Abstract
The opioid antagonist naltrexone (NTX) is used to treat alcohol dependence and may reduce alcohol consumption by selectively blocking opioid receptors. In rat experiments, discrepancy exists across studies regarding the potency of NTX to reduce ethanol consumption. One cause of this discrepancy may be the use of different routes of NTX administration (e.g., intraperitoneal vs. subcutaneous). The purpose of this study was to directly compare the effects of intraperitoneal and subcutaneous injections of NTX on ethanol self-administration. Rats pressed a lever for a sweetened ethanol solution (10% wt/vol in 0.1% saccharin) during 20 min daily sessions. One group received intraperitoneal injections of 1, 3, 10, and 30 mg/kg NTX before the sessions. Another group received subcutaneous injections of 0.03, 0.1, 0.3, and 1 mg/kg NTX before the sessions. The group that received subcutaneous NTX was also tested with a single intraperitoneal injection of 0.3 mg/kg NTX. Naltrexone significantly reduced ethanol self-administration, and NTX was more potent when administered via subcutaneous injection versus intraperitoneal injection. Ethanol intake (g/kg) was significantly reduced after subcutaneous injection of NTX 0.1 mg/kg and higher. In contrast, ethanol intake was significantly reduced after intraperitoneal injection of NTX 3 mg/kg and higher. A comparison of the NTX ED50 values showed that subcutaneous NTX was approximately 30-fold more potent than intraperitoneal NTX. For the subcutaneous 0.3 mg/kg NTX dose, a detailed bin analysis showed that responding during the first 2 min after injection was similar to that during the first 2 min after a saline injection while responding after NTX decreased in subsequent bins. These findings suggest that researchers should carefully consider the route of NTX administration when discussing potency and selectivity of NTX's effects on ethanol-related behaviors in rats. These findings further support the notion that NTX acts by terminating responding early rather than reducing the initial responding.
Keywords: Ethanol, Naltrexone, Naloxone, Intraperitoneal, Subcutaneous
Introduction
The opioid antagonist naltrexone (NTX) effectively reduced alcohol craving and relapse rates in early clinical trials with alcohol-dependent patients (O'Malley et al., 1992; Volpicelli et al., 1992). Since then, multicenter clinical trials and meta-analyses have confirmed that alcohol-dependent individuals experience some benefit from treatment with NTX (Anton et al., 2006; Pettinati et al., 2006). Other opioid antagonists, such as nalmefene, may produce benefits as well (Drobes et al., 2004; Mason et al., 1999). Opioid antagonists are hypothesized to reduce alcohol consumption by blocking the rewarding effects associated with an alcohol-stimulated increase in endogenous opioid activity (for reviews see Gianoulakis, 2001; Modesto-Lowe and Fritz, 2005).
The specific opioid receptor subtype that mediates the effects of ethanol and the ability of opioid antagonists to reduce ethanol consumption continue to be explored. The evidence in the literature is complicated and points to the involvement of multiple opioid receptor subtypes. For example, μ-selective antagonists such as CTOP, naloxonazine, and β-funaltrexamine (β-FNA) reduced ethanol consumption in rat studies (Hyytia and Kiianmaa, 2001; Krishnan-Sarin et al., 1998; Mhatre and Holloway, 2003; Stromberg et al., 1998). In addition, δ-selective antagonists such as ICI 174,864, naltrindole, and naltriben reduced ethanol intake in some studies (Froehlich et al., 1991; Krishnan-Sarin et al., 1995a,b). Even the κ-opioid receptor may be involved in ethanol consumption. Walker and Koob (2008) suggested that the κ receptor plays a role in the aversive properties of alcohol dependence, as they showed that nor-binaltorphimine decreases ethanol consumption only during ethanol withdrawal. Thus, ethanol consumption may be modulated by one or more opioid receptor subtypes.
NTX, along with naloxone, is more commonly used in animal experiments and is typically considered a “nonselective” antagonist. NTX and naloxone have the ability to bind to all three opioid receptors but do so at different concentrations. In vitro experiments using guinea pig ileum, mouse vas deferens, rat brain, or monkey brain show that NTX and naloxone preferentially bind to the μ receptor at low concentrations and then bind to δ or κ receptors at higher concentrations (Childers et al., 1979; Emmerson et al., 1994; Paterson et al., 1984; Takemori and Portoghese, 1984). This binding profile has been applied as rationale for the effects of systemically injected NTX or naloxone on ethanol self-administration in rodents. For example, Mhatre and Holloway (2003) indicated that low doses of NTX (<1 mg/kg) occupy μ receptors, whereas doses up to 20 mg/kg occupy μ- and δ receptors. They indicate that doses greater than 20 mg/kg additionally occupy κ receptors. However, the potency of NTX or naloxone to reduce ethanol consumption varies dramatically according to the literature. For instance, some studies show that NTX doses as low as 0.1 mg/kg reduced ethanol consumption or ethanol-reinforced responding (Holter and Spanagel, 1999; Hyytia and Kiianmaa, 2001; June et al., 1998), whereas other studies show that acute injections as high as 3 or 30 mg/kg failed to significantly reduce ethanol-reinforced responding (Bienkowski et al., 1999; Williams, 2007). The observed potency differences may be influenced by many experimental variables, such as the self-administration paradigm (operant responding vs. free access), strain of rat (outbred vs. ethanol-preferring rats), or route of administration (intraperitoneal vs. subcutaneous injection).
