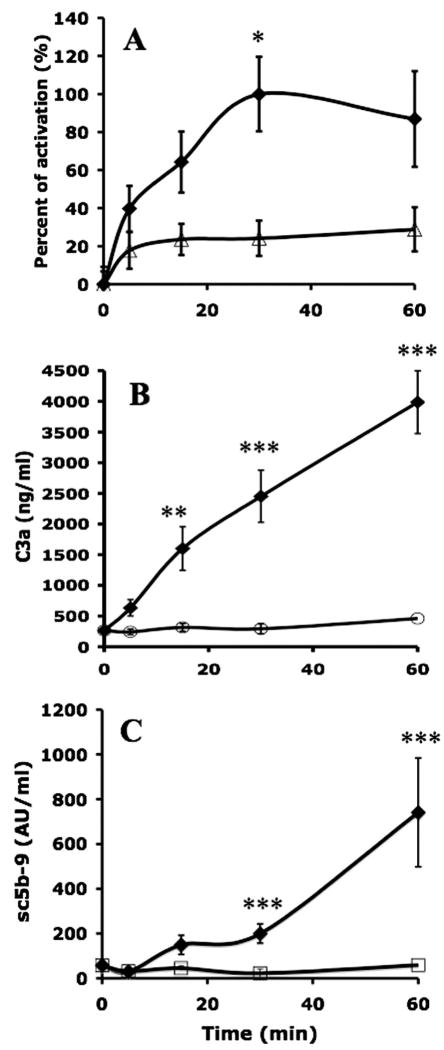
For kinetic analyses, islets were incubated in ABO-compatible plasma for 0, 5, 15, 30, or 60 min in the presence or absence of the complement inhibitor Compstatin (10 μM), and then analyzed by COPAS (Fig. 2). In the absence of Compstatin, C3b/iC3b fragments were detected on the islets after as little as 5 min, and the binding of C3b/iC3b continued to increase over time. Addition of Compstatin significantly reduced the binding of C3b/iC3b to the islets (Fig. 2A). Assessment of C3a and sC5b-9 in the supernatants of the cultured islets confirmed this picture (Fig. 2B,C).
FIGURE 2.
Human islets (n=5) incubated in ABO-compatible hirudin-treated plasma (n=5) for 30 min in the presence (open symbols) or absence (filled symbols) of 10 μM Compstatin. (A) The binding of C3b/iC3b as a percentage of the maximum value; values for the generation of (B) C3a and (C) sC5b-9 are given as mean±SEM (*P<0.05, **P<0.01, ***P<0.001).
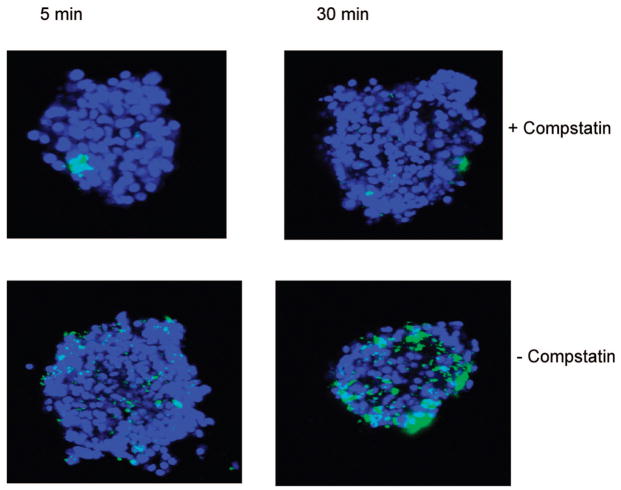
Examination of plasma-incubated islets by confocal microscopy confirmed that binding of C3b/iC3b had already occurred after 5 min, and it further demonstrated that after 30 min, the islets were covered by C3b/iC3b (Fig. 3). In contrast, addition of Compstatin to the islets totally prevented C3b/iC3b binding at both 5 and 30 min.
FIGURE 3.
Confocal microscopic images of islets incubated in ABO-compatible hirudin-treated plasma human in the presence or absence of 10 μM Compstatin at 5 and 30 min. The islets were stained for C3b/iC3b. One hundred islets from five different donors/recipient combinations were investigated.
