Abstract
Purpose
The authors determined the role of the protein kinase C (PKC) isoforms cPKCα and nPKCε in EGF-stimulated proliferation of cultured rat and human conjunctival goblet cells.
Methods
Rat and human conjunctivas were minced, and goblet cells were allowed to grow. Passage 1 cells were serum starved for 24 to 48 hours and were incubated with the PKC inhibitors calphostin C and Gö 6983 (10−10-10−7 M) for 20 minutes before stimulation with EGF (10−7 M) for 24 hours. The presence and localization of PKC isoforms in cultured rat goblet cells were determined by Western blot analysis and immunofluorescence microscopy, respectively. Cultured rat goblet cells were serum starved and incubated with adenoviruses containing genes for dominant-negative cPKCα(Ad DNPKCα, 104 pfu), dominant-negative nPKCε(Ad DNPKCε, 104 pfu), and wild-type cPKCα(Ad WTPKCα, 107 pfu), and proliferation was measured.
Results
In rat goblet cells, EGF-stimulated proliferation was completely inhibited by calphostin C, whereas Gö 6983 inhibited proliferation by 53% ± 15%. In human goblet cells, EGF-stimulated proliferation was completely inhibited by calphostin C. PKCα, -βI, -βII, -δ, -ε, -ι/λ, -θ, -γ, and -ζ were found in cultured rat goblet cells. Ad DNPKCα and Ad DNPKCε inhibited EGF-stimulated proliferation in rat goblet cells by 78% ± 6% and 92% ± 8%, respectively. Incubation with Ad WTPKCα alone significantly increased proliferation.
Conclusions
cPKCα and nPKCε play key roles in conjunctival goblet cell proliferation.
The protein kinase C (PKC) superfamily of lipid-regulated serine/threonine protein kinases includes 10 different isoforms.1 Specific isoforms play critical roles in the signal transduction pathways that regulate cell proliferation, transformation, differentiation, and secretion. The PKC isoforms can be divided into three classes based on structure and cofactor requirements. Classical or conventional PKCs (cPKCα, -βI, -βII, and -γ) are activated by Ca2+ and diacylglycerol. Novel PKCs (nPKCδ, -ε, -η, and -θ) are activated by diacylglycerol but not Ca2+. Atypical PKCs (aPKCζ and -ι/λ) are unresponsive to diacylglycerol and Ca2+.
It is well established that PKC isoforms play important roles in cell proliferation2–5 and are cell type specific.6 Overexpression of PKCα has been shown to increase proliferation in thymocytes, MCF-7 cells, and U87 cells.7 In contrast, in intestinal, pancreatic, and mammary cells, PKCα has been shown to have an antiproliferative effects.6 nPKCε increases the proliferation of lactotrophs through ERK1/28 and promotes survival in lung cancer cells.6 Indeed, nPKCε has been shown to be an oncogene.4
The conjunctiva is an epithelium that surrounds the cornea and lines the eyelids. We previously showed that conjunctival goblet cells in vivo contained at least seven PKC isoforms.9 Cholinergic agonists, which are known to activate PKC, stimulate mucin secretion, as do phorbol esters.10 In this tissue, the phorbol ester PMA activates the nonreceptor tyrosine kinases Pyk2 and Src to phosphorylate the EGF receptor, which then activates ERK1/2 to cause secretion.10
Large oligomeric mucins, such as MUC5AC and MUC5B (two gel-forming mucins), are produced in the airway, gastrointestinal tract, and ocular surface and protect these wet-surfaced mucosa from the external environment.11,12 Gel-forming mucins are synthesized and secreted by goblet cells located in the wet-surfaced epithelia in response to stimuli from the extracellular environment. The amount of mucin produced by the goblet cells is dependent on the number of cells present (proliferation or differentiation), the amount of mucin synthesized and stored in the secretory granules (synthesis), and the release of mucin from the secretory granules (secretion). Each tissue has its own unique response, leading to an increase or a decrease in mucin production.
