Abstract
Background
The aging process occurs at variable rates both among and within species and may be related to the variability in oxygen consumption and free radical production impacting oxidative stress. The current study was designed to test whether nonagenarians have a relatively low metabolic rate and whether it is associated with low levels of oxidative stress relative to age.
Methods
Resting metabolic rate (RMR) and markers of oxidative stress to lipids, proteins, and DNA were measured in three groups of individuals aged 20–34 (n = 47), 60–74 (n = 49), and ≥90 years (n = 74).
Results
RMR, adjusted for fat-free mass, fat mass, and sex, was lower in both older groups when compared to the young group (p ≤ .0001). There were no significant differences in urinary isoprostanes, serum protein carbonyls, or DNA fragmentation between groups, and RMR was not related to any markers of oxidative stress.
Conclusions
This study confirms an age-related decline in RMR independent of changes in body composition but surprisingly did not show an accumulation of oxidative damage with increasing age. Our data challenge the theory that RMR is a significant determinant of oxidative stress and therefore contributes to the aging process.
There are currently more than 300 theories attempting to explain the aging process (1). However, there is still no agreement regarding the causes or mechanisms of biological aging. Two complementary, closely related theories are the Rate of Living Theory and the Free Radical/Oxidative Stress Hypothesis of Aging. Combined, these theories state that free radicals produced during cellular metabolism react with biomolecules to produce oxidative damage, which accumulates with age (2,3). There is support for both of these theories. For example, free radical production and metabolic rate are inversely related to life span (4,5). However, there is a discrepancy in the results between intra- and interspecies animal models (2,5–7). In addition, it is now well known that free radicals have important roles in various physiological functions (8). It appears that only when free radicals are in excess (excessive production and/or insufficient removal), can they accumulate and cause damage to cells and tissue contributing to disease and the aging process (8). However, it is unknown if there is a relationship between resting metabolic rate (RMR) and oxidative stress in humans and whether these are related to the aging process.
Reactions with oxygen can produce a number of free radicals known as reactive oxygen species (ROS) (9–12). ROS attack lipids, proteins, and DNA generating a number of products that affect normal cell functioning (13–15). However, organisms have adapted a number of mechanisms to protect from free radical production (10,16). The function of many proteins, such as antioxidant enzymes, includes removing free radicals from cells and tissues (11). Recently, uncoupling proteins (UCPs) have been shown to be activated in response to excessive free radicals in the mitochondrial matrix (17). By inducing uncoupling, UCPs help to reduce the release of electrons from the electron transport chain, thereby attenuating the production and accumulation of free radicals (18). However, despite evolved defense systems, cross-sectional studies demonstrate an accumulation of oxidative stress with age in both animals and humans (19–21)
Early studies reported a direct relationship between metabolic rate and oxidative damage and a negative relationship with both of these to maximum life span across species (22,23). In contrast, Speakman and colleagues (7) reported a positive relationship between oxygen consumption and maximum life span in mice, and Selman and colleagues (24) showed that calorically restricted rats exhibited energy expenditures higher than predicted on the basis of body weight. However, unlike in humans, it is notoriously difficult to measure metabolic rate in rodents, and results do not differentiate RMR from thermogenesis and physical activity, all components of total daily energy expenditure (TDEE) (26). Free radical production from the electron transport chain is highest in the resting state (RMR) when the demand for energy is low (27,28). Therefore, one would assume that free radical production and oxidative stress would be related specifically to RMR rather than to TDEE. Unfortunately, there are very few studies that have investigated the relationship between energy expenditure and oxidative stress and none that have examined the relationship with RMR in humans and have looked at the effect of age on this relationship (29).
Individuals achieving extreme old age (> 90 years) have been thought of as “models of successful aging” (30). A few studies have documented that centenarians and nonagenarians may be protected against oxidative stress (31–33). However, to our knowledge, no one has examined both RMR and oxidative damage in the oldest old. Therefore, the purpose of this study was to test whether “healthy” nonagenarians have relatively low metabolic rates compared to old (60- to 74-year-old) and young (20- to 34-year-old) individuals and whether such a low metabolic rate is associated with relatively low levels of oxidative damage.
