Summary
Objective
An age-related decline in chondrocyte production of OP-1 (BMP-7) may contribute to cartilage loss in osteoarthritis. This study was designed to determine if increased methylation of the OP-1 promoter might serve as a mechanism for the age-related decline in OP-1 expression.
Methods
Human articular chondrocytes were isolated from cartilage obtained after death from tissue donors (ages 19-86 years) without a known history of arthritis. DNA was obtained from isolated chondrocytes in primary culture and analyzed for OP-1 promoter methylation by PCR after bisulfite treatment. Cultured cells were treated with the DNA methyltransferase inhibitor 5-azacytidine and OP-1 production was measured in the media by ELISA. RNA was isolated to measure expression of IGF-1, the IGF-1 receptor, aggrecan, and OP-1 by real-time PCR.
Results
Methylation of the OP-1 promoter was detected in chondrocytes isolated from tissue obtained from older adults and there was a positive correlation between age and OP-1 methylation status (n=22, R2=0.277, p=0.014). Inhibition of methylation in cultured cells with 5-azacytidine increased chondrocyte production of OP-1 protein and increased the expression of the IGF-1, the IGF-1 receptor, aggrecan, and OP-1 genes but not GAPDH.
Conclusion
Age-related methylation of the OP-1 promoter may contribute to a decrease in OP-1 production in cartilage and a decrease in expression of OP-1 responsive genes such as IGF-1, the IGF-1 receptor, and aggrecan.
Introduction
The loss of articular cartilage that is a characteristic feature of osteoarthritis (OA) is strongly associated with aging but the mechanisms by which aging contributes to the development of OA are not completely defined1. Since chondrocytes are responsible for both the synthesis and breakdown of the articular cartilage, processes which cause chondrocytes to exhibit either decreased matrix synthesis and/or increased production of matrix degrading enzymes will lead to cartilage loss. Growth factors, including insulin-like growth factor-1 (IGF-1) and osteogenic protein-1 (OP-1), are important regulators of chondrocyte anabolic activity and so alterations in the levels or activity of these growth factors with aging could contribute to an imbalance in matrix synthesis and degradation.
Aging has been associated with a decline in levels of growth factors. Although systemic levels of IGF-1 decline with aging, studies to date have not determined if IGF-1 levels in cartilage change with age. However, about a 4-fold reduction in the level of OP-1 in cartilage has been observed in adult humans between the ages of 35 and 75 years2. This was correlated with an age-related reduction in OP-1 RNA levels suggesting reduced OP-1 gene transcription with aging. An age-related reduction in the chondrocyte synthetic response to IGF-I has been observed3,4 while the synthetic response to OP-1 with aging has not been determined. Importantly, chondrocytes from older adults and from OA cartilage respond better to a combination of IGF-I and OP-1 in terms of matrix synthesis5,6 and inhibition of stimulated matrix metalloproteinase expression7. In the latter study, evidence was provided for a stimulatory effect of OP-1 on IGF-1 and IGF-1 receptor expression indicating that an age-related loss of OP-1 could potentially have a negative effect on the IGF-1 system in cartilage.
The present study was undertaken in order to determine the mechanism for the age-related reduction in expression of OP-1 by chondrocytes. Recent studies have demonstrated the importance of epigenetic regulation of gene transcription, including the role of DNA methylation which can be altered with aging8. DNA methyltransferases serve to regulate transcription of specific genes through the methylation of cytosine residues most commonly found in a 5′-CG-3′ or the “CpG” configuration. We noted that the OP-1 promoter has a large number of CpG rich regions, including 27 CG pairs in the first 250 bp 5′ to the transcription start site, and so set-out to test the hypothesis that increased methylation of the OP-1 promoter with aging could be responsible for the age-related reduction in OP-1 expression.