Goblet cells in the conjunctiva are responsible for production of the large soluble mucin MUC5AC, the major soluble mucin of the tear film.13 Ocular mucin is increased in allergy and inflammation but decreased in diseases of impaired corneal sensitivity such as herpes keratitis and anesthetic cornea.14,15 Thus, the amount of ocular surface mucin is highly regulated. In the conjunctiva, the regulation of goblet cell mucin synthesis has not been investigated. It is known that the parasympathetic neuro-transmitters acetylcholine, vasoactive intestinal peptide and the nerve growth factor (NGF) and its family member brain-derived nerve factor (BDNF) induce goblet cell secretion.16,17 It is also known that the epidermal growth factor (EGF) family of growth factors stimulates conjunctival goblet cell proliferation when measured in primary cell culture.18,19
EGF and its family members TGFα and HB-EGF are stimuli of conjunctival goblet cell proliferation.10 EGF activates the EGF receptor and stimulates the ERK1/2 pathway, translocating ERK1/2 to the nucleus and causing proliferation in rat and human goblet cells.10 In the present study, we investigated the role of PKC isoforms in EGF stimulation of goblet cell proliferation and found that EGF activation of cPKCα and nPKCε induces cell proliferation.
Materials and Methods
Human EGF was purchased from PeproTech (Rocky Hill, NJ). Calphostin C and Gö 6983 were from EMD (Madison, WI). Cell proliferation reagent WST-8 came from Dojindo Molecular Technologies (Gaithersburg, MD). RPMI 1640 media, L-glutamine, penicillin-streptomycin, and trypsin-EDTA solution were from Lonza (Walkersville, MD); fetal bovine serum was from Hyclone Laboratories (Logan, UT).
Antibodies
Antibodies specific to the individual anti-rabbit PKC isoforms and horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The secondary antibody used for immunofluorescence microscopy was Cy3 conjugated to rabbit IgG and was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The secondary antibody for Western blot analysis was anti-rabbit IgG conjugated to HRP and was purchased from Millipore (Billerica, MA). A monoclonal antibody against β-actin was purchased from Sigma Chemical (St. Louis, MO).
Animals
Male Sprague-Dawley rats weighing between 125 and 150 g were obtained from Taconic Farms (Germantown, NY). Rats were anesthetized with CO2 for 1 minute and decapitated, and the nictitating membranes and fornix were removed from both eyes and minced. The procedure for removal of the conjunctiva was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Isolation and Culture of Goblet Cells
Human conjunctival tissue was obtained from patients during ocular surgery according to a protocol that adhered to the tenets of the Declaration of Helsinki. The protocol was approved by the Massachusetts Eye and Ear Infirmary and Schepens Eye Research Institute Human Subjects Internal Review Boards. The tissue, which was normally discarded during surgery, was donated by seven patients (five men, two women; average age, 68 years) after informed consent. Diagnoses for the patients were retinal detachment (four patients), vitrectomy for age-related macular degeneration with subretinal hemorrhage (one patient), vitrectomy for aqueous misdirection (one patient), and vitrectomy for nonclearing vitreous hemorrhage (one patient). The tissue was immediately placed in Optisol-GS (Bausch & Lomb, Rochester, NY) for transportation to the laboratory. Before culture, the tissue was placed in 1× phosphate-buffered saline (PBS) consisting of 3× (300 μg/mL) penicillin-streptomycin.
Goblet cells from rat and human conjunctival pieces were grown in organ culture, as described previously.20,21 Briefly, minced pieces of conjunctiva were placed in culture with RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 μg/mL penicillin-streptomycin. After cells were observed growing out from the plug, the plug was removed and goblet cells were allowed to grow. Cells were identified as goblet cells by the following characteristics: their morphology as visualized by light microscopy, their positive staining with the lectin Ulex europaeus agglutinin and antibody to cytokeratin 7 viewed by immunofluorescence microscopy, and their negative staining for the stratified squamous cell markers the lectin Griffonia (Bandeiraea) simplicifolia lectin I and antibody to cytokeratin 4. First-passage goblet cells were used in all experiments.
