Abstract
Objective
We previously reported that men with schizophrenia had reduced volumes of the posterior nasal cavity bilaterally. Since the nasal cavities develop in conjunction with both the palate and ventral forebrain, this could represent a simple marker of embryological dysmorphogenesis contributing to schizophrenia. The current study expands on this finding by examining a larger sample of both male and female patients and unaffected 1st-degree relatives, to determine the gender distribution of this abnormality and the extent to which it may be genetically mediated.
Method
A measurement of nasal volume and geometry was acquired by acoustic rhinometry for 85 schizophrenia patients, 25 unaffected first-degree relatives of schizophrenia probands and 66 healthy comparison subjects.
Results
Male patients had smaller posterior nasal volumes than both male control subjects and male relatives. However, female patients did not differ from either female controls or female family members. Unaffected first-degree relatives did not differ from same-sex control subjects. These findings persisted after covarying for height and smoking history, and were unrelated to clinical symptomatology or antipsychotic medication usage.
Conclusion
Posterior nasal cavity volume decrement appears to be a specific developmental craniofacial abnormality that may reflect an early disruption in embryological development in males with schizophrenia. Although further study is needed, this may be a marker of a “second hit” that distinguishes genetically vulnerable men who go on to develop the illness from those who do not.
Keywords: schizophrenia, nasal volume, neurodevelopment, embryogenesis, genetic vulnerability
1. Introduction
Schizophrenia is thought to be a neurodevelopmental brain disorder, but without a clear etiology or pathognomonic biological markers. Neurodevelopmental disorders (e.g., Down’s syndrome, velo-cardio-facial syndrome) often include abnormalities of both cerebral and craniofacial morphogenesis, as these are intimately linked during embryological development. In schizophrenia, there is a small increase in gross midline abnormalities such as cleft palate and cavum septum pelucidum and, inconsistently, evidence of subtle craniofacial dysmorphogenesis (Waddington et al., 1999b). Recent studies in our laboratory have identified anatomical and functional deficits in the olfactory neural system in patients (Moberg et al., 1999; Turetsky et al., 2000, 2003a). Since olfactory structures develop in conjunction with both the palate and ventral forebrain, we hypothesized that there might be structural abnormalities of the nasal cavity, which could represent specific markers of embryological dysmorphogenesis underlying schizophrenia.
Our initial investigation (Moberg et al., 2000), which included only men, found that male patients had reduced posterior nasal cavity volumes, which could not be explained by differences in stature, smoking, or vascular engorgement of the nasal tissue. Embryologically, the nasal cavities develop through invagination of the nasal placodes, which are derived from neural crest tissue. This occurs between the 6th and 11th weeks of embryogenesis, in conjunction with palatal and nasal septal fusion. Although subsequent development results in overall enlargement, there is no substantial reshaping of the cavities (Larsen, 2001). The etiology of the schizophrenia nasal volume decrement, and when it occurs, are both unknown. However, given the time course of nasal cavity development and its overlap with a period of substantial embryological risk for schizophrenia (Cantor-Graae et al., 1994; Gallagher et al., 1999; Hulshoff et al., 2000), this volume decrement could denote a disturbance in early embryological development that contributes to schizophrenia vulnerability.
The fact that 1st-degree relatives of schizophrenia patients exhibit a pattern of olfactory and craniofacial abnormalities similar to that observed in patients (Deutsch et al., 2000; Roalf et al., 2006) suggests that this may be an endophenotypic marker of a genetic “first hit” that leaves an individual vulnerable to subsequent pathology (Maynard et al., 2001). However we do not know whether nasal cavity abnormalities are, in fact, present in either women with schizophrenia or unaffected family members. If this is a genetically mediated endophenotype, then we might expect to observe similar deficits in both of these groups. We have now addressed this question by examining nasal cavity volume in a larger sample that included both male and female patients and controls, as well as unaffected 1st-degree relatives of both genders.
2. Methodology
2.1 Subjects
The sample included 85 patients with DSM-IV diagnosis of schizophrenia, 66 healthy comparison subjects, and 25 unaffected 1st-degree relatives of schizophrenia patients. These 176 subjects were derived from 156 independent families. All healthy comparison subjects were unrelated individuals. Among the patients, there were two mother-son pairs who were both affected. The unaffected family members consisted of 10 parents, 14 siblings and 1 child of a schizophrenia proband, derived from 19 independent families. The patient sample included the affected probands from 12 of these 19 families. Patients and family members were recruited from the outpatient psychiatric facilities at the Hospital of the University of Pennsylvania and through community outreach at community mental health centers and family support programs. All patients were stable outpatients at the time of testing. Control subjects were recruited through advertisements in community newspapers and neighborhood bulletin boards.
