Abstract
Calorie restriction (CR) delays the development of age-associated disease and increases lifespan in rodents, but the effects in humans remain uncertain.
Purpose
Determine the effect of 6 months of CR with or without exercise on cardiovascular disease (CVD) risk factors and estimated 10-year CVD risk in healthy non-obese men and women.
Methods
Thirty-six individuals were randomized to one of three groups for 6 months: Control, 100% of energy requirements; CR, 25% calorie restriction; CR+EX, 12.5% CR + 12.5% increase in energy expenditure via aerobic exercise. CVD risk factors were assessed at baseline, 3 and 6 months.
Results
After 6 months, CR and CR+EX lost approximately 10% of body weight. CR significantly reduced triacylglycerol (-31 ± 15 mg/dL) and factor VIIc (-10.7 ± 2.3%). Similarly CR+EX reduced triacylglycerol (-22 ± 8 mg/dL) and additionally reduced LDL-C (-16.0 ± 5.1 mg/dL) and DBP (-4.0 ± 2.1 mmHg). In contrast, both triacylglycerol (24 ± 14 mg/dL) and factor VIIc (7.9 ± 2.3%) were increased in the control group. HDL-cholesterol was increased in all groups while hsCRP was lower in the Controls vs. CR+EX. Estimated 10-year CVD risk significantly declined from baseline by 29% in CR (P< 0.001) and 38% in the CR+EX (P<0.001) while remaining unchanged in the Control group.
Conclusions
Based on combined favorable changes in lipid and blood pressure, caloric restriction with or without exercise that induces weight loss favorably reduces risk for CVD even in already healthy non-obese individuals.
Keywords: caloric restriction, exercise, cardiovascular risk factors, nutritional intervention, weight loss, aging
INTRODUCTION
Prolonged caloric restriction (CR) has been suggested as an anti-aging strategy in the belief that it will extend lifespan and improve quality of life. While data are convincing in shorter-lived species 1, whether calorie restriction extends lifespan in humans is not known. Heart disease and stroke are the number one and three in the causes of death in the USA 2, hence delaying the progression of atherosclerotic cardiovascular disease maybe one potential mechanism by which CR promotes longevity. The risk factors for CVD including blood lipids, blood pressure, hemostatic factors, inflammatory markers and endothelial function are all worsened with aging 3-6. At least a portion of these age-related changes appear to be secondary to increases in adiposity and/or reductions in physical activity 7, 8 and, therefore, may be amenable to improvements through prolonged caloric restriction and/or increased physical activity.
It is well established that obesity is associated with increased CVD mortality 9 and weight loss in obese individuals by CR is associated with improvements in CVD risk factors 10 and a lowering of coronary heart disease event rates 11. Increased physical activity, primarily through aerobic exercise also reduces the risk for atherosclerotic disease, acute cardiovascular events, stroke and Type 2 diabetes mellitus 12, 13. Thus, it would be expected that increasing physical activity as a means to achieve a negative energy balance in conjunction with CR would provide benefits at least equivalent (or superior) to that of simply reducing caloric intake.
The Comprehensive Assessment of the Long Term Effects of Reducing Intake of Energy (CALERIE) study examined the potential health benefits of CR in sedentary, non-obese, healthy individuals. While the primary aim of the study was to determine the impact of CR on biomarkers of longevity and metabolic adaptation 14 the secondary aims were to evaluate the changes in risk factors for type 2 diabetes mellitus 15 and CVD. We hypothesized that six months of CR would improve markers for CVD and that changes in CVD risk factors would be similar whether the energy deficit was produced by combining exercise with CR or by CR alone.
METHODS
Study Participants
Forty-eight healthy, non-smoking male (25-50y) and female (25-45y), overweight participants (25 ≤ BMI < 30) were recruited to participate in a 6-month intervention 14. Participants were excluded if they had a history of CVD, elevated blood pressure (>160/90 mmHg), high fasting blood glucose (>126 mg/dL), chronic medications (except oral contraceptives), smoking, regular exercise (more than twice a week), abnormal thyroid function or abnormal ECG. The study was approved by the Pennington Center Institutional Review Board and the CALERIE Data Safety Monitoring Board. All subjects provided written informed consent.