Methods
Tissue Acquisition and Cell Culture
In order to study normal tissue and avoid the potential effects of degenerative changes or OA, which are very common in the knees of older adults, normal articular cartilage was obtained from the ankle joints (talus) of approximately 31 tissue donors through the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL.). Tissue was also obtained from the knee joint of three donors in order to do limited ankle-knee comparisons. The donors had no known history of arthritis. Each joint was graded on a modified Collin's scale (0-4) for gross evidence of damage as described9. Only normal or nearly normal ankle tissue (grade 0 or 1) was used while the knee tissue from one donor was grade 2. Cartilage was dissected from the talus of the ankle joint or from the femur of the knee joint and cells were isolated by enzymatic digestion as previously described5. Chondrocytes were cultured in high density monolayers for 96 hours in the presence of 10% fetal bovine serum prior to DNA isolation for methylation specific PCR. In other experiments, freshly isolated chondrocytes were cultured for 120 hours in media with 10% serum in the presence of 0-10μM 5-aza-2′-deoxycytidine (5-azacytidine) (Sigma-Aldrich, St. Louis, MO) in order to inhibit DNA methylation10. We chose to treat the cells for this time period because, based on our experience with cultured human chondrocytes, this is the time when the cells will proliferate in monolayer culture after isolation and proliferation is necessary for optimal inhibition of DNA methylation by 5-azacytidine.
Analysis of OP-1 Promoter Methylation
OP-1 promoter methylation was quantitated using methylation-specific PCR with DNA collected from the short-term (96 hour) high density monolayer cultures. We were not able to design the primers for traditional bisulfite sequencing PCR due to the GC-rich sequence of the OP-1 promoter (62% GC). The following primers, interrogating 4 CG pairs, were designed to hybridize with CG pairs only if methylated:
Forward (-46 to -21): 5′GGT TTG TTG GTTGTT TTT TTT ATT C3′
Backward (+75 to +100): 5′CGA ACC CCC GCC CCC TAC TCG ATA C3′.
DNA was treated with sodium bisulfite as previously described11, amplifed with the primers, and the product quantitated using real time PCR on a Corbett Rotor-Gene real-time PCR unit (Corbett Life Sciences, Mortlake, Australia). Results were standardized to loading controls, consisting of similar amplification of an adjacent sequence lacking CG pairs:
Forward(-824 to -800);5′AAT TTT AGT TTG GTA GGT TTT GGA G 3′
Backward(-391 to -366); 5′ ATC TTA CCC CTC AAT CCC TAT ATC 3′
OP-1 ELISA
OP-1 was measured in media samples from cells treated with 5-azacytidine using an ELISA previously described in detail2. This chemiluminescent (Supersignal ELISA Femto Maximum Sensitivity Substrate from Pierce, Rockford, IL) sandwich ELISA uses two antibodies to OP-1 for detection and purified human recombinant OP-1 (Stryker Biotech, Hopkinton, MA) as standard and is sensitive down to the pg/ml range of OP-1. The data, expressed as relative light units, were obtained using a chemiluminescent ELISA plate reader. ELISA assays were performed in quadruplicate and results were normalized to the total DNA in each sample well measured using the picogreen assay as previously described5.
Real time-PCR for IGF-1, IGF-1R, OP-1, and aggrecan
Total cellular RNA was isolated using the RNeasy® Mini kit (Qiagen). Reverse transcription (RT) was carried out with 2μg total cellular RNA using the ThermoScript™ RT-PCR System (Invitrogen, Carlsbad, CA) for the first strand cDNA synthesis. The cDNA was amplified using the MyiQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Relative mRNA expression was determined using the ΔΔCT method, as detailed by the manufacturer's guidelines (Bio-Rad). GAPDH was used as a control and was not observed to change with the treatments tested. The primer sequences used are listed in Table 1.
Table 1.
Primer sequences for Real-time PCR
| Gene | Primer sequences(forward/reverse)(5′-3′) | Size(bp) | Annealing temperature(°C) | Reference Accession no. |
|---|---|---|---|---|
| GAPDH | TCGACAGTCAGCCGCATCTTCTTT GCCCAATACGACCAAATCCGTTGA |
148 | 55 | EMBL:NM_002046 |
| Aggrecan | TCTTGGAGAAGGGAGTCCAACTCT ACAGCTGCAGTGATGACCCTCAGA |
89 | 55 | EMBL:NM_013227 |
| IGF-1 | TGGTCCTGGAGTTGGTAGATTGCT CACCCATGCATTTGTGGCTCTTGA |
124 | 55 | NM_000618.2 |
| IGF-1R | TGATCCTGGATGCGGTGTCCAATA TGGTCTTCTCACACATCGGCTTCT |
111 | 55 | NM_000875.3 |
| OP-1 | AGGGCTGGCTGGTGTTTGACAT AGAGCTGCAGGCCCAGGTTGT |
81 | 58 | NM_001719.1 |
Results
OP-1 DNA methylation was evaluated using DNA samples from untreated chondrocyte cultures analyzed by methylation specific PCR, using primers designed for the OP-1 promoter. This revealed methylated DNA in articular chondrocyte samples isolated from a 74-year old tissue donor but not in a chondrocyte DNA sample from a 20-year old donor (Fig.1A). In contrast, primers specific for unmethylated DNA amplified the sample from the young donor and resulted in a weak band in the sample from the old donor (Fig.1A) but the primers tested were not found to give consistent and reliable results. Therefore, only methylation-specific primers were used for further analysis. Samples of articular chondrocyte DNA from an additional 20 normal appearing ankle joints were analyzed and, as expected with human samples, there was heterogeneity in the levels of OP-1 promoter methylation but a positive correlation between age and the ratio of methylated to unmethylated DNA was found (Fig.1B).