In the present study, we used a self-administration model with an ethanol solution (10% wt/vol) that was sweetened with 0.1% saccharin. Although the addition of saccharin may confound our ability to draw conclusions about manipulations that are specific to ethanol alone, the sweetened ethanol solution has many benefits for researchers using the self-administration model. First, ethanol intakes (g/kg) are maintained at a higher level in outbred rats compared to solutions containing ethanol alone. The average intakes in the present experiments were close to 1 g/kg during a 20 min period in an outbred strain of rats that had free access to food and water in the home cage. Although the added sweetener enhances the palatability of the ethanol solution, consumption is likely maintained by the pharmacological properties of ethanol as demonstrated by a behavioral economic analysis (Heyman, 1997; Heyman et al., 1999). A second benefit of using a sweetened alcohol solution is the reduced training time by avoiding the time spent “fading out” the sweetener. A third benefit is that the model has face validity compared to the human condition. Humans consume ethanol in beer, wine, or mixed drinks, which allows palatability to play a role in alcohol consumption and, therefore, may influence effects of pharmacotherapy.
The purpose of this study was to examine the route of administration by directly comparing the effects of intraperitoneal and subcutaneous injections of NTX on ethanol self-administration in outbred rats. Although these experiments are not highly novel, they do fill a gap in the literature. The “common knowledge” that route of administration influences potency is not clearly supported by published empirical evidence. The literature demonstrates a lack of a standard for antagonist route of administration. Many articles make comparisons of the effective antagonist doses across studies without mentioning the differing type of injection.
Materials and methods
Animals and housing
Male Long-Evans rats weighing 126—150 g (Charles River, Wilmington, MA) upon arrival to the laboratory were individually housed and maintained in a temperature- and humidity-controlled room with a 12-h light/dark cycle with lights on at 9:00 a.m. Operant experiments were conducted six days per week during the light cycle between 10:00 a.m. and 2:00 p.m. The rats had free access to food and water in their home cages except during initial training for the lever-press response. The experimental protocol was approved by Institutional Animal Care and Use Committee, and the animals were treated in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Apparatus
Experiments were conducted in standard operant chambers (Med Associates, St. Albans, VT) housed in melamine sound-attenuated cubicles. Each chamber (30.5×24.2×29.2 cm) contained a rolled-edge standard lever approximately 7 cm from the grid floor on the right wall near the rear of the chamber. A single-cup receptacle, placed on the center of the right wall approximately 3 cm from the grid floor, was fitted to receive food pellets from a modular dispenser and fluid deliveries from a syringe pump via 18-gauge stainless steel tubing connected to the receptacle. A white stimulus light was located above the cup receptacle. The house light was on the center of the left wall near the top of the chamber. Operant chambers were controlled with programs written in Med-PC MedState Notation version IV (Med Associates).
Training
Training for the lever-press response started after food depriving the rats for 24 h. Rats began lever pressing for 45-mg food pellets (Bio-Serv #F0021, Frenchtown, NJ) during shaping sessions that lasted 10—20 min and were conducted twice per day for up to 4 days. When the rats acquired the lever-press response, 20% sucrose was substituted for the food pellets for a single 20-min session to allow the rats to transition from solid food reinforcers to fluid reinforcers. On the following day, the rats responded for 5% ethanol (wt/vol) mixed in 0.1% saccharin (wt/vol). Thirty seconds before the start of the session, the rats were placed in the operant chamber during which time the chamber was dark, and responses on the lever had no consequence. When the operant session began, the house light was illuminated, and the rats could earn fluid reinforcers by responding on a continuous reinforcement schedule such that every response resulted in a reinforcer. Fluid reinforcers were delivered by activating the syringe pump fitted with a 30-ml syringe for 2.5 s to allow a fluid volume of 0.1 ml per reinforcer. The accuracy of volume delivery was confirmed during all sessions by measuring the fluid remaining in the syringe at the end of each session. During the fluid delivery and for an additional 1.5 s (total 4 s), the house light was extinguished, and responding had no further consequence. As the ethanol delivery began, a clicking sound was presented, and the stimulus light over the receptacle cup flashed until the end of the 4-s interval. Just before placing the rat in the chamber, six drops of peppermint extract (McCormick, Sparks, MD) were put into the bottom of the waste pan inside the chamber to serve as an olfactory discriminative stimulus signaling ethanol availability for the session. The ethanol-paired auditory/ visual stimuli and the olfactory discriminative stimulus were used, because these rats were run concurrently with rats in an experiment for which these stimuli were essential. Exposure to the same stimulus conditions allowed consistency in the operant room. Over the next couple of weeks, the ethanol concentration was gradually increased such that the final operant schedule consisted of a 20-min response period during which 10% ethanol mixed in 0.1% saccharin was available under an fixed ratio 1 (FR1) schedule of reinforcement. Under the final operant schedule, the rats were placed in the chamber 10 min before the start of the session. None of the lights in the chamber were illuminated at this time. Rats were allowed to self-administer the ethanol solution for approximately 2 weeks under the above conditions. A minimum average of 0.5 g/kg ethanol consumption was required for the rats to be included in the study, of 30 rats, a total of eight failed to reach this criterion. Due to limited laboratory space and concurrently running experiments, the rats were tested sequentially, with the first group (n=11) receiving intraperitoneal NTX injections. The second group was exposed to similar training procedures but received subcutaneous NTX injections (n=11).
