1. Summary
Adenovirus vectors have been shown to be highly effective as vaccine platforms capable of inducing both humoral and cell mediated immune responses. An Ad serotype 5 vector containing unique deletions in the E2b region (Ad5 [E1-, E2b-]) has been reported to have several advantages over conventional Ad5 vectors deleted in only the E1 region (Ad5 [E1-]), including increased carrying capacity and diminished viral late gene expression. Here, we evaluated a novel Ad5 [E1-, E2b-] vector utilizing the E.C7 cell line for viral packaging. Its’ effectiveness as a potential vaccine platform as compared to the currently utilized Ad5 [E1-] based platform was assessed in both Ad5 naïve and Ad5 immune mice. We employed the HIV-1 Gag gene as the antigenic transgene expressed by the novel vector. Cellular expression of the Gag was confirmed by Western Blot analysis. Dose response studies using three intradermal immunizations of 107 to 1010 virus particles (VP) of each construct revealed that immunization with 1010 VP resulted in the maximum immunological response. Multiple immunizations of Ad naïve BALB/c mice with an Ad5 [E1-, E2b]-gag vaccine resulted in higher ELISpot CMI responses as compared to mice immunized with an Ad5 [E1-]-gag vaccine. More importantly, multiple immunizations of Ad5 immune BALB/c mice with an Ad5 [E1-, E2b]-gag vaccine resulted in significant increases in ELISpot CMI responses when compared to Ad5 immune mice vaccinated with an Ad5 [E1-]-gag vector. Preliminary studies in three Ad5 immune non-human primates (NHP) demonstrated that vaccination with Ad5 [E1-, E2b-]-gag induced elevated levels of interferon-γ and IL-2 secreting lymphocytes as assessed by ELISpot assays. These studies indicate that the novel Ad5 [E1-, E2b-] viral vector can be utilized as a potential vaccine platform to induce elevated CMI responses as compared to current generation Ad5 [E1-] viral vectors even in the presence of pre-existing Ad5 immunity.
Keywords: Adenovirus 5, Vaccine, Cell Mediated Immunity
2. Introduction
Important diseases such as HIV/AIDS, malaria, tuberculosis and Leishmaniasis afflict hundreds of millions of people and are potentially candidates for vaccine based preventative or treatment strategies. These vaccines need to be capable of not only large scale, cGMP compliant production, but also provide for safe and effective induction of both antigen specific humoral and cell mediated immune (CMI) responses. Vaccination using current generation recombinant Adenovirus serotype 5 (Ad5) vector vaccines deleted at the E1 or the E1 and E3 regions (Ad5 [E1-]) have been reported to have promise as vaccine platforms for the prevention and treatment of diseases. Ad5 viruses are ideal for vaccine applications because of their propensity to induce robust humoral and CMI responses, which has been demonstrated in murine, canine and non-human primate models as well as in human clinical trials [1–5, 6]. A barrier to the widespread use of current generation Ad5 vector platforms is pre-existing Ad5 immunity present in a high percentage of potential vaccinees. It has been reported that 40 to 60% of humans have detectable levels of neutralizing antibody (NAb) against Ad5 [7].
The adverse effect of Ad5 immunity on the level of B- and T- cell responses to transgene products expressed by Ad5 [E1-] vectors has been reported in animal models and in humans [8, 9, 10, 11]. Results from the Merck STEP HIV-1 vaccine trial demonstrated that the Ad5 [E1-] vector containing HIV-1 transgene inserts (MrkAd5) failed to significantly inhibit trended HIV-1 infection. This trial involved immunization of approximately 3,000 healthy uninfected volunteers with MrkAd5, which consisted of three current generation vectors each expressing an HIV-1 gene: gag, pol, or nef, respectively. Vaccinees who had high titers of Ad5 antibodies (Ad5>1:200) prior to immunization with MrkAd5 tended to have a higher incidence of HIV-1 infection than those without pre-existing Ad5 immunity (Ad5<1:18). The robustness of the CMI to the HIV-1 antigens was also weaker in the Ad5 immune individuals [8]. Vaccinees with pre-existing Ad5 immunity from exposure to wild-type Ad5 could have activated a memory immune response against the Ad5 vectors. This immune reactivation may have cleared the vaccine, eliminating the development of a robust CMI response against the inserted Gag, Pol and Nef products.
The presence of Ad5 immunity has resulted in a variety of immunization protocols designed to overcome this limitation. Although there is evidence that increasing vaccine dosage can increase induction of desired CMI responses in Ad-immune animals [12], it can result in unacceptable adverse effects and also increased immunity against the vector. Investigators have explored development of Ad based vaccines derived partially or completely from other serotypes or species, such as chimpanzee AdC68 and AdC1 [10, 13, 14], under the premise that NAb and CMI against the serotype or non-human primate viruses are not present in the human vaccinee. Several important issues with these approaches includes that it has been demonstrated that Ad5 specific NAb and CD8+ T cells cross react between alternate serotypes of human and chimpanzee Ad vectors [9, 10, 15]. Once an alternate serotype is utilized the vaccinee would mount a serotype specific immune response, a condition that would terminate the future use of that Ad vector for boosting or re-immunization. Furthermore, it has been confirmed that a number of alternative serotype Ad vectors have novel innate and adaptive immune response profiles which may increase safety concerns with their use [15–17]. Such concerns prompt continued efforts to improve the properties of the safe, well-characterized Ad5 platform.