Fig. 1.
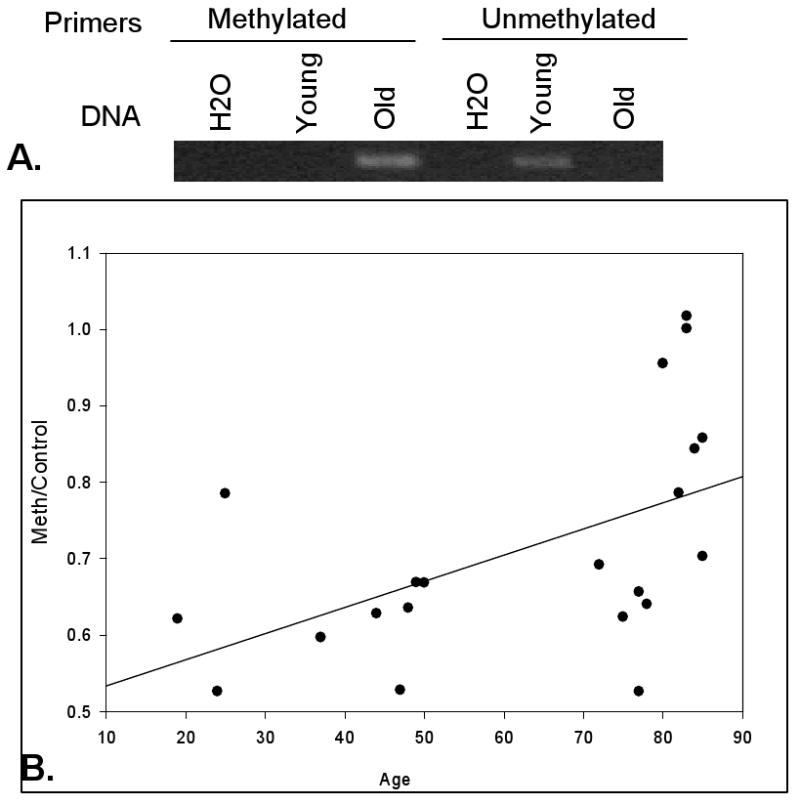
Age-related increase in methylation of OP-1 promoter DNA. (A). Methylation specific PCR for OP-1 using DNA isolated from cultures of young (20 years-old) and old (74 years-old) donor chondrocytes. DNA was amplified using primers recognizing sequences corresponding to methylated and unmethylated regions of bisulfite treated DNA. Samples with H2O substituted for DNA were used as a control to exclude primer-dimer formation. (B). Relationship of age to OP-1 promoter methylation status. The graph shows the ratio of PCR products obtained using the methylation specific primers normalized to results with primers from the unmethylated region (n=22, R2=0.277, p=0.014).