All subjects received a semi-structured psychiatric interview (DIGS, Diagnostic Interview for Genetic Studies) and the Family Interview for Genetic Studies (FIGS). Patients were excluded for any concurrent Axis I diagnosis other than schizophrenia. Healthy comparison subjects were excluded for any history of an Axis I diagnosis, Axis II Cluster A (schizotypal, schizoid, or paranoid) personality disorder, or family history of an Axis I psychotic disorder. Family members were excluded for any Axis I psychotic disorder or prodromal symptoms of a psychotic illness, but were not excluded on the basis of an Axis II diagnosis. Across the three groups, subjects were excluded for any history of a neurological disorder, including head trauma with loss of consciousness, any lifetime history of substance dependence, history of substance abuse within the preceding six months, or any medical condition that might affect cerebral functioning. Subjects were excluded for any obvious cranio-facial trauma or abnormality, including septal deviation, and for any acute respiratory condition, cold, or allergy. Written informed consent was obtained after the procedures had been fully explained.
Demographic characteristics of the three groups are presented in Table 1. There were significant differences in gender [χ2(2)=6.82, p=.03] and racial composition [χ2(2)=23.20, p=.00001] across the three groups. The family member sample had a greater preponderance of women, and the patient sample had proportionally more African Americans, than either of the other two groups. The groups also differed in age [F(2,173)=6.24, p=.002]. In post-hoc comparisons, patients were significantly younger than both family members (p<.0001) and controls (p=.03). The latter two groups did not differ from each other (p=.17). There was an overall group difference in smoking status [χ2(2)=11.33, p=.003]. As expected, the patient sample contained more active smokers than the control sample [χ2(1)=9.51, p=.002]. However there were no differences between unaffected family members and either patients [χ2(1)=2.42, p=.12] or controls [χ2(1)=0.37, p=.54]. Importantly, the three groups were equivalent in height [F(2,173)=0.42, p=.66], a measure that is typically used to account for any nasal volume differences that can be attributed simply to differences in physical size.
Table 1.
Sample Characteristics mean (±sd)
| Patients |
Controls |
Relatives |
|
|---|---|---|---|
| Gender | |||
| Male (#) | 61 | 45 | 11 |
| Female (#) | 24 | 21 | 14 |
| Race | |||
| Caucasian (#) | 27 | 47 | 13 |
| African American (#) | 58 | 19 | 12 |
| Age (years) | |||
| Range | 19–60 | 19–84 | 18–78 |
| Mean (sd) | 35.0 (10.1) | 40.3 (19.0) | 46.5 (17.8) |
| Height (inches) | |||
| Range | 59–76 | 59–77 | 61–76 |
| Mean (sd) | 68.7 (4.0) | 68.4 (4.3) | 69.4 (4.2) |
| Smoking | |||
| Smoker (#) | 32 | 9 | 5 |
| Nonsmoker (#) | 53 | 57 | 20 |
Descriptive clinical information and standardized rating scale measures for patients are presented in Table 2, separately for men and women. Male patients were younger than female patients [t(83)=2.9, p<.01]. Although initial results suggested that male patients also had a significantly earlier age of onset of illness - defined as the earliest evidence of psychotic symptomatology associated with functional decline - this was not robust to adjustments for variance differences between the two groups. There were no differences in duration of illness, medicated vs. unmedicated status, or daily antipsychotic dosage among the patients taking medications. The Brief Psychiatric Rating Scale (BPRS) (Overall & Gorham, 1980), the Scale for Assessment of Negative Symptoms (SANS) (Andreasen, 1983) and Scale for Assessment of Positive Symptoms (SAPS) (Andreasen, 1984) were obtained at the time of testing. Ratings were completed by trained investigators with inter-rater reliability > .90. BPRS items were summed to form an index of overall symptom severity. SANS global ratings for five negative symptom subscales (Affective Flattening, Alogia, Anhedonia, Avolition, Attention) and SAPS global ratings for four positive symptom subsales (Hallucinations, Delusions, Bizarre Behavior, Formal Thought Disorder) assessed specific dimensions of psychotic symptomatology. Overall, these ratings suggested a relatively mild level of acute symptomatology, without any evidence of gender differences.
