Summary
10-formyl tetrahydrofolate is a key metabolite in C1 carbon metabolism, arising through the action of formate-tetrahydrofolate ligase (FTL) and/or 5,10-methenyltetrahydrofolate cyclohydrolase/5,10-methylene tetrahydrofolate dehydrogenase (DHCH). Leishmania major possesses single DHCH1 and FTL genes encoding exclusively cytosolic proteins, unlike other organisms where isoforms occur in the mitochondrion as well. Recombinant DHCH1 showed typical NADP+-dependent methylene tetrahydrofolate DH and 5,10-methenyltetrahydrofolate CH activities, and the DH activity was potently inhibited by a substrate analog 5,10-CO-THF (Ki 105 nM), as was Leishmania growth (EC50 1.1 μM). Previous studies showed null ftl− mutants were normal, raising the possibility that loss of the purine synthetic pathway had rendered 10-CHO-THF dispensable in evolution. We were unable to generate dhch1− null mutants by gene replacement, despite using a wide spectrum of nutritional supplements expected to bypass DHCH function. We applied an improved method for testing essential genes in Leishmania, based upon segregational loss of episomal complementing genes rather than transfection; analysis of ~1400 events without successful loss of DHCH1 again established its requirement. Lastly, we employed ‘genetic metabolite complementation’ using ectopically expressed FTL as an alternative source of 10-CHO-THF; now dhch1− null parasites were readily obtained. These data establish a requirement for 10-CHO tetrahydrofolate metabolism in L. major, and provide genetic and pharmacological validation of DHCH as a target for chemotherapy, in this and potentially other protozoan parasites.
Keywords: trypanosomatid protozoa, C1-THF metabolism, 1 carbon transfer, chemotherapy, formyl methionyl-tRNA
Introduction
Leishmania are important pathogens infecting millions of people worldwide. The symptoms of these infections range from skin lesions and disfiguring necrosis in the face, to lethal pathology in the liver and spleen, depending on the Leishmania species involved. Although lasting immunity is possible, no vaccine is presently available, and the drugs used in chemotherapy suffer from various deficiencies, such as toxicity, high cost and emerging resistance (Kedzierski et al., 2006, Mishra et al., 2007). Consequently, the development of alternative therapies is an urgent priority.
Folate derivatives are essential cellular cofactors in the synthesis of thymidine, purines and the amino acids glycine and methionine, in the metabolism of histidine and serine (Nzila et al., 2005, Christensen & MacKenzie, 2006) and for mitochondrial protein synthesis through the formylation of initiator methionyl-tRNAMet (Kozak, 1983). The metabolism of reduced folate cofactors is of particular interest in drug discovery, since dihydrofolate reductase (DHFR) can be efficiently targeted in anti-tumor and anti-microbial chemotherapy (Nzila et al., 2005, Schweitzer et al., 1990, Then, 2004). While current antifolates are ineffective against Leishmania because DHFR inhibition is bypassed by pteridine reductase 1 (PTR1), which is relatively insensitive (Bello et al., 1994), combined inhibitor strategies may overcome this (Hardy et al., 1997, Cavazzuti et al., 2008). In this work we explore a potential new arena for chemotherapy, the enzymes responsible for the synthesis of 10-formyl tetrahydrofolate (10-CHO-THF).
Three known enzyme activities mediate production of 10-CHO-THF (Fig. 1) (Christensen & Mackenzie, 2008, Christensen & MacKenzie, 2006, Appling, 1991). In one pathway formate is added directly onto THF in an ATP-dependent reaction by formate-tetrahydrofolate ligase (FTL, EC 6.3.4.3) (Rabinowitz & Pricer, 1962). Alternatively, 10-CHO-THF arises in two steps from 5,10-methylene-tetrahydrofolate (5,10-CH2-THF), beginning with oxidation to 5,10-methenyl-tetrahydrofolate (5,10-CH=THF) by a NADP+ or NAD+-dependent methylenetetrahydrofolate dehydrogenase (DH, EC 1.5.1.5 or 1.5.1.15, respectively) (Moore et al., 1974, Hatefi et al., 1957). 5,10-CH=THF is then hydrolyzed to 10-CHO-THF by methenyltetrahydrofolate cyclohydrolase (CH, EC 3.5.4.9). These enzymes occur physically in various combinations, with monofunctional enzymes such as a human, bacterial and plant FTLs, bifunctional enzymes with dehydrogenase and cyclohydrolase activities (referred to as DHCH enzymes), or trifunctional enzymes with all three activities (referred to as the C1-THF synthases) (Christensen & Mackenzie, 2008, Christensen & MacKenzie, 2006, Appling, 1991, Nour & Rabinowitz, 1991, Tan et al., 1977). Adding to this complexity, multiple genes often encode these activities in a single organism, with humans expressing both a trifunctional C1-synthase in the cytoplasm (MTHFD1), and a bifunctional DHCH (MTHFD2) and a monofunctional FTL (MTHFD1L) targeted to the mitochondrion (Christensen et al., 2005b). Other eukaryotes express differing combinations of both cytosolic and organellar DHCH and FTLs (Christensen & MacKenzie, 2006, Hanson & Roje, 2001).
FIG. 1. 10-formyl-THF metabolism in Leishmania.
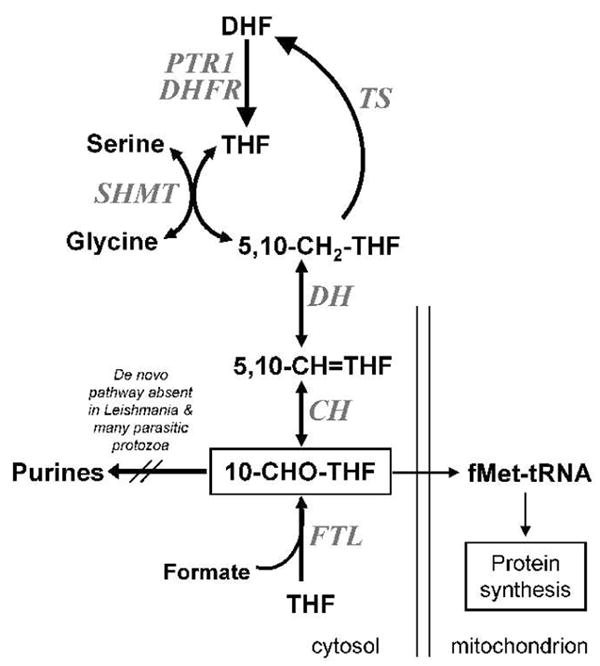
Following synthesis through the activity of dihydrofolate reductase (DHFR, encoded by the bifunctional DHFR-TS) or pteridine reductase 1 (PTR1), tetrahydrofolate (THF) can enter C1 metabolic pathways by direct formylation in an ATP-dependent reaction by formate-tetrahydrofolate ligase (FTL, LmjF30.2600). Alternatively, THF is converted into 5,10-methylene-tetrahydrofolate (5,10-CH2-THF) through the activity of serine hydroxymethyltransferase (SHMT). 10-CHO-THF can be generated in two steps from 5,10-methylene-tetrahydrofolate (5,10-CH2-THF), through oxidation to 5,10-methenyl-tetrahydrofolate (5,10-CH=THF) by a NADP+-dependent methylenetetrahydrofolate dehydrogenase (DH, LmjF26.0320), and hydrolysis to 10-CHO-THF by methenyltetrahydrofolate cyclohydrolase (CH, LmjF26.0320). In L. major the DH and CH activities are contained in the single bifunctional protein DHCH1, which like FTL is localized exclusively to the cytosol (Vickers et al., 2008). While 10-CHO-THF is used in the de novo purine synthetic pathway in many organisms, this pathway is absent in Leishmania and many parasitic protozoans (Carter et al., 2008). Current data suggest 10-CHO-THF may be utilized in the mitochondrion for methionyl-tRNA formylation, the only unique function thus far uncovered by genomic and metabolic studies in Leishmania.
The two products of these enzymes have several known functions. 5,10-CH=THF is best-known as an intermediate leading to 10-CHO-THF (Hanson & Roje, 2001, Christensen & MacKenzie, 2006, Appling, 1991), although it also occurs in some DNA photolyases, and functions in mammalian histidine catabolism (Weber, 2005, Stanger, 2002). These pathways have not been described in Leishmania and their properties in other organisms suggest they are unlikely to be essential. 10-CHO-THF acts as a formyl donor in purine biosynthesis, and in bacteria and eukaryotic organelles for formylation of the initiator methionyl-tRNAMet to produce fMet-tRNAMet (Appling, 1991, Christensen & MacKenzie, 2006, RajBhandary, 1994, Varshney et al., 1993, Kozak, 1983). Interestingly, many protozoans including trypanosomes and Leishmania lack the de novo purine pathway and rely exclusively on salvage (Carter et al., 2008), eliminating the need for 10-CHO-THF in this capacity. The importance of initiator Met-tRNAMet formylation is the subject of some controversy. While thought originally to be essential in bacteria, recent data suggest that is not always the case (Newton et al., 1999, RajBhandary, 2000). Mitochondrial initiator Met-tRNAMet formylation is dispensable in the yeast Saccharomyces cerevisae but may be required in humans; even when not essential, the consequences of its loss vary widely (Li et al., 2000, Spencer & Spremulli, 2004, Vial et al., 2003). Studies in the protozoal parasite Trypanosoma brucei, a member of the protozoal subfamily Trypanosomatidae to which Leishmania belongs, show that Met-tRNA is imported into the mitochondrion and then formylated, where it is used for mitochondrial protein synthesis (Tan et al., 2002, Charriere et al., 2005). RNAi knockdown of the formyl-Met-tRNAMet transferase (FMT) shows little phenotype. However, negative results in RNAi knockdowns sometimes can arise through incomplete inhibition, a particular problem in cofactor metabolism where even low levels of THF-dependent metabolites can fulfill normal metabolic needs. Importantly, simultaneous RNAi knockdown of the T. brucei FMT with the mitochondrial initiation factor IF2 yield significant growth inhibition (Charriere et al., 2005), possibly signifying the importance of fMet-tRNAMet for essential mitochondrial protein synthesis.
Previously we and others used database mining to survey the 10-CHO-THF synthetic pathway in Leishmania and other trypanosomatids (Vickers et al., 2008, Opperdoes & Coombs, 2007). The L. major genome encodes an active monofunctional FTL and a predicted bifunctional DHCH encoded by the DHCH1 gene. Both proteins were localized exclusively in the cytoplasm consistent with the absence of a predicted N terminal mitochondrial targeting sequence, as shown by immunoblotting of cellular fractions as well as GFP fusions to DHCH1 (Vickers et al., 2008). Unexpectedly, ftl− null mutants showed normal viability and virulence. As noted above, the absence of purine synthesis mitigates the need for 10-CHO-THF in Leishmania, and potentially this could reduce or even eliminate this requirement globally.
Here we characterize the role of DHCH1 in L. major metabolism through enzymatic, genetic knockout, and pharmacological tests. First we show that DHCH1 encodes a functional protein with NADP-dependent DH and CH activities, with kinetic properties similar to those seen in other organisms. We used both traditional transfection-based and a new plasmid segregational approach to establish that DHCH1 was essential. Notably, over-expression of FTL enabled the recovery of dhch1− mutants, establishing through ‘genetic metabolite complementation’ that 10-CHO-THF is essential. Lastly we showed that Leishmania growth and DHCH1 activity can be inhibited by a specific DHCH inhibitor. In total these data provide genetic and pharmacological validation of DHCH as a target for anti-parasitic chemotherapy.