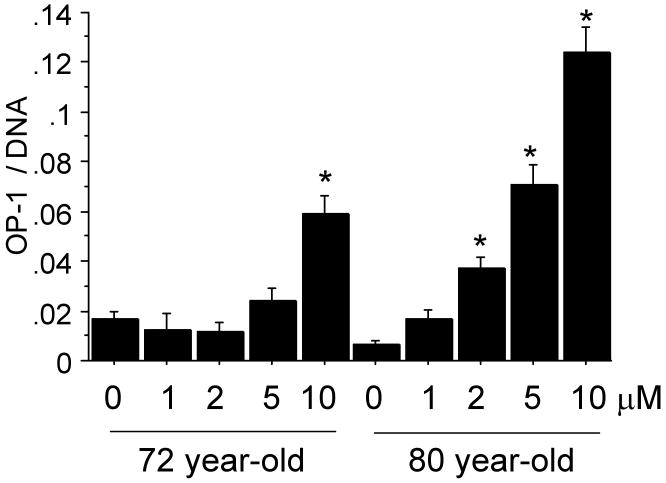
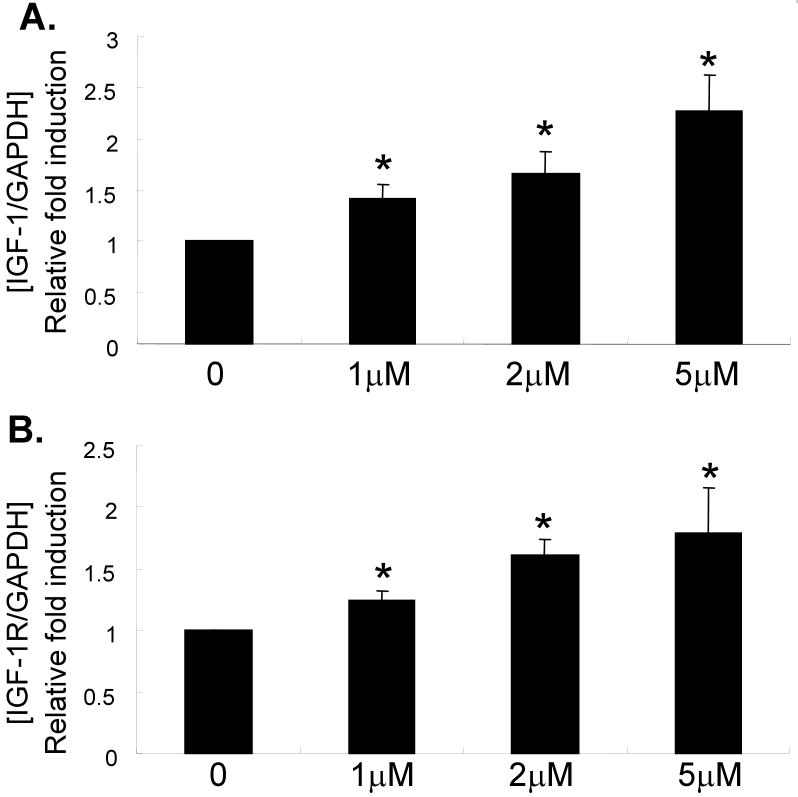
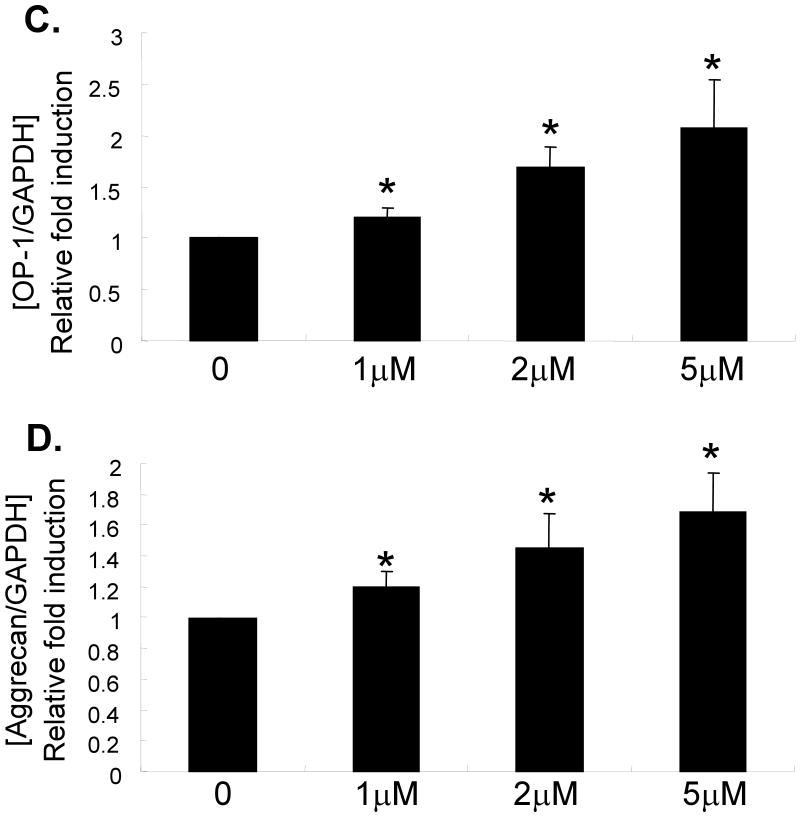
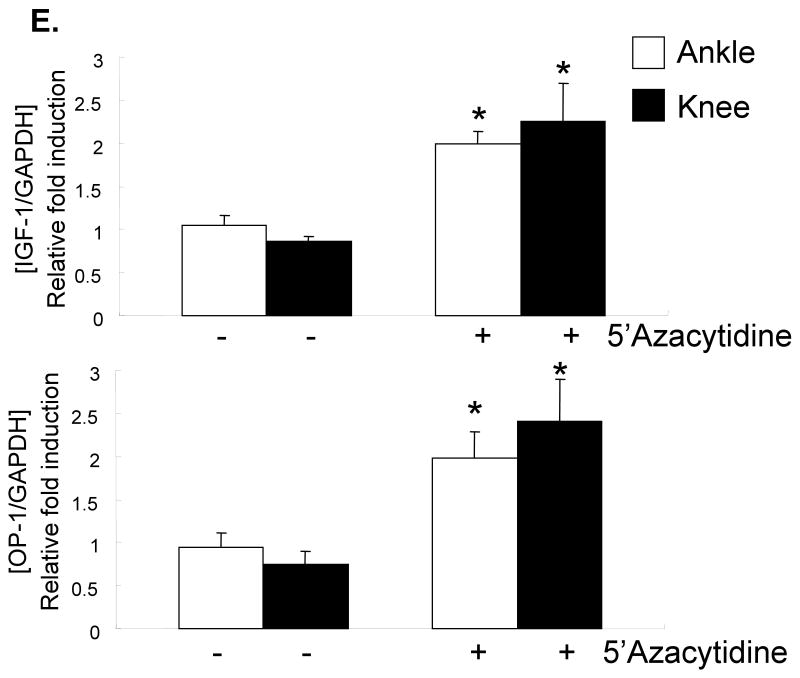
Chondrocyte cultures from 72 year-old and 80 year-old donors, treated with 0-10μM 5-azacytidine, were used to measure OP-1 production by ELISA. There was a dose dependent increase in OP-1 production indicating that inhibition of DNA methylation resulted in increased OP-1 expression (Fig.2). This was further tested by treating cells from 6 additional older (71-82 years) donors and measuring RNA levels by real-time PCR with primers specific for OP-1 and aggrecan, as well as IGF-1 and the IGF-1 receptor, genes we had previously shown7 were regulated by OP-1. Treatment with 5-azacytidine increased expression of IGF-1, the IGF-1 receptor, OP-1 and aggrecan (Fig.3A-D). Chondrocytes also obtained from the knee joints of 2 donors were compared to those from ankle joints and similar effects of 5-azacytidine on IGF-I and OP-1 expression were observed (Fig.3E).
Fig. 2.
Treatment of chondrocytes with 5-azacytidine (0-10μM) increases the production of OP-1. An ELISA was used to measure OP-1 levels in the media from cells of two different donors aged 72 and 80 years. OP-1 results were normalized to the DNA content measured in the cell-layer. *p<0.001 vs control
Fig. 3.
Effects of 5-azacytidine on chondrocyte gene expression. Primary chondrocytes isolated from donors with ages of 71-82 years were treated in culture with the indicated doses of 5-azacytidine for 120 hours. RNA was isolated and used for real-time-PCR with primers specific for IGF-1, the IGF-1 receptor (IGF-1R), OP-1, aggrecan, or GAPDH as a control. (A-D) Results using cells cultured from the ankle joint (n=6) and (B) a comparison of cells cultured from ankle (n=7) and knee cartilage (n=2). *p<0.05.
Discussion
To our knowledge this is the first report of DNA methylation in the OP-1 (BMP-7) promoter. The finding that OP-1 promoter methylation was increased in cells from older adults and that inhibition of methylation in cultured cells increased OP-1 expression are consistent with our hypothesis that increased OP-1 promoter methylation can be responsible for an age-related decrease in OP-1 expression previously found in chondrocytes2. Because OP-1 is a potent anabolic growth factor for articular chondrocytes, an aging-related increase in OP-1 promoter methylation that leads to decreased expression may contribute to cartilage loss seen with aging and in particular with the progression of OA in older adults.