Study design
Participants were randomized into one of four groups for 24 weeks: Control=healthy weight maintenance diet, CR=25% caloric restriction from baseline energy requirements, CR+EX=12.5% caloric restriction and 12.5% increase in energy expenditure through structured aerobic exercise and LCD=low calorie diet (890kcal/day) to rapidly achieve 15% weight loss. Because of the different rate and extent of weight loss (rapid over 3 months) and different macronutrient composition, we did not include the LCD group in this analysis. Study outcomes were assessed during a 5-day inpatient stay at baseline and during weeks 12 (M3) and 24 (M6) of intervention.
Energy Prescription
Individual values used to prescribe the daily energy content during the intervention were calculated at baseline from total daily energy expenditure assessed during two 14-day periods by doubly labeled water and changes in body weight during a 2-week period when participants consumed all meals prepared by our metabolic kitchen 14.
Diets and diet delivery
All diets were based on the American Heart Association guidelines; 30% fat, 15% protein and 55% carbohydrate and provided the RDA for all essential vitamins and minerals. During weeks 1-12 and 23-24 of the intervention, participants consumed only foods prepared by our metabolic kitchen. During weeks 13-22 participants self-selected a diet based on their individual calorie target. Multivitamin and mineral supplements (including calcium) were not permitted.
Exercise
Except for participants in CR+EX, other participants were not permitted to modify their physical activity pattern. The CR+EX group increased their energy expenditure by 12.5% above baseline by undergoing supervised aerobic exercise, 5 days per week. The exercise time necessary to expend the 12.5% calorie target was determined for each individual by indirect calorimetry (V-max, Sensormedics, Yorba Linda, CA) and exercise sessions were monitored by heart rate (Polar S-610, Polar Beat, Port Washington, NY) 14. Participants self-selected their exercise intensity which ranged from 47-76% VO2max (Women: 47-70% VO2max and Men: 48-76% VO2max). The energy expenditure target for the exercise intervention was 403 ± 63 kcal per session for women and 569 ± 118 kcal per session which at the self-selected exercise intensity 53 ± 11 and 45 ± 14 min per session for women and men, respectively.
Behavioral Intervention
Commencing at baseline, participants attended weekly meetings to teach subjects how to adhere to their meal and exercise plans and to boost motivation and morale. Emphasis was placed on teaching participants the skills necessary to modify eating behavior and comply with the inventions during the out-patient phase of the study.
Analytical Methods
Serum lipids were analyzed using a Beckman-Coulter Synchron CX7 (Brea, CA). Total cholesterol (Total-C) was assayed by the cholesterol esterase/oxidase/peroxidase method, triacylglycerols (TG) by the GPO-Trinder method and HDL-cholesterol (HDL-C) by an assay from Trinity Biotech (Jamestown, NY). LDL-cholesterol (LDL-C) was calculated using the Friedwald equation. The coefficient of variation for the above assays is less than 2.0%.
Factor VII and fibrinogen were assayed on an Instrumentation Laboratory ACL 3000+ (Lexington, MA). Factor VII coagulant activity was assayed by determining the ability of test plasma to correct the clotting time of factor VII-deficiency plasma and expressed relative to a serial dilution of pooled plasma. Fibrinogen was measured following a standard protocol 16. C-reactive protein (hsCRP) was measured by automated immunoassay with chemiluminescent detection on a DPC-2000 instrument with reagents supplied by the instrument manufacturer.
Measurements of systolic (SBP) and diastolic (DBP) blood pressure were taken twice 5 min apart in a quiet room at thermo-neutrality from the participant’s right arm with a manual sphygmomanometer by a certified staff member after 10 min seated rest.
Brachial artery ultrasound images were obtained using a multi-frequency 7.5 MHz linear array transducer as previously described 17. Images were acquired in the longitudinal plane just proximal to the olecranon process of the elbow by a single trained operator. The Doppler signal was obtained by placing the gate in the center of the vessel, and using an angle of incidence of < 60 degrees. Induction of hyperemia was accomplished by inflation of a blood pressure cuff to 300 mmHg for 5 min on the non-dominant arm. Images were analyzed using the Brachial Analyzer software package (MIA Vascular Tools, Coralville, Iowa) to obtain baseline artery diameter, maximum post-release change in diameter (absolute and percentage), and time to maximum post release diameter change.
Estimates of 10-Year CVD Risk
Ten-year CVD risk was calculated using the gender-specific equations developed by Anderson et al 18. These equations rely upon values for total and HDL cholesterol (expressed as their ratio), systolic blood pressure, age and gender. Because smoking, presence of diabetes, and abnormal ECG were exclusionary, these risk factors were set to zero for all participants. Relative risk estimates at months 3 and 6 were taken as the ratio of the 10-year risk at these times to the baseline 10-year risk.