Minimizing the number of viral genes in an Ad5 vaccine vector can reduce the number of Ad5 viral proteins expressed, possibly reducing the potential of Ad5 encoded viral proteins from impacting host immune responses [18]. An advanced generation of Ad5 vectors with unique deletions of the E1, E2b and E3 regions (Ad5 [E1-, E2b-]) has previously been described and utilized to allow for improved gene transfer in a number of clinically relevant applications [19–23]. The E2b region encodes the viral DNA polymerase (pol) and the pre-terminal protein (pTP) genes, which are required for Ad based genome replication, as well as late gene expression which encode all the viral structural proteins. Lack of E2b functionality completely prevents Ad5 replication even in the presence of supra-normal levels of the E1 transactivator [19–23]. Removal of the E2b region results in a 10,000-fold reduction in the expression of Ad late genes occurs due to the cis-activation of the Ad major late promoter [20]. As a result of this unique biology, it has been demonstrated in vivo that Ad5 [E1-, E2b-] vectors lead to an increased quantity and/or allow for sustained transgene expression in a number of animal models [23]. Ad5 [E1-, E2b-] vectors also display reduced acute toxicities when directly compared to Ad5 [E1-] vectors [24,25]. Specifically, Ad5 [E1-, E2b-] vectors were reported to have decreased toxicity in multiple cell types, including hepatocytes, myocytes, and cochlear hair cells found in the inner ear [26–29].
We hypothesized that the reduction of toxic Ad5 gene expression and/or induction of immune responses to these gene products would result in improved CMI responses to Ad5 [E1-, E2b-] expressed antigenic targets. Furthermore, this might allow for improved vaccine efficacy in the presence of Ad5 immunity since Ad5 [E1-, E2b] deleted vectors may provoke a decreased Ad5 specific immune response in this context. This would allow for focused CMI responses to the desired transgene antigen. We report herein the capability of an Ad5 [E1-, E2b-] vector to be utilized as a vaccine platform that induces CMI responses. Studies were performed to directly compare Ad5 [E1-, E2b-] and Ad5 [E1-] vectors’ propensities to induce CMI responses in Ad5 naïve and Ad5-immune mice. Ad5 [E1-, E2b-] and Ad5 [E1-] vectors were constructed containing the HIV-1 Gag gene insert as a transgene. We choose the HIV-1 model due to the importance of CMI responses in controlling the disease [30–32] and also because of the use of recombinant Ad5 viral vectors as a vaccine platform in HIV-1 clinical trials [31, 32]. CMI was assessed by total number of T cells secreting IFN-γ and IL-2 as determined by ELISpot analysis. We found that the novel Ad5 [E1-, E2b-] viral vector can induce increased levels of antigen specific CMI responses following immunization when compared to Ad5 [E1-] vectors in both Ad5 naïve and Ad5 immune animals despite the presence of high levels of Ad5 neutralizing activity. The propensity of this vector to illicit robust CMI in Ad5-immune animals also enabled for homologous boosting. Based upon the numerous improvements noted, we undertook a pilot study in which Ad5-hyper immune non-human primates (NHP) were immunized with Ad5 [E1-, E2b-]-gag. The immunogenicity of this novel vaccine platform in Ad5 immune vaccinees was confirmed in the NHP model.
3. Materials and Methods
3.1. Animals
Specific pathogen-free, BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) ages 6 to 8 weeks were housed in animal facilities at the Infectious Disease Research Institute (Seattle, Washington) and all procedures were conducted according to IACUC approved protocols. Cynomolgus macaques were housed at the Southern Research Institute (SRI, Frederick, MD) and all protocols were reviewed and approved by appropriate animal care and biosafety committees before initiation of the study. NHP peripheral blood mononuclear cells (PBMC) and serum samples were collected by SRI and shipped overnight to Etubics Corporation (Seattle, WA) for analysis.
3.2. Vaccine Vectors
Using the HXB Gag gene (Genbank Accession # K03455) derived from pVRC3900 (kindly provided by the Vaccine Research Center, NIAID) [33] the Ad5 [E1-]-gag and [E1-, E2b-]-gag vector platforms were constructed, and vaccines were purified according to previously published procedures [19]. Briefly, the HXB Gag cDNA was sub-cloned into the E1 region of the Ad5 [E1-] or Ad5 [E1-, E2b-] vectors using a previously described homologous recombination based system and Gag protein production was placed under the expressional control of a cytomegalovirus (CMV) enhancer/promoter element. The replication deficient virus was then propagated in the HEK-293 (Ad5 [E1-]) or E.C7 (Ad5 [E1-, E2b-]) packaging cell lines, CsCl2 purified, and titered as described previously [26–29]. The infectious unit and particle numbers of the Ad5 [E1-]-gag and Ad5 [E1-, E2b-]-gag were compared. Infectious titers were determined on 293 cell monolayers which had plaque-forming titers of 3.0 × 1010 and 5.0 × 1010 PFU/ml for the Ad5 [E1-]-gag and Ad5 [E1-, E2b-]-gag virus preparations, respectively. The manufacturing particle concentrations were determined spectrophotometrically and were 1.1 × 1012 virus particles/ml for both viral lots. Thus, the ratios of particle number to PFU were similar for both virus lots, 36 versus 22 VP/PFU, respectively.
3.3. Immunization
Mice were injected with Ad5 [E1-]-gag or Ad5 [E1-, E2b-]-gag intradermally into a hind footpad and NHP were immunized intradermally in a hind leg below the knee. Ad5 vector constructs were delivered at a dose of 1010 virus particles (VP) to both mice and NHP unless otherwise noted. Doses were administered in 25 μl of injection buffer (20 mM HEPES with 3% sucrose) and control mice were injected with injection buffer alone.
3.4. Western Blot Analysis
Human lung carcinoma cells (A-549) (ATCC number CCL-185), (106) were infected at a MOI of 200 VP per cell. Vehicle treated A-549 cells served as a mock-infected control group. Cells were harvested 44 h post infection and lysed by four freeze-thaw cycles. The cell lysate was then separated on a 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane (GE Healthcare, Piscataway, NJ). Membranes were then blocked with TBS containing 5% (w/v) blocking reagent (GE Healthcare, Piscataway, NJ) for 2 hours at room temperature and sequentially incubated with mouse anti-p24 antibody (1:2000) (Advanced Bioscience Laboratories, Gaithersburg, MD) and goat anti-mouse-HRP conjugated antibody (1:5000) (KPL) for an hour at room temperature. Positive reactivity was visualized by chemiluminescence using an ECL Western Blotting analysis system (GE Healthcare, Piscataway, NJ) according to the manufacturer’s specifications.
