Abstract
We evaluated the associations between glycemic therapies and prevalence of diabetic peripheral neuropathy (DPN) at baseline among participants in the Bypass-Angioplasty-Revascularization-Investigation-2-Diabetes (BARI 2D) trial on medical and revascularization therapies for coronary artery disease (CAD) and on insulin-sensitizing versus insulin-providing treatments for diabetes. 2368 patients with type 2 diabetes and CAD were evaluated. DPN was defined as clinical examination score >2 using the Michigan Neuropathy Screening Instrument (MNSI). DPN odds ratios across different groups of glycemic therapy were evaluated by multiple logistic regression, adjusted for multiple covariates including age, sex, HbA1c, diabetes duration. 51% BARI 2D subjects with valid baseline characteristics and MNSI scores had DPN. After adjusting for all variables, use of insulin was significantly associated with DPN (OR1.57, 1.15, 2.13). Patients on sulfonylurea or combination of sulfonylurea/metformin/TZD had marginally higher rates of DPN than the metformin/TZD group. This cross-sectional study in a cohort of patients with type 2 diabetes and CAD showed association of insulin use with higher DPN prevalence, independent of disease duration, glycemic control and other characteristics. The causality between a glycemic control strategy and DPN cannot be evaluated in this cross-sectional study, but continued assessment of DPN and randomized therapies in BARI 2D trial may provide further explanations on the development of DPN.
Keywords: diabetic peripheral neuropathy, type 2 diabetes, coronary artery disease, Michigan Neuropathy Screening Instrument, glycemic control therapy
Introduction
In the United States, more than 23 million people 20 years of age and older have diabetes (www.diabetes.org; www.cdc.gov) and the incidence is increasing by 5% per year. Diabetic peripheral neuropathy (DPN) is the most common chronic complication of diabetes and is the leading cause of diabetes-related hospital admissions and nontraumatic amputations (Pirart, 1977; Boulton et al., 2005). DPN leads to major physical disability, poor quality of life (Vileikyte et al., 2005), high mortality (Boulton et al., 2005), and estimated total annual costs of $22 billion (www.diabetes.org, www.cdc.gov). Despite its high morbidity (Boulton et al., 2005), results from most randomized clinical trials assessing the efficacy of various therapeutic agents have been disappointing, most likely due to the complexity of mechanisms involved in its pathogenesis (Pop-Busui et al., 2006). Therefore to date no effective treatment exists for DPN, other than control of hyperglycemia (Diabetes Control and Complications Trial Research Group, 1995; Ohkubo et al., 1995).
The initial screening and diagnosis of DPN in clinical practice depend on assessment of subjective complaints. A variety of validated scales and composite scores semi-quantitatively assess sensation, strength and reflexes and are frequently used as the primary outcome measure in clinical trials (Dyck et al., 1997; Dyck, 2003). The Michigan Neuropathy Screening Instrument (MNSI) is a validated clinical screening instrument for the assessment of DPN designed to balance the contribution of motor and sensory findings (Feldman et al., 1994) and has been widely used in clinical trials and longitudinal cohort studies including the Diabetes Control and Complications Trial/Epidemiology of Diabetes Intensive Control study (DCCT/EDIC) (Brown et al., 2004; Martin et al., 2006). The examination score of the MNSI was established to achieve high specificity (95%) and sensitivity (80%), with a positive predictive value of 97% and a negative predictive value of 74% (Feldman et al., 1994).
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial is a National Institutes of Health-sponsored randomized clinical trial that evaluates treatment efficacy for patients with type 2 diabetes mellitus (T2DM) and angiographically documented, stable coronary artery disease (CAD) (Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group, 2008). One of the unique hypotheses of the BARI 2D trial is that insulin-providing agents will differ from insulin-sensitizing agents in their effects on cardiovascular outcomes (Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group, 2008). DPN as assessed by the MNSI at baseline and annual examinations thereafter is one of the secondary outcomes followed in the BARI 2D cohort.
Intensive glycemic control can reduce the incidence of neuropathy in both type 1 and type 2 diabetic subjects (Diabetes Control and Complications Trial Research Group, 1995; Ohkubo et al., 1995), but different anti-diabetic agents may have other effects besides lowering glucose. For instance, animal studies reported beneficial effects of the insulin sensitizers thiazolidinediones and metformin on markers of experimental DPN via prevention of increased oxidative stress, inflammation and signaling in the protein kinase C pathway (Yamagishi et al., 2008) and prevention of neuronal apoptosis (El-Mir et al., 2008). However, whether different anti-diabetic therapies could play a role in DPN prevalence in T2DM or whether insulin sensitizers may be protective is unknown. The association of diabetic therapies and the prevalence of DPN have been reported in population studies (Franklin et al., 1994; Savage et al., 1997; el-Shazly et al., 1998), but those findings from over ten years ago would not reflect the use of newer drugs such as metformin and/or thiazolidinediones for glycemic control. Therefore, in this large, ethnically diverse cohort of patients with type 2 diabetes in the BARI 2D trial, we examined the association of prior diabetes therapy and the prevalence of DPN at baseline.
Materials and Methods
The BARI2D trial design and patient population have been described elsewhere (Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group, 2008). Briefly, a total of 2368 patients, with T2DM and angiographically documented CAD, were recruited from 49 international clinical sites and randomized to early revascularization and aggressive medical therapy versus aggressive medical therapy alone and to insulin-sensitizing versus insulin-providing treatments for diabetes. All participants signed an informed consent and study was approved by Institutional Review Boards at all participating institutions.
Glycemia treatments
Upon enrollment, the current diabetes management regimen was recorded (within 6 months) and laboratory data were obtained on all participants (Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group, 2008). The glycemic control medication for the present analysis was categorized into five mutually exclusive classes: 1) no diabetes medication; 2) insulin sensitizers (IS) only, which included metformin and/or thiazolidinediones (Met/TZD); 3) insulin providers (IP) only without insulin, which included sulfonylurea and/or meglitinide and/or phenylalanine derivative (SU for all); 4) combination of IS and IP without insulin (Met/TZD+SU); and 5) insulin alone or in combination with any other diabetes medication(s) (Insulin). The neutral agents, alpha glucosidase inhibitors, were not categorized separately from IS or IP drugs since only 26 patients took these drugs at baseline, and they were equally distributed among the five groups.
Neuropathy screening
The MNSI consists of a brief symptom questionnaire of 15 “yes or no” questions on foot sensation and a clinical examination (Feldman et al., 1994). The clinical examination portion of the MNSI, the most objective portion, is a simple 8-point score evaluating ankle reflexes, light touch and vibration sensation in the great toe and foot appearance. One point per side is assigned for abnormalities of foot appearance (deformities, dry skin, calluses, infection and ulcers) and 1 point per side (in 0.5 point increments) for abnormalities of ankle reflexes, light touch or vibration at the great toe. BARI 2D nurse-coordinators were trained in a central session and certified to perform the MNSI in all BARI 2D subjects at the enrollment visit. DPN was defined operationally as a score greater than 2.0 on the MNSI examination, thresholds defined by prior validation studies (Feldman et al., 1994; Brown et al., 2004; Martin et al., 2006). A score >2 requires at least one abnormality in reflexes, vibration or light touch.