Results
Enzymatic activity of recombinant L. major DHCH1
Previously we used database mining of trypanosomatid genomes to identify gene LmjF26.0320 as DHCH1 as the only functional DHCH in Leishmania major (Vickers et al., 2008). A hexahistidine-tagged DHCH1 was expressed in E. coli and the recombinant protein was purified by metal-affinity chromatography (Supplementary Figure S1) (Vickers et al., 2008). Dehydrogenase activity was measured by following the conversion of 5,10-CH2-THF to 5,10-CH=THF, while the cyclohydrolase activity was monitored by following the consumption of 5,10-CH=THF, yielding 10-CHO-THF (see methods). Purified DHCH1 showed both 5,10-CH2-THF dehydrogenase and 5,10-CH=THF cyclohydrolase activities, with specific activities of 22 ± 2 and 6.3 ± 0.8 μmol min−1 mg−1, respectively. In comparison to the activities of the porcine cytosolic multifunctional C1-synthase the L. major enzyme has a slightly higher dehydrogenase activity and a lower cyclohydrolase activity, with the porcine enzyme having activities of 7.5 and 22 μmol min−1 mg−1, respectively (Tan et al., 1977).
The kinetic properties of the dehydrogenase reaction were determined and are summarized in Table 1. The reaction followed typical Michaelis-Menten kinetics and could be saturated with either substrate. In mammals, the cytoplasmic DH activity of the trifunctional C1-synthase is dependent on NADP+ (Tan et al., 1977), whereas the mitochondrial DH is dependent on NAD+ (Mejia & MacKenzie, 1988). The L. major DHCH1 dehydrogenase activity was highly dependent upon NADP+, with no activity detected with NAD+ (limit of detection 0.3 μmol min−1 mg−1, about 1% of the activity seen with NADP+). While the DH activity of human mitochondrial DHCH with NAD+ requires phosphate and magnesium ions (Christensen et al., 2005a), when tested in the presence of both 5 mM (K+)PO4 and 5 mM MgCl2 L. major DHCH1 still showed no NAD+-dependent activity (data not shown). These data are consistent with the conservation of residues in LmDHCH1 that interact with the NADP+ cofactor in the human cytosolic enzyme, notably Arg173 that interacts with the coenzyme 2’ phosphate group in the human enzyme’s active site (Allaire et al., 1998) (Fig. S2). In comparison to the human enzymes, the Leishmania DHCH1 dehydrogenase activity appears most similar to that of the NADP+-dependent cytoplasmic C1 synthase, with a lower Km for the coenzyme and lower turnover than the human mitochondrial DHCH MTHFD2 (Pawelek & MacKenzie, 1998).
TABLE 1.
Kinetic constants of recombinant Leishmania major DHCH1
| Substrate | Km (app) (μM) | kcatc (s−1) | kcat/Km (M−1 s−1) × 105 |
|---|---|---|---|
| 5,10-CH2-THF a | 120 ± 10 | 14 ± 1 | 1.2 |
| NADP+b | 38 ± 6 | 10 ± 1 | 2.6 |
Measured with 1 mM NADP+ as the fixed substrate.
Measured with 250 μM 5,10-CH2-THF as the fixed substrate.
Calculated assuming one active site per polypeptide.
All values given with the standard error of fit.
Attempts to generate DHCH1 knockout by ‘classic’ double replacements
Leishmania are predominantly diploid and thus inactivation of most genes requires two rounds of gene replacement (Cruz et al., 1991). While some chromosomes show aneuploidy in some WT Leishmania strains, preliminary CGH data suggest that Chromosome 26 bearing DHCH1 is disomic in the L. major line studied here (E. Kruvand and S. Beverley, in preparation). Thus, we constructed two targeting fragments, with the DHCH1 ORF replaced by ones encoding puromycin (PAC) or hygromycin B (HYG) resistance, flanked by ~ 1 kb of 5′ and 3′ sequence (Fig. 2A). Electroporation of either fragment separately into WT L. major followed by plating on selective media yielded transfectant colonies efficiently at control frequencies (not shown). Analysis of 20 clonal transfectant lines showed that all were heterozygotes (DHCH1/Δdhch1::PAC or DHCH1/Δdhch1::HYG, abbreviated hereafter as PAC/+ or HYG/+) as expected (Fig 2A, Supplementary Figure S3, and data not shown). These studies used PCR tests with primer pairs containing one located outside of the targeting fragment to either the 5′ or 3′ side, partnered with a marker-specific primer (Fig. S3, 2B, 2C and data not shown).
FIG. 2. Attempts to generate dhch1− null mutants by ‘classic’ double replacements.
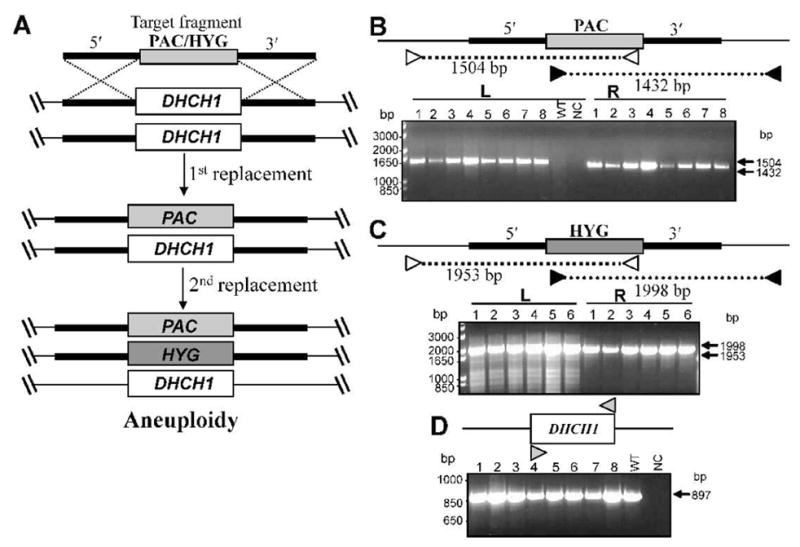
A. Planned deletion of the DHCH1 ORF by homologous gene replacement using targeting fragments with PAC or HYG resistance markers. In the first round, parasites were transfected with either construct separately; in this diagram the round starting with PAC is shown. Heterozygous replacement lines were obtained with high efficiency. In the second round, the +/PAC heterozygote (formally, Δdhch1::PAC/DHCH) was transfected with the HYG targeting fragment, with the expectation of yielding homozogous dhch1− null mutants. Instead, only aneuploid parasites showing both the two planned replacements but retaining WT DHCH1 were recovered (formally, Δdhch1::PAC/DHCH/Δdhch1::SAT/Δdhch1::PAC/DHCH1). B. PCR tests of planned PAC replacements at DHCH1. Homologous replacement was visualized by PCR amplification using a primer directed against sequences located with the PAC ORF, and a primer located on the DHCH1 chromosome outside the targeting fragment (indicated by heavy line) on the 5′ (white arrows) or 3′ (black arrow) side (SMB2731/2558 and SMB2771/2557, Table S1). The sizes of the predicted PCR products are shown. WT, wild-type DNA; NC, negative control (no DNA template), #1–8, eight independent lines with PAC replacements at DHCH1. C, PCR tests of planned HYG replacements at DHCH1. This experiment is similar to that described in panel B, except that primers were directed against sequences located in the HYG targeting fragment (SMB2731/2562 and SMB2771/2561, Table S1). #1–6, six independent lines with HYG replacements at DHCH1. D. PCR tests showing retention of DHCH1 in second round transfectants. Amplifications were performed using primers directed against sequences within the L. major DHCH1 ORF (SMB3125/3126, Table S1). Lines # 1 – 4 represent double replacement with PAC/HYG and lines # 5 – 8 with HYG/PAC.
Next the HYG/+ or PAC/+ heterozygotes were submitted to a second round of electroporation with the remaining targeting fragment (PAC or HYG respectively) and plated on semisolid media containing both puromycin and hygromycin B. PCR tests of 12 doubly resistant clonal transfectant lines showed that each contained both the planned HYG and PAC DHCH1 replacements (Fig 2B, 2C). Nonetheless, the clonal transfectants retained a copy of DHCH1, as revealed by PCR amplification of an intact DHCH1 ORF (Fig. 2D), and grew normally (data not shown). This finding of successful ‘double replacement’ accompanied by retention of the WT gene has been seen previously in attempts to target other essential genes in Leishmania, arising through generation of aneuploid or tetraploid parasites with additional chromosomes bearing the target gene (Balana-Fouce et al., 2008, Cruz et al., 1993, Dumas et al., 1997, Ilgoutz et al., 1999a, Vergnes et al., 2005). Analysis of the DNA content of the doubly-targeted lines above by flow cytometry showed WT DNA contents (data not shown), suggested that these were likely to be aneuploids, bearing at least one ‘WT’ chromosome along with the planned HYG and PAC replacements (Fig. 2A).
Attempts to generate DHCH1 knockout by double replacement using different nutritional supplements
We attempted to bypass the consequences of DHCH1 ablation through metabolic complementation. In these studies, the 2nd round transfections above were repeated, but with parasites grown and plated on media containing various supplements potentially able to bypass the DHCH1 requirement. First we included metabolites known to participate and/or arise in C1 folate metabolism, including glycine, serine, formate, tetrahydrofolate, thymidine and folinic acid. Second, parasites were grown with the supplements above plus the C1 folates 5,10-CH=THF and 10-formylfolic acid (10-CHOFA), the first being an intermediate metabolite formed through the DH activity of DHCH1 and the second constituting a potential precursor to 10-formyl-THF. While the ideal supplement would have been 10-CHO-THF, this metabolite is very unstable; however, under the mildly alkaline pH of Leishmania culture media (7.4), spontaneous isomerization of 5,10-CH=THF to 10-CHO THF occurs over time (Rabinowitz, 1963, Brouwer et al., 2007). Lastly, since the expected role of 10-CHO-THF in Leishmania arises through its role in mitochondrial protein synthesis, we attempted to bypass this potential requirement by performing transfections in the presence of pyruvate and uridine, two metabolites that have been proposed to bypass the need for mitochondrial DNA and presumably the proteins encoded therein (Sen et al., 2007).
In total, 65 independent clonal lines were analyzed, all of which yielded the presumptive aneuploidy phenotype by the PCR assay described above (data not shown), i.e. parasites bearing both the planned HYG and PAC replacements while retaining at least one copy of the DHCH1 ORF, without the recovery of any dhch1− null mutants.
Deletion of chromosomal DHCH1 in the presence of ectopically expressed DHCH1
An important control was whether successful targeting could be achieved in the presence of ectopic DHCH1 expression, as this would render unlikely the possibility that molecular events neither anticipated nor tested in our strategy above had occurred. First, DHCH1 was expressed from the multicopy episomal pXNG4 vector, which bears a nourseothricin (SAT) resistance marker (D. Scott and S. Beverley, in preparation). DHCH assays of WT protein extracts showed a specific activity of 1 nmol−1 min−1 mg, a value not significantly different than the assay background in controls lacking NADP+, whereas WT/pXNG4-DHCH1 transfectants showed a specific activity of 31 nmol−1 min−1 mg, at least 30-fold greater. pXNG4-DHCH1 was then introduced into the HYG/+ or PAC/+ heterozygotes described previously, and the second chromosomal allele in these lines targeted by transfection as before (Fig. 3A). To visualize the chromosomal DHCH1 allele, PCR using a forward primer located 5′ and outside of the DHCH1 targeting fragment and a reverse primer located within the DHCH1 ORF was used (Fig. 3B).