Table 2.
Patient Clinical Measures mean (±sd)
| MALE | FEMALE | |
|---|---|---|
| Age | 33.1 (10.0) | 39.8 (9.0) ** |
| Age of Onset | 20.8 (4.5) | 24.7 (10.6) |
| Duration of Illness | 12.0 (9.1) | 15.2 (9.6) |
| Medicated (#) | 46 | 18 |
| Unmedicated (#) | 15 | 6 |
| Antipsychotic dosage (CPZ equivalents) | 326 (183) | 385 (241) |
| Brief Psychiatric Rating Scale (BPRS) | 29.2 (7.1) | 33.1 (11.3) |
| Negative Symptom Scale (SANS) | ||
| Affect | 1.7 (1.3) | 1.4 (1.1) |
| Alogia | 1.0 (1.2) | 0.9 (1.0) |
| Avolition | 1.8 (1.4) | 1.6 (1.2) |
| Anhedonia | 2.3 (1.2) | 2.2 (1.2) |
| Attention | 1.1 (2.0) | 1.8 (3.0) |
| Positive Symptom Scale (SAPS) | ||
| Hallucinations | 1.4 (1.6) | 1.4 (1.9) |
| Delusions | 1.6 (1.6) | 1.7 (1.7) |
| Bizarre Behavior | 0.3 (0.8) | 0.7 (1.0) |
| Formal Thought Disorder | 1.1 (1.3) | 0.9 (1.2) |
male-female difference: p<.01, 2-tailed uncorrected
2.2 Experimental Procedure
Subjects refrained from smoking for approximately 1 hour prior to assessment. Volumetric and morphologic measurements of each nasal cavity were acquired using a Hood Laboratories Eccovision™ Acoustic Rhinometer (AR) (Figure 1). This device operates on the same principle as ultrasound or sonar devices. An acoustic pulse is transmitted into the nasal cavity, and this is reflected off of the cavity walls and transmitted back to the device. A comparison of the time-lagged reflected wave provides a measurement of acoustic impedance, which is proportional to the cross-sectional area of the nasal cavity. By summing these cross-sectional area measurements along the length of the nasal cavity, a volume measurement can be obtained.
Figure 1.
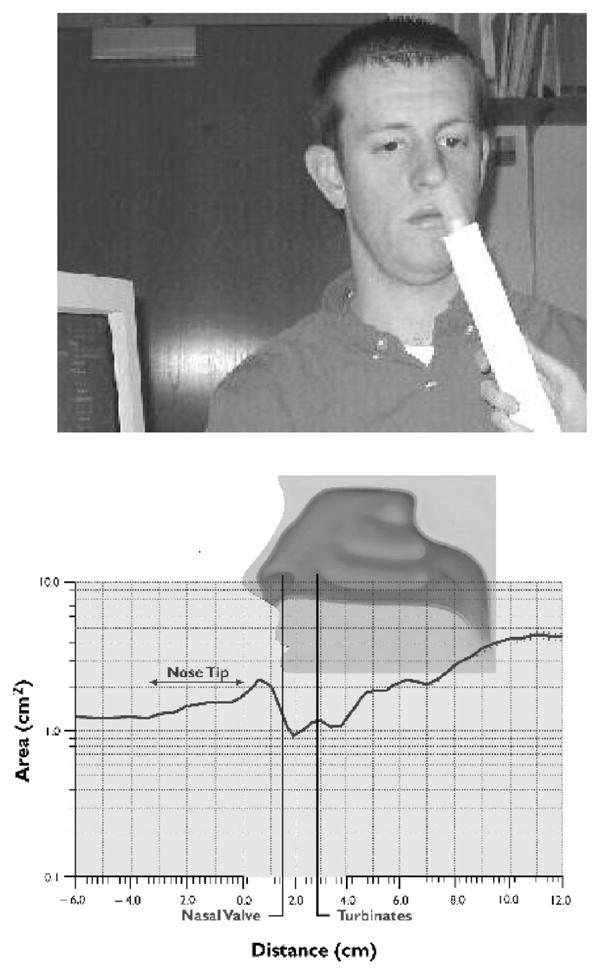
Top: Illustration of acoustic rhinometry data acquisition. Bottom: Rhinogram showing the area distance curve that is the data output of the acoustic rhinometry procedure.