Statistical methods
The primary outcome was the presence of DPN as defined by a MNSI clinical score >2. Relevant baseline characteristics of demographics, lifestyle, clinical history, and laboratory measures were evaluated for the association with DPN, using Chi-square tests and Student’s t-tests to test the significance at an alpha level of 0.05. Logistic regression models were built to evaluate the association of DPN with glycemic control therapies. To test our hypothesis, patients on Met/TZD only were selected as the reference group. A model was built to estimate the unadjusted odds ratios of DPN by glycemic control medication, without any other covariates in the model. The following covariates were then added to the model: demographic characteristics (sex, age, race/ethnicity), current cigarette use and alcohol consumption as categorical variables and hemoglobin A 1C (HbA1c), diabetes duration, body mass index (BMI), systolic blood pressure, high density lipoprotein cholesterol (HDLc), low density lipoprotein cholesterol (LDLc) and triglycerides as continuous variables. Although age and duration of DM are statistically correlated, they are not collinear and independently contribute to the model. Significance was evaluated at a 0.05 level for all odds ratio estimates. SAS v.9.1.3 software (Cary, NC) was used for all data processing and statistical analysis.
Results
Among the 2368 patients enrolled in the BARI 2D trial, 2314 patients had valid baseline MNSI clinical scores, specified glycemic control strategies, and at least 80% baseline information available. This group formed the basis of the present analysis.
The demographic and clinical characteristics show a cohort with an average age of 62.4 ± 8.9 years, with predominance of males (71%) and of non-Hispanic whites (66%) (Table 1). 43% of these subjects reported more than 10 years diabetes duration (mean: 10.4 ± 8.7 years) and 61% had an HbA1c above target (i.e., ≥ 7%) at baseline (HbA1c mean ± SD: 7.7 ± 1.6).
Table 1.
Association of the presence of neuropathy and baseline characteristics.
HbA1c=hemoglobin A 1C, BMI=body mass index; HDLc=high density lipoprotein, LDLc=low density lipoprotein
| Characteristic | Category | N | Neuropathy % | p-value |
|---|---|---|---|---|
| Age | < 50 years | 195 | 35.4% | <0.001 |
| 50-59 years | 725 | 47.4% | ||
| 60-69 years | 892 | 52.6% | ||
| ≥ 70 years | 502 | 58.0% | ||
| Sex | Women | 682 | 46.2% | 0.005 |
| Men | 1632 | 52.6% | ||
| Race/Ethnicity | White non Hispanic | 1518 | 50.9% | 0.026 |
| Black non Hispanic | 392 | 55.4% | ||
| Hispanic | 293 | 47.4% | ||
| Others | 111 | 40.5% | ||
| HbA1c | < 7% | 906 | 47.7% | 0.021 |
| ≥ 7% | 1403 | 52.6% | ||
| Diabetes Duration | < 5 years | 767 | 43.0% | <0.001 |
| 5-9 years | 541 | 51.9% | ||
| 10-14 years | 401 | 57.1% | ||
| ≥ 15 years | 595 | 55.5% | ||
| BMI | < 30 kg/m2 | 1014 | 49.2% | 0.208 |
| ≥ 30 kg/m2 | 1300 | 51.8% | ||
| History of Hypertension | No | 401 | 42.6% | <.001 |
| Yes | 1885 | 52.1% | ||
| Blood Pressure (mmHg) * | ≤ 130/80 | 1097 | 50.1% | 0.569 |
| > 130/80 | 1210 | 51.3% | ||
| Urine Albumin/Creatinine Ratio | ≤ 30 | 1450 | 48.1% | <0.001 |
| > 30 | 697 | 57.2% | ||
| HDL (mg/dl) * | > 40 men/ > 50 women | 558 | 47.8% | 0.112 |
| ≤ 40 men/ ≤ 50 women | 1750 | 51.7% | ||
| LDL (mg/dl) * | < 100 | 1346 | 51.3% | 0.498 |
| ≥ 100 | 928 | 49.9% | ||
| Triglycerides (mg/dl) * | < 150 | 1165 | 50.3% | 0.704 |
| ≥ 150 | 1147 | 51.1% | ||
| Current Cigarette Use | No | 2028 | 51.1% | 0.287 |
| Yes | 285 | 47.7% | ||
| Alcohol Consumption | Not regular | 1696 | 51.9% | 0.101 |
| Regular | 376 | 46.0% | ||
| Binge | 242 | 49.2% |
79% of patients were taking at least one kind of lipid lowering agent at baseline. 96% of patients took at least one kind of antihypertensive agent at baseline. Lipid lowering agents include statins, fibrates, niacin, bile acid sequestrants, omega-3 fatty acid, and cholesterol absorption inhibitor. Antihypertensive agents include beta blockers, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers, and diuretics.
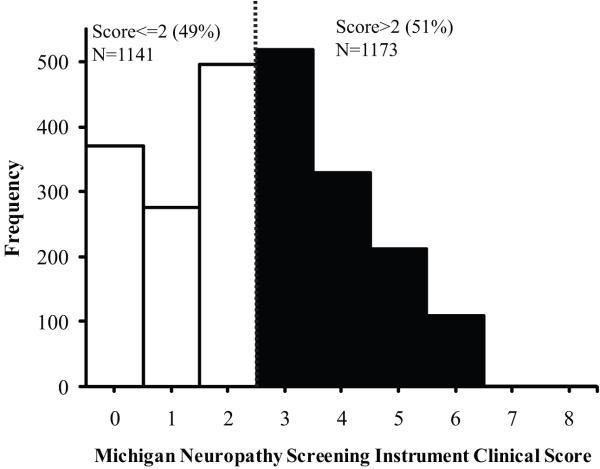
At baseline the MNSI clinical score ranged from 0 to 8. 1173 out of 2314 subjects (51%) had a MNSI clinical score >2 consistent with DPN (Fig. 1). In this cohort, the higher prevalence of DPN was significantly associated with older age, male sex, black non-Hispanic ethnicity, longer diabetes duration, HbA1c ≥ 7%, history of hypertension and albuminuria (p<0.05) (Table 1). On the other hand, none of the lipid variables, current cigarette use or ethanol intake was significantly associated with the prevalence of DPN (Table 1). Of note, 79% of the subjects were on treatment for dyslipidemia. The mean diabetes duration and HbA1c correlated with each other in the different glycemic control therapy groups with the shortest duration and lowest mean HbA1c values in subjects without a diabetes drug and the longest duration and highest HbA1c values in the subjects treated with insulin (Table 2).
Figure 1.
Distribution of MNSI clinical score in the BARI 2D cohort at baseline. Scores with 0.5 increments were rounded up to the next integer.
Table 2.
Clinical characteristics and unadjusted odds ratio of DPN by glycemic control group.