FIG. 3. Deletion of chromosomal DHCH1 in the presence of ectopic DHCH1.
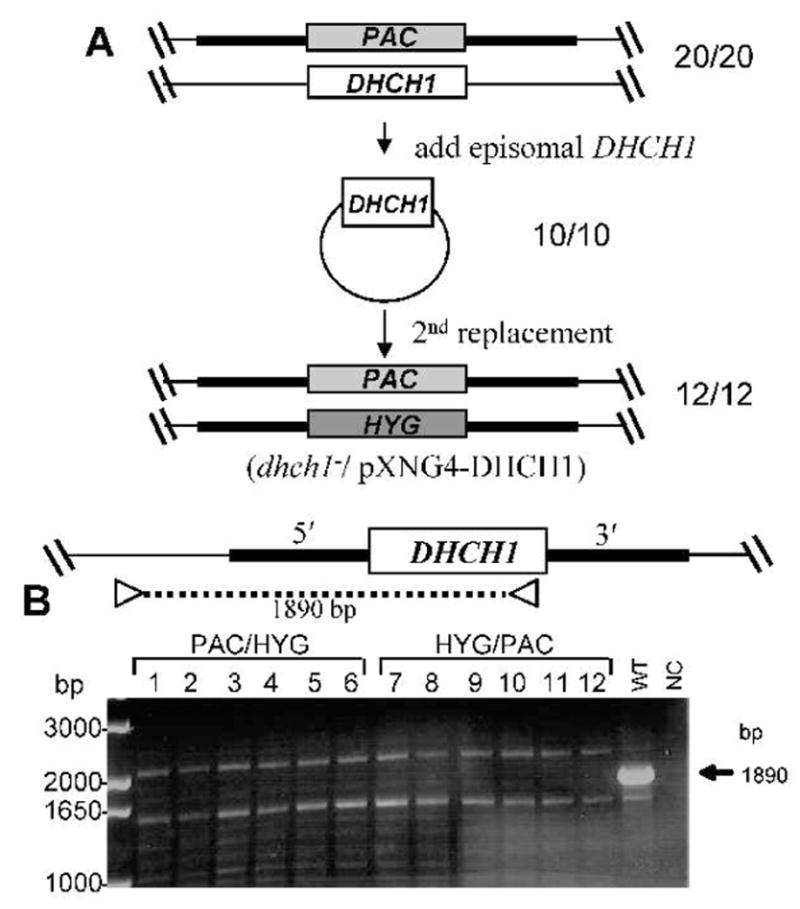
A. Scheme for the deletion of chromosomal DHCH1 in the presence of ectopically expressed DHCH1. First, a pXNG4-DHCH1 episomal expression construct was transfected into a DHCH1/Δdhch1::PAC heterozygote described in Fig. 2A; 10/10 transfectants analyzed were confirmed to have the predicted genotype depicted. These DHCH1/Δdhch1::PAC [pXNG4-DHCH1] lines were then transfected with the HYG DHCH targeting fragment and selected for resistance to all three antibiotics; of the 12 clonal lines tested, all showed the planned replacement of the chromosomal DHCH1 locus (formally, Δdhch1::HYG DHCH1/Δdhch1::PAC [pXNG4-DHCH1]).
B – PCR tests showing loss of the chromosomal DHCH1. To distinguish the ectopic DHCH1 from the chromosomal locus, DNA from candidate lines was subjected to PCR tests using a using a primer directed against sequences located with the DHCH1 ORFt, and a primer located on the DHCH1 chromosome outside of the targeting fragment on the 5′ side (SMB2731/3126,, Table S1). WT, wild-type DNA control; NC, negative control lacking any DNA template. The arrow marks the size of the predicted fragment found in WT DNA, which is absent in the chromosomal dhch1− knockouts.
In contrast to the results above with parasites lacking ectopic DHCH1 expression, the chromosomal DHCH1 was now readily lost in all 12 independent clonal transfectants tested (Fig. 3B). PCR tests showed that these lines contained both the HYG and PAC Δdhch1− replacements, while retaining the DHCH1 ORF borne on pXNG4-DHCH1 (data not shown). Thus, in the presence of ectopically expressed DHCH1, both chromosomal DHCH1 alleles could be successfully eliminated, yielding parasites that were genetically dhch1−/+pXNG4-DHCH1. These methodological controls further support the conclusion that under the conditions tested, DHCH1 is essential.
Genetic metabolite complementation permits the recovery of DHCH1 replacement
A requirement for DHCH1 was somewhat surprising, since Leishmania possess a second cytoplasmic pathway for 10-CHO-THF synthesis through formyl tetrahydrofolate ligase (FTL), whose enzymatic activity has been verified (Vickers et al., 2008) (Fig. 1). This could indicate that DHCH1 has an unanticipated function, or alternatively, that the activity of the FTL pathway was insufficient for metabolic needs in the absence of DHCH1. Indeed, evidence that the FTL pathway is of secondary importance comes from the fact that ftl− mutants could be readily generated without any apparent effect on growth in vitro or virulence in animal infections (Vickers et al., 2008).
Thus we asked whether provision of 10-CHO-THF by over-expression of FTL using the multicopy episomal vector pXNG4-FTL could bypass the requirement for DHCH1 (Fig. 4A). pXNG4-FTL was introduced into the heterozygous +/PAC or +/HYG lines, and FTL over-expression was confirmed by western blot analysis (Supplementary Figure S4). These parasites were then submitted to second round of targeting as before. Analysis of 12 independent transfectant lines showed that all now lacked the DHCH1 ORF entirely, and western blot analysis with a polyclonal anti-DHCH1 antiserum confirmed a complete loss of DHCH1 protein (Fig. 4B, C). The ability of elevated FTL expression to enable parasites to readily dispense with DHCH1 is strong evidence of genetic metabolite complementation, presumably through the product of FTL activity, 10-CHO-THF, and in turn argues strongly that the essential role of DHCH1 is the provision of this metabolite.
FIG. 4. Deletion of chromosomal DHCH1 in the presence of ectopic FTL overexpression.
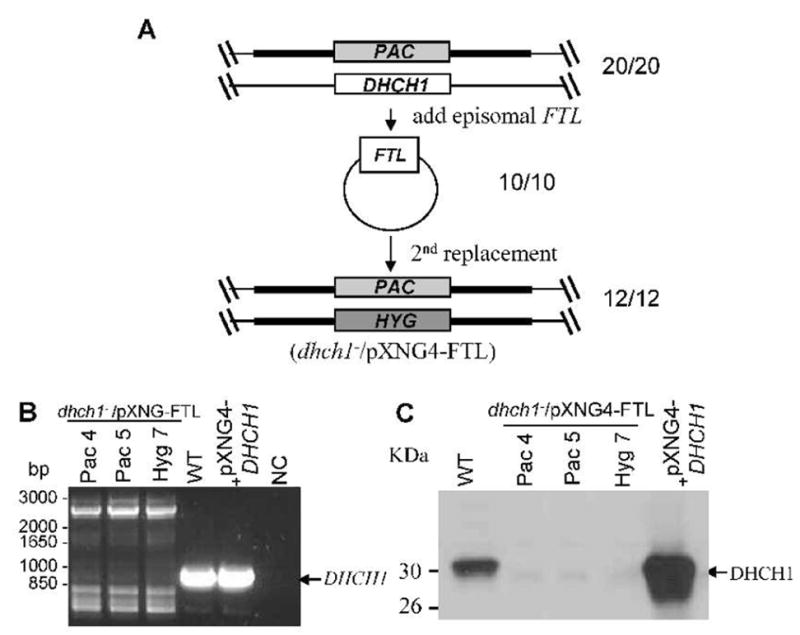
A. Scheme for the deletion of chromosomal DHCH1 in the presence of ectopically expressed FTL. A pXNG4-FTLepisomal expression construct was transfected into a DHCH1/PAC (DHCH1/Δdhch1::PAC) heterozygote described in Fig. 2A; 10/10 transfectants analyzed had the predicted genotype depicted. These DHCH1/PAC/+FTL lines (DHCH1/Δdhch1::PAC [pXNG4-FTL]) were then transfected with the HYG DHCH1 targeting fragment as described in the legend to Fig. 3A; of the 12 clonal lines tested, all showed the planned replacement of the chromosomal DHCH1 locus and were thus dhch1−/+FTL (Δdhch1::HYG DHCH1/Δdhch1::PAC [pXNG4-FTL]).
B – PCR tests showing loss of the chromosomal DHCH1. This was performed as described in the legend to Fig. 3B. NC, negative control lacking DNA; WT, WT DNA; pXNG-DHCH1, WT transfected with pXNG-DHCH1. Pac4, Pac5, and Hyg7 are three different lines showing an absence of the predicted chromosomal DHCH1 PCR product (shown by arrow). C. Western blot showing loss of DHCH1 expression in dhch1−/+FTL transfectants. The lines are as described in part B, extracts from which were probed with polyclonal rabbit antisera against DHCH1 (1:3,000) (Vickers et al., 2008).
Forcing loss of pXNG4-DHCH1 by negative selection
As a final test probing the essentiality of DHCH1 in WT Leishmania, we applied a method building on ‘plasmid shuffling’ approaches common in yeast genetics. Plasmid segregation based approaches have the advantage of allowing the separation of tests of gene function from transfection, which is relatively inefficient (D. Scott and S.M. Beverley, in preparation). In our studies this utilized two features of the episomal pXNG4 vector, namely the presence of both a negative selective marker (a modified HSV thymidine kinase gene) and a positive GFP fluorescence marker (Fig. 5A). The starting point was the dhch1−/pXNG4-DHCH1 parasite described earlier, where the chromosomal DHCH1 was ablated and parasite growth maintained by the episomal pXNG4-DHCH1 (Fig. 3, 5A).
FIG. 5. Plasmid segregational tests of DHCH1 essentiality.