METHODS
Participants included in this study represent a subset from an ongoing population-based study called the Louisiana Healthy Aging Study (LHAS) initially funded by the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund and now by a Program Project from the National Institute on Aging. The overall aim of the LHAS is to determine whether characteristics of an individual’s metabolism predispose to long (or not so long) life with the retention of physical and cognitive functionality that is associated with healthy aging. A total number of 877 participants will be included in the main project, which is related to the genetics of aging and longevity. After individuals are screened for the main project, eligibility is then determined for other projects including the present study (metabolic rate and oxidative stress) and studies of physical and cognitive functionality. Randomly selected individuals (from voter registration lists and the Medicare Beneficiary Enrollment Data File from The Center of Medicare and Medicaid Services) from a 40-mile area surrounding Baton Rouge, Louisiana, were invited to participate in the study. After potential participant lists were randomly generated, study staff attempted to obtain contact information. Potential participants were first contacted by letter extending an invitation to participate in the study, which was then followed up with a phone call. In many instances, potential participants were not able to be contacted, e.g., contact information was unattainable, individuals had moved, or there was no response from letter or phone calls. Despite that, we have experienced an approximate 20% enrollment rate for the overall study thus far, varying considerably by decade of age. Reasons given for individuals not wanting to participate include not having time or not wanting to come to the testing facility. For this specific study, individuals were excluded if they had been diagnosed with diabetes or had elevated fasting blood sugar (> 125 mg/dL), thyroid disease, unstable cardiovascular disease, or mental health problems requiring drug treatment. Nonagenarians were excluded if they had a heart attack or stroke in the 3 months prior to testing; had severe high blood pressure or blood vessel aneurysm; were taking certain medications used for myasthenia gravis; or had uncontrolled asthma, an asthma-like condition, or emphysema/chronic obstructive pulmonary disease (COPD). According to study design and the above eligibility criteria, 170 individuals were available for data analysis. Data were collected in three groups of individuals aged 20–34 (20M/27F), 60–74 (26M/23F), and ≥90 years (38M/36F). The study was approved by the Institutional Review Board of the Pennington Biomedical Research Center, and participants provided written informed consent.
Body Composition and RMR
Weight was measured in a gown (and without shoes and without jewelry and/or a watch), to ±0.1 kg with an electronic scale (Detecto, Webb City, MO) that was checked daily with standard weights. Body composition was measured using dual-energy x-ray absorptiometry (DXA) (QDA 4500A; Hologics, Bedford, MA), and fat-free mass (FFM) and fat mass (FM) were calculated from weight and percent body fat. RMR was measured for 30 minutes following a 12-hour overnight fast using a ventilated-hood Deltatrac II metabolic cart (Sensormedics, Yorba Linda, CA). Participants were required to rest in a reclined position for 30 minutes before the start of the test, and the last 20 minutes of the 30-minute measure were used to calculate energy expenditure. The cart was calibrated before each test using room air and a known calibration gas concentration with 96% oxygen and 4% carbon dioxide.
Thyroxine and Triiodothyronine Concentrations
Fasting serum total thyroxine (T4) and total triiodothyronine (T3) levels were measured using immunoassays (DPC 2000; Diagnostic Product Corporation, Los Angeles, CA).
Lipid Damage Measured by Urinary Isoprostanes
Urinary isoprostanes are derived from the free radical oxidation of arachidonic acid, and its concentration in urine has been accepted as a marker of oxidative stress as previously described (34). Briefly, urine samples were collected the morning of the test day and prepared by adding 10 ng of [18O2]15-F2t-IsoP-M to 3 mL of water, then 0.25 mL of urine and sufficient 1 N HCl to acidify the sample to a pH=3. High-performance liquid chromatography (HPLC) was carried out on a 30-mL sample using a Surveyor MS Pump from ThermoFinnigan (San Jose, CA) with a mobile phase consisting of solvent A (5 mM ammonium acetate with 0.1% acetic acid) and solvent B (acetonitrile/methanol, 95:5). F2-IsoP-M was chromatographed on a Magic C18AQ, 3 µm 100 Å column (Michrom BioResources, Auburn, CA). Mass spectrometry was performed using a ThermoFinnigan TSQ Quantum triple quadrupole mass spectrometer (San Jose, CA) equipped with standard electrospray ionization. The inter-assay coefficient of variation was 13%, while the day-to-day coefficient of variation was 5%.
Protein Damage Measured by Protein Carbonyls
ROS react with proteins to produce cross-links and carbonyl derivatives (16). The carbonyl content in proteins was determined using a modified 2,4-dinitrophenylhydrazine (DNPH) assay according to the method of Mates and colleagues (35). Briefly, serum was treated with acidified DNPH. Proteins were then precipitated by trichloric acid and centrifuged. The pellet was dissolved in guanidine hydrochloride, and any insoluble material was removed by additional centrifugation. The carbonyl content was calculated from peak absorbance at 355–390 nm, using an absorption coefficient ε of 22,000 M−1 cm−1 (Beckman, Brea, CA). Results were expressed as nanomoles per milligram of protein. Total protein concentration was determined by the method of Bradford (36). The intra- and inter-assay coefficients of variation were 4.7% and 8.5%, respectively.