Met=metformin; TZD=thiazolidinediones; SU=sulphonylurea; DM=diabetes mellitus.
| Characteristics | Glycemic Control Medication | |||||
|---|---|---|---|---|---|---|
| Met/TZD1 | No DM drug | Sulfonylurea | Met/TZD + SU | Insulin2 | P value | |
| Number of patients | 366 | 199 | 388 | 716 | 645 | - |
| Mean Age (years) | 61.2 | 62.6 | 63.7 | 62.4 | 62.2 | 0.004 |
| Mean Duration of DM (years) | 5.3 | 3.7 | 8.2 | 10.9 | 16.3 | <0.00 1 |
| Mean HbA1c (%): | 7.1 | 6.7 | 7.4 | 7.7 | 8.3 | <0.00 1 |
| % having neuropathy | 41.5% | 43.2% | 51.8% | 49.9% | 58.4% | <0.00 1 |
| Unadjusted odds ratio (95% CI) | Reference Group | 1.07 (0.76, 1.52) |
1.51† (1.14, 2.02) |
1.40† (1.09, 1.81) |
1.98† (1.53, 2.57) |
- |
Among the 366 patients in the Metformin/TZD category at baseline, 274 (75%) took only metformin; 39 (11%) took only a TZD; and 53 (14%) took the combination of metformin and TZD.
Patients in the insulin category could be on insulin alone or with any other diabetes medication.
Significant at α level = 0.05.
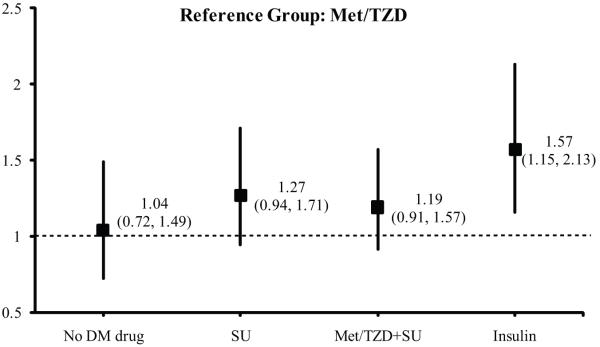
In unadjusted analysis, subjects treated only with Met/TZD had the lowest DPN prevalence compared to the other groups and were therefore used as the comparator group for further analysis. Subjects treated with insulin had the highest DPN prevalence at baseline (Table 2, p<0.001). After adjusting for all the selected covariates including HbA1c and DM duration, the use of insulin was associated with a significant increase in the rate of DPN, compared with Met/TZD group (Fig. 2). Subjects in the SU or on the SU-Met/TZD groups had insignificantly higher rate of DPN than the Met/TZD group (Fig. 2).
Figure 2.
Odds ratios for DPN by glycemic control therapy. Reference group: Met/TZD. The full model was adjusted for sex, age, race/ethnicity, duration of diabetes diagnosis, BMI, HbA1c, HDL, LDL, triglycerides, systolic blood pressure, currently cigarette use, and alcohol consumption.
To further investigate the association of insulin use and the prevalence of DPN, we analyzed the rate of DPN in all insulin users compared to all non-insulin users at the time of randomization. In a logistic regression model that simultaneously adjusted for all the covariates mentioned in the methods, the odds ratios of DPN remained over 30% higher in subjects taking insulin compared to subjects not taking insulin (OR=1.34, 95% CI 1.08, 1.67).
Given the importance of DM duration to the risk of neuropathy, we analyzed the prevalence of DPN in a subgroup of the cohort who reported a diabetes duration ≥10 years. Based on this subgroup analysis, insulin use was associated with about a two-fold higher prevalence of DPN compared to the Met/TZD group (OR 1.91 95% CI 1.0, 3.6). In this subgroup insulin users had also higher prevalence of DPN as compared with all noninsulin users (OR 1.32 95% CI 0.99, 1.76).
Discussion
In this ethnically diverse and large cohort of patients with T2DM and angiographically confirmed CAD, we observed a high prevalence of DPN (51%) using a validated, easy to use clinical instrument. In addition, after controlling for age, sex, race/ethnicity, diabetes duration, HbA1c and all other described variables, insulin use at baseline was the glycemic control strategy that was independently associated with a significantly higher prevalence of DPN. On the other hand, a lower prevalence of DPN was seen in the Met/TZD group as compared with all other diabetes medication groups, although it did not reach statistical significance.
In general, the development of diabetic complications is related to the degree of metabolic control that is determined by multiple factors. Until recently, hyperglycemia, the common clinical expression of both types of diabetes, has been regarded as the major culprit initiating the cascade of metabolic and molecular abnormalities resulting in degenerative phenomena and progressive neurological deficits. This concept alone, however, does not account for the results from trials designed to achieve optimal glycemic control but only show a partial reduction in the development of complications in general, and on neuropathy in particular (Diabetes Control and Complications Trial Research Group, 1995; 1998; Azad et al., 1999). These data therefore suggest that factors other than hyperglycemia are involved in the pathophysiology of DPN and that such factors may account for the differences in the prevalence and progression of DPN.
At study entry in BARI 2D, the use of insulin was significantly associated with higher rates of DPN in a cross-sectional analysis even after adjusting for many identified confounders such as HbA1c and duration of diabetes. Some prior and smaller observational studies reported similar associations. For instance, a cross sectional population study of 890 T2DM subjects from the Denver metropolitan area reported that the use of insulin was significantly associated with the presence of all microangiopathic complications, but especially with DPN, as compared with oral hypoglycemic agents. The association continued to remain quite strong after adjusting for multiple variables including diabetes duration, HbA1c and sex (Savage et al., 1997). In the San Luis Valley Diabetes Study of 277 T2DM subjects, insulin use was a strong independent factor associated with DPN (Franklin et al., 1994). In a European case-control cohort of 1300 subjects with diabetes, treatment with insulin was significantly associated with the presence of lower limb complications, in spite of similar prevalence of other complications or comorbidities (el-Shazly et al., 1998). The current study extends these prior observations on diabetes and DPN to a much larger and well-characterized population of over 2000 T2DM patients recruited through the BARI 2D trial. The population is also well-represented by black non-Hispanics (17%) and Hispanics (13%).
As for the other epidemiologic studies, the cross-sectional results presented here cannot address causality between insulin use and DPN. The reasons why a certain glycemic control therapy was prescribed are not known. The presence of DPN may have prompted the initiation of insulin therapy to forestall worsening DPN and other microvascular complications. If insulin therapy was added after the failure of diet and various oral hypoglycemic agents singly or in combination, then the insulin use cohort could have had several periods of poor glycemic control before the therapy was intensified. Also, the association of insulin use and DPN must be taken in the context that moderately severe CAD was a selection criterion for the BARI 2D cohort. The development of DPN may be affected by the presence of atherosclerosis and possible peripheral artery disease.