Statistical Analysis
SAS Version 9.12 (SAS Institute, Cary, NC) was used for data analysis. Changes from baseline at M3 and M6 were analyzed by a repeated measures approach with respect to treatment and time and treatment × time interaction, with baseline values included as covariates. A Bonferroni adjustment was used for all pair-wise comparisons to maintain an overall Type-I error rate of <5%. S normalizing and variance-stabilizing logarithmic transformation was applied to CRP variable.
RESULTS
Study Population
The baseline characteristics of the 35 men and women who completed the 6-month CALERIE trial are summarized in Table 1. As expected, the three groups were matched for weight, BMI and age. They all had normal fasting plasma glucose concentration. Furthermore their fasting plasma insulin concentration and insulin sensitivity measured by the minimal model 15 were suggestive of normal glucose tolerance. Average baseline levels for all groups were within normal ranges for blood lipids (Total-C, HDL-C, LDL-C, TG), and blood pressure (SBP and DBP).
TABLE 1.
Participant Characteristics Obtained During the Baseline Period
| Control | CR | CR+EX | |
|---|---|---|---|
| Number (M/F) | 5/6 | 6/6 | 5/7 |
| Age, y | 38 ± 8 | 39 ± 5 | 36 ± 6 |
| Race (C/AA/O)1 | 7/4/0 | 7/4/1 | 7/4/1 |
| Weight, kg | 81.8 ± 9.3 | 80.9 ± 11.4 | 81.9 ± 10.5 |
| BMI, kg/m2 | 27.6 ± 2.0 | 27.8 ± 1.4 | 27.5 ± 1.6 |
| Total-C, mg/dL | 175 ± 33 | 177 ± 25 | 169 ± 33 |
| LDL-C, mg/dL | 110 ± 32 | 107 ± 24 | 105 ± 28 |
| HDL-C, mg/dL | 38 ± 15 | 41 ± 9 | 44 ± 8 |
| TG, mg/dL | 134 ± 65 | 146 ± 113 | 98 ± 66 |
| SBP, mmHg | 113 ± 12 | 111 ± 7 | 111 ± 10 |
| DBP, mmHg | 74 ± 10 | 72 ± 8 | 72 ± 8 |
| Factor VIIc, % | 110 ± 5 | 111 ± 5 | 106 ± 5 |
| Fibrinogen, mg/dL | 405 ± 19 | 369 ± 29 | 354 ± 17 |
| hsCRP, mg/dL | 0.30 ± 0.08 | 0.25 ± 0.07 | 0.15 ± 0.03 |
| Homocysteine, μmol/L | 6.9 ± 0.5 | 7.5 ± 0.6 | 6.8 ± 0.4 |
| Flow-mediated dilation, % | 9.8 ± 1.9 | 11.6 ± 2.1 | 11.7 ± 2.1 |
Data are mean ± SD
Abbreviations for participants’ race: Caucasian (C), African American (AA), Asian or Latino (O).
Body Weight
Weight loss in CR and CR+EX groups continued throughout the study leading to a significantly greater reduction in body weight at M6 relative to M3 in both CR (P<0.01) and CR+EX (P<0.001), with no significant difference between the two groups. These differences were significant with respect to baseline and to changes in the Controls (-0.4% (NS); Table 2).
TABLE 2.