NTX injections
After the responding stabilized in the procedures described above, the rats received injections of NTX or the NTX vehicle (saline) 10 min before the start of the operant session approximately twice per week, such that each week the rats received one injection of NTX and one injection of saline. On other days of the week, operant sessions were conducted in the absence of injections; data from these days were considered baseline days. The first group of rats (n=11) received 1, 3, 10, and 30 mg/kg NTX via intraperitoneal injections, whereas a second group of rats (n=11) received 0.03, 0.1, 0.3, and 1 mg/kg NTX via subcutaneous injections. Thus, in each group, NTX was tested once per week, taking 4 weeks to complete the dose-effect curves. All rats received the weekly NTX doses in a random order via Latin square design. After the determination of the NTX dose—effect curve with subcutaneous injections, this group of rats also received three intraperito-neal injections of saline. After the rats were habituated to the new method of injection, they experienced a test day with an intraperitoneal injection of 0.3 mg/kg NTX. All intraperitoneal injections in these rats were given across a 2-week period.
Drugs
Ethanol solutions were prepared by mixing appropriate volumes of 95% wt/vol ethanol (Pharmco Products Inc., Brookfield, CT) and distilled water. Ethanol solutions were sweetened by adding 0.1% wt/vol sodium saccharin (Sigma Aldrich, St. Louis, MO). Sucrose solution (20% wt/vol) was made by dissolving granulated cane sugar in distilled water. All solutions were presented at room temperature. NTX HCl was supplied by the National Institute on Drug Abuse and dissolved in 0.9% saline. NTX and saline were given in a fluid volume of 1 ml/kg via intraperitoneal or subcutaneous injection.
Data analysis
Because the groups were tested sequentially, a two-tailed, between-groups t-test was applied to confirm that the average ethanol intake (g/kg) on noninjection baseline days during the injection regimen did not differ significantly between groups. The significance level for this test and all others was set at P<.05, and all data are displayed as the mean±standard error of the mean (S.E.M.).
For within-group analysis (intraperitoneal group or subcutaneous group), the intake data were analyzed using one-way repeated-measures analysis of variances (ANOVAs) that compared the average during baseline, saline, and the various injections of NTX. The baseline data included the average of the 3 days before the first NTX injection and the non-injection days during determination of the NTX dose-effect curves. The saline data included the average of the 4 weekly saline injections. If the F values were statistically significant, a Tukey's honestly significant difference (HSD) test was applied as a post hoc test for all ANOVAs.
The NTX dose that reduced ethanol intake by 50% (ED50 value) relative to saline control injections was determined for each group of rats. Ethanol intake data for each NTX dose were expressed as a percentage of the average intake after the two nearest saline control injections. ED50 values were calculated using linear regression for each individual rat. Values were averaged to obtain group ED50 values, and 95% confidence intervals (CIs) were calculated to allow comparison between groups.
A comparison was conducted for the 1 mg/kg NTX dose that was administered to both groups. A two-tailed, between-groups t-test was applied using the average intake values converted to a percentage of saline control injections. Similar data were used to conduct a within-groups test to compare the effects of 0.3 mg/kg NTX dose that was given via both subcutaneous injection and intraperitoneal injection to the same group of rats.
The data following subcutaneous injection of 0.3 mg/kg/ were analyzed in further detail. The pattern of within-session fluid deliveries following this NTX injection was compared to that after the nearest saline injection. The average data were analyzed across the 20-min session in 2-min bins using a two-way repeated measures ANOVA (injection [2] × bin [10]).