Besides methodological issues related to the cross-sectional study design, insulin use could be associated with DPN by nerve damage from exogenous insulin. Some have previously postulated that exogenous insulin therapy in T2DM might be associated with DPN through an exacerbation of obesity, fluid retention, hypertension and hyperlipidemia (Savage et al., 1997). However, several randomized clinical trials in T1DM have shown that intensive insulin therapy can prevent or delay the development of DPN compared to conventional insulin therapy (Diabetes Control and Complications Trial Research Group, 1995; Ohkubo et al., 1995). Additionally, experimental evidence suggests that, beyond improving glycemic control, insulin may have complex protective vascular actions (Pop-Busui and Stevens, 2005).
Another possible explanation for the association of insulin use with the prevalence of DPN in this cohort could be that insulin use indicates beta cell failure in a group of patients. If insulin therapy was initiated because of beta cell failure, then it may be a biologic marker of a later stage of the natural history of diabetes and more accurately reflect the severity of diabetes than the HbA1c levels or the reported duration of diabetes in patients for whom the true onset of diabetes is not clear. In addition, several mechanisms such as glucotoxicity per se and its downstream increase in oxidative stress and free fatty acids have been shown to negatively impact beta-cell function and to induce peripheral nerve damage and thus could have contributed to the association we found with insulin use and DPN (Pop-Busui et al., 2006). The decrease in C-peptide secretion that accompanies beta-cell failure may also directly contribute to neuropathy since evidence from animal models suggests that C-peptide can reverse DPN (Stevens et al., 2004). However, this still needs to be confirmed in humans.
Another interesting observation is the trend towards DPN protection observed in the Met/TZD group, which raises the question whether different pharmacologic and molecular effects of various antihyperglycemic treatments may also play a role. A large prospective clinical trial has suggested that the beneficial effect of metformin on the incidence of diabetes complications was not entirely related to its glycemic effects (UK Prospective Diabetes Study (UKPDS) Group, 1998a). For instance, metformin has pleiotropic effects with direct vascular implications, such as improvements in lipid profiles (Wu et al., 1990), prevention of oxidative stress-induced endothelial cell death (Detaille et al., 2005) and direct neuroprotective effects via inhibition of oxidative stress-related apoptotic cell death in primary neurons (El-Mir et al., 2008). On the other hand, sulfonylureas promote increases in circulating insulin levels by closing beta-cell ATP-dependent potassium channels (K+-ATP) and K+-ATP channels are widely expressed on neurons. Data from animal studies have shown that blockage of the K+-ATP channels with SU may selectively potentiate neuronal mitochondrial dysfunction and neurotoxicity by potentiating mitochondrial inhibitors (Kou et al., 2006) and glutamate-induced generation of superoxide (Yamauchi et al., 2003), although similar human data are not yet reported. In the United Kingdom Prospective Diabetes Study (UKPDS), intensive blood-glucose control by SU did not decrease the risk of DPN, whereas it substantially decreases the risk of retinopathy and nephropathy (UK Prospective Diabetes Study (UKPDS) Group, 1998b).
TZDs may provide additional beneficial vascular effects beyond glycemic improvement, such as reduction of inflammation (Sjoholm and Nystrom, 2005), oxidative stress (Chen et al., 2004) and improvement in endothelial dysfunction (Sjoholm and Nystrom, 2005), all important mechanisms involved in the pathogenesis of DPN. In addition, preclinical evidence indicates that TZDs may exert direct neuroprotective effects after cerebral ischemia and motor neuron loss, myelin and microglial activation after spinal cord injury by inhibition of NF-kappaB and JNK activation and oxidative stress-mediated neuronal damage (Xing et al., 2007; Rosa et al., 2008; Yu et al., 2008).
In summary, the findings of this cross-sectional, baseline study in a cohort of patients with T2DM and confirmed CAD suggest that insulin use was associated with a higher prevalence of DPN and that the use of Met/TZD had a trend towards protection, independent of disease duration, glycemic control and other characteristics. A better understanding of the role of diabetes medications and other factors in the development of DPN is likely at the conclusion of the BARI 2D trial because the analysis will include standardized annual DPN exams on a well-characterized population who received aggressive medical therapy and insulin-sensitizing or insulin-providing agents in a controlled and randomized fashion for the five years of the trial.
Acknowledgements
Clinical Trial Registration No: NCT00006305 clinicaltrials.gov. These studies were supported by NHLBI and NIDDK: NHLBI Nos. U01 HL061746, U01 HL061748, U01 HL063804. NIDDK No. U01 HL061744. The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial is sponsored by the National Heart, Lung and Blood Institute (NHLBI) and receives substantial funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The BARI 2D Trial is coordinated by the Epidemiology Data Center at the University of Pittsburgh, Graduate School of Public Health. BARI 2D receives significant supplemental funding from GlaxoSmithKline, Bristol-Myers Squibb Medical Imaging, Inc., Astellas Pharma US, Inc., Merck & Co., Inc., Abbott Laboratories, Inc., and Pfizer, Inc. BARI 2D also receives generous support from Abbott Laboratories Ltd., MediSense Products, Bayer Diagnostics, Beckton, Dickinson and Company, J.R. Carlson Laboratories, Inc., Centocor, Inc., Eli Lilly and Company, LipoScience, Inc., Merck Sante, Novartis Pharmaceuticals Corporation, and Novo Nordisk, Inc.