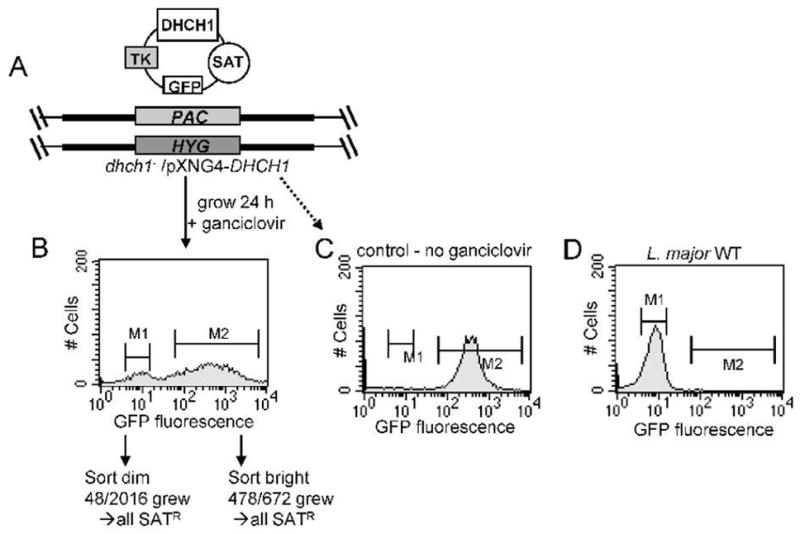
A. Starting dhch1−/+pXNG4-DHCH1 transfectant. The structure of one of the lines generated as described in Fig. 3A is shown, additionally showing the TK (ganciclovir sensitivity), GFP and SAT resistance markers encoded on the pXNG4-DHCH1 vector. B. Forced loss of pXNG4-DHCH1 by negative selection and sorting. The line depicted in Panel A was grown for 24 hr in the presence of ganciclovir but in the absence of nourseothricin. Fluorescent (bright) and non-fluorescent (dim) parasites were assigned using the M1 and M2 gates shown, and single cells were sorted into 96-well plates containing M199 medium and/or various supplements (see methods). With ‘bright’ cells, 478/672 (71 %) of the wells yielded growth. In contrast, only 48/2016 of wells inoculated with ‘dim’ cells (2.4 %) showed growth; all of these continued to grow in the presence of nourseothricin (SAT resistance) establishing retention of the pXNG-DHCH1 episome. C. Retention of pXNG4-DHCH1 in the absence of ganciclovir selection. The line shown in panel A was grown 24 hr without ganciclovir prior to GFP flow cytometry. D. WT L. major subjected to GFP flow cytometry.
dhch1−/pXNG4-DHCH1 parasites were grown briefly (24 h, or ~3 doublings) in the absence of nourseothricin (selective for the SAT marker of episomal pXNG4) but in the presence of ganciclovir, which through the action of HSV TK selects against parasites bearing pXNG4. Over this period little effect on parasite growth was seen. Then, parasites were subjected to flow cytometry, to assess plasmid copy number by following expression of the GFP marker. Two populations of cells were evident following this treatment: first, ‘bright’ cells showing strong fluorescence (>200 FU; M2 gate in Fig. 5B) and presumably high copy numbers of pXNG4-DHCH1; and ‘dim’ cells, showing background fluorescence (2–20 FU; M1 gate in Fig. 5B), presumably either lacking pXNG4-DHCH1, or bearing just a few copies. Controls showed that in the absence of ganciclovir the dhch1−/+pXNG4-DHCH1 parasites retained high GFP fluorescence, while WT cells showed background fluorescence only (Fig. 5C, 5D). As our previous results indicated that DHCH1 was essential, we expected that the ‘bright’ cells retaining pXNG4-DHCH1 would be viable, while the ‘dim’ cells would suffer a growth disadvantage and perhaps perish.
We used flow cytometry to select single cells into individual wells of a 96 well microtiter plate, focusing on the ‘dim’ (M1) or ‘bright’ (M2) parasites from the M1 population shown in Fig. 5B. We inoculated parasites into plates containing M199 media containing puromycin and hygromycin B (to guarantee retention of dhch1− cells) but lacking ganciclovir or nourseothricin (pXNG4 markers). Several experiments were performed with media supplemented additionally with the various metabolites described earlier.
In these studies, 478/672 of the wells inoculated with a single ‘bright’ parasite grew out (71 %), a value similar to that seen with controls probing the effects of sorting, recovery and growth of single cells in the cells (50 – 80%). In contrast, in wells receiving a single ‘dim’ parasite, growth was seen in only 48/2016 (2.4 %) (Fig. 5B). Importantly, all 48 ‘dim’ parasite lines retained the pXNG4-DHCH1 plasmid, as judged by their ability to grow in the presence of nourseothricin (SATR) and/or DHCH1 PCR tests. We presume these cells arose either by imperfect sorting, and/or from cells originally expressing low levels of GFP due to the presence of only one or a few copies of pXNG4-DHCH1. After correcting for the intrinsic plating efficiency, we estimate that in total approximately 1430 cells (71% of 2016) had been scored for their ability to grow in the absence of DHCH1. Thus plasmid shuffling enabled us to extend the stringency of our selection from a total of 65 events scored by the ‘classic’ transfection based approach, to a value more than 20-fold higher.
Interaction of L. major DHCH1 with novel antifolates
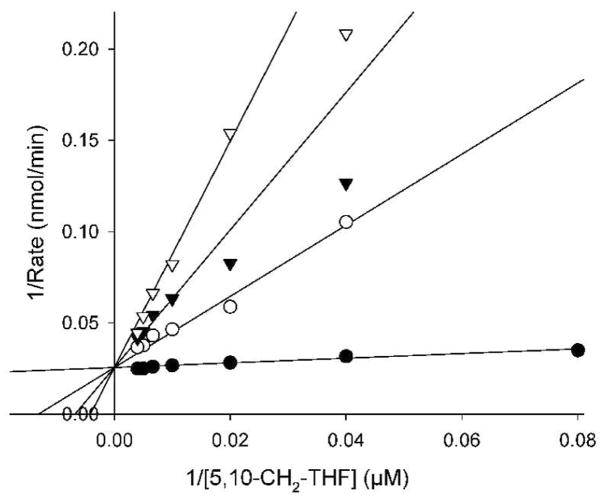
To corroborate the conclusion that DHCH activity was essential for Leishmania growth, we turned to pharmacological tests. The substrate analogue 5,10-CO-THF ((2S)-2-[[4-[(6aR)-3-amino-1,9-dioxo-5,6,6a,7-tetrahydro-4H- imidazol[3,4-f]pteridin-8-yl]benzoyl]amino]pentanedioic acid) was previously shown to be a potent inhibitor of the DHCH activity of the human cytosolic C1-synthase (Tonkinson et al., 1998, Temple et al., 1982). We predicted that this compound should also be a potent inhibitor of the Leishmania DHCH1, based on the overall sequence conservation, including the residues shown to intact with this inhibitor in structural studies of the human C1-synthase DHCH domain (Schmidt et al., 2000) (Fig. S2). Indeed, 5,10-CO-THF was a potent inhibitor of the DH activity of recombinant Leishmania DHCH1, with the data fitting to a simple competitive model with respect to 5,10-CH2-THF, giving a Ki of 105 ± 2 nM (Fig. 6). This was about 10-fold higher than the Ki of 18 ± 5 nM seen for the DH activity of the human cytosolic C1-synthase (Schmidt et al., 2000). While 5,10-CO-THF was shown previously to be a weak inhibitor of the human DHFR (Ki > 100 μM) (Temple et al., 1982), 100 μM 5,10-CO-THF did not inhibit the activity of Leishmania DHFR-TS (data not shown) nor FTL (Vickers et al., 2008). L. major DHCH1 was also not inhibited by pteridine analogues shown previously to inhibit L. major DHFR or PTR1, including methotrexate or hydrophobic diamino-quinazoline, -pyrimidine or -pteridine analogues (compound 34: 2,4-diamino-6-(3,4-dichlorophenoxy)-quinazoline); compound 70; 2,4-diamino-6-benzyl-5-(3-phenylpropyl)-pyrimidine and compound 25 (2,4-diamino-6,7-diisopropylpteridine) (Hardy et al., 1997, Bello et al., 1994) (tested at 50 μM; data not shown).
FIG. 6. Inhibition of L. major DHCH1 by 5,10-CO-THF.
The dehydrogenase activity of purified recombinant DHCH1 (1.3 μg was assayed in the presence of 1 mM NADP+ and varying concentrations of 5,10-CH2-THF substrate (12.5 to 250 μM) in the presence of no (●), 1.5 μM (○), 3 μM (▼) or 5 μM (▽) inhibitor. The lines show a global fit of these data to a simple competitive mode of inhibition.
Toxicity of folate analogues to cell lines
5,10-CO-THF inhibited L. major growth with an EC50 of 1.1 ± 0.04 μM (Table 2). Parasites overexpressing DHCH1 (WT/pXNG4-DHCH1 or dhch1−/+pXNG4-DHCH1) showed 4–5 fold resistance (p < 0.0001 and p < 0.0003, respectively; Table 2), consistent with the studies of purified DHCH above. Parasites overexpressing FTL (WT/pXNG4-FTL or dhch1−/pXNG4-FTL) also showed 3-fold resistance (p < 0.0001 and p < 0.0002, respectively; Table 2), implying that FTL activity could act as a metabolic by-pass for DHCH inhibition. Consistent with the enzyme inhibition data, DHCH1 or FTL overexpressors did not show resistance to methotrexate or two of the hydrophobic antifolates tested above (compounds 34 and 70; Table 2). Interestingly, even in the absence of a DHCH target (dhch1−/pXNG4-FTL line), growth was inhibited with an EC50 in the μM range (Table 2), suggesting 5,10-CO-THF has other targets in Leishmania. FTL can be excluded as it is not sensitive to 5,10-CO-THF, and while the bridged structure of the inhibitor does not closely resemble that of 10-CHO-THF, it seems probable that the target could be other folate-dependent enzymes or transporters within the cell.
TABLE 2.
Effects of 5,10-CO-THF and antifolates compound on growth of FV1 promastigote lines grown in M199 culture medium
| L. major FV1 Lines | 5,10-CO-THF EC50 (μM) | MTX EC50 (nM) | Compound 34 EC50 (nM) | Compound 70 EC50 (nM) |
|---|---|---|---|---|
| WT | 1.1 ± 0.04 * | 36 ± 1 | 680 ± 90 | 440 ± 50 |
| dhch1−/pXNG4-DHCH1 | 4.5 ± 0.5 | 41 ± 2 | 760 ± 80 | 400 ± 50 |
| dhch1−/pXNG4-FTL | 3.3 ± 0.3 | 40 ± 2 | 540 ± 60 | 420 ± 40 |
| WT/pXNG4-DHCH1 | 5.1 ± 0.8 | n.d. | n.d. | n.d. |
| WT/pXNG4-FTL | 3.7 ± 0.5 | n.d. | n.d. | n.d. |
Compound 5,10-CO-THF is ((2S)-2-[[4-[(6aR)-3-amino-1,9-dioxo-5,6,6a,7-tetrahydro-4H- imidazo[3,4-f]pteridin-8-yl]benzoyl]amino]pentanedioic acid); MTX: Methotrexate; Compound 34 is (2,4-diamino-6-(3,4-dichlorophenoxy)-quinazoline); Compound 70 is (2,4-diamino-6-benzyl-5-(3-phenylpropyl)-pyrimidine).
EC50 for 5,10-CO-THF differs significantly between WT and all other lines tested (p < 0.0001 to p < 0.0003). n.d. not determined.
Discussion
We show here that L. major expresses an active bifunctional NADP+-dependent DHCH encoded by DHCH1 (Table 1). Previous studies have localized DHCH1 protein to the parasite cytosol, consistent with the predicted lack of an N terminal mitochondrial targeting sequence and the NADP dependency of the DH activity (Vickers et al., 2008). Attempts to generate dhch1− null mutants by homologous replacement were unsuccessful, despite analysis of a large number of independent events and the use of metabolic supplements shown in other organisms to rescue DHCH-deficiency. Instead, these studies invariably yielded parasites bearing the two planned replacements, but also bearing extra copies of DHCH1 that most likely arose through aneuploidy. The unusual Leishmania phenotype of the recovery of planned replacements, but with retention of the target gene through aneuploidy or tetraploidy, is widely used as an indication that a gene is gene essential in this organism. As a technical control, chromosomal dhch1− replacements were readily obtained after ectopic expression of DHCH1. Lastly, we applied a plasmid shuffling approach that allowed us to increase the number of events analyzed 20 fold: from 65 by classic transfectional approaches, to more than 1,400 through negative selection and cell sorting. However, we were still unable to recover a viable dhch1− null mutant (the virtues and potential of the ‘plasmid shuffle’ methodology in Leishmania are discussed further below). Thus we are confident in concluding that DHCH1 is an essential gene in L. major under these circumstances.