Effect of caloric restriction alone or in combination with exercise on change in weight, body composition, and risk factors for CVD
| Month 3 |
Month 6 |
|||||
|---|---|---|---|---|---|---|
| Control | CR | CR+EX | Control | CR | CR+EX | |
| Δ Weight, kg | -0.3 ± 0.7 | -5.8 ± 0.4c,† | -4.6 ± 0.4c,* | -0.4 ± 0.9 | -8.2 ± 0.8c,†,y | -8.1 ± 0.8c,†,z |
| (% Δ) | (-0.4 ± 0.9) | (-7.2 ± 0.4) | (-5.6 ± 0.5) | (-0.5 ± 1.2) | (-10.2 ± 0.9) | (-9.9 ± 0.9) |
| Δ SBP, mmHg | 0.5 ± 1.8 | 1.2 ± 2.2 | -2.3 ± 2.2 | 1.70 ± 2.0 | -2.7 ± 1.7 | -1.7 ± 2.3 |
| (% Δ) | (0.6 ± 1.6) | (1.3 ± 2.0) | (-1.9 ± 2.0) | (1.5 ± 1.6) | (-2.3 ± 1.5) | (-1.1 ± 2.1) |
| Δ DBP, mm HG | -0.9 ± 1.2 | 0.3 ± 1.9 | -1.7 ± 2.3 | -0.7 ± 1.2 | -2.1 ± 1.7 | -4.0 ± 2.1a |
| (% Δ) | (-1.2 ± 1.7) | (0.8 ± 2.6) | (-1.4 ± 3.6) | (-0.9 ± 1.7) | (-2.4 ± 2.4) | (-4.7 ± 2.8) |
| Δ Factor VIIc, % | 2.2 ± 4.0 | -7.5 ± 2.2a | -6.5 ± 1.7 | 7.9 ± 2.3a | -10.7 ± 2.3b,* | -3.3 ± 5.8 |
| (% Δ) | (2.1 ± 3.6) | (-6.7 ± 2.0) | (-6.3 ± 1.6) | (7.1 ± 2.2) | (-9.8 ± 2.4) | (-2.4 ± 5.9) |
| Δ Fibrinogen, mg/dL | -18 ± 24 | -4 ± 27 | -13 ± 17 | -50 ± 22 | -6 ± 27 | -2 ± 14 |
| (% Δ) | (-2.4 ± 5.7) | (9.6 ± 15.7) | -2.8 ± 4.7) | (-12.1 ± 5.6) | (8.4 ± 15.4) | (-0.3 ± 4.2) |
| Δ Ln(hsCRP), ln(mg/dL) | -0.13 ± 0.20 | -0.43 ± 0.10b | -0.36 ± 0.14a | -0.39 ± 0.12a | -0.14 ± 0.22 | -0.48 ± 0.17b |
| (% Δ)1 | (12 ± 31) | (-31 ± 7) | (-24 ± 9) | (-27 ± 8) | (29 ± 53) | (-27 ± 14) |
| Δ Homocysteine, μmol/L | -0.03 ± 0.18 | 0.02 ± 0.34 | 0.07 ± 0.28 | 0.05 ± 0.22 | -0.04 ± 0.23 | -0.07 ± 0.32 |
| (% Δ) | (-1.1 ± 2.5) | (1.8 ± 3.9) | (1.5 ± 4.4) | (2.2 ± 3.5) | (0.0 ± 2.7) | (1.0 ± 4.5) |
Data are mean ± SEM. Percent change from baseline are shown in parenthesis; Control, n=11; CR, n=12, CR+EX, n=12
Percent change calculated from non-transformed data.
Statistically significant from baseline:
P < 0.05;
P < 0.01;
P < 0.0001
Statistically significant from Control:
P < 0.01;
P <0.0001
Statistically significant from Month 3:
P < 0.01;
P < 0.001
Blood Lipids
Significant reductions from baseline in LDL-C were observed in CR+EX at both M3 and M6 (P<0.001). LDL-C was not significantly changed in either the Control or CR groups (Figure 1). At M6, the reduction in LDL-C in CR+EX was significantly different compared to Controls, but not to CR. HDL-C was unchanged at M3, but was significantly increased at M6 in all groups. There were no significant differences in HDL-C between the groups at any time point. TG concentrations increased (P<0.05) in the Control group but decreased at M3 (P<0.05) and M6 (P<0.001) in CR and CR+EX groups. At both time points, changes in TG levels in CR and CR+EX were significantly different (P<0.05 to P<0.001) when compared to the Control group, but not when compared to each other.
Figure 1.