DNA Damage Measured by Single Cell Gel Electrophoresis (Comet Assay)
Single cell gel electrophoresis is a sensitive method to examine DNA damage and repair at individual cell level. Modification of the method with the use of repair enzymes, such as those used to repair damage produced by ROS, have proved useful in assessing oxidative damage to DNA. DNA fragmentation was measured by single cell gel electrophoresis as described by Deutsch and colleagues (38). Whole blood cells were washed with phosphate-buffered saline and dimethyl sulfoxide and centrifuged. The blood pellet was suspended in low-melting-point agarose and spread onto two commercially available slides (Trevigen, Gaithersburg, MD), and the slides were treated as per the manufacturer’s instructions. Two slides were prepared for each blood sample, one for performing the standard Comet assay for detecting single-stranded breaks and abasic sites in DNA, and one for measuring oxidized DNA bases such as 8-deoxyguanine revealed by the Fpg (Escherichia coli formamidopyrimidine-DNA glycosylase) FLARE (Fragment Length Analysis using Repair Enzymes) assay. The Fpg enzyme solution was added to the agarose on the slide for the FLARE assay and placed at 37°C for 55 minutes. The slide for the Comet assay was placed in a drawer at room temperature during this time. All slides were then immersed in alkaline buffer, washed, and subjected to electrophoresis for 10 minutes at 17 volts. The slides were then fixed, stained with SYBR green dye (Trevigen), and viewed under an ultraviolet microscope (Nikon Microphot FXA, Hamamatsu high resolution, 512 lines, Image I AT software, FITC 3 filter). The extent of DNA damage was determined by calculating the comet tail moment, which is the integrated density in the comet tail multiplied by the distance from the center of the nucleus to the center of mass of the tail of 25 cells using freely available software (Herbert M Geller; http://www2.umdnj.edu/~geller/lab/comet.htm). Because the rapidly changing intensities of individual cells are difficult to control, a large variance within each experiment is unavoidable (39). Therefore, a normalizing and variance-stabilizing logarithmic transformation was applied to the calculated tail moments.
Although there are no agreed upon reference values for the comet assay, we performed a reliability assay by measuring DNA damage in duplicate samples from 20 individuals twice as well as separate samples collected from the same participants on consecutive days. The inter- and intra-class correlation coefficients were 0.95 and 0.95, respectively, and the inter- and intra-assay coefficients of variation were 0.2% and 3.7%, respectively. We also compared DNA damage in whole blood (whole nucleated cells) and isolated lymphocytes in 20 samples. Bland–Altman analysis revealed no significant differences in DNA fragmentation using isolated lymphocytes or whole blood. The coefficient of variation was 2.9%.
Statistical Analysis
Data in the text, Table 1, and Figure 2 are provided as means ± standard error of the mean. Data analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC). A total of 170 participants were included in the analysis. However, isoprostane data were available in only 74 participants, whereas protein carbonyl data were available in 119 individuals. Multiple regression analysis was used to adjust RMR for FFM, FM, and sex. Residual values represent the difference between the measured and predicted values (see RMR section). Analysis of variance was used to assess differences between groups. Linear regression models were used to assess associations between RMR and markers of oxidative stress. Multiple regression analysis was also used to determine which factors best predicted indices of oxidative damage. Finally, analysis of covariance was used to test whether current or past history of smoking or the presence of cancer influenced the relationship between RMR and markers of oxidative stress.
Table 1.
Physical Characteristics of the Participants
| Characteristic | 20–34 Years 20 Men/27 Women |
Young vs Aged (p Value) |
60–74 Years 26 Men/23 Women |
Aged vs Nonagenarians ( p Value) |
≥ 90 Years 38 Men/36 Women |
Young vs Nonagenarians ( p Value) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 27 ± 1 | 27 ± 1 | — | 69 ± 1 | 68 ± 1 | — | 92 ± 1 | 92 ± 1 | — |
| [21–34] | [21–34] | [60–74] | [61–74] | [89–97] | [90–98] | ||||
| Weight, kg | 91.6 ± 5.0 | 72.3 ± 3.8* | NS | 90.5 ± 2.6 | 76.3 ± 2.7* | < .0001 | 71.8 ± 1.3 | 60.1 ± 2.2* | < .0001 |
| [61.2–140.7] | [52.6–127.3] | [70.8–128.2] | [50.2–95.3] | [56.4–93.3] | [36.5–88.5] | ||||
| Height, cm | 170 ± 1 | 163 ± 1 | NS | 177 ± 1 | 160 ± 1 | NS | 170 ± 1 | 157 ± 1 | NS |
| [150–190] | [148–182] | [165–187] | [143–169] | [162–181] | [147–172] | ||||
| BMI, kg/m2 | 29.1 ± 1.5 | 27.2 ± 1.4 | NS | 29.4 ± 0.9 | 28.6 ± 0.7 | < .0001 | 25.1 ± 0.4 | 24.4 ± 0.8 | .0003 |
| [21.1–47.6] | [18.8–49.5] | [24.4–43.7] | [21.3–35.0] | [20.4–30.4] | [16.1–38.7] | ||||
| Percent fat | 21.6 + 2.1 | 34.4 ± 1.5* | .0002 | 27.4 ± 1.1 | 39.0 ± 1.1* | NS | 27.5 ± 0.8 | 34.3 ± 1.2* | .02 |
| [9.4–41.2] | [19.0–47.4] | [17.8–43.7] | [24.8–45.8] | [17.6–37.2] | [19.1–47.7] | ||||
| FFM, kg | 68.5 ± 2.2 | 46.3 ± 1.6* | NS | 65.3 ± 1.6 | 46.4 ± 1.7* | < .0001 | 52.1 ± 0.8 | 38.3 ± 0.9* | < .0001 |
| [49.0–85.8] | [36.4–67.4] | [51.8–85.3] | [30.9–71.0] | [41.0–64.4] | [26.9–51.7] | ||||
| FM, kg | 20.5 ± 2.9 | 25.9 ± 2.4 | 0.02 | 25.2 ± 1.8 | 30.0 ± 1.5 | < .0001 | 19.7 ± 0.8 | 21.4 ± 1.5 | NS |
| [6.9–55.9] | [10.8–60.3] | 16.9–56.0 | [15.3–41.1] | [11.8–29.6] | [7.6–42.2] | ||||
| Men and women combined | |||||||||
| RMR (kcal/d) | 1587 ± 50* | < .0001 | 1465 ± 37* | < .0001 | 1165 ± 20* | < .0001 | |||
| RMR† | 104 ± 20 | < .0001 | −24 ± 20 | .24 | −53 ± 15 | < .0001 | |||
| T3 (ng/mL) | 164 ± 6 | .0001 | 135 ± 4 | .80 | 134 ± 4 | < .0001 | |||
| T4 (ng/mL) | 8.8 ± 0.3 | .04 | 8.0 ± 0.2 | .04 | 7.4 ± 0.2 | < .0001 | |||
Notes: Values are means ± standard error of the mean with ranges in brackets.