3.5. Adenovirus 5 Neutralization Assay
A human embryonic kidney (HEK-293) cell line (supplied by Dr. Jeffrey Chamberlain, University of Washington) was cultured at a cell concentration of 2 × 103 cells/well in 96-well plates in culture medium consisting of DMEM plus 10% fetal calf serum (FCS). After incubating for 24 hours at 37°C in 5% CO2, dilutions of heat inactivated (56 °C for 60 minutes) test sera were prepared in 100 μL volumes of culture media, mixed with 4 × 107 VP Ad5-null, and incubated for 60 minutes at room temperature for virus neutralization activity. After incubation, the samples were added to microwells containing the HEK-293 cells in triplicate and incubated for an additional 72 hours. Wells containing medium only or medium with cells plus 4 × 107 Ad5-null VP in triplicate served as controls. To quantify resultant cell killing, 40 μL of CellTiter 96® AQueouse (Promega, Madison, WI) was added to all wells and incubated for 75 minutes at 37°C in 5% CO2. Following this incubation, media was removed from each well and transferred to a second microwell plate and analyzed at 492nm using a Finstruments Microplate reader and endpoint Ad5 NAb titers were then determined.
3.6. Enzyme-Linked Immunospot (ELISpot) Assay
An ELISpot assay was used to detect HIV-1 Gag specific IFN-γ and IL-2 producing T cells from either fresh or cryopreserved mouse splenocytes previously stimulated with a recombinant p24 protein (IDRI, Seattle, WA). NHP PBMC were stimulated with a pool of 15 amino acid peptides with an 11-mer overlap (NIH). The murine ELISpot assay was run according to the manufacture’s protocols (eBioscience, San Diego, CA). For the NHP ELISpot assay, we utilized procedures previously described for IL-2 by R&D Systems, Minneapolis, MN and for IFN-γ by MABTECH, Cincinnati, OH. Mouse splenocytes or NHP PBMC were used at a concentration of 2 × 105 cells/well and reported as the number of spots per 106 cells per well. HIV-1 p24 protein (IDRI, Seattle, WA), Adenovirus 5 (Viraquest, North Liberty, IA) and carcinoembryonic antigen protein (CEA) (Aspen Bio Pharma, Castle Rock, CO), was used at a final concentration of 1 μg/well. HIV-1 Gag, Pol (NIH), Nef (NIH), herpes simplex virus glycoprotein B (HSV-gB2) (Anaspec, San Jose, CA), Cytomegalovirus (CMV-CEF21) (Anaspec, San Jose, CA), and influenza virus (Flu-CEF9) (Anaspec, San Jose, CA) peptides were utilized at 0.2 μg of each peptide/well. Cells stimulated with concanavalin A (ConA) at a concentration of 0.0625 μg/well served as a positive control. Cells were also stimulated with Adenovirus 5 (Ad5) Colored spot-forming cells (SFC) were counted using an Immunospot ELISpot plate reader (Cellular Technology, Shaker Heights, OH) and responses were considered to be positive if, 1) 50 SFC were detected/106 cells after subtraction of the negative control and, 2) SFC were ≥2-fold than those in the negative control wells. SFC quantities are reported as the number of observed spots per 106 cells per well.
3.7. Statistical analysis
Statistically significant differences in the mean cellular immune responses between groups of animals were determined by Student’s t-test (p<0.05), using GraphPad PrismR (GraphPad Software, Inc.).
4. Results
4.1. Cellular Expression of Gag by Ad5 [E1-]-gag and Ad5 [E1-, E2b-]-gag vaccines in vitro
HIV-1 Gag protein expression by the Ad5 [E1-]-gag and Ad5 [E1-, E2b-]-gag vectors was evaluated and confirmed prior to use in immunizations. A-549 cells were infected with the vaccine virus platforms and analyzed by Western blot analysis as described above. Transgene product could be detected using HIV-1 Gag antibody, indicating that cells infected with the recombinant Ad5-gag vaccines expressed HIV-1 Gag (data not shown).
4.2. Dose response assessment of Ad5 [E1-]-gag and Ad5 [E1-, E2b-]-gag vector immunizations
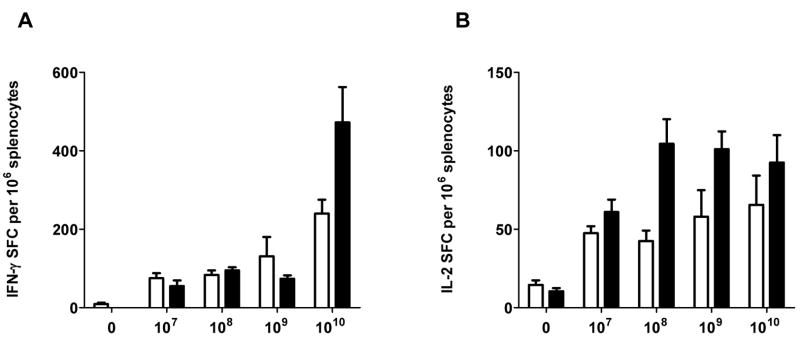
Optimal immunizing dose was determined by evaluating dose effect on CMI response induction. Groups of naïve mice were immunized three times at two week intervals using 107, 108, 109, or 1010 VP of Ad5 [E1-, E2b-]-gag or Ad5 [E1-]-gag. We chose to employ a three-immunization vaccination regime because it has been used in recent Ad5 based clinical trials [7]. Fourteen days after the third vaccination, CMI responses were determined by IFN-γ and IL-2 ELISpot assays as described in the material and methods section. ELISpot IFN-γ responses were detected after three immunizations with 107, 108,109 and 1010 VP of Ad5 [E1-, E2b-]-gag or Ad5 [E1-]-gag but the levels of IFN-γ secreting splenocytes were significantly elevated after three immunizations with 1010 VP of either vector (Figure 1A). Mice that received 1010 VP of Ad5 [E1-, E2b-]-gag had a statistically significant higher number splenocytes secreting IFN-γ than mice that were immunized with Ad5 [E1-]-gag at that same dose. Low levels of ELISpot IL-2 responses were detected after three immunizations of Ad5 [E1-]-gag at all four doses but increasing the dose made no statistical improvement in CMI response. Mice immunized with 107 VP of Ad5 [E1-, E2b]-gag exhibited a low level of IL-2 secretion, which increased and remained elevated after three immunizations of 108, 109 or 1010 VP of Ad5 [E1-, E2b]-gag (Figure 1B). These results indicated that the optimal dose to be used in a three administration immunization regime in this murine model is 1010 VP of Ad5 [E1-, E2b-]-gag or Ad5 [E1-]-gag.