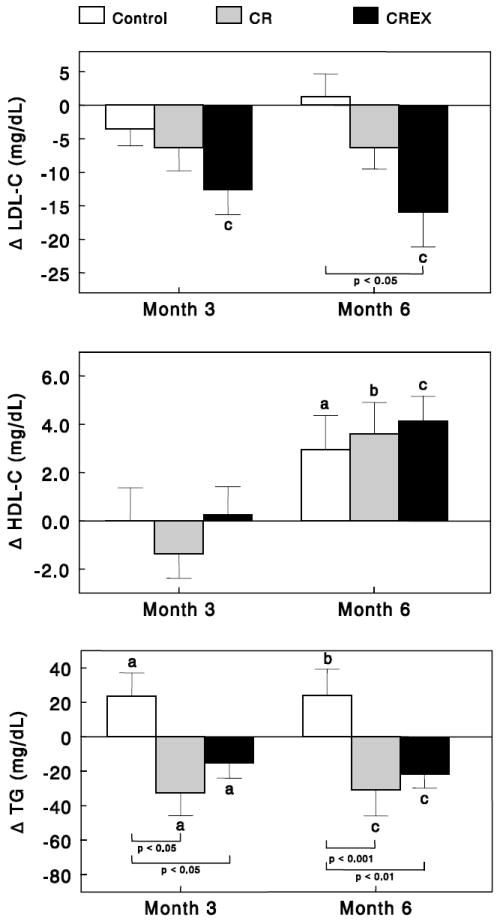
Effects of caloric restriction alone or in combination with exercise on serum lipid levels. Data are changes from baseline for (Control group: n=11; CR group: n=12; and CR+EX group: n=12) at months 3 and 6. Error bars are SEM. Statistically significant from baseline: aP < 0.05; bP < 0.01; cP < 0.001.
Blood Pressure
At M6, DBP was significantly reduced relative to baseline in CR+EX (P<0.05; Table 2). However, there was no effect of either intervention on SBP.
Hemostasis Factors, Homocysteine and Markers of Inflammation
Factor VIIc was reduced in CR at M3 (P<0.05) and M6 (P<0.01), while it remained unchanged in CR+EX (Table 2). In contrast, factor VIIc in the Control group was significantly elevated from baseline at M6 (P<0.05). The factor VIIc reduction in CR was significantly (P<0.01) different from the Control group at M6. Fibrinogen and Homocysteine concentrations were not changed in the Control or the intervention groups over the course of the study. hsCRP levels were significantly reduced in CR+EX at both time points and in CR at M3 only (P<0.01). hsCRP was reduced in the Control group at M6 only (P<0.05) . However, there were no differences in the change in hsCRP at M3 or M6 between the two interventions and the Control groups.
Brachial Artery Flow Mediated Dilation
Brachial artery flow-mediated dilation (BA-FMD), a marker of endothelial function, was not significantly changed relative to baseline in any group across time (Figure 2). Furthermore, there were no significant effects of either intervention group on endothelial function.
Figure 2.
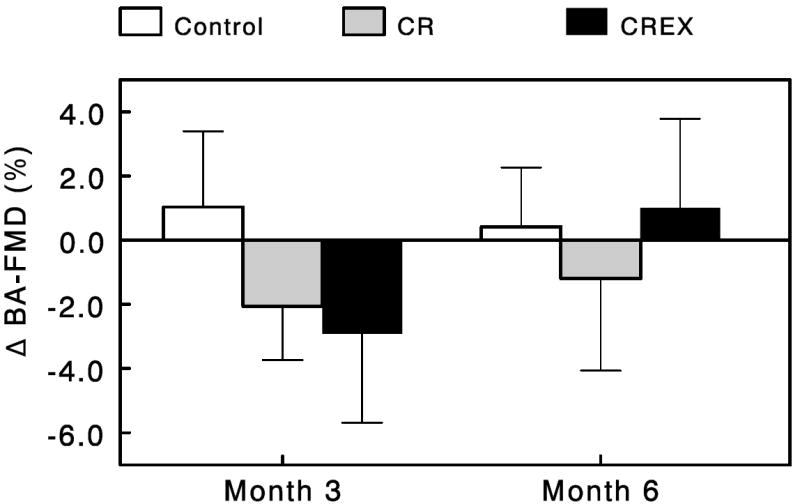
Effects of caloric restriction alone or in combination with exercise on brachial artery flow-mediated dilation (BA-FMD). Data are change from baseline for the Control group (n=11), CR group (n=12) and CR+EX group (n=12) at months 3 and 6. Error bars are SEM.
Estimated 10-Year CVD Risk
Estimated 10-year CVD risk at baseline was low in all groups (Control, 3.4 ± 1.3%; CR, 2.5 ± 0.6%; CR+EX, 1.5 ± 0.6%) due primarily to the relatively young age and good health of the study population. Caloric restriction had a substantial effect on estimated CVD risk (Figure 3). Expressed as estimated risk relative to baseline values, relative risk levels at M6 were significantly reduced in CR and CR+EX (P<0.001). The reduction in relative risk was achieved more quickly in the CR+EX group in that it was already significantly reduced at M3 and tended to be larger at M6 compared to the CR group. Relative risk in the Control group was unchanged.