Sex differences.
Adjusted for FFM, FM, and sex.
BMI = body mass index; FFM = fat-free mass; FM = fat mass; T3 = total triiodothyronine; T4 = fasting serum total thyroxine; RMR = resting metabolic rate; NS = not significant.
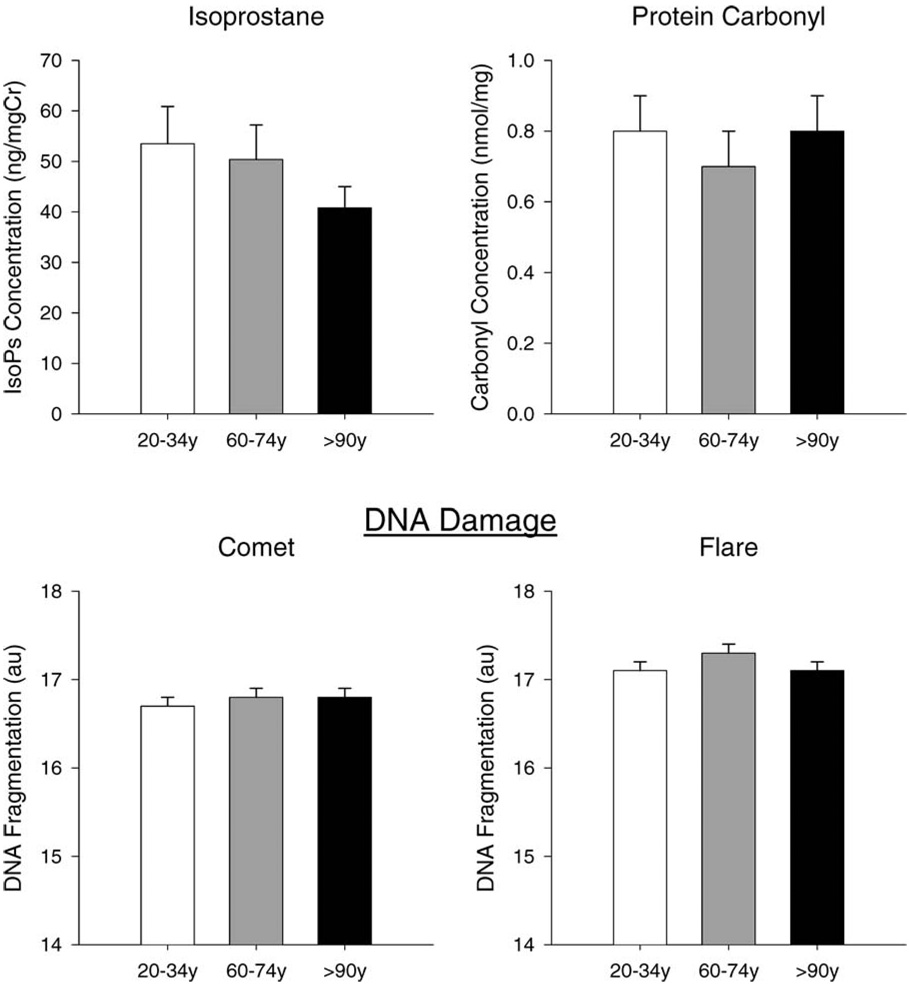
Figure 2.
Markers of oxidative stress in the three different age groups. DNA fragmentation, n = 170; protein carbonyls, n = 119; urinary isoprostanes, n = 74. There were no significant differences in isoprostanes, protein carbonyls, or DNA fragmentation by the Comet or Fragment Length Analysis using Repair Enzymes (FLARE) assay between the three age groups.