Results
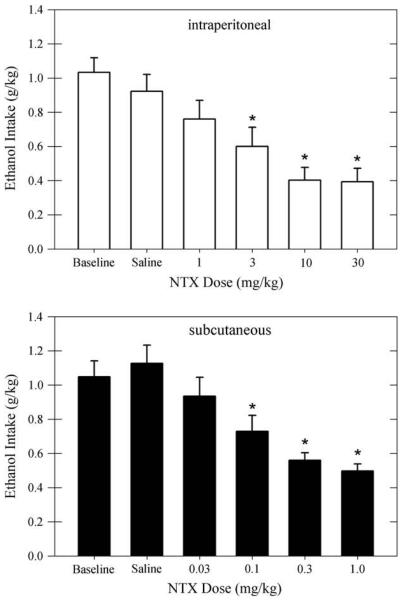
The effects of NTX, given via intraperitoneal or subcutaneous injection, on ethanol intake (g/kg) are shown in Fig. 1. For the group of rats receiving intraperitoneal injections of NTX, the average ethanol intake during baseline and after saline injections was 1.03±0.09 and 0.92±0.10 g/kg, respectively. For the group of rats receiving subcutaneous injections of NTX, the average ethanol intake during baseline and after saline injections was 1.05±0.09 and 1.13±0.11 g/kg, respectively. The between-groups t-test confirmed that the ethanol intake was similar across groups before beginning NTX injections (t[20]=−0.12,P=.91). Intraperitoneal injections of NTX caused a dose-related reduction of ethanol intake (F[5, 50]=13.91, P<.001). The post hoc tests showed that NTX significantly reduced ethanol intake relative to baseline and saline after NTX doses of 3 mg/kg and greater (P<.05). The reduction of ethanol intake was similar after 10 and 30 mg/kg, whereas both doses reduced ethanol intake more than the 3 mg/kg dose (P<.05). Subcutaneous injections of NTX also reduced ethanol intake in a dose-related manner (F[5, 50]=22.28, P<.001). For subcutaneous injections, NTX significantly reduced ethanol intake at doses 0.1 mg/kg and greater compared to baseline and after saline injections (P<.01). The reductions after 0.3 and 1 mg/kg were similar, whereas the intake after 1 mg/kg was less than that after 0.03 and 0.1 mg/kg (P<.05).
Fig. 1.
Average ethanol intake (g/kg) is shown during baseline and after intraperitoneal injections of saline or 1–30 mg/kg NTX (upper panel, open bars). Similar data are shown for rats receiving subcutaneous injections of saline or 0.03–1 mg/kg NTX (lower panel, filled bars). Data are shown for both groups of rats (n=11 per group) as means±standard error of the mean. *P<.05 compared to baseline and saline control. NTX=naltrexone.
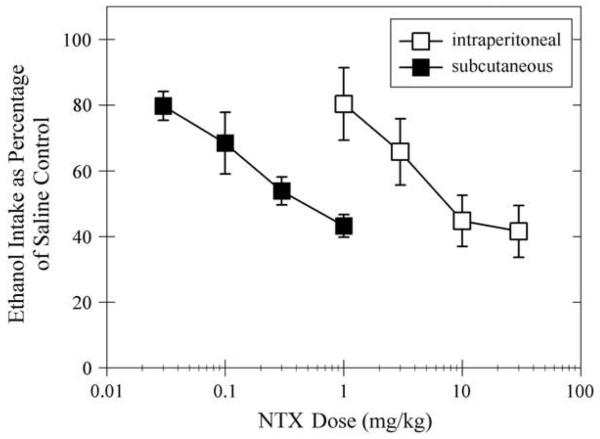
When NTX's effect on ethanol intake was expressed as a percentage of the ethanol intake after saline injections (Fig. 2), the data showed similar reductions across groups for 3 mg/kg intraperitoneal and 0.1 mg/kg subcutaneous (66±10% and 68±10%, respectively) doses. These doses seemed to be the threshold doses that caused significant reductions of ethanol self-administration. A comparison of the dose—effect curves via ED50 calculation with 95% CIs demonstrates that the curves were significantly different from each other (i.e., CI were not overlapping). The ED50 value and 95% CIs was 7.4 mg/kg (4.8—11.6) for intraperitoneal injection and 0.25 mg/kg (0.16—0.39) for subcutaneous injection. The effect of NTX appeared to plateau at the higher intraperitoneal doses; the ethanol intake after 10 mg/kg was 45±8% of saline control and 42±8% of saline control after 30 mg/kg.
Fig. 2.
Effect of NTX on ethanol intake is shown when expressed as a percentage of the ethanol intake following saline control injections for intraperitoneal injections (open squares) and subcutaneous injections (filled squares). Data are shown for both groups of rats (n=11 per group) as means±standard error of the mean. NTX=naltrexone.
Only 1 mg/kg NTX was given to both groups of rats. The average ethanol intake was 0.76±0.11 mg/kg for the intraperitoneal group, whereas the average intake was 0.50±0.04 mg/kg for the subcutaneous group (Fig. 1). When the data are expressed as a percentage of the intake after saline control, the intraperitoneal dose reduced ethanol intake to 80±11% of saline control. The subcutaneous dose reduced ethanol intake to 43±3% of saline control (Fig. 2). Between-groups t-tests show significant differences for ethanol intake when expressed as a percentage of saline control (t[20]=3.21, P<.005).