Measurement of Goblet Cell Proliferation
Human or rat conjunctival goblet cells in primary culture were trypsinized, and 200 cells/well were seeded on 48-well culture plates. Cells were grown to subconfluence for 24 to 48 hours and were serum starved in RPMI with 0.35% bovine serum albumin (BSA) for 24 hours (rat) or 48 hours (human) before the start of the experiment. Cells were then pretreated for 20 minutes with no additions or with the inhibitors calphostin C or Gö 6983 at 10−10 to 10−7 M. EGF (10−7 M) was then added for 24 hours. Either inhibitor was present for the entire 24-hour incubation time. Incubation was terminated by the removal of supernatant, and cell proliferation was determined by WST-8 assay, a colorimetric assay for the quantification of cell number and viability. This assay is based on the cleavage of the tetrazolium salt WST-8 by mitochondrial dehydrogenases in viable cells. Absorbance was read at 465 nm after 60-minute incubation at 37°C.
Western Blot Analysis of PKC Isoforms
The presence of PKC isoforms in rat goblet cells was determined by Western blot analysis. Cultured goblet cells grown in RPMI 1640 and 10% FBS were removed from the culture dishes by scraping and were homogenized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS and 1 mM EDTA) protease inhibitors (phenylmethylsulfonyl fluoride 0.6 mM, aprotinin 0.2 U/mL, and sodium orthovanadate 100 nM). Cells were sonicated and centrifuged at 2000g for 15 minutes at 4°C. Twenty micrograms of proteins in the supernatant were separated by SDS-PAGE on a 10% gel and transferred onto nitrocellulose membranes. The nitrocellulose membranes were blocked overnight at 4°C in 5% nonfat dried milk in buffer containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween-20 (TBST) and then were incubated with primary antibody for 1 hour at room temperature at a dilution of 1:500 or overnight at 4°C, followed by incubation with HRP-conjugated secondary antibody at 1:5000. Immunoreactive bands were detected by the enhanced chemiluminescence method. Homogenized conjunctiva and brain served as the positive controls.
Immunofluorescence Microscopy of PKC Isoforms
The cellular location of PKC isoforms was analyzed by immunofluorescence microscopy. Primary cultures of rat conjunctival goblet cells were trypsinized, seeded onto glass coverslips, and grown to subconfluence in RPMI medium supplemented with 10% FBS. Cells were fixed with ice-cold 4% paraformaldehyde. Fixed cells were blocked in 3% BSA and 0. 3% Triton X-100 for 1 hour before incubation for 1.5 hours at room temperature with primary antibody at a dilution of 1:100 and 1 hour at room temperature with secondary antibody at a dilution of 1:300. The same antibodies were used in Western blot analysis and immunofluorescence experiments. Coverslips were mounted on glass slides using PVA/DABCO mounting medium, and the cells were viewed under a microscope (Eclipse E80i; Nikon, Tokyo, Japan) with a microscope digital camera (SPOT; Diagnostic Instruments Inc., Sterling Heights, MI). Omission of primary antibody served as the negative control.
Expression of Wild-Type and Dominant-Negative PKC Isoforms
Replication defective adenovirus (Ad) constructs expressing green fluorescent protein (Ad GFP), wild-type cPKCα(Ad WT PKCα), dominant-negative cPKCα(Ad DN PKCα), and dominant-negative nPKCε(Ad DN PKCε) were used. Virus constructs were a kind gift from George King (Joslin Diabetes Center, Boston, MA). First-passage goblet cells were serum starved for 24 hours and then incubated with Ad constructs at either 1 × 107 pfu (Ad GFP), 1 × 107 pfu (Ad WTPKCα), or 1 × 104 pfu (Ad DN PKCα and Ad DN PKCε) for 24 hours. The amount of PKC isoform synthesized was determined by Western blotting using an antibody for the appropriate PKC isoform. The amount of protein loaded was standardized with the use of an antibody for actin at a dilution of 1:1000. For measurement of proliferation, cells were serum starved for 24 hours before Ad was added. Proliferation was measured after 24 hours using WST-8, as described. Blots were scanned and analyzed by NIH ImageJ (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Data Presentation and Statistical Analysis
Data are expressed as the fold increase over the basal value, which was standardized to 1.0. Results are expressed as the mean ± SEM. Data were analyzed by Student’s t-test. P < 0.05 was considered statistically significant.