RESULTS
Body Composition
Participant characteristics by age and sex are displayed in Table 1. The three groups will be referred to as young (20–34 years), aged (60–74 years), and nonagenarians (≥90 years). Three of the young individuals were current smokers, and four had a history of smoking. Two of the aged individuals were current smokers, and seven had a history of smoking. None of the nonagenarians were currently smoking, but 13 had a history of smoking. Three of the nonagenarians had been diagnosed with prostate cancer, two with breast cancer, one with ovarian cancer, and seven had been treated for skin cancer. Analysis of covariance revealed that neither smoking status nor the presence or history of cancer influenced relationships between the study variables. Nonagenarians weighed less than the young and aged groups (p < .001), resulting in lower body mass index (p < .003). There were also differences in percent body fat between the young and aged groups (p < .0002) and between the young group and the nonagenarians (p = .02). As expected, nonagenarians had significantly less FFM (p < .0001) than the two younger groups. However, the aged group had more FM than the young group (p = .02).
RMR
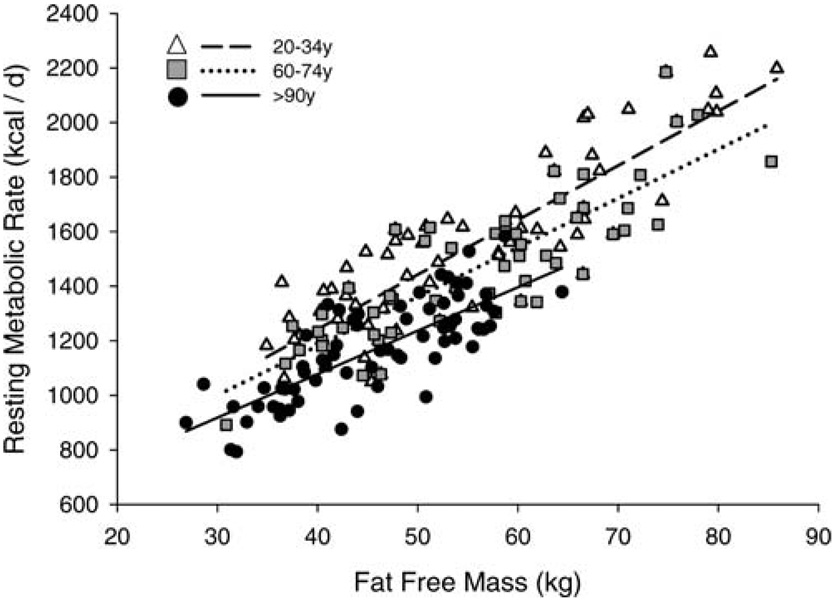
Absolute resting metabolic values are also displayed in Table 1. As expected, absolute RMR was significantly different between the three groups (p < .0001). RMR was related to FFM (Figure 1) and sex. After adjustment for FFM, FM, and sex, RMR was lower in the aged group and the nonagenarians than in the young group (p < .0001). There was, however, no difference in RMR between the aged group and the nonagenarians. RMR was related to total serum T3 concentrations (r=0.31, p < .0001), and T3 was significantly lower in the aged group and in the nonagenarians (p < .0001) compared to the young group. Serum total T4 concentrations were significantly different among all three age groups.
Figure 1.
Relationship between resting metabolic rate (RMR) and fat-free mass (FFM). There was a significant effect of age (p < .001) on the relationships between RMR and FFM.
Markers of Oxidative Stress
Values for markers of oxidative stress to lipids, proteins, and DNA are displayed in Figure 2. There were no significant differences between men and women in any markers of oxidative stress. Unexpectedly, there were no differences in urinary isoprostane concentrations (p = .2), serum protein carbonyl concentrations (p = .3), or spontaneous DNA damage (comet) (p = .3) among the three age groups. There was a trend for increased DNA damage measured by the FLARE assay in the aged participants compared to either nonagenarians or young individuals (both, p = .1). Contrary to our hypothesis, there were no relationships between absolute or adjusted RMR and any of the markers of oxidative stress. In addition, multiple regression analysis revealed that RMR, adjusted for FFM, FM, and sex, was not a determinant of the variation in urinary isoprostanes, serum protein carbonyls, or DNA fragmentation.
DISCUSSION
To our knowledge, the Louisiana Healthy Aging Study is the first study to examine the relationship between RMR and oxidative stress in humans. The results from this study support previous research that RMR declines with age even after adjustment is made for differences in body weight, body composition, and sex. However, this study does not provide evidence for an accumulation in oxidative stress with age, nor does it support the theory that energy metabolism is directly involved in the aging process through the production of free radicals and accumulation of oxidative damage to lipids, proteins, and DNA.
There is still considerable controversy over whether oxidative damage increases with age. Although a number of studies have been conducted in animals, studies in humans are too scarce to conclude whether an accumulation of oxidative damage also occurs with aging. In addition, the accumulation of oxidative stress seems to be dependent on the choice of markers of oxidative stress as well as the type of tissue studied. Both factors make it difficult to come to a conclusion on the effect of age on oxidative stress. For example, oxidative damage to DNA and proteins was increased in skeletal muscle of older patients and was decreased in blood cells after calorie restriction (21,25,40). In contrast, data regarding lipid peroxidation are more confusing. Studies using malondialdehyde (MDA) as a marker of lipid peroxidation have indicated an increase in lipid peroxidation as a result of increasing age (41,42). However, data from the Framingham Study actually observed a decrease in isoprostanes, a more reliable marker of lipid peroxidation, in individuals ranging from 33 to 88 years old (43).