Abbreviations
- DPN
diabetic peripheral neuropathy
- MNSI
Michigan Neuropathy Screening Instrument
- BARI 2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes
- T2DM
type 2 diabetes mellitus
- CAD
coronary artery disease
- HbA1c
hemoglobin A 1C
- BMI
body mass index
- HDLc
high density lipoprotein
- LDLc
low density lipoprotein
- Met
metformin
- TZD
thiazolidinediones
- SU
sulphonylurea
- DM
diabetes mellitus
- BP
blood pressure
- OR
odds ratio
APPENDIX A: BARI 2D INVESTIGATORS
BARI 2D, Coordinating Center, University of Pittsburgh, Pittsburgh, PA
Principal Investigator: Katherine M. Detre, MD, DrPH†, Sheryl F. Kelsey, PhD
Co-Principal Investigator: Maria Mori Brooks, PhD
Co-Investigators: David Kelley, MD, Trevor J. Orchard, MBBCh, MMedSci, Jamal Rana, MD, PhD, Stephen B. Thomas, PhD, Kim Sutton Tyrrell, RN, DrPH, Richard Holubkov, PhD
Coordinator: Frani Averbach, MPH, RD
Administrative Coordinators: Sharon W. Crow, BS, Joan M. MacGregor, MS, Scott M. O’Neal, MA, Kathleen Pitluga, BA, Veronica Sansing, BA, Mary Tranchine, BS
Statisticians: Regina Hardison, MS, Kevin Kip, PhD, Jiang Lu, MS, Manuel Lombardero, MS
Data Managers: Sue Janiszewski, MSIS, Darina Protivnak, MSIS, Sarah Reiser, BS
System Programmers: Stephen Barton, ASB, Yulia Kushner, BS, BA, Owen Michael, ASB
System Support: Jeffrey P. Martin, MBA, Christopher Kania, BS, Michael Kania, BS, Jeffrey O’Donnell, BS
Consultant: Rae Ann Maxwell, RPh, PhD
Office of Study Chair, Mayo Clinic Foundation, Rochester, Minnesota
Robert L. Frye, MD, Professor of Medicine
Program Office, National Heart, Lung and Blood Institute, Bethesda, MD, National Institutes of Health, Bethesda, MD
Project Officer: Suzanne Goldberg, RN, MSN
Deputy Project Officer: Yves Rosenberg, MD, MPH
NHLBI Officers: Patrice Desvigne-Nickens, MD, Abby Ershow, ScD, David Gordon, MD, PhD, Dina Paltoo, PhD, MPH
Co-Funded by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
Program Director for Diabetes
Complications: Teresa L. Z. Jones, MD
List of Participants, Clinical Centers
University of Sao Paulo Heart Institute, São Paulo, Brazil
Principal Investigators: Whady Hueb, MD, Cardiology, José Ramires, MD, Cardiology, Neuza Lopes, MD, Cardiology, Bernardo Wajchenberg, MD, Diabetology
Investigators: Eulogio E Martinez, MD, Sergio A Oliveira, MD
Coordinator: Roberto Betti, MD
Toronto General Hospital/University Health Network, Toronto, Canada
Principal Investigators: Leonard Schwartz, MD, Cardiology, George Steiner, MD, Diabetology
Investigators: Alan Barolet, MD, Yolanda Groenewoud, MD
Coordinators: Kathy Camelon, RD, CDE, Lisa Mighton, RN, CDE
Texas Health Science at San Antonio/South Texas Veterans Health Care System, San Antonio, TX
Principal Investigators: Robert O’Rourke, MD, Cardiology, Janet Blodgett, MD, Diabetology
Investigators: Edward Sako, MD, PhD
Coordinators: Judith Nicastro, RN, Robin Prescott, MSN
Mayo Clinic-Rochester (Vanguard Site), Rochester, MN
Principal Investigators: Charanjit Rihal, MD, Cardiology, Frank Kennedy, MD, Diabetology
Investigators: Gregory Barsness, MD, Amanda Basu, MD, Alfredo Clavell, MD, Robert Frye, MD, David R. Holmes, Jr, MD, Amir Lerman, MD, Charles Mullaney, MD, Guy Reeder, MD, Robert Rizza, MD, Hartzell Schaff, MD, Steven Smith, MD, Virend Somers, MD, Thoralf Sundt, MD, Henry Ting, MD, R Scott Wright, MD
Coordinators: Pam Helgemoe, RN, Diane Lesmeister, Deborah Rolbiecki, LPN
Mexican Institute of the Social Security, Mexico City, Mexico
Principal Investigators: Luis Lepe-Montoya, MD, Cardiology, Jorge Escobedo, MD, FACP, Diabetology
Investigators: Rafael Barraza, MD, Rubén Baleón, MD, Arturo Campos, MD, Paula García, MD, Carlos Lezama, MD, Carlos Miramontes, MD, Salvador Ocampo, MD, Joaquín V. Peñafiel, MD, Arquímides Valdespino, MD, Raúl Verdín, MD, Héctor Albarrán, MD, Fernando Ayala, MD, Eduardo Chávez, MD, Héctor Murillo, MD
Coordinators: Luisa Virginia Buitrón, MD, Beatriz Rico-Verdin, MD, PhD
University Hospitals of Cleveland/CASE Medical School (Vanguard Site), Cleveland, OH
Principal Investigators: Dale Adler, MD, Cardiology, Austin Arthur Halle, MD, Cardiology, Faramarz Ismail-Beigi, MD, PhD, Diabetology
Investigators: Suvinay Paranjape, MD
Coordinators: Stacey Mazzurco, RN, Karen Ridley, RN, BSN
Memphis VA Medical Center/University of Tennessee, Memphis, TN
Principal Investigators: Kodangudi Ramanathan, MD, Cardiology, Solomon Solomon, MD, Diabetology
Investigators: Darryl Weinman, MD, Cardiology, Barry Wall, MD, Nephrology
Coordinators: Lillie Douglas, RN, Tammy Touchstone, RN, BSN
Montréal Heart Institute/Hôtel-Dieu-CHUM (Vanguard Site), Montréal, Canada
Principal Investigators: Martial Bourassa, MD, Cardiology, Jean-Claude Tardif, MD, Cardiology, Jean-Louis Chiasson, MD, Diabetology, Marc Andre Lavoie, MD, Diabetology
Coordinators: Hélène Langelier, BSC, RD, Suzy Foucher, RN, BA, Johanne Trudel, RN, BSc
Albert Einstein College of Medicine/Montefiore, Bronx, NY
Principal Investigators: Scott Monrad, MD, Cardiology, Vankeepuram Srinivas, MD, Cardiology, Joel Zonszein, MD, Diabetology
Investigators: Jill Crandall, MD
Coordinators: Helena Duffy, ANP, CDE, Eugen Vartolomei, MD
Fuqua Heart Center/Piedmont Hospital, Atlanta, GA
Principal Investigators: Spencer King, III, MD, Cardiology, Carl Jacobs, MD, Cardiology, David Robertson, MD, Diabetology
Coordinators: Jennifer LaCorte, RN, BSN, CCRN, Melinda Mock, RN, BSN, MA, Marty Porter, PhD
University of Alabama at Birmingham (Vanguard Site), Birmingham, AL
Principal Investigators: William Rogers, MD, Cardiology, Fernando Ovalle, MD, Diabetology, David Bell, MBBCh, Diabetology