Results
Effect of Inhibition of PKC on EGF-Stimulated Rat and Human Goblet Cell Proliferation
We previously showed that EGF at 10−7 M caused a significant increase in ERK activation and inhibition of ERK inhibited goblet cell proliferation.19,22 To determine whether PKC is also involved in this process, rat goblet cells were preincubated with the PKC inhibitors calphostin C and Gö 6983 at 10−10 to 10−7 M and were stimulated with EGF at 10−7 M. Calphostin C did not alter basal goblet cell proliferation (Fig. 1A). In the absence of inhibitor, EGF significantly stimulated proliferation 1.4 ± 0.1-fold above basal (n = 7; Fig. 1A). Increasing concentrations of calphostin C inhibited EGF-stimulated proliferation in a concentration-dependent manner with a significant inhibition of 100% obtained at 10−8 M calphostin C. Calphostin C at 10−7 M, with or without EGF, decreased proliferation below basal levels, suggesting a toxic effect at this concentration. Gö 6983 increased basal levels of proliferation (n = 5; Fig. 1B). EGF significantly stimulated proliferation 3.8 ± 0.6-fold above basal, which was decreased by Gö 6983 in a concentration-dependent manner, with maximum inhibition of 53% ± 15% obtained at 10−7 M.
Figure 1.
Inhibition of PKC blocks EGF-stimulated cultured rat goblet cell proliferation. Cultured rat goblet cells were serum starved for 24 hours before the addition of the PKC inhibitors calphostin C (A) or Gö 6983 (B) at the indicated concentrations for 20 minutes and before the addition of EGF (10−7 M) for 24 hours. Goblet cell proliferation was determined using WST-8. Data are mean ± SEM from either seven (calphostin C) or five (Gö 6983) independent experiments. *Significant difference from basal; #significant difference from EGF.
The effect of calphostin C was tested on human conjunctival goblet cells. Calphostin C alone increased basal goblet cell proliferation with significant increases at 10−8 and 10−7 M (n = 4; Fig. 2). EGF significantly stimulated proliferation 1.7 ± 0.2-fold above basal. Increasing concentrations of calphostin C blocked EGF-stimulated proliferation in a concentration-dependent manner, with 100% inhibition obtained at 10−6 M. In these cells calphostin C at 10−5 M, with or without EGF, decreased proliferation below basal levels.
Figure 2.

Inhibition of PKC blocks EGF-stimulated cultured human goblet cell proliferation. Cultured human goblet cells were serum starved for 48 hours before the addition of the PKC inhibitor calphostin C at the indicated concentrations for 20 minutes and before the addition of EGF (10−7 M) for 24 hours Goblet cell proliferation was determined using WST-8. Data are mean ± SEM from four independent experiments. *Significant difference from basal; #significant difference from EGF.
Data from Figures 1 and 2 suggest that EGF activates PKC to stimulate conjunctival goblet cell proliferation in rats and humans.
Identification of PKC Isoforms in Rat Conjunctival Goblet Cells
The presence of PKC isoforms in rat conjunctival goblet cells was determined by Western blot analysis and confirmed by fluorescence microscopy. With the use of Western blot analysis, we found PKCα, -βI, -βII, -δ, -ε, and -ι/λ in rat conjunctival goblet cells (n = 3; Fig. 3). PKCγ, -θ, and -ζ were not detected. Each PKC isoform identified was present as a major band at the appropriate molecular weight. For some isoforms, additional minor bands were detected. Each PKC isoform, including PKCγ, -θ, and -ζ, was also detected in the positive controls rat conjunctiva and brain. Given that we previously showed that PKCη was not present in conjunctival goblet cells,9 we did not determine whether it was present in cultured goblet cells. We also detected the closely related protein kinase D (formally known as PKCμ; data not shown).
FIGURE 3.
Presence of PKC iso-forms in cultured rat goblet cells. Cultured rat goblet cells (G), rat conjunctiva (C), and rat brain (B) were homogenized. Proteins were separated by SDS-PAGE, and Western blot analysis was performed with antibodies specific to the PKC isoforms. Blots are representative of three independent experiments.