In the current study, there were no differences in any markers of oxidative stress among the three groups. Such results directly contradict the well accepted theory that oxidative stress increases with age. There was a trend for the aged individuals to have more DNA damage than either of the other groups; this trend supports the idea that nonagenarians may be protected from oxidative damage to DNA. In healthy individuals, the accumulation of oxidative stress may be small and may require larger studies to observe age-related differences. In addition, one of the requirements of the study was that individuals had to be able to come to the testing facility to be tested. Participants with diabetes were also excluded. Therefore, it is possible that the study design selected healthier older participants and that this may mask or dilute any differences between groups or relationships that otherwise would have been detected. Furthermore, it is recognized that the accumulation of oxidative stress is greater in postmitotic tissues (such as skeletal muscle and brain) than in other cell types with faster turnover rates (such as blood), which may partially account for the fact that there were no differences detected between groups with regard to protein carbonyls in serum (20). However, a recent study in rats reported that 8-OhdG significantly increased with age in peripheral lymphocytes as well as in heart, skeletal muscle, brain, liver, and intestine (44). The current study used the Comet assay in whole blood, a generally accepted method to assess DNA fragmentation in humans, but did not reveal any differences between groups. Our validation studies of the assay provide a strong reliability of the test and also show that using only lymphocytes or all nucleated cells in blood did not change the outcome.
There is still disagreement as to whether RMR declines with age independent of changes in body weight and body composition and whether such a decrease contributes to the aging process and determination of life span. Recent studies in a group of mice demonstrated that those in the upper quartile of metabolic intensity had 17% greater resting oxygen consumption and lived 36% longer than animals in the lower quartile (7). However, the measurement of RMR in animals, especially rodents, is notoriously difficult when compared to measurement in humans in whom strict cooperation can be obtained during the procedure. Therefore, it may not be possible to apply conclusions from rodent studies to humans when it comes to aging and energy metabolism. In humans, earlier observations of a decline in RMR with age were later supported by reports of lower RMR in older individuals when compared to younger individuals even after adjusting for FFM (45–48). Others have reported that the decline in RMR may be due to the decline in cell mass only. The decline in physical activity with age has also been implicated as a potential cause for the reduction in RMR with age, even if there is no clear relationship between RMR and maximal oxygen consumption, an index of the level of physical activity (49,50).
In the current study, RMR was lower in each of the older groups compared to the young group, and this situation persisted after adjustment for body weight and composition. The relationship between RMR and total T3 serum concentrations suggests that one of the determinants of the variability in RMR may be the activity of the thyroid axis. Possible other reasons for this decline in RMR with aging include lower sympathetic nervous system (SNS) activity, lower sensitivity to the activity of the SNS, smaller organ size, or lower overall energy intake. Although neither were measured in the present study, previous studies have indicated that SNS activity is related to energy expenditure and that the decline in RMR in older men may be directly due to a decline in energy intake (49–52).
Contrary to our hypothesis, RMR was not a significant determinant of urinary isoprostane concentrations, serum protein carbonyl concentrations, or DNA fragmentation. Loft and colleagues (29) found a positive relationship between 24-hour oxygen consumption and urinary excretion of 8-oxodG in women. However, free radical production does not necessarily increase in direct proportion to total oxygen consumption because electron leak is not constant across tissues. In addition, free radical production is highest in the resting state when adenosine triphosphate (ATP) demand is low (53). Therefore, one would expect a direct relationship between markers of oxidative stress and RMR rather than total energy expenditure, which is the sum of RMR, the thermic effect of food, and the energy needed to sustain physical activity as observed by Loft and colleagues (29). These discrepancies indicate that a number of factors, not just energy expenditure, contribute to the production of free radicals.
While our data in a large cohort covering the ages from 21 to 98 years do not directly support the Rate of Living/oxidative stress hypothesis, they do not refute it. Although there was no relationship between RMR and markers of oxidative stress, nonagenarians had significantly lower RMR with no significant differences in oxidative stress. Could the lower RMR in nonagenarians be an adaptive response in an effort to attenuate the age-related accumulation of oxidative stress? This question could not be answered by the present study design. Furthermore, our cross-sectional study design lends itself to limitations because it is impossible to know who from the younger individuals will live beyond 90 years. Because we did not directly measure free radical production, antioxidant concentrations, or antioxidant intake, it is impossible to determine if older individuals produce more free radicals but also have an enhanced capacity to defend against them. In addition, we did not control for the presence of some diseases of aging or medication usage (hypertension and dyslipidemia). Recent data indicate that many disease states are associated with oxidative stress and that the medications used to treat these conditions can also influence oxidative stress; these factors may have affected the current results (54,55). In short, prospective studies of the role of metabolic rate as a determinant of the level of oxidative stress later in life, or even better of mortality, are needed to answer the relevance of the “Rate of Living Theory” of aging. Such studies would indicate whether individuals with a lower metabolic rate throughout life have less oxidative damage and therefore live longer.