Consequences of Complement Attack on Islet Cells
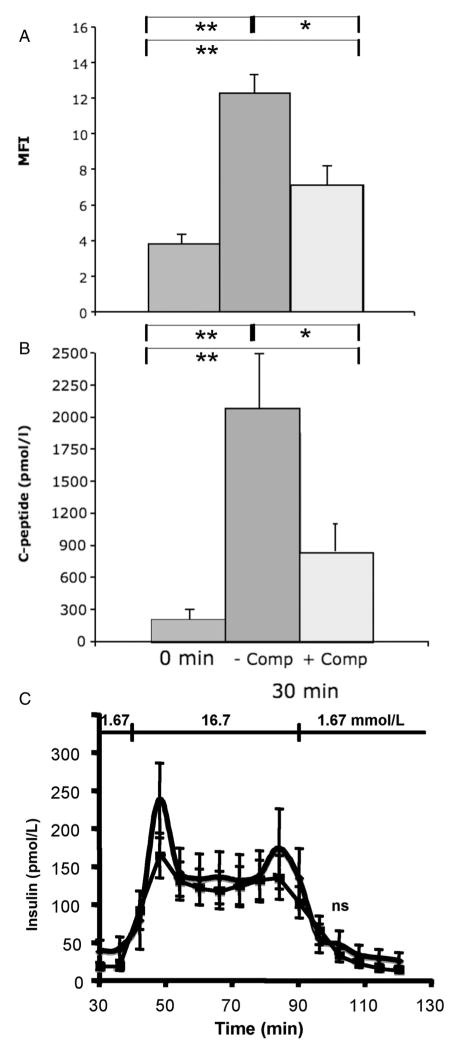
To determine whether complement activation led to lysis of islet cells, the islets were incubated in ABO-compatible plasma at 37°C and stained with PI (Fig. 4A). COPAS analysis showed a significant increase in PI staining after 30 min. This staining was significantly reduced in the presence of 10 μM Compstatin. After incubation of the islets at 37°C for 24 hr, no binding of annexin V was detected.
FIGURE 4.
Human islets incubated in ABO-compatible hirudin-treated ABO-compatible plasma for 30 min in the presence (dark grey) or absence (light grey) of 10 μM Compstatin. The islets were stained with PI (A) and analyzed by COPAS, and the presence of C-peptide in the supernatants was determined by enzyme-linked immunosorbent assay (B) (n=7; mean±SEM; *P<0.05, **P<0.01). In (C) islets incubated in the presence (squares) and absence (diamonds) of ABO-compatible plasma for 30 min were challenged by glucose and the release of insulin assessed (n=6; ns=non significant).
Similar results were obtained when the supernatants were analyzed for C-peptide as a marker for islet destruction (Fig. 4B). A significant release of C-peptide was found after incubation of the islets for 30 min at 37°C. This release was significantly reduced in the presence of 10 μM Compstatin.
Islets were also incubated in ABO-compatible plasma for 30 min and challenged with glucose in a dynamic perfusion system. Although there was a tendency towards less insulin release in the plasma-treated islets, there was no statistically significant difference between islets incubated in the presence or absence of plasma (Fig. 4C).
To exclude Compstatin toxicity, islets were cultured in the presence of 10 μM Compstatin for 24 hr and showed no significant difference in insulin release, compared with those cultured in medium alone (not shown).
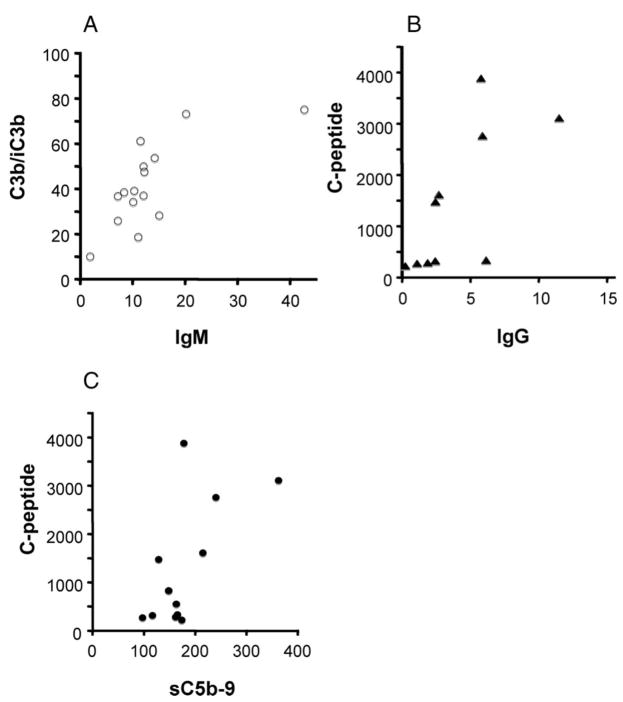
Correlation Between Binding of IgM, Complement Activation, and Release of C-Peptide
Significant correlations were found between the binding of IgM and C3b/iC3b, between IgG and C-peptide release, and between sC5b-9 and C-peptide release (Fig. 5).