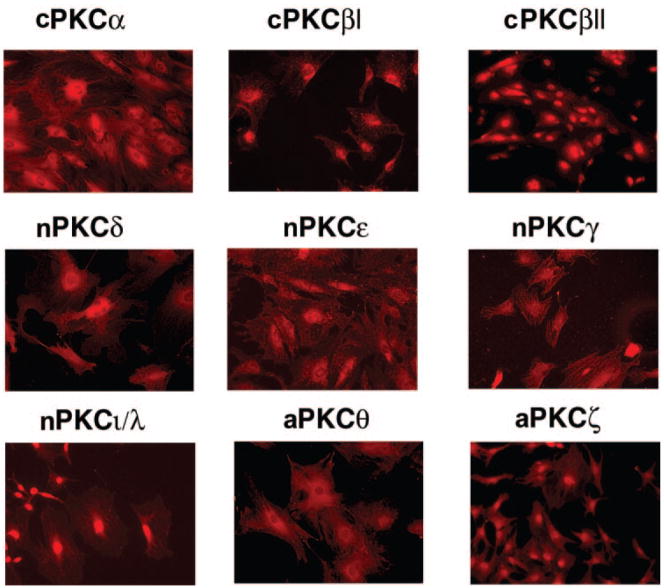
The cellular location of each PKC isoform was determined in cultured goblet cells. All PKC isoforms were detected in the goblet cells by immunofluorescence (n = 3; Fig. 4). PKCα, -βI, -ε, -γ, and -θ were detected in the cytoplasm. PKCβII, -δ, -ι/λ, and -ζ were found predominantly in the nuclei, with some expression in the cytoplasm. PKCγ, -θ, and -ζ were detected by immunofluorescence microscopy but not by Western blot analysis. Thus, multiple PKC isoforms of the classical, novel, and atypical subtypes are present in conjunctival goblet cells.
Figure 4.
Localization of PKC isoforms in cultured rat goblet cells. Cultured rat goblet cells were grown on glass coverslips, and immunofluorescence was performed with antibodies specific to the PKC isoforms. Images are representative of three independent experiments. Original magnification, 200×.
Effect of Inhibition of cPKCα and nPKCε on EGF-Stimulated Rat Conjunctival Goblet Cell Proliferation
Adenovirus-containing GFP was used to measure the effectiveness of adenovirus transduction of conjunctival goblet cells. Cells were incubated with Ad GFP (1 × 107 pfu) for 24 hours. With the use of microscopy, we found that all cells expressed GFP and, hence, were transduced by adenovirus (Fig. 5).
Figure 5.
Cultured rat goblet cells can be transduced by adenovirus containing green fluorescent protein. Cultured rat goblet cells grown on glass coverslips and serum starved for 24 hours before incubation with adenovirus containing the gene for green fluorescent protein (1 ×107 pfu) for 24 hours. Cells were viewed by fluorescence microscopy. Original magnification, 200×.