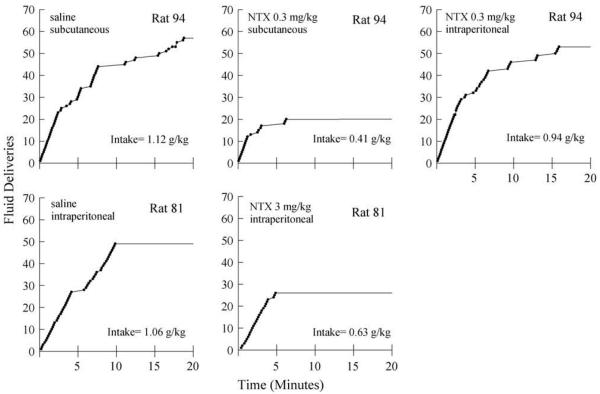
Naltrexone 0.3 mg/kg was given to the same group of rats via subcutaneous and intraperitoneal injections. When the data are expressed as a percentage of the intake after saline control, the subcutaneous dose reduced ethanol intake to 54±4% of saline control (figure not shown). The ethanol intake following the intraperitoneal dose was 88±10% of saline control. The within-group t-test confirmed a significant difference for the effect of these doses delivered via different routes of administration (t[10]=−2.82, P<.05). Fig. 3 shows cumulative records of ethanol fluid deliveries for a typical rat from this experimental group (upper panels). Following subcutaneous saline control injections, the rat obtained approximately half of the total session fluid deliveries within the first 10 min of the session (left upper panel). Fluid deliveries were also obtained during the remainder of the session at a slower rate. The pattern of fluid deliveries was similar after the third intraperitoneal injection (data not shown). Following subcutaneous 0.3 mg/kg NTX, the pattern of fluid deliveries began in a manner similar to that after saline. However, the rate of fluid deliveries slowed and virtually stopped within the first 5 min of the session (middle upper panel). Following intraperitoneal 0.3 mg/kg NTX (right upper panel), the pattern of fluid deliveries was much more similar to that during the saline control condition (i.e., the rate of fluid deliveries appeared to slow only after 10 min, and the total session intake was similar to that during the saline control condition). For comparison purposes, the lower panels of Fig. 3 show the cumulative records for a rat in the intraperitoneal injection group following an intraperitoneal injection of saline and 3 mg/kg NTX. The pattern of fluid deliveries for this rat during the saline control condition was similar to that for the rat in the subcutaneous group; most fluid deliveries were obtained within the first 10 min of the operant session. Following 3 mg/kg NTX, the pattern of fluid deliveries was similar to that after 0.3 mg/kg NTX for the rat in the subcutaneous group. After these doses of NTX, both rats obtained fluid deliveries within the first few minutes of the session, but then responding stopped earlier than that during the saline control conditions.
Fig. 3.
Cumulative records of fluid deliveries (closed circles) are shown for a typical rat following subcutaneous injections of saline (left upper panel) and 0.3 mg/kg NTX (middle upper panel) and following an intraperitoneal injection of 0.3 mg/kg NTX (right upper panel). In the bottom panels, the cumulative records for a different rat are shown following an intraperitoneal injection of saline (left lower panel) and an intraperitoneal injection of 3 mg/kg NTX (middle lower panel). NTX=naltrexone.
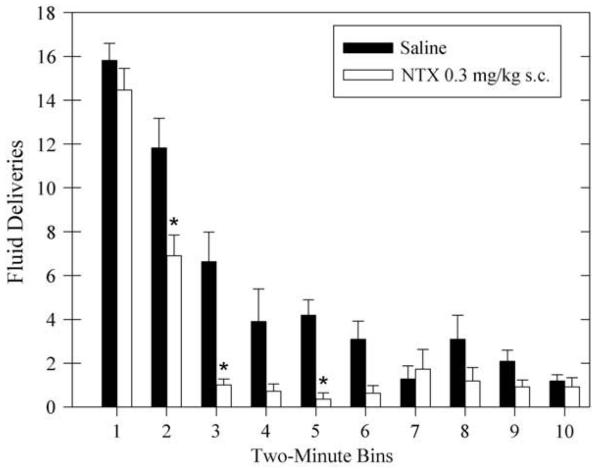
Fig. 4 quantitatively demonstrates that responding stopped earlier in the session following an effective dose of NTX. The average fluid deliveries following subcutaneous injection of 0.3 mg/kg NTX (29±2) was compared to that after saline injection (53±6) across the session in 2-min bins. The analysis showed a main effect of injection (F[1,10]=19.08; P<.005), a main effect of bin (F[9,90]=70.23; P<.001), an interaction effect (F[9,90]=4.78; P<.001). Subsequent post-hoc comparisons showed that the number of fluid deliveries during the first 2 mins after NTX injection were not significantly different from those after saline injection (P=0.99). However, the number of fluid deliveries after NTX was significantly less than that after saline for the second, third, and fifth bins (all P<.01).
Fig. 4.