FIGURE 5.
Correlation between the binding of (A) IgM and C3b/iC3b (ρ=0.67; P=0.01); (B) IgG and C-peptide (ρ=0.83; P=0.01); and (C) sC5b-9 and C-peptide (ρ=0.62; P=0.04) to the islets.
DISCUSSION
In the present study, we have characterized the complement activation triggered by human pancreatic islet preparations incubated in ABO-compatible human plasma. In line with previous results published by Titus et al. (13), we found deposition of immunoglobulins and complement fragments on the islet surface. The presence of IgG, IgM, C1q, C4, C9, and MBL was examined on intact pancreatic islets by COPAS as well as by confocal microscopy. In particular, IgG, IgM, C4, C3, and C9 gave a strong signal, whereas MBL protein was not detected. Deposition of C3b/iC3b was observed within 5 min, and after 30 min in plasma, and the islets were more or less covered with C3b/iC3b. The generation of fluid-phase complement products C3a and sC5b-9 that we observed underscores the likelihood that the deposition of complement fragments on the islet surface is the result of complement activation. Also confirming this notion was the fact that the complement inhibitor Compstatin was able to abolish the deposition of C3b/iC3b on the surface of the islets and abrogate the generation of fluid-phase complement activation products.
The presence of C9 on the islet surface suggests that the membrane attack complex (MAC) had been formed. The MAC is known to mediate cell lysis, a reaction that is a type of necrotic cell death; however, apoptosis followed by secondary necrosis has also been described (20). The staining of the islet preparations with PI and the release of C-peptide into the supernatant both support the concept that the islets were damaged by complement attack. It should be pointed out that because the islet preparations are not pure, only release of C-peptide is a specific marker of insulin-producing cells, and that damaged exocrine cells contribute to the PI staining. Also, the fact that the addition of Compstatin reduced PI staining and decreased the release of C-peptide provides further confirmation that complement-mediated lysis is involved in this damage to the islet cells.
There was no sign of apoptosis, as reflected by Annexin-V staining.
Our COPAS analysis of complement regulator expression confirmed the results of previous studies on single cells (21), which indicated that DAF (CD55), CD59, and MCP (CD46) are poorly expressed on the islet surface. This absence of regulators would explain the pronounced sensitivity of these cells to attack by complement.
The presence of significant levels of IgG, IgM, C4, C3, and C9 on the surface of the islets suggests that complement activation is mediated by immunoglobulins and proceeds through the classical pathway to the formation of MAC. Supporting this chain of events is the correlation we found (a) between the binding of IgM and the deposition of C3b/iC3b on the surface of the islets and (b) between the formation of soluble C5b-9 and release of C-peptide.
In the present study we have used blood plasma from normal ABO-compatible donors; therefore, the involvement of natural antibodies with other specificities must be considered. Such antibodies are present at low concentrations in normal blood plasma. To which antigen(s) present on the islet surface the antibodies are binding is not yet clear. Among the candidate antigens are extracellular matrix proteins, which are present on both endocrine and exocrine tissue after digestion with collagenase during the isolation procedure of the islets. One intriguing explanation for our findings would be that natural antibodies are recognizing laminin and collagen (22). Laminin is a well-known autoantigen and has been implicated in many autoimmune diseases (23, 24). Guilbert et al. (25) have shown that human sera also contain antibodies that bind to collagens. Also, natural antibodies to a number of autoantigens have been described, including thyroglobulin, DNA, tubulin, and myosin (26). Finally, oxidative stress is known to affect the lipid membrane of cells, and naturally occurring antibodies directed against these altered cell membranes can activate the complement system (27).