Notably, we were able to completely remove DHCH1 following ectopic expression of FTL, which provides an alternative metabolic route to 10-CHO-THF synthesis via the formate tetrahydrofolate ligase activity. This form of ‘genetic metabolic complementation’ suggests that parasite dependency on DHCH1 activity arises through the provision of its ultimate metabolite of both FTL and DHCH1, 10-CHO-THF. Hypothetically, the active metabolite could be 5,10-CH=THF, as these two metabolites interconvert at significant rates under physiological conditions, which also precludes measurement of their individual abundance in the cell (Brouwer et al., 2007). However, as yet there is no known essential role in metabolism for 5,10-CH=THF, other than as an intermediate in 10-CHO-THF synthesis, and we thus favor the conclusion that 10-CHO-THF is the relevant metabolite.
Interestingly, the levels of WT FTL activity appear insufficient to rescue the dhch1− parasites, consistent with our previous finding that null mutant ftl− parasites were phenotypically normal when grown in vitro and are fully infective to susceptible mice (Vickers et al., 2008). Hypothetically, it is possible that elevated FTL synthesis or other alterations in 10-CHO-THF metabolism could bypass the requirement for DHCH1 under some circumstances, and indeed in other studies we have been able to detect second site mutations including FTL gene amplification in other selections forcing loss of DHCH1 (S. Murta and S. Beverley, unpublished data).
What is the role of 10-CHO-THF in Trypanosomatid metabolism?
In mammals, known roles of 10-CHO-THF include the provision of formyl groups in purine biosynthesis, as an intermediate C1-THF interconversions involved in amino acid metabolism (glycine, methionine, histidine, and serine), or the production of fMet- tRNAMet in the mitochondrion (Appling, 1991, Christensen & MacKenzie, 2006). However, Leishmania are well known to be purine auxotrophs as their genome lacks the purine biosynthetic enzymes glycinamide ribonucleotide (GAR) and phosphoribosylaminoimidazolecarboxamide (AICAR) transformylases (EC 2.1.1.2 and 2.1.2.3 respectively) (Carter et al., 2008). Similarly, several studies have established that Leishmania still require the amino acids arising through the C1-pathway for normal growth (Scott et al., 2008, Vickers et al., 2008). Thus under the conditions tested, formylation of the initiator Met-tRNAMet currently stands as the only candidate essential metabolic role. As discussed earlier, the extent to which fMet- tRNAMet synthesis is required is a matter of some debate, with differences seen in studies both within and between species in both eukaryotes and prokaryotes. However, the conclusion that Leishmania require fMet-tRNAMet would be consistent with the finding that the related trypanosomatid parasite T. brucei requires formylated initiator methionyl-tRNAMet for mitochondrial protein synthesis for survival (Tan et al., 2002, Charriere et al., 2005). This would then suggest that cytoplasmic 10-CHO-THF arising from either FTL or DHCH1 activity in the cytosol must then be transported to the mitochondrion. While generally it is thought that metabolites other than reduced folates are the principal route of communication for cytosolic-mitochondrial C1-dependent pathways in mammalian cells (Appling, 1991), some studies point to trafficking of C1 tetrahydrofolates between the cytosol and mitochondrion in plants (Prabhu et al., 1998, Hanson et al., 2000).
The interpretations above rest upon the strength and accuracy of current genome-based models of Trypanosomatid metabolomes. Given the substantial number of hypothetical proteins of unknown function encoded in Trypanosomatid genomes (Peacock et al., 2007, Ivens et al., 2005, El-Sayed et al., 2005, Berriman et al., 2005), one cannot exclude the possibility that Leishmania possess novel essential pathways that require 10-CHO-THF. Indeed, the L. major genome predicts a highly degenerate pseudogene (pseDHCH2) and DHCH2-related sequences are completely absent in the L. braziliensis and African trypanosome genomes (Peacock et al., 2007, Ivens et al., 2005, El-Sayed et al., 2005, Berriman et al., 2005). In contrast, seemingly intact DHCH2s occur in L. infantum, L. mexicana, Crithidia fasciculata and Trypanosoma cruzi, whose predicted proteins show conservation of DHCH active site residues (Vickers et al., 2008). Interestingly, a GFP tagged LiDHCH2 is localized to the mitochondrion when expressed in L. donovani (Vickers et al., 2008), although we have not been able to demonstrate DHCH activity associated with over-expression of WT LiDHCH2 (data not shown). Potentially the occurrence of a catalytically active DHCH2 could signify an increased importance of mitochondrial 10-CHO-THF synthesis in some trypanosomatid species. In the future, direct studies of mitochondrial fMet-tRNAMet synthesis mediated by FMT and whether DHCH2 is enzymatically active in those trypanosomatids that possess it will test these hypotheses.
Plasmid segregation and shuffling as an improved test of gene function
Currently, the standard approach to determining if a gene is essential in Leishmania is an inability to delete both chromosomal alleles by homologous gene replacement (Cruz et al., 1991, Barrett et al., 1999). Fortunately, these studies are facilitated by the fact that homologous recombination occurs far more frequently than non-homologous insertions in trypanosomatids (Beverley, 2003, Clayton, 1999). Ignoring technical failures, the general experience of most investigators has been that attempts to delete essential genes in Leishmania yield parasites bearing the planned allelic replacements but containing additional gene copies that arise via aneuploidy or polyploidy (Cruz et al., 1993, Dumas et al., 1997, Ilgoutz et al., 1999b, Vergnes et al., 2005, Balana-Fouce et al., 2008). This is often considered a diagnostic criterion, especially when accompanied by successful replacement in the presence of an ectopic gene.
One challenge of the gene replacement-based approach is the relative inefficiency of transfection (10−4 or less), limiting the number of events that can be scored. For example, here we analyzed 65 events targeting the last copy of DHCH1, a number exceeding that typically reported in tests of Leishmania gene function. Other factors impinging on replacement-based approaches include that transfection efficiencies can vary greatly depending on the locus and targeting construct used (Papadopoulou & Dumas, 1997), and that survivors can also emerge through mutations unrelated to targeted gene (unpublished data). In contrast, in the plasmid shuffling approach, functional tests arise through segregation of an ectopically expressed gene borne on a plasmid bearing both positive (GFP) and negative (TK) markers, in a line previously engineered to lack the gene of interest (Fig. 5). Following a brief period of culture to allow segregation of the episomal vector, a large number of candidate ‘segregants’ can be obtained through appropriate manipulations of the GFP or TK markers, and rapidly tested. This allowed us to rapidly score ~1400 such segregants for DHCH1, at least 20 times the number achieved by traditional methods, and if necessary many more could have been tested. Thus we believe that the plasmid segregation method provides a significant improvement to tests of essential gene function in Leishmania, as first established in the yeast Saccharomyces cerevisiae (Forsburg, 2001). In the future one can envisage a number of other applications based on the approach of ‘plasmid shuffling’.
DHCH1 and 10-CHO-THF metabolism as a target for anti-parasitic chemotherapy
L. major folate metabolism shows many differences from that of their human hosts, including a bifunctional dihydrofolate reductase-thymidylate synthase (DHFR-TS) (Coderre et al., 1983), a novel pteridine reductase (PTR1) (Bello et al., 1994), a large and complex family of folate and biopterin transporters (Ouellette et al., 2002), and cytosolic but not mitochondrial synthesis of 10-CHO-THF (Vickers et al., 2008), amongst others. These factors contribute to profound differences in antifolate sensitivity; for example, the relative insensitivity of PTR1 to many antifolates and its ability to act as a metabolic bypass of DHFR inhibition accounts for the relative lack of efficacy of these drugs against Leishmania (Bello et al., 1994, Nare et al., 1997).
While some folate metabolizing enzymes are essential in Leishmania (DHFR-TS, folylpolyglutamyl-synthase) (Cruz & Beverley, 1990, El Fadili et al., 2002), others tested appear largely or wholly dispensable for growth and virulence, including methylene tetrahydrofolate reductase and the glycine cleavage complex (Scott et al., 2008, Vickers et al., 2006). This may reflect the ability of the parasite to salvage sufficient quantities of many C1-THF dependent metabolites such as amino acids and purines (Carter et al., 2008, Scott et al., 1987), particularly in the mammalian stages where the parasite reside within phagolysosomes that are postulated to be rich in free amino acids (McConville et al., 2007). In contrast, we were unable to rescue the requirement for DHCH1 with any of a wide array of metabolites known or postulated to rescue DHCH deficiency. This could reflect many factors including a deficiency in a non-salvageable metabolite, a conclusion consistent with an essential requirement for mitochondrial fMet-tRNAMet.
For a parasite enzyme to be a good drug target it must be both essential for parasite survival, and dispensable, absent, or substantially different in the host (Fairlamb, 2002). In mammals both the cystosolic and mitochondrial forms of DHCH (MTHFD1 and MTHFD2 respectively) are essential for early development (Di Pietro et al., 2002, Brody et al., 2002, Parle-McDermott et al., 2005). However, human fibroblasts lacking either enzyme are viable, but are purine or glycine auxotrophs (Christensen et al., 2005b, Patel et al., 2003), and 10-CHO-THF metabolism is dispensable in the yeast S. cerevisiae when grown in rich media (West et al., 1996). This suggests that the adult tissues of the human host may be less dependent upon DHCH activity than Leishmania. In contrast, the 10-CHO-THF pathway is simplified in many parasitic protozoans, where many species synthesize a single cytosolic DHCH and FTL (L. major, L. braziliensis), while others synthesize just a cytosolic DHCH (African trypanosomes) (Vickers et al., 2008). Preliminary genomic analysis suggest that like African trypanosomes, the malaria parasite P. falciparum also possess a single route to 10-CHO-THF synthesis, via a bifunctional DHCH (Nzila et al., 2005). These data suggest that the relative simplicity of the 10-CHO-THF pathway in parasitic protozoa relative to their human host may render them more sensitive to chemotherapeutic interventions.
While few DHCH inhibitors have been developed, L. major DHCH1 activity and promastigote growth in vitro were sensitive to the substrate analog 5,10-CO-THF (Temple et al., 1982). The specificity of this inhibitor was supported by the findings that DHCH1 overexpressors are less susceptible, and it fails to inhibit DHFR or FTL enzymatic activity. However, over-expression of FTL did not completely overcome 5,10-CO-THF inhibition suggesting it could have other targets beyond DHCH1.
Consistent with the genetic studies above, DHCH inhibitors have little activity or toxicity against mammalian cells in vitro, or in mouse cancer models, at concentrations below 10 μM (Tonkinson et al., 1998, Temple et al., 1982). In preliminary studies we found modest activity of 5,10-CO-THF against Leishmania amastigotes in macrophages (EC50 ~ 5 μM), although host cell toxicity was seen above 10 μM (S. Hickerson and S. Beverley, unpublished data). These results are encouraging since 5,10-CO-THF is actually more selective for the host than parasite enzymes (Ki = 18 v. 105 nM respectively; (Schmidt et al., 2000) Fig. 6). While highly conserved, numerous differences between Leishmania DHCH1 and the human cytosolic and mitochondrial DHCHs (Fig. S2) could be exploited to design more selective and potent inhibitors. DHCH inhibitors have not received a high priority in cancer chemotherapy programs due to the poor efficacy for the first ones tested, although a number of antifolates targeting 10-CHO-THF utilizing enzymes such as GAR transformylase have been developed (Zhang et al., 2003). Possibly, similar pharmacophores may hold promise in targeting 10-CHO-THF metabolism in Leishmania and other Trypanosomatids.