Figure 1. Dose titration of Ad5 [E1-]-gag and Ad5 [E1-, E2b-]-gag and resultant CMI responses.
Naïve BALB/C mice (n=5/group) were immunized three times using doses of 107, 108, 109 or 1010 VP of Ad5 [E1-, E2b-]-gag. Fourteen days after the final immunization splenocytes from the mice were assessed by ELISpot analysis. The greatest induction of CMI was achieved by using 1010 VP of the vector. White = Ad5 [E1-]-gag; Black = Ad5 [E1-, E2b-]gag. The error bars depict the standard error of the mean (SEM).
4.3. CMI response to a single immunization in Ad5 naïve mice
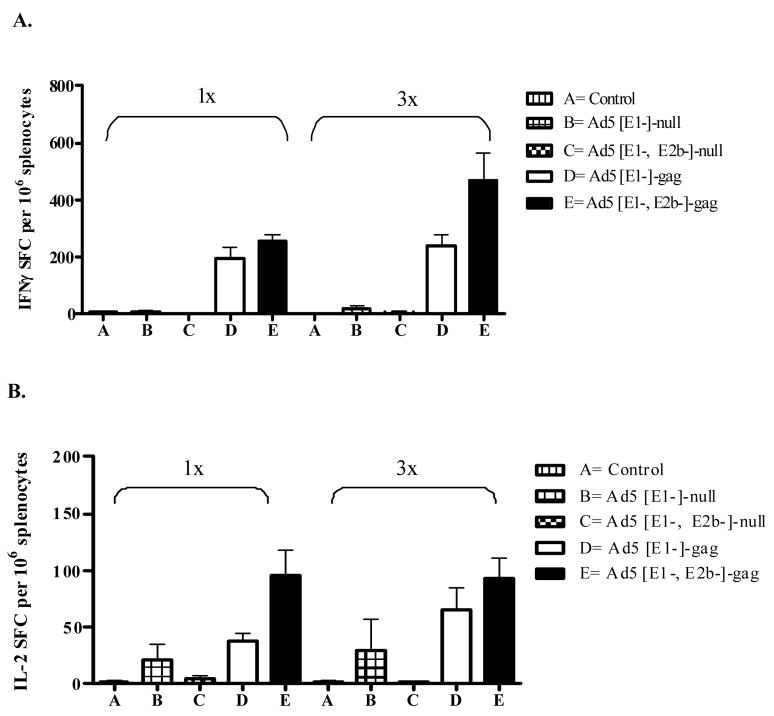
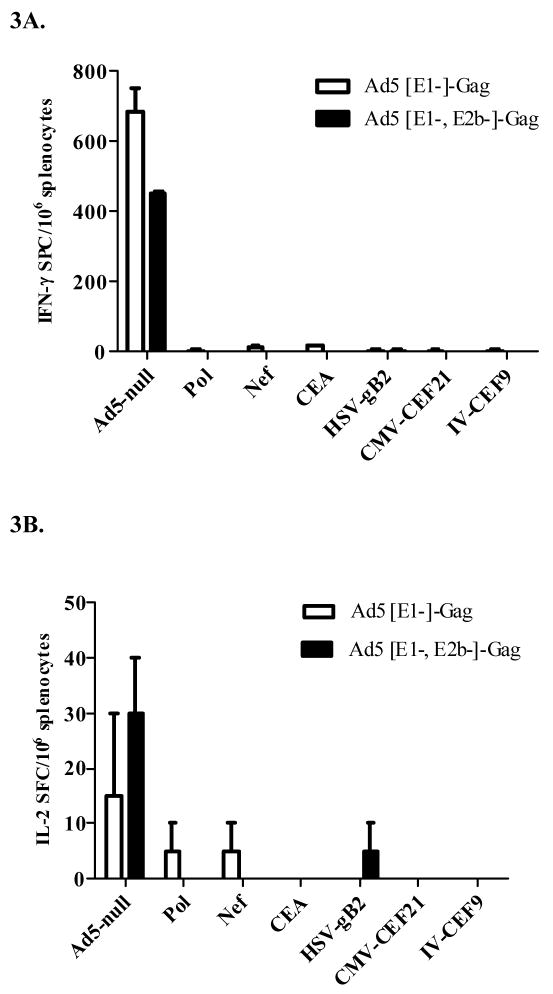
To assess the induction of CMI following a single immunization, naïve BALB/c mice were immunized once with 1010 VP of Ad5 [E1-, E2b-]-gag or Ad5 [E1-]-gag. Fourteen days later, Gag-specific CMI responses were determined by IFN-γ and IL-2 ELISpot assays as described above. Mice immunized once with Ad5 [E1-, E2b-]-gag demonstrated a higher trend of IFN-γ secreting splenocytes than mice immunized once with Ad5 [E1-]-gag, but the difference was not significant (Figure 2A); however there was a statistically significant higher (p=0.029) number of splenocytes secreting IL-2 in mice immunized once with Ad5 [E1-, E2b-]-gag (Figure 2B). Splenocytes from the vaccinated mice were also assessed for non-specific cytokine secretion by stimulation with multiple irrelevant antigens including the HIV-1 antigens Pol and Nef, carcinoembyronic antigen (CEA), Herpes Simplex Virus glycoprotein B (HSV-gB2), Cytomegalovirus (CMV-CEF21) and influenza virus (IV-CEF9). No non-specific IFN-γ or IL-2 secretion was detected in splenocytes from mice vaccinated with either vector (Figure 3). Splenocytes were also stimulated with Ad5-null to confirm positive vaccination (Figure 3).