In our previous studies in which human islets were perfused in whole blood rather than hirudin-treated plasma, C1q, C3b/iC3b, C5b-9, IgG, and IgM were not found on the islets (7). The reason for this difference in results may be technical, reflecting the use of less-sensitive techniques in the earlier studies; however, our failure to detect these proteins more likely reflects the fact that, when incubated with whole blood, the islets become concealed by platelets and fibrin surrounding the islets. The damage that we have detected in the present investigation correlates with the extremely rapid destruction that we observed by PET/CT immediately after infusion of the islets (2, 3). These observations suggest the occurrence of a double-peaked innate immune response against the islets during allotransplantation: First, a rapid complement attack mediated by natural antibodies is elicited, followed by a secondary slower coagulation reaction triggered by tissue factor. Both these events are likely to be of significance in terms of causing the damage to the islets. The first complement attack may also contribute to the second phase by stimulating the release of tissue factor from the cytoplasm of the cells, and thus, further potentiate and amplify the IB-MIR (8).
Complement activation is of great importance in bridging innate immunity and specific immune responses. In allogeneic transplantation, C3 is one of the essential factors that trigger rejection in mice (28–30) and humans (31). The robust correlation between rejection and the deposition of C4d on the endothelial surface of transplanted organs supports this notion (32). A number of experiments have shown that binding of the C3 fragment C3dg to an antigen acts as a strong adjuvant to promote both humoral and cell-meditated immune responses. The magnitude of this adjuvant effect is similar to that of Freund’s adjuvant, and C3 fragments have been put forward as potential components of vaccines (33, 34). It is therefore reasonable to expect that complement activation and C3dg binding will trigger an immune response that can lead to an adaptive immune response against a graft.
One possible way to avoid the damage caused by complement attack would be to develop techniques to remove the natural antibodies from the plasma, but such an approach would be cumbersome and time-consuming. Instead, the identification of Compstatin as an inhibitor of the antibody-mediated complement attack on the islets makes this substance a prime candidate for eliminating complement damage to the islet graft. Compstatin is a cyclic peptide that binds to C3, inhibiting the cleavage of C3 into C3a and C3b by the convertases. This potential drug candidate has been shown to be very effective in inhibiting complement with, as far we know, no side effects (35). Studies in nonhuman primates will elucidate the usefulness of Compstatin in allogeneic islet transplantation.
Acknowledgments
The authors acknowledge Susanne Lindblom for excellent technical assistance, and Dr. Deborah McClellan for excellent editorial assistance.
This work was supported by National Institute of Health (5U01-A1065192, GM-62134, AI068730, and GM069736), Swedish Research Council Grants (2006-5595 and 15244), and by grants from the Natural Sciences Faculty, University of Kalmar.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Eich T, Eriksson O, Lundgren T. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med. 2007;356:2754. doi: 10.1056/NEJMc070201. [DOI] [PubMed] [Google Scholar]
- 3.Eich T, Eriksson O, Sundin A, et al. Positron emission tomography (PET): A real time tool to quantify early islet engraftment in a pre-clinical large animal model. Transplantation. 2007;84:893. doi: 10.1097/01.tp.0000284730.86567.9f. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 6.Berman DM, Cabrera O, Kenyon NM, et al. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation. 2007;84:308. doi: 10.1097/01.tp.0000275401.80187.1e. [DOI] [PubMed] [Google Scholar]