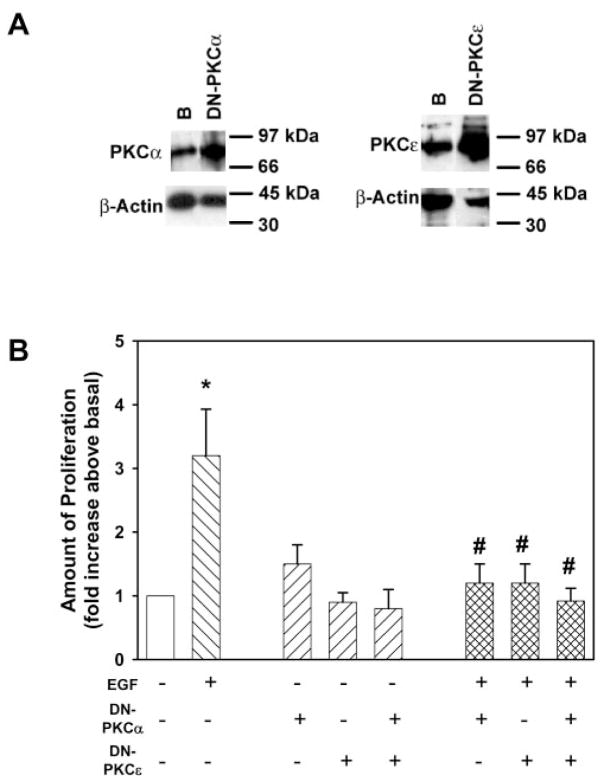
To determine whether individual PKC isoforms were used to stimulate goblet cell proliferation, cPKCα or nPKCε activity was prevented in rat conjunctival goblet cells with Ad DN PKCα and Ad DN PKCε. Through Western blot analysis, we found that these viruses caused a 4.0-fold and a 4.8-fold over-expression of cPKCα and nPKCε, respectively (n = 3; Fig. 6A). EGF (10−7 M) stimulated goblet cell proliferation 3.2 ± 0.7-fold above basal (n = 5; Fig. 6B). Incubation of Ad DN PKCα and Ad DN PKCε, alone or together, did not alter basal goblet cell proliferation (Fig. 6B). Expression of Ad DN PKCα and Ad DN PKCε significantly blocked EGF-stimulated goblet cell proliferation by 78% ± 6% and 92% ± 8%, respectively. Transduction with Ad DN PKCα and Ad DN PKCε together completely blocked EGF-stimulated goblet cell proliferation. These data show that EGF uses two PKC isoforms, cPKCα and nPKCε, to stimulate rat conjunctival goblet cell proliferation.
Figure 6.
Expression of dominant-negative cPKCα and nPKCε inhibit EGF-stimulated cultured rat goblet cell proliferation. Cultured rat goblet cells were serum starved for 24 hours before incubation with EGF (10−7 M) or adenovirus containing either the gene for dominant-negative cPKCα(DN-PKCα) or nPKCε(DN-PKCε; 1 × 104 pfu) for 24 hours. Western blot analysis was performed and shown (A) to ensure that transduction had occurred. Western blot analysis was performed for the presence of β-actin to indicate the total amount of protein in each sample. Blots are representative of three independent experiments. Goblet cell proliferation was measured by WST8 (B). Data are mean ± SEM of five independent experiments. Open bar: untreated cells; left hatched bars: cells treated with EGF; right hatched bars: cells treated with virus; crosshatched bars: cells treated with both. *Significant difference from basal; #significant difference from EGF.
Effect of Overexpression of cPKCα on Rat Conjunctival Goblet Cell Proliferation
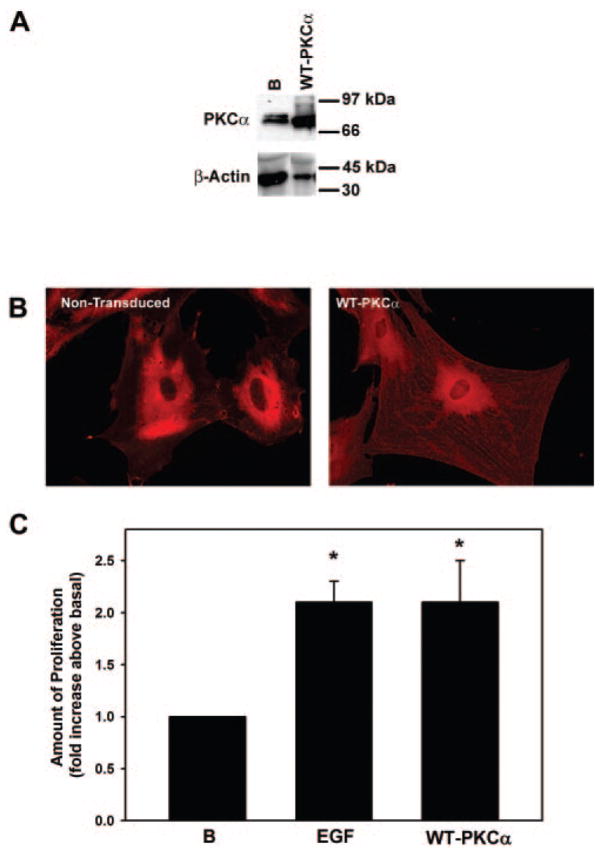
Adenovirus-expressing wild-type cPKCα was used to determine whether overexpression of this PKC isoform alone could stimulate goblet cell proliferation. With the use of Western blot analysis, we found that Ad WT PKCα was overexpressed by 14.0-fold (Fig. 7A). With fluorescence microscopy, the overexpressed Ad WT PKCα was located in the perinuclear area, similar to the location of cPKCα in nontransduced cells (Fig. 7B). In addition, cPKCα was present on filaments throughout the cytoplasm in cells treated with Ad WT PKCα. When cultured goblet cells were incubated for 24 hours with Ad WT PKCα, goblet cell proliferation was increased 2.1 ± 0.4-fold above basal, the same proliferative response as for EGF (n = 5; Fig. 7C). Thus, overexpression of cPKCα alone can stimulate rat conjunctival goblet cell proliferation.