Disposable silastic nosepieces were used and selected for each individual based upon the size of the nasal aperture. A sterile water soluble lubricating jelly (Medline; Mundelein, IL, USA) was swabbed around the edge of the nosepiece to create a good seal, decreasing any potential interference of the acoustic signal. The participants were asked to sit in a comfortable position as the trained examiner placed the AR wave tube into the tip of the nostril, while being careful not to place any unnecessary pressure on the nasal aperture. The AR wave tube was aligned near the midline and about 45° off vertical; this angle was minimally adjusted to produce a stable acoustic trace. Two separate AR measurements were collected from each nostril and the mean volume was computed for two measures: volume of the nasal cavity and the minimum cross-sectional area (i.e., a measure of the size of the nasal cavity at its narrowest point, which is sensitive to nasal obstruction or vascular engorgement). Consistent with standard practice (Clement and Gordts, 2005; Hilberg and Pedersen, 2000; Hilberg, personal communication) and our own previous study (Moberg et al., 2000), total nasal volume was divided into anterior (0–3.5mm) and posterior (3.5–5.5mm) compartments. This break-point typically denotes the anterior end of the inferior turbinate; the nasal cavity anterior to this location is vulnerable to mucosal congestive changes caused by infection, pollutants or smoke irritation, while the region posterior to this location, being more cartilaginous, is relatively resistant to such changes. The posterior cavity is similarly less sensitive to racial and/or ethnic differences in nasal structure.
Subjects also underwent standardized psychophysical assessment of their olfactory abilities. A single staircase, forced-choice odor detection task was used to estimate basal detection sensitivity to phenyl ethyl alcohol (PEA). The ability to identify odors was assessed by the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al., 1984), a standardized forty item forced-choice test. The results of this olfactory testing have already been published (Roalf et al., 2006). These measures will be considered here only for correlative analyses to examine associations between olfactory functioning and nasal volume.
2.3 Statistical Analysis
Since the patient and family member groups were not strictly independent samples, group differences in volume and minimum cross-sectional area were assessed using the Generalized Linear Latent and Mixed Models (GLLAMM) algorithm implemented in Stata 9.0 (StataCorp; College Station, TX, USA), with subject and family identification as hierarchically nested random-effects factors. This effectively accounted for any shared variance between individual members of the same family. Group (patient/control/relative), gender, race, nostril (right/left) and compartment (anterior/posterior) were included as fixed-effects predictors. The significance levels of individual model parameters were assessed using the Wald test statistic with a χ2 distribution. Significant group differences and interactions were parsed by post-hoc computation of appropriate linear combinations of the model coefficients, along with their associated z-statistic and p-value. Post-hoc contrasts were subject to Bonferroni corrected p-values of p<.05 to minimize Type I errors from multiple comparisons.
3. Results
The initial analysis revealed significant group X gender [χ2(2)=9.58, p=.008] and group X compartment [χ2(2)=18.23, p=.0001] interactions for nasal volume. There was no effect of nostril [χ2(1)=0.14, p=.71] and no interaction between nostril and group [χ2(2)=3.42, p=.18]. There was, similarly, no effect of race [χ2(1)=0.08, p=.78] and no interaction between race and group [χ2(2)=0.07, p=.96] on nasal volume. For the minimum cross sectional area, there was a significant effect of race [χ2(1)=10.23, p=.001], but no main or interaction effects of group.
Given the significant group X gender and group X compartment interactions for nasal volume, we examined the paired group contrasts within each nasal compartment, separately for males and females. These post-hoc comparisons revealed two significant group differences: male patients had smaller posterior compartment volumes than both male control subjects (z=4.39, corrected p<.0001) and male 1st-degree relatives (z=3.40, corrected p=.004). On a percentage basis, the male patients had posterior compartment volume reductions of 25% and 31% relative to male controls and male family members, respectively. These results were not altered by covarying for height or smoking status. None of the other contrasts reached even the uncorrected threshold of p<.05. In particular, there were no group differences among the female subjects, and no differences between unaffected family members and control subjects of either gender. Mean anterior and posterior compartment volumes are presented in Figure 2, separately for men and women in each group.
Figure 2.