Average fluid deliveries are shown after subcutaneous injection of saline (filled bars) or subcutaneous injection of 0.3 mg/kg NTX (open bars) within each 2-min bin during the operant session. Data are shown for 11 rats as means±standard error of the mean. *P<.01 compared to saline control within the same bin. NTX=naltrexone.
Discussion
The main findings of these experiments clearly demonstrate that the potency of NTX to reduce self-administration of an ethanol solution is far greater when injected subcutaneously than when injected intraperitoneally. Via both routes of injection, NTX caused dose-related reductions of ethanol intake, and direct comparison of the dose—effect curves via comparison of ED50 values showed that subcutaneous NTX was approximately 30-fold more potent than intraperitoneal NTX. Cumulative records of the fluid deliveries obtained during the session and the bin analysis support other literature that show that the effective doses of NTX (subcutaneous or intraperitoneal) are characterized by early cessation of responding rather than delayed responding at the start of the operant session.
The route of opioid antagonist administration may have a substantial impact on preclinical experiments, although it is rarely emphasized in the literature regarding the effects of antagonists on self-administration of ethanol. Subcutaneous injections of 0.1—0.3 mg/kg naloxone (Hyytia and Kiianmaa, 2001; Schwarz-Stevens et al., 1992) or 0.1 mg/kg NTX (Holter and Spanagel, 1999; June et al., 1998) reduced ethanol-reinforced responding. Similar potency is observed in experiments where rats are allowed to consume ethanol during nonoperant conditions. For example, 0.1 mg/kg NTX reduced ethanol consumption when rats had limited access to ethanol from bottles in the home cage (Stromberg et al., 1998). Intraperitoneal antagonist injections reduce ethanol-reinforced responding or ethanol consumption at doses that are at least 10-fold larger than the effective doses given via subcutaneous injection. In experiments wherein intraperitoneal antagonist dose—effect curves were identified, 1 mg/kg naloxone was required to reduce ethanol-reinforced responding (Hyytia and Sinclair, 1993), and 1 mg/kg NTX was required to reduce ethanol drinking from bottles in the home cage (Hill and Kiefer, 1997). It is important to note that intraperitoneal naloxone at 1 mg/kg does not have selective effects on ethanol consumption; this dose reduced bottle consumption of 15% sucrose, 0.6% saccharin, and water (Cichelli and Lewis, 2002). In other studies, even larger intraperitoneal doses of NTX or naloxone (3 mg/kg or higher) had a marginal effect or no effect on responding for ethanol (Bienkowski et al., 1999; Sharpe and Samson, 2001; Williams, 2007). Because the potency differences for NTX or naloxone across different routes of administration may differ by more than 10-fold, caution should be used when making comparisons of effective NTX doses across studies following systemic injections.
Our study is one of the only studies to directly compare the influence of route of opioid antagonist administration in rats. Only one other study has conducted a similar comparison (Mucha and Iverson, 1984). In that study, subcutaneous injections of 0.5 mg/kg naloxone produced a large conditioned place aversion after only three pairings, and the aversion was greater than that observed after similar naloxone doses given via intraperitoneal injection. One explanation for this effect is that compounds injected via the intraperitoneal route are subject to first-pass metabolism by the liver due to absorption through portal circulation (Lukas et al., 1971), and NTX undergoes substantial metabolism by the liver (Crabtree, 1984). In our experiments, we show clear potency differences based on route of administration while controlling for NTX pretreatment time. In the literature, a broad range of pretreatment times exist. For example, naloxone has been given as an intraperitoneal injection immediately before an operant session in an appetitive-consumption model (Sharpe and Samson, 2001), and NTX has been given as long as 70 min before a drinking session (Perfumi et al., 2003). In our experiments, NTX was given 10 min before the operant session. Although we did not have the opportunity to measure blood levels of NTX, this pretreatment time should allow rapid entry of NTX into systemic circulation even via the intraperitoneal route. For instance, in one study, an intraperitoneal injection of 3 mg/kg NTX given 10 min before the session caused a dramatic rightward shift of the dose—effect curve for intravenous heroin self-administration in rats (Martin et al., 1996). Our data are consistent with the above study; a dose of 3 mg/kg given intraperitoneally significantly reduced ethanol intake, and the pattern of fluid deliveries illustrated effects within the first 5 min of the operant session. Although the route of administration may influence the potency differences observed in the literature, other variables may play a role as well.