Figure 2. Multiple immunizations induces greater CMI response.
Naïve BALB/C mice (n=5/group) were immunized once or three times at fourteen day intervals with 1010 VP of Ad5 [E1-]-null, Ad5 [E1-, E2b-]-null, Ad5 [E1-, E2b-]-gag, Ad5 [E1-]-gag or injection buffer alone (control). Fourteen days after the final immunization splenocytes were assessed for (A) IFN-g or (B) IL-2 secreting splenocytes by ELISpot analysis. The error bars depict the SEM.
Figure 3. CMI against Ad5 [E1-, E2b]-gag and Ad5 [E1-]-gag does not to cross react with other antigenic targets.
Splenocytes from BALB/c mice immunized once or three times at fourteen day intervals with 1010 VP of Ad5 [E1-, E2b-]-gag or Ad5 [E1-]-gag. Fourteen days after the final immunization splenocytes from the mice were assessed by ELISpot for non specific (A) IFN-g or (B) IL-2 secretion by stimulation with the non-immunizing antigens HIV-1 Pol, HIV-1 Nef, carcinoembryonic antigen (CEA), Herpes Simplex Virus glycoprotein B (HSV-gB2), Cytomegalovirus (CMV-CEF21), and influenza virus (IV-CEF9). Splenocytes were also stimulated with Ad5-null to confirm positive vaccination. Values for control mice were less than 10 spots. White = Ad5 [E1-]-gag; Black = Ad5 [E1-, E2b-]-gag. The error bars indicate the SEM.
4.4 CMI response to multiple immunizations in Ad5 naïve mice
To determine if the Ad5 [E1-, E2b-] platform could out perform the current generation Ad5 [E1-] platform in a homologous boost immunization regime, mice were immunized three times at two week intervals intradermally with 1010 VP of Ad5 [E1-, E2b-]-gag or Ad5 [E1-]gag. Splenocytes were harvested fourteen days after the final vaccination and evaluated by IFN-γ and IL-2 ELISpot. Mice immunized three times with Ad5 [E1-]-gag did not have statistically significant elevated levels of IFN-γ (p=0.39) or IL-2 (p=0.20) secreting splenocytes as compared to mice injected once with Ad5 [E1-]-gag (Figure 2A). There was no immune “boost” achieved after multiple immunizations with the current generation platform. Conversely, mice immunized three times with Ad5 [E1-, E2b-]-gag did demonstrate a Gag specific immunologic boost after homologous immunizations, with a statistically significant higher number of splenocytes secreting INF-γ (p=0.046) after three immunizations as opposed to once (Figure 2B). These mice did not have a significant boost in IL-2 secretion with multiple immunizations but the levels remained elevated. The immunity to Ad5 as a result of the primary vaccination did not affect the robustness of immune response induced by subsequent vaccinations. These results demonstrate the ability utilize the Ad5 [E1-, E2b-] platform in homologous vaccination regimens.
4.5. CMI response induction in Ad5 immune mice
To test our hypothesis that the Ad5 [E1-, E2b-] platform would outperform the current generation Ad5 [E1-] vector in the presence of Ad5 immunity, we first developed an Ad5 immune murine model. In order to render animals immune to the Ad5 virus, naïve mice were immunized once, twice or three times with 1010 VP of an Ad5 [E1-]-null (vector containing no transgene insert) to induce Ad5 neutralizing antibody (NAb). Fourteen days after the final immunization of the Ad5 [E1-]-null vector, Ad5 NAb titers were measured by the Ad5 neutralization assay described above. As shown in Figure 4, Ad5 NAb titers averaged 1:25 after one immunization, 1:350 after two immunizations and 1:3500 after 3 immunizations. Based on these results, mice were given two immunizations with Ad [E1-]-null to be considered “Ad5 immune”. Please note in the Merck STEP trial participants with an Ad5 antibody titer >1:200 were considered to have “high” pre-existing Ad5 antibody titers. BALB/c mice were pre-immunized twice with 1010 VP Ad5 [E1-]-null to induce Ad5 NAb endpoint titers of ≥1:200 which was confirmed in Ad5 NAb assays. These mice were then immunized three times at two week intervals with 1010 VP of Ad5 [E1-, E2b-]-gag or Ad5 [E1-]-gag. Splenocytes were collected 14 days after the final immunization and then assessed by IFN-γ and IL-2 ELISpot as described above. As shown in Figure 5, Ad5 immune mice immunized with Ad5 [E1-, E2b-]-gag exhibited significantly higher levels of IFN-γ (p=0.004) and IL-2 (p=0.003) secreting splenocytes as compared to Ad5 immune mice immunized with Ad5 [E1-]-gag. These results indicate the superiority of the Ad5 [E1-, E2b-] vector in eliciting CMI responses to the desired transgene in Ad5 immune vaccinees as compared to the Ad5 [E1-] vector in this model.
Figure 4. Induction of Ad5 NAb after immunization with Ad5-null in mice.
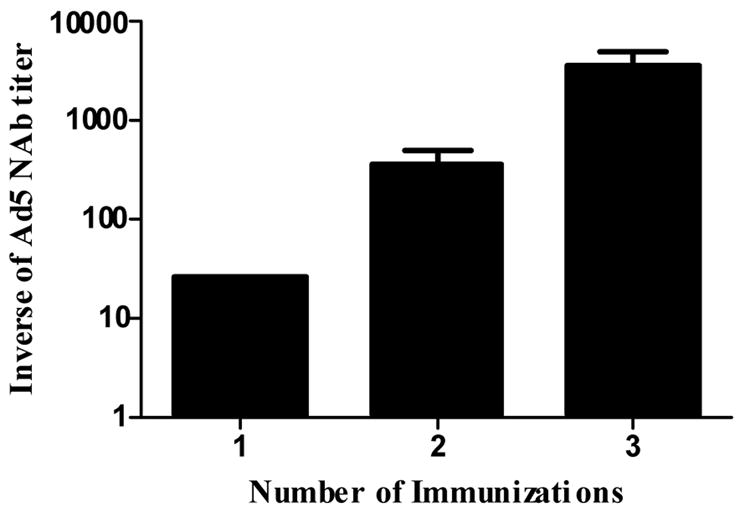
Circulating Ad5 Neutralizing antibody titers present in serum from mice immunized one, two or three times with 1010 VP of Ad5-null. Note the increasing levels of NAb after each immunization. The error bars indicate the SEM.