EXPERIMENTAL PROCEDURES
Reagents
(6-R,S)-Tetrahydrofolate (THF) and 5,10-CH=THF were obtained from Schircks Laboratories (Jona, Switzerland). (2S)-2-[[4-[(6aR)-3-amino-1,9-dioxo-5,6,6a,7-tetrahydro-4H- imidazo[3,4-f]pteridin-8-yl]benzoyl]amino]pentanedioic acid (5,10-CO-THF) was custom synthesized by SAFC Pharma, a division of Sigma-Aldrich Corporation, according to the method of Temple et al. (Temple et al., 1982); the compound was 96.2% pure by high-performance liquid chromatography. Methotrexate and compound 25 (2,4-Diamino-6,7-diisopropylpteridine) were purchased from Sigma, the folate analogues compound 34 (2,4-diamino-6-(3,4-dichlorophenoxy)-quinazoline) and compound 70 (2,4-diamino-6-benzyl-5-(3-phenylpropyl)-pyrimidine) were as described (Hardy et al., 1997). Hygromycin B (HYG) was purchased from Calbiochem (EMD Biosciences, San Diego, CA) and nourseothricin (SAT) was from Werner BioAgents (Jena, Germany). All other chemicals and reagents were of analytical grade and purchased from Sigma-Aldrich.
Parasite growth and drug inhibition
All studies used derivatives of L. major clone Friedlin V1 promastigotes, grown at 27°C in M199 medium (US Biologicals) supplemented with 40 mM 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES) pH 7.4, 50 μM adenosine, 1 μg ml−1 biotin, 5 μg ml−1 hemin, 2 μg ml−1 biopterin and 10 % (v/v) heat-inactivated fetal calf serum. Growth was determined by seeding parasites at 5 × 105 cells ml−1 at various concentrations of drug and counting cells using a model Z1 Coulter counter when cultures reached late-log phase. The EC50 value is the concentration of drug required to decrease growth by 50%.
Cloning of L. major DHCH1
The DHCH1 coding sequence was amplified with recombinant Pfu DNA polymerase (Stratagene) using L. major genomic DNA template, prepared by the LiCl mini-prep method (Medina-Acosta & Cross, 1993), using primers SMB3528 and SMB3529, which added 5′ NdeI and 3′ BamHI sites, respectively. The amplified product was cloned into pCR.blunt (Invitrogen), yielding pCR.blunt. DHCH1 (B6255) and the NdeI-BamHI fragment transferred into the corresponding sites of pET15b (Novagen), yielding pET15b-LmjDHCH1 (B6026).
Expression, purification and enzymatic assays of recombinant L. major DHCH1
Protein was expressed in E. coli from pET15b-LmjDHCH1 and purified by metal-affinity chromatography, as described in supplemental methods. During the purification and storage of this protein the inclusion of 10% glycerol in all buffers was required to prevent loss of activity. In this manner, the enzyme could be flash-frozen with no loss in activity. DHCH activities were measured spectrophotometrically, using a temperature-controlled Beckman DU-640 spectrophotometer, essentially as described by Tan et al. (Tan et al., 1977) following either the formation of 5,10-CH=THF from 5,10-CH2-THF (DH activity) or the conversion of 5,10-CH=THF to 10-CHO-THF (CH activity). 5,10-CH2-THF dehydrogenase assays were carried out in a 0.5 ml volume at 27°C and contained 25 mM MOPS, pH 7.3, 250 μM THF, 2.5 mM formaldehyde, 1 mM NADP+ and 30 mM 2-mercaptoethanol. Assays were preincubated for 5 min to allow formation of 5,10-CH2-THF and reactions initiated by the addition of enzyme. The reactions were then stopped after 5 min by the addition of an equal volume of 1 M HCl and the 5,10-CH=THF produced quantified at 350 nm, using a extinction coefficient of 24.9 mM−1 cm−1. DH activity was expressed as μmoles 5,10-CH=THF produced per minute. Formaldehyde stocks were freshly-prepared from paraformaldehyde, and THF dissolved just before use in 250 mM triethanolamine-Cl, pH 7, 40 mM 2-mercaptoethanol. Cyclohydrolase activity was measured in 0.5 ml assays at 27°C containing 25 mM MOPS, pH 7.3, 30 mM 2-mercaptoethanol and 50 μM 5, 10-CH=THF. The hydrolysis of 5,10-CH=THF was followed at 355 nm, and the non-enzymatic rate subtracted.
Enzyme inhibition was measured using the dehydrogenase assay, with stocks of hydrophobic diaminoquinazolines and diaminopteridines dissolved in DMSO, and 5,10-CO-THF dissolved in 250 mM triethanolamine-Cl, pH 7, 40 mM 2-mercaptoethanol, as with THF, and stored at −80 °C. 5,10-CO-THF was custom synthesized by Sigma-Aldrich Chemical Pvt. Ltd. (Bangalore, India); the authenticity of the procuct was supported by infrared spectroscopy and proton NMR and it was estimated to be greater than 96% pure. Data from 5,10-CO-THF inhibition assays were fitted to competitive, non-competitive, uncompetitive and mixed inhibition models by non-linear regression using Sigmaplot. The DHFR activity of the bifunctional L. major DHFR-TS was measured as described (Hardy et al., 1997). Activity in all other drug assays was expressed as the percentage of a no-drug DMSO control.
DHCH1 replacement constructs
Fusion PCR (Szewczyk et al., 2006) was used to generate the two deletion constructs, which contained either puromycin (PAC) or hygromycin (HYG) drug-resistance cassettes between the 5′ and 3′ LmjDHCH1 flanking sequences (Szewczyk et al., 2006). Briefly, the flanking sequences and drug resistance cassettes were amplified separately by PCR using primers that produce overlapping ends. The 5′ and 3′ LmjDHCH1 flanking sequences (743 and 726 bp), were amplified using the primers SMB2772/2656 and 2657/2773 that added a SphI and BamHI site, respectively. PAC and HYG drug-resistance cassettes (600 and 1026 bp) were amplified using the primers SMB2557/2558 and SMB2561/2562, respectively that added a linker sequence. Each deletion construct was then made by mixing the purified PCR products for the flanking sequences and a drug-resistance cassette and using these products as templates in a second round of PCR. The two PCR reactions to generate the PAC and HYG constructs both used the external primers SMB2772 and 2773, and fused the flanking sequences to the correct drug resistance cassette. These two deletion constructs were then cloned into pGEM-T (Promega), yielding pGEM-T.5′. PAC.3′. LmjDHCH1 (B4827) and pGEM-T.5′. HYG.3′. LmjDHCH1 (B4827). The sequences of these constructs were confirmed by restriction mapping and sequencing.
DHCH1 replacement and transfections
DHCH1-targeting fragments were digested with SphI and BamHI, and the linear targeting fragments containing PAC (2.1 kb) or HYG (2.5 kb) were transfected separately into wild-type (WT) L. major FV1 promastigotes as described (Robinson & Beverley, 2003). Heterozygous clones, DHCH1/Δdhch1::PAC or DHCH1/Δdhch1::HYG (referred as PAC/+ or HYG/+), were isolated by plating on semisolid M199 medium containing 30 μg ml−1 puromycin or 50 μg ml−1 hygromycin B, respectively. Heterozygous PAC/+ or HYG/+ clones were transfected with deletion constructs containing HYG or PAC cassettes. The parasites were plated in semisolid M199 medium containing 30 μg ml−1 puromycin and 50 μg ml−1 hygromycin. In attempts to rescue the growth of DHCH1−/− homozygotes several different mixtures of different supplements were added to the plating medium. The basic supplements were: glycine (1 mg ml−1), serine (0.1 mg ml−1), formate (5 mM), THF (5 μM), thymidine (10 μg ml−1) and folinic acid (10 μg ml−1). Additional supplements were added to the basic mixture in other plates, these were: 5,10-CH=THF (10 μM), 10-formylfolic acid 10-CHOFA (10 μM) or, alternatively, uridine (0.1 mg ml−1) and sodium pyruvate (1 mM). DNA contents of permeabilized RNAse treated cells were measured by propidium iodide staining using flow cytometry (Cruz et al., 1993).
PCR primers for assessing chromosomal DHCH1 or PAC or HYG replacements
Generation of the planned PAC or HYG replacements was confirmed by PCR, using a primer located 5′ (SMB2731) or 3′ (SMB2771) of the DHCH1 ORF targeting fragment and a primer located within the PAC or HYG genes (SMB2557/2558 or SMB2561/2562). The loss of the DHCH1 ORFs was confirmed using primers specific for the chromosomal DHCH1 allele (SMB3125/3126) or using a forward primer located in the chromosome 5′ (SBM2731) of the DHCH1 ORF targeting fragment and a reverse primer located within the DHCH1 gene (SMB3126).
pXNG4 expression constructs
DHCH1 and FTL ORFs were amplified with primers (SMB3125/3126 and SMB3123/3124, respectively) from L. major genomic DNA, adding flanking BglII sites, and a CCACC sequence preceding the initiation codon. The DHCH1 and FTL ORFs were inserted into pGEM-T (Promega) yielding pGEM-DHCH1 (B6029) and pGEM-FTL (B6030); the DHCH1 and FTL ORFs were then extracted by BglII digestion and inserted into the BglII site of pXNG4 (B5840) to create pXNG4-DHCH1 (B6031) and pXNG4-FTL (B6032).
These plasmids were transfected into either wild-type L. major FV1, or heterozygote lines (DHCH1/Δdhch1::PAC or DHCH1/Δ dhch1:: HYG) and transformants isolated as before, using plating media containing 100 μg ml−1 nourseothricin and either 30 μg ml−1 puromycin or 50 μg ml−1 hygromycin. The presence of plasmid was confirmed by PCR and FACS analysis for the presence of SAT and GFP markers, respectively. Three colonies over-expressing FTL or DHCH1 were submitted to second replacement using either the PAC or HYG targeting fragments. The parasites were isolated by plating on semi-solid M199 media containing 100 μg ml−1 nourseothricin, 30 μg ml−1 puromycin and 50 μg ml−1 hygromycin.
Single-cell sorting
Prior to flow cytometry, dhch1−/pXNG4-DHCH1 lines were incubated with 50 μg ml−1 ganciclovir in M199 media for 24 h. Single cells sorting was then performed based upon their GFP fluorescence as described in the Results. Cells were resuspended in phosphate-buffered saline, and filtered through CellTrics 50 μm filters to remove clumps (Partec). Single cells were then recovered using a Dako MoFlo high-speed cell sorter and placed into individual wells of 96-well plates, each containing 150 μl M199 medium. Plates were incubated at 27° C for 1–2 weeks and parasite growth was scored.