Although there is accumulating evidence that altered DNA methylation may contribute to the aging phenotype8, 13, very few studies have examined the role of DNA methylation in the regulation of chondrocyte gene expression14. Changes in DNA methylation were not found to directly regulate aggrecan expression in normal aged or OA cartilage15. However, Roach et al16 reported that a decrease in the methylation of promoter sites in several matrix degrading enzymes (MMP-3, MMP-9, MMP-13, and ADAMTS-4) could be detected in OA chondrocytes. Similar to our findings, this latter study also noted a high degree of variability in promoter methylation in both the normal older adult cartilage samples and the OA samples.
It is not unexpected to find that some promoters exhibit increased methylation while others are decreased in the same cell-type or even the same disease state since multiple factors control methylation of specific promoters. These factors include interactions of the promoters with transcription factors which may influence subsequent methylation or demethylation. For example, it is possible that demethylation of the MMP promoters observed in OA chondrocytes is related to cytokine-stimulation of NFκB14 which can bind MMP promoters but would not be expected to interact with the OP-1 promoter. In addition, regional modification of histones may also play a role17.
Another interesting finding in the present study was that IGF-1, IGF-1 receptor, and aggrecan RNA levels appeared to increase after inhibition of DNA methylation. Although these cells do not produce enough IGF-1 in culture to measure IGF-I protein levels (unpublished observation), the finding was reproducible at the RNA level in cultures established from 6 older donors. Although we did not examine the IGF-1 promoter using methylation specific PCR, the effect was not likely due to a direct effect of demethylation of the IGF-1 promoter since it does not contain CpG islands. Because OP-1 can upregulate IGF-1 expression, as well as IGF-1 receptor and aggrecan expression, it is possible that the increase in all three after 5-azacytidine treatment was due to stimulation by OP-1. Further studies will need to be performed to investigate the mechanism and consequences of OP-1 promoter methylation in chondrocytes. This work could provide important information needed to understand the link between aging, reduced growth factor activity in cartilage, and the development of OA.
Acknowledgments
Supported by NIH grants AG-16697 (RL), AG-47654 (SC), AR-42525 (BR), ES-15214 (BR), AG-25877 (BR), AR-53220 (H-JI) and Stryker Biotech grant SC-001 (SC). We would like to thank the Gift of Hope Organ and Tissue Donor Network and Dr. Arkady Margulis for providing human donor tissues and Arnavaz Hakimiyan, Xin Li, and Ailing Wu for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loeser RF., Jr Aging Cartilage and Osteoarthritis--What's the Link? Sci Aging Knowledge Environ. 2004;2004:PE31. doi: 10.1126/sageke.2004.29.pe31. [DOI] [PubMed] [Google Scholar]
- 2.Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002;1588:126–134. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 4.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 6.Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, et al. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2007;15:421–430. doi: 10.1016/j.joca.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278:25386–25394. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2:245–261. doi: 10.1016/s1568-1637(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 9.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 10.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Richardson B. Methods for Analyzing the Role of DNA Methylation and Chromatin Structure in Regulating T Lymphocyte Gene Expression. Biol Proced Online. 2004;6:189–203. doi: 10.1251/bpo89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soder S, Hakimiyan A, Rueger DC, Kuettner KE, Aigner T, Chubinskaya S. Antisense inhibition of osteogenic protein 1 disturbs human articular cartilage integrity. Arthritis Rheum. 2005;52:468–478. doi: 10.1002/art.20856. [DOI] [PubMed] [Google Scholar]
- 13.Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23:413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Roach HI, Aigner T. DNA methylation in osteoarthritic chondrocytes: a new molecular target. Osteoarthritis Cartilage. 2007;15:128–137. doi: 10.1016/j.joca.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Poschl E, Fidler A, Schmidt B, Kallipolitou A, Schmid E, Aigner T. DNA methylation is not likely to be responsible for aggrecan down regulation in aged or osteoarthritic cartilage. Ann Rheum Dis. 2005;64:477–480. doi: 10.1136/ard.2004.022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach HI, Yamada N, Cheung KS, Tilley S, Clarke NM, Oreffo RO, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 17.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]