Figure 3.
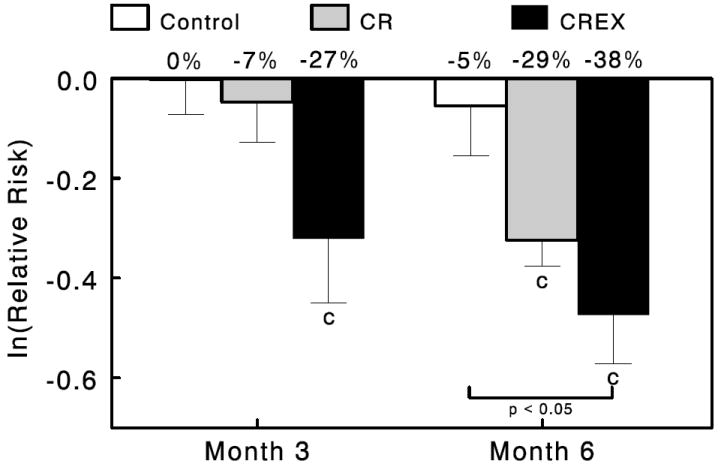
Effects of caloric restriction alone or in combination with exercise on estimated 10-year CVD risk relative to baseline. Data are the log of the ratio of month 3 and month 6 over baseline values for 10-year estimated CVD risk for the Control group (n=11), CR group (n=12) and CR+EX group (n=12) at months 3 and 6. Error bars are SEM. Numbers at the top of each bar are estimated percent reduction in risk. Statistically significant from baseline: cP < 0.001.
DISCUSSION
This randomized clinical trial examined the short-term benefits of 25% caloric restriction with and without exercise on established and emerging risk factors for CVD. We observed significant reductions in triacylglycerol (TG) in both the CR and CR+EX groups. Additionally, LDL-C, blood pressure and hsCRP were reduced in CR+EX group while factor VIIc level was reduced only with CR. Fibrinogen, homocysteine and endothelial function were not changed by either intervention, despite 10% reductions in body weight in both groups. Estimated 10-year CVD risk was significantly reduced by the interventions, with a tendency towards a greater reduction in the group assigned to caloric restriction and structured exercise.
Weight loss reduces LDL-C and TG whereas active weight loss reduces HDL-C even if HDL-C is increased once weight loss is stabilized. Based on prediction equations 19 and the degree of weight loss achieved in our study at six months, we would expect the CR group to experience a 5.8 mg/dL reduction in LDL-C, a 10.3 mg/dL reduction in TG, and a 2.1 mg/dL reduction in HDL-C if in active weight loss (or a 2.7 mg/dL increase at weight stability). For CR, the change in LDL-C (-4.1 mg/dL) was close to that predicted while HDL-C was increased (+1.2 mg/dL). The decrease in TG (-31 mg/dL) was however substantially greater than predicted. Weight loss and changes in HDL-C and TG were similar in the CR+EX group vs. the CR group. However, the reduction in LDL-C in the CR+EX group (-17 mg/dL vs. Control) was four-times greater than that observed in the CR group and much greater than predicted by weight loss alone. Such a large effect of exercise on LDL-C has not been typically observed 20, 21 and may in part reflect the carefully controlled exercise protocol.
Both atherosclerosis and CVD are recognized as inflammatory diseases 22 and consistently elevated levels of hemostatic factors (factor VIIc, fibrinogen), homocysteine and C-reactive protein (hsCRP) are associated with increased risk for CVD and cardiac events 23-25. In our study, factor VIIc levels were significantly reduced in the CR group, but not the CR+EX group. This contrasts with an increase in factor VIIc levels in the Control group at M6 providing a significant treatment effect. Changes in factor VIIc levels are known to correlate with changes in TG 26 which may in part explain the observed changes in the Control and CR groups. Homocysteine levels were not affected by the interventions confirming other data 27. hsCRP and fibrinogen are both acute phase reactants and markers of inflammation 25. There was a trend for both interventions to lower hsCRP. However, the responses were inconsistent with a large degree of variability and the data suggest that the observed favourable change in inflammatory markers was a function of an improvement in diet quality, rather than an effect of CR or CR+EX.