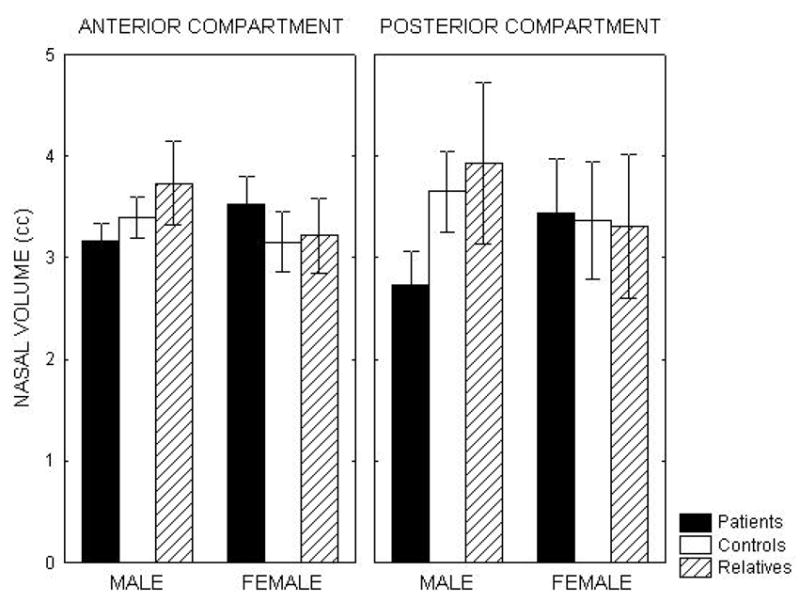
Mean nasal volumes for anterior and posterior nasal compartments in patients with schizophrenia, unaffected first-degree relatives and healthy control subjects, separated by gender. Volumes are averaged across left and right nostrils. Error bars represent 95% confidence intervals for the mean. Male patients had significantly reduced posterior compartment volumes relative to both unaffected family members and healthy controls.
We considered whether there were any relationships, among either male patients or the entire patient sample, between these posterior compartment volume measures and measures of clinical symptomatology, treatment status, or olfactory abilities. Posterior compartment volume measures were used as independent measures in a General Linear Model (GLM) with the following dependent measures: SANS and SAPS subscale ratings, BPRS total score, antipsychotic medication dosage, age of illness onset, duration of illness, UPSIT odor identification score, and PEA odor threshold level. We also examined nasal volume differences between medicated and unmedicated patients independent of dosage, using analysis of variance (ANOVA). None of these measures were associated with nasal volume, even with an uncorrected p-value <.05 for an exploratory analysis.
4. Discussion
This study replicates, in a larger sample, our previous finding that male schizophrenia patients have abnormally small posterior nasal volumes. It also demonstrates, for the first time, that female patients and unaffected first degree relatives do not share this craniofacial abnormality. The absence of any change in minimum cross-sectional area strengthens the conclusion that this is not simply a state-dependent effect of increased nasal engorgement or congestion, but a true craniofacial dysmorphology. The results therefore indicate that posterior nasal volume decrement is an abnormality that is specific to male schizophrenia patients. Since unaffected 1st-degree relatives of patients do not appear to exhibit a similar abnormality, this decrement may be environmentally, rather than genetically, mediated.
An increased prevalence of minor craniofacial anomalies in schizophrenia patients has been observed repeatedly (e.g., Donovan-Lepore et al., 2006; Green et al., 1989; Trixler et al., 2001; Waddington et al., 1999a). Early studies were limited by the use of subjective rating scales, lacking proven reliability, to assess dysmorphology (Trixler and Tenyi, 2000). However, more recent studies, employing precise quantitative anthropometric measurements of the craniofacial area, have confirmed the presence of specific morphologic anomalies (Deutsch et al, 2000; Donovan-Lepore et al., 2006; McGrath et al., 2002). Among other findings, a widening of the base of the skull has been reported and replicated (Donovan-Lepore et al., 2006; Lane et al., 1997; McGrath et al., 2002). Bony ossification of the skull base begins in week 11 of gestation, coinciding with both the growth of the fronto-nasal area and, in the brain, with the development of midline structures, including the hippocampus and entorhinal cortex (Kjaer, 1995), that also exhibit volume reductions in schizophrenia (Gur et al., 2000; Turetsky et al., 2003b). There is thus a striking convergence of findings from studies of minor physical anomalies and volumetric MRI analyses suggesting a late first-trimester disruption of embryogenesis in the etiology of schizophrenia.
Given the essentially retrospective character of this investigation, it is not clear that the nasal volume decrements that we have observed are, in fact, a reflection of such an embryological disturbance. Very little is known about the evolution of the nasal cavities through childhood and adolescence. The evidence clearly suggests that these structures enlarge as the rest of the face grows, but that there is very little actual reshaping of the cavities (Losken et al., 1994). Our data indicate that this volume decrement is unlikely to be secondary to either the chronic disease process or its treatment, as it was unrelated to measures of illness severity or the use of antipsychotic medications. Also, we would not expect a nonspecific consequence of the illness to manifest itself exclusively in male patients. Nevertheless, we cannot rule out the possibility that it represents a developmental abnormality arising later in life, rather than in utero.