Figure 7.
Expression of wild-type PKCα stimulates cultured rat goblet cell proliferation. Cultured rat goblet cells were serum starved for 24 hours before incubation with adenovirus containing the gene for wild-type PKCα (WT-PKCα; 1 × 107 pfu) for 24 hours Western blot analysis was performed to ensure that transduction had occurred. (A) Blots are representative of two independent experiments. Western blot analysis was performed for the presence of β-actin to indicate the total amount of protein in each sample. (B) Two immunofluorescence micrographs of goblet cells grown on glass coverslips and incubated with antibody specific to cPKCα(red staining). Magnification, 200×. (C) Goblet cell proliferation was measured by WST8. Data are mean ± SEM of five independent experiments. *Significant difference from basal; #significant difference from EGF.
Discussion
The present study demonstrates that EGF stimulates conjunctival goblet cell proliferation by activating cPKCα and nPKCε. EGF regulation of goblet cell proliferation has not been studied in goblet cells from tissue other than the conjunctiva, though Nadel et al.23 showed that EGF can stimulate airway goblet cell MUC5AC synthesis. In tumor cells, nPKCε is thought to be proliferative and to induce cell survival, whereas cPKCα can be antiproliferative and can cause apoptosis or proliferation, depending on the cell type.6 In contrast to cancer cells and similar to goblet cells, in corneal endothelial cells, serum stimulation of cell proliferation uses cPKCα and nPKCε to stimulate proliferation.24 It has been shown that EGF induces the translocation of cPKCα and the induction of cell proliferation in corneal epithelial cells.25 In conjunctival goblet cells, cPKCα and nPKCε play major roles in the stimulation of proliferation.
In this study, we showed that cultured conjunctival goblet cells contain multiple PKC isoforms in addition to cPKCα and nPKCε. The roles of the other PKC isoforms in cell proliferation were not directly determined. Interestingly, inhibition of EGF-stimulated proliferation with the PKC inhibitors Gö 6983 in rat goblet cells and calphostin C in human goblet cells increased basal proliferation. It is possible that additional PKC isoforms play a negative role in proliferation (i.e., they inhibit proliferation by acting as a mechanism to stop proliferation). Removing this inhibitory pathway would increase proliferation. Therefore, it is possible that multiple PKC isoforms play a role, positive or negative, in goblet cell proliferation.
cPKCα is a classical isoform and is activated by Ca2+ and diacylglycerol, whereas nPKCε is a novel isoform and is activated by diacylglycerol only. Inhibition of either cPKCα or nPKCε using dominant-negative adenovirus blocked EGF-stimulated proliferation. Inhibition of both PKC isoforms caused even greater inhibition than inhibition of each of the PKC isoforms alone. These results imply that cPKCα and nPKCε have separate or incompletely overlapping mechanisms of action. In conjunctival goblet cells, cPKCα and nPKCε had similar subcellular localizations. In goblet cells within the intact conjunctiva, these two isoforms were also present in similar locations.9 However, nPKCε, but not cPKCα, is known to have an actin-binding domain.26 Actin polymerization is well known to play a role in cell proliferation because depolymerization causes cell cycle arrest in the G1 phase.27 Thus, the activation of nPKCε, but not cPKCα, could stimulate cell proliferation by inducing actin polymerization in a Ca2+-independent manner. Differential activators of the two PKC isoforms plus differential actin binding could provide the basis for the distinct pathways activated by the two PKC isoforms.