Endothelial dysfunction, an early event in CVD, can be assessed by measures of flow-mediated endothelium-dependent vasodilation of the brachial artery (BA-FMD). Impaired BA-FMD predicts coronary endothelial dysfunction and is influenced by plasma lipoprotein levels 28, Type 2 diabetes 29, insulin resistance 30 and hypertension 31. Thus, many of the risk factors targeted by caloric restriction could commonly impact endothelial function. However, despite generally favorable changes in many of the CVD risk factors and insulin sensitivity 15, we did not observe an improvement in BA-FMD in either intervention group. Previous studies of the effects of caloric restriction and weight loss on BA-FMD are controversial with some studies showing improved endothelial function 32 while others have not 27, 33 and may be reflective of health of the studied population. It is conceivable that an effect of the interventios could be identified with a longer intervention period and a larger sample size.
While the changes in individual risk factors were generally modest, when combined in a single prediction equation for CVD risk 18, the overall effect of caloric restriction was impressive. A 32% reduction in 10-year CVD risk was predicted based upon the combined changes in total cholesterol, HDL-C and systolic blood pressure. All participants in the CR and CR+EX groups experienced some degree of CVD risk reduction relative to baseline, while only 5 of 11 participants in the Control groups experienced such reductions. Furthermore, although not statistically significant, the addition of exercise appeared to be associated with a more favorable CVD risk profile than caloric restriction alone. By study design, we did not examine the effects of energy deficit by exercise alone, but a recent study published by Fontana et al. observed a similar reduction in CVD risk between by exercise alone as compared to CR alone 34. Several caveats must be considered when interpreting these changes. First, our population was relatively young and included 4 participants under the age of 30 even if the equations were generated for individuals 30-74 years of age. Furthermore, the risk equations take into consideration only changes in serum lipids and blood pressure. Newer equations, currently available for women only 35, also consider the effects of hsCRP on CVD risks. However, when applied to women only, the equation still predicted a 25-30% reduction in 10-year CVD risk in the CR and CR+EX groups while it was essentially unchanged in the Control group (+2%).
The lack of clear independent effect of training on CVD risk factors was of surprise to us. Yates T et al. 36 systematically reviewed all the controlled trials to determine the independent effect of exercise on glucose levels and risk of type 2 diabetes in people with prediabetes (IGT and/or IFG). They concluded that the contribution of physical activity independent of dietary or weight loss changes to the prevention of type 2 diabetes in people with prediabetes was equivocal. However studies such as the Diabetes Prevention Program clearly identified that intensive lifestyle changes including increased physical activity reduced the incidence of diabetes in persons at high risk 13. Finally I a prospective epidemiology study, low physical fitness was found to be a strong and independent predictor of CVD and all-cause mortality 37. However, in a randomized clinical trial of graded dose of exercise training in previously sedentary, overweight or obese postmenopausal women with elevated blood pressure, Church et al did not find exercise training to reduce blood pressure despite observing a graded increase in fitness across doses of exercise 38. Taken together, while exercise is clearly protective against premature mortality and morbidity the mechanisms responsible for this are unclear with many studies observing common CVD risk factors not improving in response to exercise.
The design of this study was unique from several perspectives. Firstly, care was extended in establishing baseline energy requirements and tailoring the subsequent intervention to achieve a 25% deficit in energy intake in each participant. Second, all estimates of CVD risk factors were obtained while participants were consuming diets of identical macronutrient composition, albeit at different absolute amounts. Importantly this approach allows us to isolate the effects of changes in caloric intake independent of changes in diet composition, a variable known to strongly influence several CVD risk factors. Finally, the interventions were carefully delivered and monitored. As a result, both intervention groups (CR and CR+EX) had gradual and almost identical reductions in body weight throughout the course of the study. However, there are some limitations that should be noted when interpreting these results. First, our study sample size was relatively small limiting our power to detect between group differences. Second, our study was only six months in length. Caloric restriction is viewed as a lifestyle change extending over years. However, the data are generally consistent with the benefits observed with longer caloric restriction in self selected individuals engaging in CR 39.
Acknowledgments
The authors want to thank the remaining members of the Pennington CALERIE Research Team including: James DeLany, Enette Larson-Meyer, Marlene Most, Steven Anton, Emily York-Crowe, Catherine Champagne, Paula Geiselman, Jennifer Howard, Jana Ihrig, Brenda Dahmer, Kim Landry, Anthony Alfonso, Darlene Marquis, Connie Murla, Aimee Stewart, and Andy Deutsch. Our gratitude is extended to the excellent staffs of the Outpatient Clinic, Inpatient Clinic, Metabolic Kitchen and Clinical Chemistry Laboratory. Thanks also to Claudia Van Skiver for developing the behavioral treatment manual and training the staff on how to use the HMR energy counting system. Finally, our profound gratitude goes to all the volunteers who spent so much time in participating in this very demanding research study.