Whether this developmental disturbance reflects a familial genetic vulnerability or an environmentally-mediated process is also not entirely clear. While some studies have reported the presence of minor physical anomalies in unaffected relatives of schizophrenia patients (Gourion et al., 2004), others have failed to observe such abnormalities, even among unaffected twins from discordant monozygotic twin pairs (Cantor-Graae et al., 1994). Consistent with this latter finding, the Maudsley family study reported an increased prevalence of minor physical anomalies among sporadic cases of schizophrenia, but not among patients with a family history of illness (Griffiths et al., 1998). Our finding that the otherwise healthy first-degree relatives of patients did not have reduced nasal volumes is similarly consistent with the hypothesis that this is an environmentally mediated abnormality.
The absence of a significant volume decrement in unaffected family members, however, does not rule out the possibility that this is a genetically mediated deficit. There are several reasons why a genetic abnormality might not have been observed in these subjects. One is simply the lack of adequate statistical power. Our family member sample was relatively small, particularly when it was segregated by gender. Nevertheless, we have observed robust deficits in other schizophrenia markers using comparable sample sizes (Turetsky et al., 2000; Roalf et al., 2006). Furthermore, the mean volumes that we observed in this sample were actually larger than those observed in the healthy control subjects (see Figure 2). This effectively rules out inadequate power as an explanation. A second possibility is a selection bias in the recruitment of our family members. Only a subset of 1st-degree relatives will share a genetic vulnerability with their ill patient proband. It is conceivable that those relatives who remain committed to the welfare of a psychotic family member, to the point of engaging in research, will be those with the least genetic loading for schizophrenia spectrum symptomatology. While we cannot disprove such a possibility, we would note, again, that these same family members have documented deficits in other physiological and olfactory measures. So we would expect to see at least some evidence of an abnormality if there were a strong genetic contribution. Our hypothesis that this is an environmentally mediated deficit is also supported, indirectly, by the lack of association between the nasal volume decrement and other olfactory impairments. Odor identification and threshold sensitivity deficits have been found repeatedly in the unaffected 1st-degree relatives of schizophrenia patients (Kopala et al., 2001; Roalf et al., 2006). These appear to be endophenotypic markers of genetically-mediated disease vulnerability. The lack of association with these olfactory measures implies a different etiology for the nasal volume deficit, although not specifically an environmental one.
The gender specificity of this volume decrement is striking. Although the female patient sample was smaller than the male sample, there is no indication that this gender difference is simply a question of statistical power, as patients had the largest volumes among the 3 female groups. Sex differences have been previously observed in both the clinical presentation of schizophrenia (Gur et al., 1996) and volumetric measurements of specific midline brain structures (Gur et al., 2004). With respect to minor physical anomalies, there is some evidence, based on qualitative rating scales, that these are more prominent in male, rather than female, schizophrenia patients (Akabaliev and Sivkov, 2003; Griffiths et al., 1998). Importantly, the prevalence of such anomalies also appears to be greater in healthy males, compared to healthy females (Akabaliev and Sivkov, 2003). Male fetuses are known to be more susceptible to intrauterine insults than females (Gualtieri and Hicks, 1985). It has also been proposed that male schizophrenia patients are more likely to exhibit a congenital variant of schizophrenia, reflecting abnormal prenatal development (Castle and Murray, 1985; Murray et al., 1992). The gender specificity of our finding, therefore, is both consistent with and supportive of the idea that these anomalies reflect environmental disruptions of intrauterine development.