Summary
The current study supports previous findings of an age-related decline in RMR that cannot be fully explained by changes in FFM or FM. Organ size and tissue size/metabolism determinations may warrant further research to investigate precise mechanisms underlying the age-related decline in RMR. Interestingly, nonagenarians may be protected from the age-related increase in oxidative damage to DNA, but the reduced RMR could not be directly implicated in the mechanism of oxidative stress because no relationship was found between metabolic rate and oxidative damage.
ACKNOWLEDGMENTS
This research was supported by the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund [HEF(2001–06)-02], by the National Institute on Aging (P01AG022064), and by the National Institute of General Medical Sciences (GM42056 and GM15431).
Members of the Louisiana Healthy Aging Study: Meghan Allen, Arturo M. Arce, Mark A. Batzer, Lauri O. Byerley, Pauline Callinan, Cathy M. Champagne, Katie E. Cherry, Yu-wen Chiu, James P. DeLany, Melissa J. deVeer, Devon A. Dobrosielski, Andrea Ermolao, Elizabeth T. Fontham, Paula J. Geiselman, Valentina Greco, Sibte Hadi, Tiffany Hall, Karri Hawley, Scott W. Herke, Hui-Chen Hsu, Sangkyu Kim, Beth Kimball, Christina King-Rowley, Kim Landry, Li Li, Hui-Yi Lin, Kay Lopez, John D. Mountz, Emily Olinde, Kim Pedersen, Jennifer C. Rood, Henry Rothschild, Ryan A. Russell, Donald Scott, Jennie Silva, Nicole Standberry, L. Joseph Su, Jessica Thomson, Crystal Traylor, Cruz Velasco-Gonzalez, Jerilyn A. Walker, Xui Yun Wang, Michael A. Welsch, David A. Welsh, Robert H. Wood, and Pili Zhang.
We acknowledge and thank everyone working on the Louisiana Healthy Aging Study from four sites, i.e., the Pennington Biomedical Research Center, Louisiana State University in Baton Rouge; Louisiana State University Health Sciences Center in New Orleans; and the University of Alabama in Birmingham. Most of all, we thank all the participants who enrolled in the Louisiana Healthy Aging Study.
REFERENCES
- 1.Austad SN. Theories of aging: an overview. Aging (Milano) 1998;10:146–147. [PubMed] [Google Scholar]
- 2.Greenberg JA. Organ metabolic rates and aging: two hypotheses. Med Hypotheses. 1999;52:15–22. doi: 10.1054/mehy.1997.0619. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- 5.Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1985;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- 6.Adelman R, Saul RL, Ames BN. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci U S A. 1988;85:2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speakman JR, Talbot DA, Selman C, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 9.Arnaiz SL, Travacio M, Llesuy S, Boveris A. Hydrogen peroxide metabolism during peroxisome proliferation by fenofibrate. Biochim Biophys Acta. 1995;1272:175–180. doi: 10.1016/0925-4439(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 10.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 11.Strobel HW, Coon MJ. Effect of superoxide generation and dismutation on hydroxylation reactions catalyzed by liver microsomal cytochrome P-450. J Biol Chem. 1971;246:7826–7829. [PubMed] [Google Scholar]
- 12.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton ML, Guo Z, Fuller CD, et al. A reliable assessment of 8-oxo-2-deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA. Nucleic Acids Res. 2001;29:2117–2126. doi: 10.1093/nar/29.10.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow J, Hill K, Burk R, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University Press; 1999. [Google Scholar]
- 17.Murphy MP, Echtay KS, Blaikie FH, et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- 18.Brand MD. Uncoupling to survive? The role of mitochondria in-efficiency in human ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 19.Fano G, Mecocci P, Veccheet J, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton ML, Van Remmen H, Drake JA, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mecocci P, Fano G, Fulle S, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;3–4:303–308. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 22.Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- 23.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selman C, Phillips T, Staib JL, et al. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev. 2005;126:783–793. doi: 10.1016/j.mad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravussin E, Swinburn BA. Energy metabolism. In: Stunkard AJ, Wadden TA, editors. Obesity: Theory and Therapy. New York: Raven Press; 1993. pp. 97–123. [Google Scholar]
- 27.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 29.Loft S, Astrup A, Buemann B, Poulsen HE. Oxidative DNA damage correlates with oxygen consumption in humans. FASEB J. 1994;8:534–537. doi: 10.1096/fasebj.8.8.8181672. [DOI] [PubMed] [Google Scholar]
- 30.Hitt R, Young-Xu Y, Silver M, Perls T. Centenarians: the older you get, the healthier you have been. Lancet. 1999;354:652. doi: 10.1016/S0140-6736(99)01987-X. [DOI] [PubMed] [Google Scholar]