FUNDING National Institutes of Health (U01 AG20478 to E.R.); National Health and Medical Research Council of Australia (ID 349553 to L.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JLJ, Jones DW, Materson BJ, Oparil S, Wright JT. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung and Blood Institute, National Institute of Health; 2004. pp. 04–5230. [Google Scholar]
- 4.Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, Howard WJ, Hunninghake DB, Illingworth DR, Luepker RV. The Third Report on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Bethesda, MD: National Heart, Lung and Blood Institute, National Institute of Health; 2001. pp. 02–5215. [Google Scholar]
- 5.Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. Bmj. 1996;312:1061–1065. doi: 10.1136/bmj.312.7038.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. Jama. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 8.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 10.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 11.Eilat-Adar S, Eldar M, Goldbourt U. Association of intentional changes in body weight with coronary heart disease event rates in overweight subjects who have an additional coronary risk factor. Am J Epidemiol. 2005;161:352–358. doi: 10.1093/aje/kwi045. [DOI] [PubMed] [Google Scholar]
- 12.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 17.Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flow-mediated dilation. Med Sci Sports Exerc. 2002;34:960–965. doi: 10.1097/00005768-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 19.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 20.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med. 1993;95:131–140. doi: 10.1016/0002-9343(93)90253-l. [DOI] [PubMed] [Google Scholar]
- 21.Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, Superko HR, Fortmann SP, Albers JJ, Vranizan KM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 22.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Manson JE, Buring JE, Shih J, Matias M, Hennekens CH. Homocysteine and risk of cardiovascular disease among postmenopausal women. Jama. 1999;281:1817–1821. doi: 10.1001/jama.281.19.1817. [DOI] [PubMed] [Google Scholar]
- 24.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 26.Bruckert E, Carvalho de Sousa J, Giral P, Soria C, Chapman MJ, Caen J, de Gennes JL. Interrelationship of plasma triglyceride and coagulant factor VII levels in normotriglyceridemic hypercholesterolemia. Atherosclerosis. 1989;75:129–134. doi: 10.1016/0021-9150(89)90169-x. [DOI] [PubMed] [Google Scholar]
- 27.Clifton PM, Keogh JB, Foster PR, Noakes M. Effect of weight loss on inflammatory and endothelial markers and FMD using two low-fat diets. Int J Obes (Lond) 2005;29:1445–1451. doi: 10.1038/sj.ijo.0803039. [DOI] [PubMed] [Google Scholar]
- 28.Clarkson P, Celermajer DS, Donald AE, Sampson M, Sorensen KE, Adams M, Yue DK, Betteridge DJ, Deanfield JE. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol. 1996;28:573–579. doi: 10.1016/0735-1097(96)82380-1. [DOI] [PubMed] [Google Scholar]
- 29.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 30.Balletshofer BM, Rittig K, Stock J, Lehn-Stefan A, Overkamp D, Dietz K, Haring HU. Insulin resistant young subjects at risk of accelerated atherosclerosis exhibit a marked reduction in peripheral endothelial function early in life but not differences in intima-media thickness. Atherosclerosis. 2003;171:303–309. doi: 10.1016/j.atherosclerosis.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Iiyama K, Nagano M, Yo Y, Nagano N, Kamide K, Higaki J, Mikami H, Ogihara T. Impaired endothelial function with essential hypertension assessed by ultrasonography. Am Heart J. 1996;132:779–782. doi: 10.1016/s0002-8703(96)90311-7. [DOI] [PubMed] [Google Scholar]
- 32.Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Caselli A, Caballero AE, Economides PA, Veves A, Horton ES. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26:2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 33.Brook RD, Bard RL, Glazewski L, Kehrer C, Bodary PF, Eitzman DL, Rajagopalan S. Effect of short-term weight loss on the metabolic syndrome and conduit vascular endothelial function in overweight adults. Am J Cardiol. 2004;93:1012–1016. doi: 10.1016/j.amjcard.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 35.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 36.Yates T, Khunti K, Bull F, Gorely T, Davies MJ. The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia. 2007;50:1116–1126. doi: 10.1007/s00125-007-0638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, Jr, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. Jama. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 38.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. Jama. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 39.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]