Investigators: Vijay K Misra, MD, William B Hillegass, MD, Raed Aqel, MD
Coordinators: Penny Pierce, RN, BSN, Melanie Smith, RN, BSN, Leah Saag RN, Ashley Vaughn RN, Dwight Smith RN, Tiffany Grimes RN, Susan Rolli RN, Roberta Hill RN, Beth Dean Barrett RN, Clarinda Morehead LPN, Ken Doss
Northwestern University Medical School, Chicago, IL
Principal Investigators: Charles J. Davidson, MD, Cardiology, Mark Molitch, MD, Diabetology
Investigators: Nirat Beohar, MD
Coordinators: Lynne Goodreau, RN, Elaine Massaro, MS, RN, CDE, Fabiola Arroyo, CCT
Na Homolce Hospital, Prague, Czech Republic
Principal Investigators: Lenka Pavlickova, MD, Cardiology, Petr Neužil, MD, PhD, Cardiology, Štěpánka Stehlíková, MD, Diabetology
Investigators: Jaroslav Benedik, MD
Coordinators: Liz Coling
University of Ottawa Heart Institute/Ottawa Hospital-Riverside Campus, Ottawa, Canada
Principal Investigators: Richard Davies, MD, Cardiology, Christopher Glover, MD, Cardiology, Michel LeMay, MD, Cardiology, Thierry Mesana, MD, Cardiology, Teik Chye Ooi, MD, Diabetology, Mark Silverman, MD, Diabetology, Alexander Sorisky, MD, Diabetology
Coordinators: Colette Favreau, RN, Susan McClinton, BScN
New York Medical College/Westchester Medical Center, Valhalla, NY
Principal Investigators: Melvin Weiss, MD, Cardiology, Irene Weiss, MD, Diabetology
Investigators: Leo Saulle, MD, Harichandra Kannam, MD
Coordinators: Joanne C. Kurylas, RN, CDE, Lorraine Vasi, RN
Emory University, Atlanta, GA
Principal Investigators: John Douglas Jr., MD, Cardiology, Ziyad Ghazzal, MD, Cardiology, Laurence Sperling, MD, Cardiology, Spencer King, III, MD, Cardiology, Priya Dayamani, MD, Diabetology, Suzanne Gebhart, MD, Diabetology
Investigators: Sabreena Basu, MD, Tarek Helmy, MD, Vin Tangpricha, MD, PhD
Coordinators: Pamela Hyde, RN, Margaret Jenkins, RN, CDE
Washington Hospital Center/Georgetown University Medical Center, Washington, DC
Principal Investigators: Kenneth Kent, MD, Cardiology, William Suddath, MD, Cardiology, Michelle Magee, MD, Diabetology
Coordinator: Patricia Julien-Williams, CNP, Vida Reed, RN, CDE, Carine Nassar, RD, MD, CDE
Quebec Heart Institute/Laval Hospital, Sainte-Foy, Canada
Principal Investigators: Gilles Dagenais, MD, Cardiology, Claude Garceau, MD, Diabetology
Coordinator: Dominique Auger, RN
University of British Columbia/Vancouver Hospital, British Columbia, Canada
Principal Investigators: Christopher Buller, MD, Cardiology, Tom Elliott, MBBS, Diabetology
Investigators: Krishnan Ramanathan, MD
Coordinators: Rebecca Fox, PA, MSc, Daniella Kolesniak, MD
NYU School of Medicine, New York, NY
Principal Investigators: Michael Attubato, MD, Cardiology, Frederick Feit, MD, Cardiology, Stephen Richardson, MD, Diabetology
Investigators: Ivan Pena-Singh, MD, James Slater, MD
Coordinator: Angela Amendola, PA, Bernardo Vargas, BS, Susan Cotton Gray, NP, Dallas Regan, MSN, NP
Lahey Clinic Medical Center (Vanguard Site), Burlington, MA
Principal Investigators: Nicholas Tsapatsaris, MD, Cardiology, Bartholomew Woods, MD, Cardiology, Gary Cushing, MD, Diabetology
Investigators: Martin Rutter, MD, Premranjan Singh, MD
Coordinators: Gail DesRochers, RN, Gail Woodhead, RN, Deborah Gannon, MS, Nancy Shinopulos Campbell, RN
University of Virginia, Charlottesville, VA
Principal Investigators: Michael Ragosta, MD, Cardiology, Ian Sarembock, MD, Cardiology, Eugene Barrett, MD, Diabetology
Investigators: Eric Powers, MD
Coordinators: Linda Jahn, RN, MEd, Karen Murie, RN
University of Minnesota/Minnesota Veterans Research Institute, Minneapolis, MN
Principal Investigators: Gladwin Das, MB, BS, MD, Cardiology, Gardar Sigurdsson, MD, Cardiology, Carl White, MD, Cardiology, John Bantle, MD, Diabetology
Investigators: J. Bruce Redmon, MD
Coordinators: Christine Kwong, MPH, RD, CDE
St. Luke’s/Roosevelt Hospital Center, New York, NY
Principal Investigators: Jacqueline Tamis-Holland, MD, Cardiology, Jeanine Albu, MD, Diabetology
Investigators: Judith S. Hochman, MD, James Slater, MD, James Wilentz, MD
Coordinators: Sylvaine Frances, PA, Deborah Tormey, RN
University of Florida, Gainesville, FL
Principal Investigators: Carl Pepine, MD, Cardiology, Karen Smith, MD, Cardiology, Laurence Kennedy, MD, Diabetology
Coordinators: Karen Brezner, CCRC, Tempa Curry, RN
Saint Louis University, St. Louis, MO
Principal Investigators: Frank Bleyer, MD, Cardiology, Stewart Albert, MD, Diabetology
Investigators: Arshag Mooradian, MD
Coordinator: Sharon Plummer, NP
University of Texas at Houston, Houston, TX
Principal Investigators: Francisco Fuentes, MD, Cardiology, Roberto Robles, MD, Cardiology, Victor Lavis, MD, Diabetology
Investigators: Jaime Gomez, MD
Coordinators: Carol Underwood, BSN, RN, CCRC, Maria Selin Fulton, RN, CDE, Julie Gomez Ramirez, BSN, RN, Jennifer Merta, MA, Glenna Scott, RN
Kaiser-Permanente Medical Center, San Jose, CA
Principal Investigators: Ashok Krishnaswami, MD, Cardiology, Lynn Dowdell, MD, Diabetology
Coordinator: Sarah Berkheimer, RN
Henry Ford Heart & Vascular Institute, Detroit, MI
Principal Investigators: Adam Greenbaum, MD, Cardiology, Fred Whitehouse, MD, Diabetology
Coordinators: Raquel Pangilinan, BSN, RN, Kelly Mann, RN, BSN, CDE
Boston Medical Center, Boston, MA
Principal Investigators: Alice Jacobs, MD, Cardiology, Elliot Sternthal, MD, Diabetology
Investigators: Susana Ebner, MD
Coordinator: Paula Beardsley, LPN
Fletcher Allen Health Care (Vanguard Site), Colchester, VT
Principal Investigators: David Schneider, MD, Cardiology, Richard Pratley, MD, Diabetology