- 7.Bennet W, Sundberg B, Groth CG, et al. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 8.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells triggers thrombotic reactions detrimental in clinical islet transplantation. Lancet. 2002;360:2039. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 9.Piemonti L, Leone BE, Nano R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: Relevance in human islet transplantation. Diabetes. 2002;51:55. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 10.Waeber G, Calandra T, Roduit R, et al. Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1997;94:4782. doi: 10.1073/pnas.94.9.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson H, Goto M, Dufrane D, et al. Low molecular weight dextran sulfate: A strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006;6:305. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 12.Ozmen L, Ekdahl KN, Elgue G, et al. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: Possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 13.Titus TT, Horton PJ, Badet L, et al. Adverse outcome of human islet-allogeneic blood interaction. Transplantation. 2003;75:1317. doi: 10.1097/01.TP.0000064517.98252.00. [DOI] [PubMed] [Google Scholar]
- 14.Lakey JR, Warnock GL, Shapiro AM, et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8:285. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- 15.Ricordi C, Lacy PE, Finke EH, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 16.Andersson J, Sanchez J, Ekdahl KN, et al. Optimal heparin surface concentration and antithrombin binding capacity as evaluated with human non-anticoagulated blood in vitro. J Biomed Mater Res A. 2003;67:458. doi: 10.1002/jbm.a.10104. [DOI] [PubMed] [Google Scholar]
- 17.Katragadda M, Magotti P, Sfyroera G, et al. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J Med Chem. 2006;49:4616. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez LA, Hatch EW, Armann B, et al. Validation of large particle flow cytometry for the analysis and sorting of intact pancreatic islets. Transplantation. 2005;80:729. doi: 10.1097/01.tp.0000179105.95770.cd. [DOI] [PubMed] [Google Scholar]
- 19.Ekdahl KN, Nilsson B, Pekna M, et al. Generation of iC3 at the interface between blood and gas. Scand J Immunol. 1992;35:85. doi: 10.1111/j.1365-3083.1992.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonfoco E, Krainc D, Ankarcrona M, et al. Apoptosis and necrosis: Two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennet W, Bjorkland A, Sundberg B, et al. Expression of complement regulatory proteins on islets of Langerhans: A comparison between human islets and islets isolated from normal and hDAF transgenic pigs. Transplantation. 2001;72:312. doi: 10.1097/00007890-200107270-00026. [DOI] [PubMed] [Google Scholar]
- 22.Bei R, Mentuccia D, Trono P, et al. Immunity to extracellular matrix antigens is associated with ultrastructural alterations of the stroma and stratified epithelium basement membrane in the skin of Hashimotos thyroiditis patients. Int J Immunopathol Pharmacol. 2006;19:661. doi: 10.1177/039463200601900322. [DOI] [PubMed] [Google Scholar]
- 23.Blank M, Gisondi P, Mimouni D, et al. New insights into the autoantibody-mediated mechanisms of autoimmune bullous diseases and urticaria. Clin Exp Rheumatol. 2006;24(1 suppl 40):S20. [PubMed] [Google Scholar]
- 24.De-Gennaro LA, Lopes JD, Mariano M. Autoantibodies directed to extracellular matrix components in patients with different clinical forms of periodontitis. J Periodontol. 2006;77:2025. doi: 10.1902/jop.2006.060104. [DOI] [PubMed] [Google Scholar]
- 25.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779. [PubMed] [Google Scholar]
- 26.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44:103. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury P, Zhou W, Sacks SH. Complement in renal transplantation. Nephron Clin Pract. 2003;95:c3. doi: 10.1159/000073012. [DOI] [PubMed] [Google Scholar]
- 29.Peng Q, Li K, Patel H, et al. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176:3330. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 30.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 31.Brown KM, Kondeatis E, Vaughan RW, et al. Influence of donor C3 allotype on late renal-transplantation outcome. N Engl J Med. 2006;354:2014. doi: 10.1056/NEJMoa052825. [DOI] [PubMed] [Google Scholar]
- 32.Feucht HE. Complement C4d in graft capillaries—The missing link in the recognition of humoral alloreactivity. Am J Transplant. 2003;3:646. doi: 10.1034/j.1600-6143.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 33.Barrault DV, Steward M, Cox VF, et al. Efficient production of complement (C3d)3 fusion proteins using the baculovirus expression vector system. J Immunol Methods. 2005;304:158. doi: 10.1016/j.jim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Rickert RC. Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol. 2005;17:237. doi: 10.1016/j.coi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Soulika AM, Khan MM, Hattori T, et al. Inhibition of heparin/protamine complex-induced complement activation by Compstatin in baboons. Clin Immunol. 2000;96:212. doi: 10.1006/clim.2000.4903. [DOI] [PubMed] [Google Scholar]