If this is correct, then reduced nasal volume may be a sensitive indicator of a prenatal “second hit”, i.e., an early environmental insult that interacts with a predisposing genetic susceptibility to produce schizophrenia. Conclusions, at this point, concerning the specific timing and etiology of the abnormality must be considered speculative. In addition, the specificity of this finding to schizophrenia, as opposed to other psychotic conditions, has yet to be demonstrated. If, however, this is a marker of an early developmental disturbance predisposing towards schizophrenia, it leads to an important testable hypothesis: among male children with high genetic risk, reduced posterior nasal volume will distinguish those who subsequently develop the disorder from those who do not. There is evidence to suggest that minor physical anomalies can, in fact, discriminate between at-risk individuals and help predict outcome. In a prospective study of 81 children between the ages of 11 and 13 who had a parent diagnosed with schizophrenia, 31% of those with 3 or more minor physical anomalies subsequently developed a schizophrenia spectrum disorder, compared to only 12% of those with 0–2 physical anomalies (Schiffman et al., 2002). This is equivalent to a test sensitivity of 71% and specificity of 58%. This was based on a subjective assessment of the simple presence or absence of the anomalies, without regard to either severity of the deformity or inter-rater reliability; sensitivity and specificity might improve considerably with the use of an objective quantitative measure of dysmorphology, such as nasal volume. Although these findings are preliminary, given the current interest in identifying ultra-high risk individuals for early treatment intervention or possible disease prevention (Larsen et al., 2006), and the ease and rapidity with which nasal volume can be assessed at the bedside or in clinic, further investigation of both the predictive utility of this simple measure is clearly warranted.
Acknowledgments
Special thanks to the Hofmann Trust for their support of this research through the National Alliance for Research on Schizophrenia and Depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akabaliev VH, Sivkov ST. Sexual dimorphism in minor physical anomalies in schizophrenic patients and normal controls. Comprehensive Psychiatry. 2003;44:341–348. doi: 10.1016/S0010-440X(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Cantor-Graae E, McNeil TF, Torrey EF, Quinn P, Bowler A, Sjostrom K, Rawlings R. Link between pregnancy complications and minor physical anomalies in monozygotic twins discordant for schizophrenia. Am J Psychiatry. 1994;151:1188–93. doi: 10.1176/ajp.151.8.1188. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychol Med. 1991;21:565–75. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- Clement PAR, Gordts F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology. 2005;43:169–179. [PubMed] [Google Scholar]
- Deutsch CK, Hobbs K, Price SF, Gordon-Vaughn K. Skewing of the brain midline in schizophrenia. NeuroReport. 2000;11:3985–3988. doi: 10.1097/00001756-200012180-00016. [DOI] [PubMed] [Google Scholar]
- Donovan-Lepore AM, Jaeger J, Czobor P, Abdelmessih S, Berns SM. Quantitative craniofacial anomalies in a racially mixed schizophrenia sample. Biol Psychiatry. 2006;59:349–353. doi: 10.1016/j.biopsych.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiology & Behavior. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Gallagher BJ, 3rd, McFalls JA, Jr, Jones BJ, Pisa AM. Prenatal illness and subtypes of schizophrenia: the winter pregnancy phenomenon. J Clin Psychol. 1999;55:915–22. doi: 10.1002/(sici)1097-4679(199907)55:7<915::aid-jclp12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Gourion D, Goldberger C, Bourdel MC, Bayle FJ, Loo H, Krebs MO. Minor physical anomalies in patients with schizophrenia and their parents: prevalence and pattern of craniofacial abnormalities. Psychiatry Res. 2004;125:21–28. doi: 10.1016/j.psychres.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Gaier DJ, Ganzell S, Kharabi F. Minor physical anomalies in schizophrenia. Schizophrenia Bull. 1989;15:91–99. doi: 10.1093/schbul/15.1.91. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Sigmundsson T, Takei N, Frangou S, Birkett PB, Sharma T, Reveley AM, Murray RM. Minor physical anomalies in familial and sporadic schizophrenia: the Maudsley family study. J Neurol Neurosurg Psychiatry. 1998;64:56–60. doi: 10.1136/jnnp.64.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri CT, Hicks RE. An immunoreactive theory of selective male affliction. Behav Brain Sci. 1985;8:427–441. [Google Scholar]