Figure 5. CMI response induction in Ad5 immune mice.
BALB/c mice were pre-immunized 2 times with 1010 VP Ad5 null to induce NAb endpoint titers of 1/200. They were then immunized three times with 1010 VP Ad5 [E1-]-gag or Ad5 [E1-, E2b-]-gag. Splenocytes were collected 14 days after the final immunization and assessed for (A) IFN-γ and (B) IL-2 secreting splenocytes by ELISpot. Note the significantly (P<0.005) higher levels of IFN-γ and IL-2 secreting cells in mice that were immunized with Ad5 [E1-, E2b-]-gag. White = Ad5 [E1-]-gag; Black = Ad5 [E1-, E2b-]-gag. The error bars depict the SEM.
4.6. CMI response induction in Ad5 immune cynomolgus macaques
Due to the encouraging results from the Ad5 immune murine study, a small preliminary study was performed in Ad5 immune NHP. Three Cynomolgus macaques were injected with a single does of 1010 VP viable wild type Ad5. Wild type Ad5 was used to create Ad5 immunity that mimics the Ad5 immune response found in humans who acquired the virus naturally in their environment. Ad5 NAb titers were measured 30 days after administration and the NHP titers were ≥1:50 (data not shown), representing “medium” Ad5 immunity. The Ad5 immune NHP were then immunized three times on days 0, 27, and 58 with Ad5 [E1-, E2b-]-gag (1010 VP/dose). Peripheral blood mononuclear cells (PBMC) from individual NHP were collected and CMI responses were assayed 32 days after the final immunization with Ad5 [E1-, E2b-]-gag. ELISpot analysis indicate that all three NHP responded similarly with an average frequency of 223 ± 70 SFC/106 PBMC producing INF-γ, and 207.1 ± 94 SFC/106 PBMC producing IL-2 (Figure 6). These values were significantly (P<0.05) elevated when compared to their baseline values. The Ad5 viral NAb titers were monitored throughout the immunization regime. Ad5 NAb titers increased following each vaccination and ranged from 1:1000 to 1:20,000 at the termination of this study (data not shown). These results indicate that a CMI response was elicited by the Ad5 [E1-, E2-] platform to the desired transgene antigen even in the presence of Ad5 immune responses in this NHP model.
Figure 6. CMI response induction in Ad5 immune cynomolgus macaques.
Peripheral blood mononuclear cells (PBMC) from individual NHP were collected and CMI responses were assayed 32 days (Day 90) after the final immunization with Ad5 [E1-, E2b-]-gag. Note the significantly (P<0.05) elevated levels of (A) IFN-γ and (B) IL-2 secreting cells from the PBMC sample taken after the vaccination protocol as compared to a baseline sample (Day –8) taken before vaccinations. The error bars depict the SEM.
5. Discussion
The need for successful vaccine prophylaxis against difficult to treat indications and infectious pathogens such as human immunodeficiency virus (HIV), H5N1 influenza, malaria, certain bioterrorism agents and cancers is evident. A plethora of investigations have been conducted utilizing live viral based vector vaccines due to their propensity to elicit significant cell mediated immunity [1,34]. Among the cadre of recombinant viral vectors, Ad5 based platforms appear to have a number of advantages, including the direct infection of antigen-presenting (dendritic) cells [35–38], induction of a 5–10 fold greater CMI response than other vectors such as modified Vaccinia Ankara (MVA) based vectors [11, 39, 38]; and live Ad-vectors provide a higher protective effectiveness on a per-cytotoxic T cell basis [39]. Unfortunately, the commonality of human exposure to Ad5 has resulted in widespread Ad NAb titers that may reduce the effectiveness of these immunizing vectors [6, 40–43]. The use of alternate Ad serotypes to avoid this challenge may not be satisfactory for a number of reasons including recent findings that Ad serotypes have novel biodistribution and innate immune response profiles and cross reactivity between serotypes [9, 17, 44]. Also, once that alternate serotype is used you face the same anti-vector immunity challenge currently hindering Ad5 platforms.
To overcome the challenge of pre-existing Ad5 immunity, the propensity of a novel Ad5 [E1-, E2b-] vector to successfully induce CMI immune responses in both Ad5 naïve and Ad5 immune animals was investigated and compared with CMI responses observed with a current generation Ad5 [E1-] vector. Ad5 [E1-, E2b-] and Ad5 [E1-] vectors were constructed to express the HIV-1 Gag gene as a “target” antigen. In Ad5 naïve mice the new Ad5 [E1-, E2b-] platform performed as well as, if not better than the potent current generation Ad5 [E1-]-vector. A striking difference between the two platforms was not expected in Ad5 naïve animals because there was no Ad5 immunity present to interfere with either vector’s delivery of the transgene. Also, extended transgene expression observed when using the Ad5 [E1-, E2b-] platform did affect the CMI response due to the short time course of the experiment. When the two vaccines were employed a homologous immunization regime the Ad5 [E1-, E2b-] platform elicited an immune “boost” after re-immunization where the Ad5 [E1-] platform did not. The immune response against the Ad5 [E1-] vector induced by the primary vaccination appeared to hinder subsequent vaccinations with that platform. The capability to re-immunize a vaccinee with the same vector platform may reduce safety concerns due to cross reactivity between vectors and also can simply immunization regimes.