Variables related to motivational level may alter the potency of NTX. One motivational factor may be related to hunger or the feeding status of the subjects. In food-restricted rats (approximately 85% of free-feeding weight), an intraperitoneal injection of 30 mg/kg NTX had minimal effects on responding to ethanol (Williams, 2007). In studies that directly compared the effects of opioid antagonists on consummatory behavior in food-restricted rats versus free-feeding rats, naloxone was at least 10-fold less potent in the food-restricted rats (Levine et al., 1995; Rudski et al., 1994). Rats that experienced repeated cycles of water restriction also showed resistance to the consumption-decreasing effects of NTX; 10–30 mg/kg reduced water consumption by no more than 40% (White and Holtzman, 2001). Another motivational factor may be the taste or palatability of the solution. In studies of consummatory behavior, sensitivity to naloxone was increased when rats were feeding on sweet food or chow versus normal chow (Giruado et al., 1993; Levine et al., 1995). Although palatability influences the effects of opioid antagonist on consumption of food, the issue is not mentioned as frequently in studies regarding ethanol self-administration. However, palatability plays a role in these experiments due to the many different ethanol solutions used in the studies. For example, some researchers have explored the effects of opioid antagonists on sweetened ethanol solutions (e.g., Gardell et al., 1997) or on lower concentrations of ethanol (e.g., Stromberg et al., 2002). Stromberg et al (2002) used a 6% vol/vol ethanol solution. This ethanol concentration equates to approximately 4.5% wt/vol ethanol, which may be more palatable than the 10% wt/vol solution often cited in the preclinical literature. At least one author calls for researchers to conform to a standardized way to calculate ethanol concentrations (Brick, 2006). More recently, the addition of a sweetener to an ethanol solution has been proposed as part of a model for binge drinking (Ji et al., 2008) and for adolescent drinking behavior (Maldonado et al., 2008). Thus, palatability should be considered when examining the effects of opioid antagonists on ethanol consumption. Although palatability may play a role in the NTX potency differences observed across different studies in the literature, we controlled for palatability by administering subcutaneous and intraperitoneal NTX while rats were consuming the same sweetened ethanol solution.
In summary, opioid antagonists such as NTX are often used in preclinical work exploring the effects of ethanol. Inferences are often made about opioid receptor selectivity following systemic injections of NTX in rodents. The work presented here indicates that caution should be used when making these inferences and that the route of NTX administration should be considered an important variable when designing experiments using opioid antagonists.
Acknowledgments
This study was supported by grant 7 R15 AA015147 from the National Institute on Alcohol Abuse and Alcoholism. We thank Jasmine Schimmel for assistance with data collection.
References
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W, Koros E. Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur. J. Pharmacol. 1999;374:321–327. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Brick J. Standardization of alcohol calculations in research. Alcohol Clin. Exp. Res. 2006;30:1276–1287. doi: 10.1111/j.1530-0277.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- Childers SR, Creese I, Snowman AM, Snyder SH. Opiate receptor binding affected differentially by opiates and opioid peptides. Eur. J. Pharmacol. 1979;55:11–18. doi: 10.1016/0014-2999(79)90142-0. [DOI] [PubMed] [Google Scholar]
- Cichelli MJ, Lewis MJ. Naloxone nonselective suppression of drinking of ethanol, sucrose, saccharin, and water by rats. Pharmacol. Biochem. Behav. 2002;72:699–706. doi: 10.1016/s0091-3057(02)00736-0. [DOI] [PubMed] [Google Scholar]
- Crabtree BL. Review of naltrexone, a long-acting opiate antagonist. Clin. Pharm. 1984;3:273–280. [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin. Exp. Res. 2004;28:1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Liu M-R, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta, and κ receptors in monkey brain membranes. J. Pharmacol. Exp. Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li T-K. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology. 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Whalen CA, Chattophadyay S, Cavallaro CA, Hubbell CL, Reid LA. Combination of naltrexone and fluoxetine on rats' propensity to take alcoholic beverage. Alcohol Clin. Exp. Res. 1997;21:1435–1439. [PubMed] [Google Scholar]
- Gianoulakis C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J. Psychiatry Neurosci. 2001;26:304–318. [PMC free article] [PubMed] [Google Scholar]
- Giruado SQ, Grace MK, Welch CC, Billington CJ, Levine AS. Naloxone's anorectic effect is dependent upon the relative palatability of food. Pharmacol. Biochem. Behav. 1993;46:917–921. doi: 10.1016/0091-3057(93)90222-f. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Preference for saccharin-sweetened alcohol relative to isocaloric sucrose. Psychopharmacology (Berl) 1997;129:72–78. doi: 10.1007/s002130050164. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Gendel K, Goodman J. Inelastic demand for alcohol in rats. Psychopharmacology (Berl) 1999;144:213–219. doi: 10.1007/s002130050996. [DOI] [PubMed] [Google Scholar]
- Hill KG, Kiefer SW. Naltrexone treatment increases the aversiveness of alcohol for outbred rats. Alcohol Clin. Exp. Res. 1997;21:637–641. [PubMed] [Google Scholar]