Investigators: William Cefalu, MD, Joel Schnure, MD
Coordinators: Michaelanne Rowen, RN, CCRC, Linda Tilton, MS, RD, DE
Jim Moran Heart & Vascular Institute, Fort Lauderdale, FL
Principal Investigators: Alan Niederman, MD, Cardiology, Cristina Mata, MD, Diabetology
Coordinator: Terri Kellerman, RN
Baylor College of Medicine, Houston, TX
Principal Investigators: John Farmer, MD, Cardiology, Alan Garber, MD, Diabetology
Investigators: Neal Kleiman, MD
Coordinators: Nancy Howard, RN, BSN, Debra Nichols, RN, Madonna Pool, RN, MSN
Duke University, Durham, NC
Principal Investigators: Christopher Granger, MD, Cardiology, Mark Feinglos, MD, Diabetology
Investigators: George Adams, MD, Jennifer Green, MD
Coordinators: Bernadette Druken, RN, CCRP, Dani Underwood, MSN, ANP
University of Maryland Hospital, Baltimore, MD
Principal Investigators: J. Lawrence Stafford, MD, Cardiology, Thomas Donner, MD, Diabetology
Investigators: Warren Laskey, MD
Coordinators: Dana Beach, RN
University of Chicago Medical Center, Chicago, IL
Principal Investigators: John Lopez, MD, Cardiology, Andrew Davis, MD, Diabetology
Investigators: David Faxon, MD, Sirimon Reutrakul, MD
Coordinator: Emily Bayer, RN, BSN
University of Pittsburgh Medical Center (Vanguard Site), Pittsburgh, PA
Principal Investigators: Oscar Marroquin, MD, Cardiology, Howard Cohen, MD, Cardiology, Mary Korytkowski, MD, Diabetology
Coordinators: Glory Koerbel, MSN, CDE, Lisa Baxendell, RN, Debbie Rosenfelder, BSN, CCRC, Louise DeRiso, MSN, Carole Farrell, BSN, Tina Vita, RN
Washington University/Barnes Jewish Hospital, St. Louis, MO
Principal Investigators: Richard Bach, MD, Cardiology, Ronald Krone, MD, Cardiology, Majesh Makan, MD, Cardiology, Janet McGill, MD, Diabetology
Coordinators: Carol Recklein, RN, MHS, CDE, Kristin M. Luepke, RN, MSN, Mary Jane Clifton
Mount Sinai Medical Center, New York, NY
Principal Investigators: Michael Farkouh, MD, MSc, Cardiology, Michael Kim, MD, FACC, Cardiology, Donald A. Smith, MD, MPH, Diabetology
Coordinators: Ida Guzman, RN, BSN, ANP, MS, CDE, Arlene Travis, RN
Mid America Heart Institute, Kansas City, MO
Principal Investigators: James O’Keefe, MD, Cardiology, Alan Forker, MD, Diabetology, William Isley, MD, Diabetology†
Investigators: Richard Moe, MD, PhD
Coordinators: Paul Kennedy, RN, Margaret Rosson, LPN, Aimee Long, RN
University of Michigan, Ann Arbor, MI
Principal Investigators: Eric Bates, MD, Cardiology, William Herman, MD, MPH, Diabetology, Rodica Pop-Busui, MD, Diabetology
Investigators: Claire Duvernoy, MD, Martin Stevens, MBBCh
Coordinators: Ann Luciano, RN, Cheryl Majors, BSN
Johns Hopkins Bayview Medical Center, Baltimore, MD
Principal Investigators: Sheldon H. Gottlieb, MD, Cardiology, Annabelle Rodriguez, MD, Diabetology
Coordinator: Melanie Herr, RN
Brown University/Rhode Island Hospital, Providence, RI
Principal Investigators: David Williams, MD, Cardiology, Robert J. Smith, MD, Diabetology
Investigators: J. Dawn Abbott, MD, Marc J. Laufgraben, MD
Coordinators: Mary Grogan, RN, Janice Muratori, RNP
Houston VA Medical Center, Houston, TX
Principal Investigators: Gabriel Habib, MD, MS, Cardiology, Marco Marcelli, MD, Diabetology
Investigators: Issam Mikati, MD
Coordinators: Emilia Cordero, NP, Gina Caldwell, LVN
New York Hospital Queens, Queens, NY/Lang Research Center
Principal Investigators: David Schechter, MD, Cardiology, Daniel Lorber, MD, Diabetology, Phyllis August, MD, MPH, Nephrology
Coordinators: Maisie Brown, RN, MSN, Patricia Depree, PhD, ANP, CDE
Wilhelminen Hospital, Vienna, Austria
Principal Investigators: Kurt Huber, MD, Cardiology, Ursula Hanusch-Enserer, MD, Diabetology
Investigators: Nelly Jordanova, MD
Coordinators: Dilek Cilesiz, MD, Birgit Vogel, MD
St. Joseph Mercy Hospital/Michigan Heart and Vascular Institute and the Ann Arbor Endocrinology and Diabetes, P.C., Ann Arbor, MI
Principal Investigators: Ben McCallister Jr., MD, Cardiology, Kelly Mandagere, MD, Diabetology, Michael Kleerekoper, MD, Diabetology, Robert Urbanic, MD, Diabetology
Investigators: James Bengston, MD, MPH, Bobby K. Kong, MD, Andrew Pruitt, MD, Jeffrey Sanfield, MD
Coordinators: Carol Carulli, RN, Ruth Churley-Strom, MSN
The Ohio State University Medical Center, Columbus, OH
Principal Investigators: Raymond Magorien, MD, Cardiology, Kwame Osei, MD, Diabetology
Coordinators: Cecilia Casey Boyer, RN
Mayo Clinic-Scottsdale, Scottsdale, AZ
Principal Investigators: Richard Lee, MD, Cardiology, Pasquale Palumbo, MD, Diabetology
Coordinators: Susan Roston, RN, Joyce Wisbey, RN
Core Laboratories
Angiographic Core Laboratory, Stanford University, Stanford, CA
Principal Investigator: Edwin Alderman, MD, Fumiaki Ikeno, MD
Staff: Anne Schwarzkopf†
Biochemistry Core Laboratory, University of Minnesota, Minneapolis, MN
Principal Investigator: Michael Steffes, MD, PhD
Staff: Maren Nowicki, CLS, Jean Bucksa, CLS
ECG Core Laboratory, Saint Louis University, St. Louis, MO (U01 HL061746)
Principal Investigator: Bernard Chaitman, MD
Staff: Jane Eckstein, RN, Teri Bertram, RN
Economics Core Laboratory, Stanford University, Stanford, CA (U01 HL061748)
Principal Investigator: Mark A. Hlatky, MD
Staff: Derek B. Boothroyd, PhD, Kathryn A. Melsop, MS
Fibrinolysis Core Laboratory, University of Vermont, Burlington, VT (U01 HL063804)
Principal Investigator: Burton E. Sobel, MD
Staff: Michaelanne Rowen, RN, CCRC, Dagnija Neimane, BS
Nuclear Cardiology Core Laboratory, University of Alabama at Birmingham, Birmingham, AL (Astellas Pharma US, Inc.)