Supplementary Material
Acknowledgments
We thank A. Hanson and M. Ouellette for discussions. We thank Sushil Rajan and Sigma-Aldrich Chemical Pvt. Ltd. for custom synthesis of 5,10-CO-THF. Single-cell sorting was done with the assistance of the staff of the high-speed sorter core, Alvin J. Siteman Cancer Center, Washington University Medical School. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842. This work was supported by National Institutes of Health Grant AI21903 (to S.M.B.); Brazilian agencies: CNPq and FAPEMIG (to S.M.F.M.) and European Molecular Biology Organization long term fellowship ALTF 106–2005 (to T. V.).
Abbreviations
- THF
tetrahydrofolate
- 10-CHO-THF
10-formyl tetrahydrofolate
- 5,10-CH=THF
5,10-methenyl-tetrahydrofolate
- 5,10-CH2-THF
5,10-methylene-tetrahydrofolate
- DHCH1
gene encoding Leishmania cytosolic methenyltetrahydrofolate cyclohydrolase/5,10-methylenetetrahydrofolate dehydrogenase
- FTL
formate-tetrahydrofolate ligase
- DHFR-TS
dihydrofolate reductase-thymidylate synthase
- WT
wild-type
- DH
dehydrogenase
- CH
cyclohydrolase
Footnotes
Supplemental information for “Methylene tetrahydrofolate dehydrogenase/cyclohydrolase and the synthesis of 10-CHO-THF are essential in Leishmania major”, by Silvane M. F. Murta, Tim J. Vickers, David A. Scott, and Stephen M. Beverley.
References
- Allaire M, Li Y, MacKenzie RE, Cygler M. The 3-D structure of a folate-dependent dehydrogenase/cyclohydrolase bifunctional enzyme at 1.5 A resolution. Structure. 1998;6:173–182. doi: 10.1016/s0969-2126(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Appling DR. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. Faseb J. 1991;5:2645–2651. doi: 10.1096/fasebj.5.12.1916088. [DOI] [PubMed] [Google Scholar]
- Balana-Fouce R, Garcia-Estrada C, Perez-Pertejo Y, Reguera RM. Gene disruption of the DNA topoisomerase IB small subunit induces a non-viable phenotype in the hemoflagellate Leishmania major. BMC Microbiol. 2008;8:113. doi: 10.1186/1471-2180-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett MP, Mottram JC, Coombs GH. Recent advances in identifying and validating drug targets in trypanosomes and leishmanias. Trends Microbiol. 1999;7:82–88. doi: 10.1016/s0966-842x(98)01433-4. [DOI] [PubMed] [Google Scholar]
- Bello AR, Nare B, Freedman D, Hardy L, Beverley SM. PTR1: a reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 1994;91:11442–11446. doi: 10.1073/pnas.91.24.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, Bohme U, Hannick L, Aslett MA, Shallom J, Marcello L, Hou L, Wickstead B, Alsmark UC, Arrowsmith C, Atkin RJ, Barron AJ, Bringaud F, Brooks K, Carrington M, Cherevach I, Chillingworth TJ, Churcher C, Clark LN, Corton CH, Cronin A, Davies RM, Doggett J, Djikeng A, Feldblyum T, Field MC, Fraser A, Goodhead I, Hance Z, Harper D, Harris BR, Hauser H, Hostetler J, Ivens A, Jagels K, Johnson D, Johnson J, Jones K, Kerhornou AX, Koo H, Larke N, Landfear S, Larkin C, Leech V, Line A, Lord A, Macleod A, Mooney PJ, Moule S, Martin DM, Morgan GW, Mungall K, Norbertczak H, Ormond D, Pai G, Peacock CS, Peterson J, Quail MA, Rabbinowitsch E, Rajandream MA, Reitter C, Salzberg SL, Sanders M, Schobel S, Sharp S, Simmonds M, Simpson AJ, Tallon L, Turner CM, Tait A, Tivey AR, Van Aken S, Walker D, Wanless D, Wang S, White B, White O, Whitehead S, Woodward J, Wortman J, Adams MD, Embley TM, Gull K, Ullu E, Barry JD, Fairlamb AH, Opperdoes F, Barrell BG, Donelson JE, Hall N, Fraser CM, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- Beverley SM. Protozomics: trypanosomatid parasite genetics comes of age. Nat Rev Genet. 2003;4:11–19. doi: 10.1038/nrg980. [DOI] [PubMed] [Google Scholar]
- Brody LC, Conley M, Cox C, Kirke PN, McKeever MP, Mills JL, Molloy AM, O’Leary VB, Parle-McDermott A, Scott JM, Swanson DA. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am J Hum Genet. 2002;71:1207–1215. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer VD, Zhang GF, Storozhenko S, Straeten DV, Lambert WE. pH stability of individual folates during critical sample preparation steps in prevision of the analysis of plant folates. Phytochem Anal. 2007;18:496–508. doi: 10.1002/pca.1006. [DOI] [PubMed] [Google Scholar]
- Carter NS, Yates P, Arendt CS, Boitz JM, Ullman B. Purine and pyrimidine metabolism in Leishmania. Adv Exp Med Biol. 2008;625:141–154. doi: 10.1007/978-0-387-77570-8_12. [DOI] [PubMed] [Google Scholar]
- Cavazzuti A, Paglietti G, Hunter WN, Gamarro F, Piras S, Loriga M, Allecca S, Corona P, McLuskey K, Tulloch L, Gibellini F, Ferrari S, Costi MP. Discovery of potent pteridine reductase inhibitors to guide antiparasite drug development. Proc Natl Acad Sci U S A. 2008;105:1448–1453. doi: 10.1073/pnas.0704384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charriere F, Tan TH, Schneider A. Mitochondrial initiation factor 2 of Trypanosoma brucei binds imported formylated elongator-type tRNA(Met) J Biol Chem. 2005;280:15659–15665. doi: 10.1074/jbc.M411581200. [DOI] [PubMed] [Google Scholar]
- Christensen KE, MacKenzie RE. Mitochondrial one-carbon metabolism is adapted to the specific needs of yeast, plants and mammals. Bioessays. 2006;28:595–605. doi: 10.1002/bies.20420. [DOI] [PubMed] [Google Scholar]
- Christensen KE, Mackenzie RE. Chapter 14. Mitochondrial methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase, and formyltetrahydrofolate synthetases. Vitam Horm. 2008;79:393–410. doi: 10.1016/S0083-6729(08)00414-7. [DOI] [PubMed] [Google Scholar]
- Christensen KE, I, Mirza A, Berghuis AM, Mackenzie RE. Magnesium and phosphate ions enable NAD binding to methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase. J Biol Chem. 2005a;280:34316–34323. doi: 10.1074/jbc.M505210200. [DOI] [PubMed] [Google Scholar]
- Christensen KE, Patel H, Kuzmanov U, Mejia NR, MacKenzie RE. Disruption of the mthfd1 gene reveals a monofunctional 10-formyltetrahydrofolate synthetase in mammalian mitochondria. J Biol Chem. 2005b;280:7597–7602. doi: 10.1074/jbc.M409380200. [DOI] [PubMed] [Google Scholar]
- Clayton CE. Genetic manipulation of kinetoplastida. Parasitol Today. 1999;15:372–378. doi: 10.1016/s0169-4758(99)01498-2. [DOI] [PubMed] [Google Scholar]
- Coderre JA, Beverley SM, Schimke RT, Santi DV. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983;80:2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A, Beverley SM. Gene replacement in parasitic protozoa. Nature. 1990;348:171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Cruz A, Coburn C, Beverley SM. Double targeted gene replacement for creating null mutants. Proceedings of the National Academy of Sciences. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AK, Titus R, Beverley SM. Plasticity in chromosome number and testing of essential genes in Leishmania by targeting. Proc Natl Acad Sci U S A. 1993;90:1599–1603. doi: 10.1073/pnas.90.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro E, Sirois J, Tremblay ML, MacKenzie RE. Mitochondrial NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase is essential for embryonic development. Mol Cell Biol. 2002;22:4158–4166. doi: 10.1128/MCB.22.12.4158-4166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas C, Ouellette M, Tovar J, Cunningham ML, Fairlamb AH, Tamar S, Olivier M, Papadopoulou B. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997;16:2590–2598. doi: 10.1093/emboj/16.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed NM, Myler PJ, Blandin G, Berriman M, Crabtree J, Aggarwal G, Caler E, Renauld H, Worthey EA, Hertz-Fowler C, Ghedin E, Peacock C, Bartholomeu DC, Haas BJ, Tran AN, Wortman JR, Alsmark UC, Angiuoli S, Anupama A, Badger J, Bringaud F, Cadag E, Carlton JM, Cerqueira GC, Creasy T, Delcher AL, Djikeng A, Embley TM, Hauser C, Ivens AC, Kummerfeld SK, Pereira-Leal JB, Nilsson D, Peterson J, Salzberg SL, Shallom J, Silva JC, Sundaram J, Westenberger S, White O, Melville SE, Donelson JE, Andersson B, Stuart KD, Hall N. Comparative genomics of trypanosomatid parasitic protozoa. Science. 2005;309:404–409. doi: 10.1126/science.1112181. [DOI] [PubMed] [Google Scholar]
- El Fadili A, Kundig C, Ouellette M. Characterization of the folylpolyglutamate synthetase gene and polyglutamylation of folates in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2002;124:63–71. doi: 10.1016/s0166-6851(02)00163-9. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH. Metabolic pathway analysis in trypanosomes and malaria parasites. Philos Trans R Soc Lond B Biol Sci. 2002;357:101–107. doi: 10.1098/rstb.2001.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL. The art and design of genetic screens: yeast. Nat Rev Genet. 2001;2:659–668. doi: 10.1038/35088500. [DOI] [PubMed] [Google Scholar]
- Hanson AD, Gage DA, Shachar-Hill Y. Plant one-carbon metabolism and its engineering. Trends Plant Sci. 2000;5:206–213. doi: 10.1016/s1360-1385(00)01599-5. [DOI] [PubMed] [Google Scholar]
- Hanson AD, Roje S. One-Carbon Metabolism in Higher Plants. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:119–137. doi: 10.1146/annurev.arplant.52.1.119. [DOI] [PubMed] [Google Scholar]
- Hardy LW, Matthews W, Nare B, Beverley SM. Biochemical and genetic tests for inhibitors of Leishmania pteridine pathways. Exp Parasitol. 1997;87:157–169. [PubMed] [Google Scholar]
- Hatefi Y, Osborn MJ, Kay LD, Huennekens FM. Hydroxymethyl tetrahydrofolic dehydrogenase. J Biol Chem. 1957;227:637–647. [PubMed] [Google Scholar]
- Ilgoutz SC, Mullin KA, Southwell BR, McConville MJ. Glycosylphosphatidylinositol biosynthetic enzymes are localized to a stable tubular subcompartment of the endoplasmic reticulum in Leishmania mexicana. EMBO J. 1999a;18:3643–3654. doi: 10.1093/emboj/18.13.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgoutz SC, Zawadzki JL, Ralton JE, McConville MJ. Evidence that free GPI glycolipids are essential for growth of Leishmania mexicana. EMBO J. 1999b;18:2746–2755. doi: 10.1093/emboj/18.10.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RM, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch AC, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Muller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O’Neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schafer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology. 2006;133(Suppl):S87–112. doi: 10.1017/S0031182006001831. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983;47:1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Holmes WB, Appling DR, RajBhandary UL. Initiation of protein synthesis in Saccharomyces cerevisiae mitochondria without formylation of the initiator tRNA. J Bacteriol. 2000;182:2886–2892. doi: 10.1128/jb.182.10.2886-2892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, de Souza D, Saunders E, Likic VA, Naderer T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007;23:368–375. doi: 10.1016/j.pt.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Medina-Acosta E, Cross GA. Rapid isolation of DNA from trypanosomatid protozoa using a simple ‘mini-prep’ procedure. Mol Biochem Parasitol. 1993;59:327–329. doi: 10.1016/0166-6851(93)90231-l. [DOI] [PubMed] [Google Scholar]