- Holter SM, Spanagel R. Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats. Psychopharmacology (Berl) 1999;145:360–369. doi: 10.1007/s002130051069. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin. Exp. Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Sinclair JD. Responding for oral ethanol after naloxone treatment by alcohol-preferring AA rats. Alcohol Clin. Exp. Res. 1993;17:631–636. doi: 10.1111/j.1530-0277.1993.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav. Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Grey C, Warren-Reese C, Durr LF, Ricks-Cord A, Johnson A, et al. The opioid receptor antagonist nalmefene reduces responding maintained by ethanol presentation: preclinical studies in ethanol-preferring and outbred Wistar rats. Alcohol Clin. Exp. Res. 1998;22:2174–2185. [PubMed] [Google Scholar]
- Krishnan-Sarin S, Portoghese PS, Li T-K, Froehlich JC. The delta2 opioid receptor antagonist naltriben selectively attenuates alcohol intake in rats bred for alcohol preference. Pharmacol. Biochem. Behav. 1995a;52:153–159. doi: 10.1016/0091-3057(95)00080-g. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Jing S-L, Kurtz M, Zweifel M, Portoghese PS, Li T-K, et al. The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology (Berl) 1995b;120:177–185. doi: 10.1007/BF02246191. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Wand GS, Li XW, Portoghese PS, Froehlich JC. Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacol. Biochem. Behav. 1998;59:627–635. doi: 10.1016/s0091-3057(97)00474-7. [DOI] [PubMed] [Google Scholar]
- Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am. J. Physiol. 1995;268:R248–R252. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J. Pharmacol. Exp. Ther. 1971;178:562–564. [PubMed] [Google Scholar]
- Martin TJ, Walker LE, Sizemore GM, Smith JE, Dworkin SI. Within-session determination of dose-response curves for heroin self-administration in rats: comparison with between-session determination and effects of naltrexone. Drug Alcohol Depend. 1996;41:93–100. doi: 10.1016/0376-8716(96)01245-8. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Alipour KK, Kirstein CL. Voluntary ethanol consumption differs in adolescent and adult male rats using a modified sucrose-fading paradigm. Alcohol clin. Exp. Res. 2008;32:1574–1582. doi: 10.1111/j.1530-0277.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch. Gen. Psychiatry. 1999;56:719–724. doi: 10.1001/archpsyc.56.8.719. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Holloway F. Mu1-opioid antagonist naloxonazine alters ethanol discrimination and consumption. Alcohol. 2003;29:109–116. doi: 10.1016/s0741-8329(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Fritz EM. The opioidergic-alcohol link: implications for treatment. CNS Drugs. 2005;19:693–707. doi: 10.2165/00023210-200519080-00005. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iverson SD. Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology (Berl) 1984;82:241–247. doi: 10.1007/BF00427782. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guidelines for the Care and use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington DC: 2003. [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Shottenfeld R, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. Arch. Gen. Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Paterson SJ, Corbett AD, Gillan MGC, Kosterlitz HW, McKnight AT, Robson LE. Radioligands for probing opioid receptors. J. Recept. Res. 1984;4:143–154. doi: 10.3109/10799898409042545. [DOI] [PubMed] [Google Scholar]
- Perfumi M, Santoni M, Cippitelli A, Ciccocioppo R, Froldi R, Massi M. Hypericum perforatum CO2 extract and opioid receptor antagonists act synergistically to reduce ethanol intake in alcohol-preferring rats. Alcohol Clin. Exp. Res. 2003;27:1554–1562. doi: 10.1097/01.ALC.0000092062.60924.56. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O'Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J. Clin. Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Rudski JM, Billington CJ, Levine AS. Naloxone's effects on operant responding depend upon level of deprivation. Pharmacol. Biochem. Behav. 1994;49:377–383. doi: 10.1016/0091-3057(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Schwarz-Stevens KS, Files FJ, Samson HH. Effects of morphine and naloxone on ethanol- and sucrose-reinforced responding in non-deprived rats. Alcohol Clin. Exp. Res. 1992;16:822–832. doi: 10.1111/j.1530-0277.1992.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Effect of naloxone on appetitive and consummatory phases of ethanol self-administration. Alcohol Clin. Exp. Res. 2001;25:1006–1011. [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O'Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and b-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Rukstalis MR, Mackler SA, Volpicelli JR, O'Brien CP. A comparison of the effects of 6-beta naltrexol and naltrexone on the consumption of ethanol or sucrose using a limited-access procedure in rats. Pharmacol. Biochem. Behav. 2002;72:483–490. doi: 10.1016/s0091-3057(02)00721-9. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Portoghese PS. Comparative antagonism by naltrexone and naloxone of mu, κ, and delta agonists. Eur. J. Pharmacol. 1984;104:101–104. doi: 10.1016/0014-2999(84)90374-1. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in treatment of alcohol dependence. Arch. Gen. Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Acute opioid pretreatment potentiates naltrexone-induced drinking suppression in water-deprived rats. J. Pharmacol. Exp. Ther. 2001;298:156–164. [PubMed] [Google Scholar]
- Williams KL. Development of naltrexone supersensitivity during food-maintained responding enhances naltrexone's ability to reduce ethanol-maintained responding. Alcohol Clin. Exp. Res. 2007;31:39–47. doi: 10.1111/j.1530-0277.2006.00272.x. [DOI] [PubMed] [Google Scholar]