The significant finding of the present study is the propensity of this novel Ad5 [E1-, E2b-] vector to induce CMI responses in the presence of Ad5 immunity. Animals who were first made immune to Ad5 and then immunized with the Ad5 [E1-, E2b-] platform were still mounted transgene specific CMI responses in the presence of anti-vector immune responses. As demonstrated before and re-confirmed in our studies, this was not achieved using the current generation Ad5 [E1-] platform. This was demonstrated in a NHP model, a model that has been previously reported to be resistant to induction of antigen CMI responses when Ad5 [E1-] vaccines were employed [9]. The capability to immunize individuals with Ad5 immunity with the Ad5 [E1-, E2b-] platform may not only allow for successful vaccination in Ad5 immune populations, but confers the possibility of re-immunization against other antigens utilizing the Ad5 [E1-, E2b-] expression platform within the same vaccinee.
The mechanism by which this vector overcomes pre-existing Ad5 immunity is not defined but there are likely several contributing factors, the most notable being a significant reduction of expression of viral late genes [18]. Deletion of the E2b region blocks the expression of downstream promoters resulting in the reduction of Ad5 late gene protein production and therefore a reduction of Ad5 vector epitopes. Deletion of the E1- region does not render the Ad5 virus completely replication deficient and at higher multiplicities of infection (MOIs) several Ad5 [E1-] promoters are active permitting late gene expression [18, 46, 47]. In vivo Ad5 [E1-] vectors have been reported to express Ad5 early and structural genes as well as replicate their genome in vivo [48, 49]. We suggest that the lack of Ad5 late gene expression from the Ad5 [E1-, E2b-] vectors enables cells infected with this virus to avoid deleterious host immune responses directed against the Ad5 antigens, thereby “focusing” immune responses toward the desired transgene antigen. Further studies are presently underway in an attempt to understand why this vector platform overcomes pre-existing Ad5 immunity.
Several attributes of the Ad5 [E1-, E2b-] vector that make it a favorable delivery platform for gene therapy also make it a suitable candidate for vaccine applications. The vector can be successfully propagated to high titers in the E.C7 packaging cell line and purified using conventional methods [21]. With additional deletions of the Ad5 genome, increased gene-carrying capacity is achieved enabling a 12kb cassette for transgene insertion. The increased cloning capacity of the vector allows for insertion of multiple genes [50]. This is advantageous for vaccine development against pathogens such as HIV-1, where a multigene vaccine approach can broaden antiviral immune responses without incurring immune interference [51]. The Ad5 [E1-, E2b-] vector has been reported to exhibit extended transgene expression at higher levels than first generation Ad [E1-] vectors, which results in increased protein load [23]. The increased transgene protein load may result in augmentation of the induction of an immune response to the transgene in the vaccinee and may increase the immune response to less antigenic transgene targets. Patient safety is a concern when utilizing live recombinant viral constructs, which may revert to wild type virus through recombination events. Due to the more extensive genetic deletions in the Ad5 [E1-, E2b-] vector platform, multiple recombination events are required to generate replication competent virus, thus limiting the theoretical potential of wild-type reversion
Herein we have presented the novel Ad5 [E1-, E2b-] vector vaccine platform. Our data indicates that higher levels of CMI responses can be induced to specific, non-Ad5 antigens delivered by the Ad5 [E1-, E2b-] vector as compared with a current generation Ad5 [E1-] vector vaccine platform. This novel Ad5 [E1-, E2b-] platform can induce a potent CMI response against the vectored antigens even in the presence of Ad5 immunity. This was most clearly evident in an Ad5 immune non-human primate model, a model that has been previously reported to be resistant to induction of antigen CMI responses when Ad5 [E1-] vaccines were employed [9]. In light of the recent results of the STEP trial, utilizing an Ad5 [E1-] vector vaccine developed by Merck [8], it is clear there is a need for improved viral vector vaccine platforms. Recent work indicates that alternatives proposed to overcome pre-existing Ad5 immunity, such as use of alternative serotype based Ad vaccines, may have more problems than anticipated which warrants further investigation into the well-characterized Ad5 based platform [15, 17]. Based upon the encouraging preliminary results reported herein, we believe further studies on this novel Ad5 [E1-, E2b-] vector platform are warranted including studies into the mechanism of action, challenge models as well as defining responses from distinct T cell populations.
Acknowledgments
This work was supported by NIH-NIAID grant number 1R43AI071733-01. The authors would like to thank Winston Wicomb for his support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gómez-Román VR, Robert-Guroff M. Adenoviruses as vectors for HIV vaccines. AIDS Rev. 2003;5:178–185. [PubMed] [Google Scholar]
- 2.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 3.Appaiahgari MB, Saini M, Rauthan M, Jyoti, Vrati S. Immunization with recombinant adenovirus synthesizing the secretory form of Japanese encephalitis virus envelope protein protects adenovirus-exposed mice against lethal encephalitis. Microbes Infect. 2006;8:92–104. doi: 10.1016/j.micinf.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Barratt-Boyes SM, Soloff AC, Gao W, Nwanegbo E, Liu X, Rajakumar PA, et al. Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J Gen Virol. 2006;87:139–149. doi: 10.1099/vir.0.81445-0. [DOI] [PubMed] [Google Scholar]
- 5.Cantanzaro AT, Koup RA, Roederrer M, Bailer RT, Enama ME, Moodic Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194(12):1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tims T, Briggs DJ, Davis RD, Xiang Z, Ertl HC, Fu ZF. Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titers of neutralizing antibodies. Vaccine. 2000;18:2804–2807. doi: 10.1016/s0264-410x(00)00088-8. [DOI] [PubMed] [Google Scholar]