Principal Investigators: Ami E. Iskandrian, MD
Staff: Mary Beth Schaaf, RN, BSN
Management Centers
Diabetes Management Center, Case Western Reserve University, Cleveland, OH
Director: Saul Genuth, MD
Staff: Theresa Bongarno, BS
Hypertension Management Center, Lahey Clinic Medical Center, Burlington, MA
Co-Director: Richard Nesto, MD
Hypertension Management Center, New York Hospital Queens, Queens, NY
Co-Director: Phyllis August, MD
Staff: Karen Hultberg, MS
Lifestyle Intervention Management Center, Johns Hopkins Bayview Medical Center, Baltimore, MD
Co-Director: Sheldon H. Gottlieb, MD
Lifestyle Intervention Management Center, St. Luke’s/Roosevelt Hospital Center, New York, NY
Co-Director: Jeanine Albu, MD
Staff: Helene Rosenhouse-Romeo, RD, CDE
Lipid Management Center, University of Pittsburgh, Pittsburgh, PA
Director: Trevor J. Orchard, MBBCh, MMedSci
Staff: Georgia Pambianco, MPH, Manuel Lombardero, MS
Safety Officer
Michael Mock, MD, North Canton, Ohio
Operations Committee
Chair: Robert L. Frye, MD
Members: Maria Mori Brooks, PhD, Patrice Desvigne-Nickens, MD, Abby Ershow, ScD, Saul Genuth, MD, Suzanne Goldberg, RN, MSN, David Gordon, MD, PhD, Regina Hardison, MS, Teresa L. Z. Jones, MD, Sheryl Kelsey, PhD, Richard Nesto, MD, Trevor Orchard, MBBCh, MMedSci, Dina Paltoo, PhD, MPH, Yves Rosenberg, MD, MPH
Morbidity and Mortality Classification Committee (MMCC)
Chair: Thomas Ryan, MD
Co-Chair: Harold Lebovitz, MD
Members: Robert Brown, MD, Gottlieb Friesinger, MD, Edward Horton, MD, Jay Mason, MD, Renu Virmani, MD, Lawrence Wechsler, MD
Data and Safety Monitoring Board (DSMB)
Chair: C. Noel Bairey-Merz, MD, J. Ward Kennedy, MD (former)
Executive Secretary: David Gordon, MD, PhD
Members: Elliott Antman, MD, John Colwell, MD, PhD, Sarah Fowler, PhD, Curt Furberg, MD, Lee Goldman, MD, Bruce Jennings, MA, Scott Rankin, MD
Footnotes
Deceased
References
- Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, Nuttall FQ, Comstock JP, Sawin CT, Silbert C, Rubino FA. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM) J Diabetes Complications. 1999;13:307–313. doi: 10.1016/s1056-8727(99)00062-8. [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, American Diabetes Association Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Bird SJ, Watling S, Kaleta H, Hayes L, Eckert S, Foyt HL. Natural progression of diabetic peripheral neuropathy in the Zenarestat study population. Diabetes Care. 2004;27:1153–1159. doi: 10.2337/diacare.27.5.1153. [DOI] [PubMed] [Google Scholar]
- Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am Heart J. 2008;156:528–536. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Chen J, Li D, Zhang X, Mehta JL. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone. Hypertension. 2004;44:655–661. doi: 10.1161/01.HYP.0000144400.49062.6b. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41:416–423. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- Dyck PJ. Severity and staging of diabetic polyneuropathy. In: Gries FA, Cameron NE, Low PA, Ziegler D, editors. Textbook of Diabetic Neuropathy. Thieme; Stuttgart: 2003. pp. 170–175. [Google Scholar]
- Dyck PJ, Davies JL, Litchy WJ, O’Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composit score in the Rochester Diatetic Neuropathy Study (RDNS) cohort. Neurology. 1997;49:229–239. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- El-Mir MY, Detaille D, G RV, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A, Leverve X. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008;34:77–87. doi: 10.1007/s12031-007-9002-1. [DOI] [PubMed] [Google Scholar]
- el-Shazly M, Abdel-Fattah M, Scorpiglione N, Benedetti MM, Capani F, Carinci F, Carta Q, Cavaliere D, De Feo EM, Taboga C, Tognoni G, Nicolucci A, The Italian Study Group for the Implementation of the St. Vincent Declaration Risk factors for lower limb complications in diabetic patients. J Diabetes Complications. 1998;12:10–17. doi: 10.1016/s1056-8727(97)00001-9. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- Franklin GM, Shetterly SM, Cohen JA, Baxter J, Hamman RF. Risk factors for distal symmetric neuropathy in NIDDM. The San Luis Valley Diabetes Study. Diabetes Care. 1994;17:1172–1177. doi: 10.2337/diacare.17.10.1172. [DOI] [PubMed] [Google Scholar]
- Kou J, Klorig DC, Bloomquist JR. Potentiating effect of the ATP-sensitive potassium channel blocker glibenclamide on complex I inhibitor neurotoxicity in vitro and in vivo. Neurotoxicology. 2006;27:826–834. doi: 10.1016/j.neuro.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, Stevens MJ, Feldman EL. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. see comments. [DOI] [PubMed] [Google Scholar]
- Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 (3rd and last part) Diabetes Metab. 1977;3:245–256. [PubMed] [Google Scholar]
- Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R, Stevens M. Insulin and cardiovascular disease in type 2 diabetes. In: Cheta D, editor. Vascular Involvement in Diabetes. Karger; Basel, Bucharest, Freiburg, Paris, London, New York: 2005. pp. 653–668. [Google Scholar]
- Rosa AO, Egea J, Martinez A, Garcia AG, Lopez MG. Neuroprotective effect of the new thiadiazolidinone NP00111 against oxygen-glucose deprivation in rat hippocampal slices: implication of ERK1/2 and PPARgamma receptors. Exp Neurol. 2008;212:93–99. doi: 10.1016/j.expneurol.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Savage S, Estacio RO, Jeffers B, Schrier RW. Increased complications in noninsulin-dependent diabetic patients treated with insulin versus oral hypoglycemic agents: a population study. Proc Assoc Am Physicians. 1997;109:181–189. [PubMed] [Google Scholar]
- Sjoholm A, Nystrom T. Endothelial inflammation in insulin resistance. Lancet. 2005;365:610–612. doi: 10.1016/S0140-6736(05)17912-4. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Zhang W, Li F, Sima AA. C-peptide corrects endoneurial blood flow but not oxidative stress in type 1 BB/Wor rats. Am J Physiol Endocrinol Metab. 2004;287:E497–E505. doi: 10.1152/ajpendo.00048.2004. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998a;352:854–865. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998b;352:837–853. [PubMed] [Google Scholar]
- Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28:2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- Wu MS, Johnston P, Sheu WH, Hollenbeck CB, Jeng CY, Goldfine ID, Chen YD, Reaven GM. Effect of metformin on carbohydrate and lipoprotein metabolism in NIDDM patients. Diabetes Care. 1990;13:1–8. doi: 10.2337/diacare.13.1.1. [DOI] [PubMed] [Google Scholar]
- Xing B, Liu M, Bing G. Neuroprotection with pioglitazone against LPS insult on dopaminergic neurons may be associated with its inhibition of NF-kappaB and JNK activation and suppression of COX-2 activity. J Neuroimmunol. 2007;192:89–98. doi: 10.1016/j.jneuroim.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Ogasawara S, Mizukami H, Yajima N, Wada R, Sugawara A, Yagihashi S. Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-gamma-ligand, in insulin-deficient diabetic rats. J Neurochem. 2008;104:491–499. doi: 10.1111/j.1471-4159.2007.05050.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kashii S, Yasuyoshi H, Zhang S, Honda Y, Akaike A. Mitochondrial ATP-sensitive potassium channel: a novel site for neuroprotection. Invest Ophthalmol Vis Sci. 2003;44:2750–2756. doi: 10.1167/iovs.02-0815. [DOI] [PubMed] [Google Scholar]
- Yu X, Shao XG, Sun H, Li YN, Yang J, Deng YC, Huang YG. Activation of cerebral peroxisome proliferator-activated receptors gamma exerts neuroprotection by inhibiting oxidative stress following pilocarpine-induced status epilepticus. Brain Res. 2008;1200:146–158. doi: 10.1016/j.brainres.2008.01.047. [DOI] [PubMed] [Google Scholar]