- Mejia NR, MacKenzie RE. NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase in transformed cells is a mitochondrial enzyme. Biochem Biophys Res Commun. 1988;155:1–6. doi: 10.1016/s0006-291x(88)81040-4. [DOI] [PubMed] [Google Scholar]
- Mishra J, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Curr Med Chem. 2007;14:1153–1169. doi: 10.2174/092986707780362862. [DOI] [PubMed] [Google Scholar]
- Moore MR, O’Brien WE, Ljungdahl LG. Purification and characterization of nicotinamide adenine dinucleotide-dependent methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum. J Biol Chem. 1974;249:5250–5253. [PubMed] [Google Scholar]
- Nare B, Luba J, Hardy LW, Beverley S. New approaches to Leishmania chemotherapy: pteridine reductase 1 (PTR1) as a target and modulator of antifolate sensitivity. Parasitology. 1997;114:S101–110. [PubMed] [Google Scholar]
- Newton DT, Creuzenet C, Mangroo D. Formylation is not essential for initiation of protein synthesis in all eubacteria. J Biol Chem. 1999;274:22143–22146. doi: 10.1074/jbc.274.32.22143. [DOI] [PubMed] [Google Scholar]
- Nour JM, Rabinowitz JC. Isolation, characterization, and structural organization of 10-formyltetrahydrofolate synthetase from spinach leaves. J Biol Chem. 1991;266:18363–18369. [PubMed] [Google Scholar]
- Nzila A, Ward SA, Marsh K, Sims PF, Hyde JE. Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol. 2005;21:292–298. doi: 10.1016/j.pt.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperdoes FR, Coombs GH. Metabolism of Leishmania: proven and predicted. Trends Parasitol. 2007;23:149–158. doi: 10.1016/j.pt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Ouellette M, Drummelsmith J, El-Fadili A, Kundig C, Richard D, Roy G. Pterin transport and metabolism in Leishmania and related trypanosomatid parasites. Int J Parasitol. 2002;32:385–398. doi: 10.1016/s0020-7519(01)00346-0. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B, Dumas C. Parameters controlling the rate of gene targeting frequency in the protozoan parasite Leishmania. Nucleic Acids Res. 1997;25:4278–4286. doi: 10.1093/nar/25.21.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parle-McDermott A, Pangilinan F, Mills JL, Signore CC, Molloy AM, Cotter A, Conley M, Cox C, Kirke PN, Scott JM, Brody LC. A polymorphism in the MTHFD1 gene increases a mother’s risk of having an unexplained second trimester pregnancy loss. Mol Hum Reprod. 2005;11:477–480. doi: 10.1093/molehr/gah204. [DOI] [PubMed] [Google Scholar]
- Patel H, Pietro ED, MacKenzie RE. Mammalian fibroblasts lacking mitochondrial NAD+-dependent methylenetetrahydrofolate dehydrogenase-cyclohydrolase are glycine auxotrophs. J Biol Chem. 2003;278:19436–19441. doi: 10.1074/jbc.M301718200. [DOI] [PubMed] [Google Scholar]
- Pawelek PD, MacKenzie RE. Methenyltetrahydrofolate cyclohydrolase is rate limiting for the enzymatic conversion of 10-formyltetrahydrofolate to 5,10-methylenetetrahydrofolate in bifunctional dehydrogenase-cyclohydrolase enzymes. Biochemistry. 1998;37:1109–1115. doi: 10.1021/bi971906t. [DOI] [PubMed] [Google Scholar]
- Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, Peters N, Adlem E, Tivey A, Aslett M, Kerhornou A, Ivens A, Fraser A, Rajandream MA, Carver T, Norbertczak H, Chillingworth T, Hance Z, Jagels K, Moule S, Ormond D, Rutter S, Squares R, Whitehead S, Rabbinowitsch E, Arrowsmith C, White B, Thurston S, Bringaud F, Baldauf SL, Faulconbridge A, Jeffares D, Depledge DP, Oyola SO, Hilley JD, Brito LO, Tosi LR, Barrell B, Cruz AK, Mottram JC, Smith DF, Berriman M. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu V, Chatson KB, Lui H, Abrams GD, King J. Effects of sulfanilamide and methotrexate on 13C fluxes through the glycine decarboxylase/serine hydroxymethyltransferase enzyme system in arabidopsis. Plant Physiol. 1998;116:137–144. doi: 10.1104/pp.116.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JC. Preparation and properties of 5,10-methenyltetrahydrofolic acid and 10-Formyltetrahydrofolic acid. Methods in Enzymology. 1963;6:814–815. [Google Scholar]
- Rabinowitz JC, Pricer WE., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962;237:2898–2902. [PubMed] [Google Scholar]
- RajBhandary UL. Initiator transfer RNAs. J Bacteriol. 1994;176:547–552. doi: 10.1128/jb.176.3.547-552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajBhandary UL. More surprises in translation: initiation without the initiator tRNA. Proc Natl Acad Sci U S A. 2000;97:1325–1327. doi: 10.1073/pnas.040579197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KA, Beverley SM. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128:217–228. doi: 10.1016/s0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Wu H, MacKenzie RE, Chen VJ, Bewly JR, Ray JE, Toth JE, Cygler M. Structures of three inhibitor complexes provide insight into the reaction mechanism of the human methylenetetrahydrofolate dehydrogenase/cyclohydrolase. Biochemistry. 2000;39:6325–6335. doi: 10.1021/bi992734y. [DOI] [PubMed] [Google Scholar]
- Schweitzer BI, Dicker AP, Bertino JR. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990;4:2441–2452. doi: 10.1096/fasebj.4.8.2185970. [DOI] [PubMed] [Google Scholar]
- Scott DA, Coombs GH, Sanderson BE. Folate utilization by Leishmania species and the identification of intracellular derivatives and folate-metabolizing enzymes. Mol Biochem Parasitol. 1987;23:139–149. doi: 10.1016/0166-6851(87)90149-6. [DOI] [PubMed] [Google Scholar]
- Scott DA, Hickerson SM, Vickers TJ, Beverley SM. The role of the mitochondrial glycine cleavage complex in the metabolism and virulence of the protozoan parasite Leishmania major. J Biol Chem. 2008;283:155–165. doi: 10.1074/jbc.M708014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N, Banerjee B, Gupta SS, Das BB, Ganguly A, Majumder HK. Leishmania donovani: dyskinetoplastid cells survive and proliferate in the presence of pyruvate and uridine but do not undergo apoptosis after treatment with camptothecin. Exp Parasitol. 2007;115:215–219. doi: 10.1016/j.exppara.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Spencer AC, Spremulli LL. Interaction of mitochondrial initiation factor 2 with mitochondrial fMet-tRNA. Nucleic Acids Res. 2004;32:5464–5470. doi: 10.1093/nar/gkh886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger O. Physiology of folic acid in health and disease. Curr Drug Metab. 2002;3:211–223. doi: 10.2174/1389200024605163. [DOI] [PubMed] [Google Scholar]
- Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc. 2006;1:3111–3120. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- Tan LU, Drury EJ, MacKenzie RE. Methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase. A multifunctional protein from porcine liver. J Biol Chem. 1977;252:1117–1122. [PubMed] [Google Scholar]
- Tan TH, Bochud-Allemann N, Horn EK, Schneider A. Eukaryotic-type elongator tRNAMet of Trypanosoma brucei becomes formylated after import into mitochondria. Proc Natl Acad Sci U S A. 2002;99:1152–1157. doi: 10.1073/pnas.022522299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple C, Jr, Bennett LL, Jr, Rose JD, Elliott RD, Montgomery JA, Mangum JH. Synthesis of pseudo cofactor analogues as potential inhibitors of the folate enzymes. J Med Chem. 1982;25:161–166. doi: 10.1021/jm00344a014. [DOI] [PubMed] [Google Scholar]
- Then RL. Antimicrobial dihydrofolate reductase inhibitors--achievements and future options: review. J Chemother. 2004;16:3–12. doi: 10.1179/joc.2004.16.1.3. [DOI] [PubMed] [Google Scholar]
- Tonkinson JL, Habeck LL, Toth JE, Mendelsohn LG, Bewley J, Shackelford KA, Gates SB, Ray J, Chen VJ. The antiproliferative and cell cycle effects of 5,6,7, 8-tetrahydro-N5, N10-carbonylfolic acid, an inhibitor of methylenetetrahydrofolate dehydrogenase, are potentiated by hypoxanthine. J Pharmacol Exp Ther. 1998;287:315–321. [PubMed] [Google Scholar]
- Varshney U, Lee CP, RajBhandary UL. From elongator tRNA to initiator tRNA. Proc Natl Acad Sci U S A. 1993;90:2305–2309. doi: 10.1073/pnas.90.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes B, Sereno D, Tavares J, Cordeiro-da-Silva A, Vanhille L, Madjidian-Sereno N, Depoix D, Monte-Alegre A, Ouaissi A. Targeted disruption of cytosolic SIR2 deacetylase discloses its essential role in Leishmania survival and proliferation. Gene. 2005;363:85–96. doi: 10.1016/j.gene.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Vial L, Gomez P, Panvert M, Schmitt E, Blanquet S, Mechulam Y. Mitochondrial methionyl-tRNAfMet formyltransferase from Saccharomyces cerevisiae: gene disruption and tRNA substrate specificity. Biochemistry. 2003;42:932–939. doi: 10.1021/bi026901x. [DOI] [PubMed] [Google Scholar]
- Vickers TJ, Murta SMF, Mandell MA, Beverley SM. 10-formyl-tetrahydrofolate synthesis is exclusively cytosolic in the trypanosomatid parasite. Leishmania major. 2008 doi: 10.1016/j.molbiopara.2009.03.009. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers TJ, Orsomando G, de la Garza RD, Scott DA, Kang SO, Hanson AD, Beverley SM. Biochemical and genetic analysis of methylenetetrahydrofolate reductase in Leishmania metabolism and virulence. J Biol Chem. 2006;281:38150–38158. doi: 10.1074/jbc.M608387200. [DOI] [PubMed] [Google Scholar]
- Weber S. Light-driven enzymatic catalysis of DNA repair: a review of recent biophysical studies on photolyase. Biochim Biophys Acta. 2005;1707:1–23. doi: 10.1016/j.bbabio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- West MG, Horne DW, Appling DR. Metabolic role of cytoplasmic isozymes of 5,10-methylenetetrahydrofolate dehydrogenase in Saccharomyces cerevisiae. Biochemistry. 1996;35:3122–3132. doi: 10.1021/bi952713d. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Desharnais J, Marsilje TH, Li C, Hedrick MP, Gooljarsingh LT, Tavassoli A, Benkovic SJ, Olson AJ, Boger DL, Wilson IA. Rational design, synthesis, evaluation, and crystal structure of a potent inhibitor of human GAR Tfase: 10-(trifluoroacetyl)-5,10-dideazaacyclic-5,6,7,8-tetrahydrofolic acid. Biochemistry. 2003;42:6043–6056. doi: 10.1021/bi034219c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.