- 31.Hyland P, Duggan O, Turbitt J, et al. Nonagenarians from the Swedish NONA Immune Study have increased plasma antioxidant capacity and similar levels of DNA damage in peripheral blood mononuclear cells compared to younger control subjects. Exp Gerontol. 2002;37:465–473. doi: 10.1016/s0531-5565(01)00216-9. [DOI] [PubMed] [Google Scholar]
- 32.Klapcinska B, Derejczyk J, Wieczorowska-Tobis K, Sobezek A, Sadowski-Krepa E, Danch A. Antioxidant defense in centenarians (a preliminary study) Acta Biochim Pol. 2000;47:281–292. [PubMed] [Google Scholar]
- 33.Paolisso G, Tagliamonte MR, Rizzo MR, Manzella D, Gambardella A, Varricchio M. Oxidative stress and advancing age: results in healthy centenarians. J Am Geriatr Soc. 1998;46:833–838. doi: 10.1111/j.1532-5415.1998.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 34.Davies SS, Zackert W, Luo Y, Cunningham CC, Frisard M, Roberts LJ., 2nd Quantification of dinor, dihydro metabolites of F(2)-isoprostanes in urine by liquid chromatography/tandem mass spectrometry. Anal Biochem. 2006;348:185–191. doi: 10.1016/j.ab.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Mates JM, Perez-Gomez C, Olella L, Segura JM, Blanca M. Allergy to drugs: antioxidant enzymic activities, lipid peroxidation and protein oxidative damage in human blood. Cell Biochem Funct. 2000;18:77–84. doi: 10.1002/(SICI)1099-0844(200006)18:2<77::AID-CBF851>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 36.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 37.Waterman MR. Spectral characterization of human hemoglobin and its derivatives. Methods Enzymol. 1978;52:456–463. doi: 10.1016/s0076-6879(78)52050-8. [DOI] [PubMed] [Google Scholar]
- 38.Deutsch WA, Krukraja A, Shane B, Hegde V. Phenobarbital, oxazepam and Wyeth 14,643 cause DNA damage as measured by the Comet assay. Mutagenesis. 2001;16:439–442. doi: 10.1093/mutage/16.5.439. [DOI] [PubMed] [Google Scholar]
- 39.Lovell DP, Thomas G, Dubow R. Issues related to the experimental design and subsequent statistical analysis of in vivo and in vitro comet studies. Teratog Carcinog Mutagen. 1999;19:109–119. [PubMed] [Google Scholar]
- 40.Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–1400. doi: 10.1016/j.exger.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Mecocci P, Polidori MC, Troiano L, Cherubini A, Cecchetti R, Pini G. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 42.Mutlu-Turkoglu U, Ilhan E, Oztezcan S, Kuru A, Aykac-Toker G, Uysel M. Age-related increases in plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in elderly subjects. Clin Biochem. 2003;36:397–400. doi: 10.1016/s0009-9120(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 43.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 44.Wolf FI, Fasanella S, Tedesco B, et al. Peripheral lymphocyte 8-OHdG levels correlate with age-associated increase in tissue oxidative DNA damage in Sprague-Dawley rats. Protective effects of caloric restriction. Exp Gerontol. 2005;40:181–188. doi: 10.1016/j.exger.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa S, Shimabukawa M, Iwaki M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGandy RB, Barrows CH, Spanies A, et al. Nutrient intakes and energy expenditure in men of different ages. J Gerontol. 1966;21:581–587. doi: 10.1093/geronj/21.4.581. [DOI] [PubMed] [Google Scholar]
- 47.Rothenberg EM, Bosaeus IG, Steen BC. Energy expenditure at age 73 and 78—a five year follow-up. Acta Diabetol. 2003;40 Suppl 1:S134–S138. doi: 10.1007/s00592-003-0046-6. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan L, Zurlo F, Ravussin E. Aging and energy expenditure. Am J Clin Nutr. 1991;53:821–825. doi: 10.1093/ajcn/53.4.821. [DOI] [PubMed] [Google Scholar]
- 49.Schulz LO, Nyomba BL, Alger S, Anderson TE, Ravussin E. Effect of endurance training on sedentary energy expenditure measured in a respiratory chamber. Am J Physiol. 1991;260(2 Pt 1):E257–E261. doi: 10.1152/ajpendo.1991.260.2.E257. [DOI] [PubMed] [Google Scholar]
- 50.Van Pelt RE, Dinneno FA, Seals DR, Jones PP. Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. Am J Physiol Endocrinol Metab. 2001;281:E633–E639. doi: 10.1152/ajpendo.2001.281.3.E633. [DOI] [PubMed] [Google Scholar]
- 51.Saad MF, Alger SA, Zurlo F, Young JB, Bogardus C, Ravussin E. Ethnic differences in sympathetic nervous system-mediated energy expenditure. Am J Physiol. 1991;261(6 Pt 1):E789–E794. doi: 10.1152/ajpendo.1991.261.6.E789. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz RS, Jaeger LF, Veith RC. The thermic effect of feeding in older men: the importance of the sympathetic nervous system. Metabolism. 1990;39:733–737. doi: 10.1016/0026-0495(90)90109-p. [DOI] [PubMed] [Google Scholar]
- 53.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 54.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 55.Freeman DJ, Norris J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–362. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]