- 7.Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–75. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? JEM. 2008;205:7–11. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin Sw, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007;81(12):6594–604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AO, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170(3):1416–22. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 11.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barouch DH, McKay PF, Sumida SM, Santra S, Jackson SS, Gorgone DA, et al. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J Virol. 2003;77:8729–35. doi: 10.1128/JVI.77.16.8729-8735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorner AR, Lemckert AA, Goudsmit J, Lynch DM, Ewald BA, Denholtz M, et al. Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity. J Virol. 2006;80(24):12009–16. doi: 10.1128/JVI.01749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol. 2003;171(12):6774–9. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- 15.Hartman ZC, Appledorn DM, Serra D, Glass O, Mendelson T, Clat T, et al. Replication-attenuated Human Adenoviral type 4 vectors elicit capsid dependent enhanced innate immune responses that are partially dependent upon interactions with the complement system. Virol. 2008 doi: 10.1016/j.virol.2008.01.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone D, Ni S, Li ZY, Gaggar A, DiPaolo N, Feng Q, et al. Development and assessment of human adenovirus type 11 as a gene transfer vector. J Virol. 2005;79(8):5090–104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appledorn DM, Kiang A, McBride A, Jiang H, Seregin S, Scott JM, et al. Wild-type Adenoviruses from groups A-F elicit unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Therapy. 2008 doi: 10.1038/gt.2008.18. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–33. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar-Singh R, Chamberlain JS. Encapsidated adenovirus minichromosomes allow delivery and expression of a 14 kb dystrophin cDNA to muscle cells. Hum Mol Genet. 1996;5:913–21. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- 20.Amalfitano A, Begy CR, Chamberlain JS. Improved adenovirus packaging cell lines to support the growth of replication-defective gene-delivery vectors. Proc Natl Acad Sci USA. 1996;93:3352–56. doi: 10.1073/pnas.93.8.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser MA, Amalfitano A, Kumar-Singh A, Hauschka SD, Chamberlain JS. Improved adenoviral vectors for gene therapy of Duchenne muscular dystrophy. Neuromuscul Disord. 1997;7:277–83. doi: 10.1016/s0960-8966(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, Serra D, Amalfitano A. Persistence of an [E1-, polymerase-] adenovirus vector despite transduction of a neoantigen into immune-competent mice. Hum Gene Ther. 1999;10:355–64. doi: 10.1089/10430349950018805. [DOI] [PubMed] [Google Scholar]
- 23.Schaack J. Induction and inhibition of innate inflammatory responses by adenovirus early region proteins. Viral Immunol. 2005;18:79–88. doi: 10.1089/vim.2005.18.79. [DOI] [PubMed] [Google Scholar]
- 24.Schaack J, Bennett ML, Colbert JD, Torres AV, Clayton GH, Ornelles D, et al. E1A and E1B proteins inhibit inflammation induced by adenovirus. Proc Natl Acad Sci U S A. 2004;101:3124–29. doi: 10.1073/pnas.0303709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–30. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 26.Luebke AE, Steiger JD, Hodges BL, Amalfitano A. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 2001;8:789–94. doi: 10.1038/sj.gt.3301445. [DOI] [PubMed] [Google Scholar]
- 27.Everett RS, Hodges BL, Ding EY, Xu F, Serra D, Amalfitano A. Liver toxicities typically induced by first-generation adenoviral vectors can be reduced by use of E1, E2b-deleted adenoviral vectors. Hum Gene Ther. 2003;14:1715–26. doi: 10.1089/104303403322611737. [DOI] [PubMed] [Google Scholar]
- 28.Hodges BL, Serra D, Hu H, Begy CA, Chamberlain JS, Amalfitano A. Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Med. 2000;2(4):250–9. doi: 10.1002/1521-2254(200007/08)2:4<250::AID-JGM113>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174:1037–145. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 30.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–89. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barouch DH, Nabel GJ. Adenovirus vector based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–156. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 32.Barouch DH, Yang ZY, Kong WP, Korioth-Schmitz B, Sumida SM, Truitt DM, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79:8828–34. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson HL. New hope for an AIDS vaccine. Nat Rev Immunol. 2002;2:239–250. doi: 10.1038/nri776. [DOI] [PubMed] [Google Scholar]
- 34.Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–23. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk CJ, Hartigan-O’Connor D, Mulé JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–802. [PubMed] [Google Scholar]
- 36.Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–70. [PubMed] [Google Scholar]
- 37.Kirk CJ, Mulé JJ. Gene-modified dendritic cells for use in tumor vaccines. Hum Gene Ther. 2000;11:797–806. doi: 10.1089/10430340050015419. [DOI] [PubMed] [Google Scholar]
- 38.Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 40.Harvey BG, Hackett NR, El-Sawy T, et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol. 1999;73:6729–42. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman A, Tsai V, Goudreau A, et al. Specific depletion of human anti-adenovirus antibodies facilitates transduction in an in vivo model for systemic gene therapy. Mol Ther. 2001;3:768–78. doi: 10.1006/mthe.2001.0316. [DOI] [PubMed] [Google Scholar]
- 42.Chirmule N, Propert K, Magosin S, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–83. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 43.Vincent T, Harvey BG, Hogan SM, et al. Rapid assessment of adenovirus serum neutralizing antibody titer based on quantitative, morphometric evaluation of capsid binding and intracellular trafficking: population analysis of adenovirus capsid association with cells is predictive of adenovirus infectivity. J Virol. 2001;75:1516–21. doi: 10.1128/JVI.75.3.1516-1521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone D, Ni S, Li ZY, Gaggar A, DiPaolo N, Feng Q, et al. Development and assessment of human adenovirus type 11 as a gene transfer vector. J Virol. 2005;79(8):5090–104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 46.Nevins JR. Mechanism of activation of early viral transcription by the adenovirus E1a gene product. Cell. 1981;26:213–20. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- 47.Nevins JR, Imperiale MJ, Kao HT, Strickland S, Feldman LT. Detection of an adenovirus E1a-like activity in mammalian cells. Current Topics Microbiology Immunology. 1984;113:15–19. doi: 10.1007/978-3-642-69860-6_3. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Ertl HC, Wilson JM. MHC class-I restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Nunes FA, Berensci K, Furth EE, Gonczol E, Wilson JM. Cellular Immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Prot Natl Aca Sci USA. 1994;91:4407–11. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartigan-O’Connor D, Barjot C, Salvatori G, Chamberlain JS. Generation and growth of gutted adenoviral vectors. Methods Enzymol. 2002;346:224–46. doi: 10.1016/s0076-6879(02)46058-2. [DOI] [PubMed] [Google Scholar]
- 51.Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77(23):12764–72. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]