- Gur RE, Kohler C, Turetsky BI, Siegel SJ, Kanes SJ, Bilker WB, Brennan AR, Gur RC. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol Psychiatry. 2004;55:512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Gur RE, Petty RG, Turetsky BI, Gur RC. Schizophrenia throughout life: Sex differences in severity and profile of symptoms. Schizophr Res. 1996;21:1–12. doi: 10.1016/0920-9964(96)00023-0. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–75. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Hilberg O, Pedersen OF. Acoustic rhinometry: recommendations for technical specifications and standard operating procedures. Rhinology. 2000;16:3–17. [PubMed] [Google Scholar]
- Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dingemans A, Schnack HG, van Haren NE, Pereira Ramos LM, Gispen-de Wied CC, Kahn RS. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–2. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995;53:135–143. doi: 10.3109/00016359509005963. [DOI] [PubMed] [Google Scholar]
- Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry. 2001;158:1286–90. doi: 10.1176/appi.ajp.158.8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A, Kinsella A, Murphy P, Byrne M, Keenan J, Colgan K, Cassidy B, Sheppard N, Horgan R, Waddington JL, Larkin C, O’Callaghan E. The anthropometric assessment of dysmorphic features in schizophrenia as an index of its developmental origins. Psychol Med. 1997;27:1155–64. doi: 10.1017/s0033291797005503. [DOI] [PubMed] [Google Scholar]
- Larsen TK, Melle I, Auestad B, Friis S, Haahr U, Johannessen JO, Opjordsmoen S, Rund BR, Simonsen E, Vaglum P, McGlashan T. Early detection of first-episode psychosis: the effect on 1-year outcome. Schizophr Bull. 2006;32:758–64. doi: 10.1093/schbul/sbl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen WJ. Human Embryology. 3. Churchill Livingstone: Oxford; 2001. [Google Scholar]
- Losken A, Mooney MP, Siegel MI. Comparative cephalometric study of nasal cavity growth patterns in seven animal models. Cleft Palate Craniofac J. 1994;31:17–23. doi: 10.1597/1545-1569_1994_031_0017_ccsonc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophrenia Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- McGrath J, El Saadi O, Grim V, Cardy S, Chapple B, Chant D, Lieberman D, Mowry B. Minor physical anomalies and quantitative measurements of the head and face in patients with psychosis. Arch Gen Psychiatry. 2002;59:458–464. doi: 10.1001/archpsyc.59.5.458. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: A qualitative and quantitative review. Neuropsychopharmacol. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Roalf DR, Gur RE, Turetsky BI. Smaller nasal volumes as stigmata of aberrant neurodevelopment in schizophrenia. Am J Psychiatry. 2000;161:2314–2316. doi: 10.1176/appi.ajp.161.12.2314. [DOI] [PubMed] [Google Scholar]
- Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- Overall JR, Gorham DR. The brief psychiatric rating scale. J Oper Psychiatry. 1980;11:48–64. [Google Scholar]
- Roalf DR, Turetsky BI, Owzar K, Balderston CC, Johnson SC, Brensinger CM, Gur RE, Siegel SJ, Moberg PJ. Unirhinal olfactory function in schizophrenia patients and first-degree relatives. J Neuropsychiatry Clin Neurosciences. 2006;18:389–396. doi: 10.1176/jnp.2006.18.3.389. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Ekstrom M, LaBrie J, Schulsinger F, Sorensen H, Mednick S. Minor physical anomalies and schizophrenia spectrum disorders: A prospective investigation. Am J Psychiatry. 2002;159:238–243. doi: 10.1176/appi.ajp.159.2.238. [DOI] [PubMed] [Google Scholar]
- Trixler M, Tenyi T. Problems with the Waldrop Scale. Am J Psychiatry. 2000;157:486. doi: 10.1176/appi.ajp.157.3.486. [DOI] [PubMed] [Google Scholar]
- Trixler M, Tenyi T, Csabi G, Szabo R. Minor physical anomalies in schizophrenia and bipolar affective disorder. Schizophr Res. 2001;52:195–201. doi: 10.1016/s0920-9964(00)00182-1. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Cannon TD, Gur RE. P300 subcomponent abnormalities in schizophrenia: III. Deficits in unaffected siblings of schizophrenic probands. Biol Psychiatry. 2000;47:380–390. doi: 10.1016/s0006-3223(99)00290-5. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003a;53:403–411. doi: 10.1016/s0006-3223(02)01865-6. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Roalf DR, Arnold SA, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003b;60:1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RC. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Lane A, Larkin C, O’Callaghan E. The neurodevelopmental basis of schizophrenia: Clinical clues from cerebro-craniofacial dysmorphogenesis, and the roots of a lifetime trajectory of disease. Biol Psychiatry. 1999a;46:31–39. doi: 10.1016/s0006-3223(99)00055-4. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Lane A, Scully P, Meagher D, Quinn J, Larkin C, O’Callaghan E. Early cerebro-craniofacial dysmorphogenesis in schizophrenia: A lifetime trajectory model from neurodevelopmental basis to ‘neuroprogressive’ process. Psychiat Res. 1999b;33:477–489. doi: 10.1016/s0022-3956(99)00024-2. [DOI] [PubMed] [Google Scholar]