Cultured rat conjunctival goblet cells provide an excellent model for studying human goblet cell function. In the present study, EGF-stimulated cell proliferation was inhibited by the PKC inhibitor calphostin C in human and rat goblet cells, suggesting that EGF activates PKC isoforms to cause proliferation in both species. In related studies, EGF activated the EGF receptor, which, in turn, stimulated ERK1/2 activity to induce proliferation in goblet cells from both species.22 In human and rat cultured conjunctival goblet cells, cholinergic agonists and EGF activated MAPK with a similar time dependence. This activation was receptor mediated, and cholinergic agonists transactivated the EGF receptor.22 In the present study, the concentration dependence of calphostin C in inhibiting EGF-stimulated proliferation varied between rat and human goblet cells. Human goblet cells were 100-fold less sensitive than rat goblet cells to the concentration of calphostin C, which caused 100% inhibition. Furthermore, calphostin C at 10−7 M appeared to be toxic to rat, but not human, goblet cells. This suggests that human goblet cells are more robust than rat goblet cells. Additional evidence for this is that human goblet cells require 48-hour serum starvation to reduce proliferation to minimal levels, but only 24 hours are required for rat goblet cells. In spite of these minor differences, cultured rat conjunctival goblet cells can be used as a model to study human conjunctival goblet cells because all the signaling pathways studied to date are present in human and rat goblet cells.
In conclusion, cPKCα and nPKCε play pivotal roles in conjunctival goblet cell proliferation because overexpression of PKCα and -ε isoforms increased goblet cell proliferation, and inhibition of these isoforms inhibited EGF-stimulated proliferation.
Acknowledgments
Supported by National Institutes of Health Grant EY09057.
Footnotes
Disclosure: M.A. Shatos, None; R.R. Hodges, None; Y. Oshi, None; J.A. Bair, None; D. Zoukhri, None; C. Kublin, None; K. Lashkari, None; D.A. Dartt, None
References
- 1.Chen D, Gould C, Garza R, et al. Amplitude control of protein kinase C by RINCK, a NOVEL E3 ubiquitin ligase. J Biol Chem. 2007;282(46):33776–33787. doi: 10.1074/jbc.M703320200. [DOI] [PubMed] [Google Scholar]
- 2.Simon F, Stutzin A. Protein kinase C-mediated phosphorylation of p47 phox modulates platelet-derived growth factor-induced H2O2 generation and cell proliferation in human umbilical vein endothelial cells. Endothelium. 2008;15(4):175–188. doi: 10.1080/10623320802174480. [DOI] [PubMed] [Google Scholar]
- 3.Cameron AJ, Procyk KJ, Leitges M, et al. PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int J Cancer. 2008;123(4):769–779. doi: 10.1002/ijc.23560. [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Sivaprasad U. Protein kinase Cε makes the life and death decision. Cell Signal. 2007;19(8):1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao LF, Xu YJ, Liu XS, et al. PKC promotes proliferation of airway smooth muscle cells by regulating cyclin D1 expression in asthmatic rats. Acta Pharmacol Sin. 2008;29(6):677–686. doi: 10.1111/j.1745-7254.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7(4):281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 7.Michie AM, Nakagawa R. The link between PKCα regulation and cellular transformation. Immunol Lett. 2005;96(2):155–162. doi: 10.1016/j.imlet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Petiti JP, De Paul AL, Gutierrez S, et al. Activation of PKC epsilon induces lactotroph proliferation through ERK1/2 in response to phorbol ester. Mol Cell Endocrinol. 2008;289(1–2):77–84. doi: 10.1016/j.mce.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Dartt DA, Rios JD, Kanno H, et al. Regulation of conjunctival goblet cell secretion by Ca(2+) and protein kinase C. Exp Eye Res. 2000;71(6):619–628. doi: 10.1006/exer.2000.0915. [DOI] [PubMed] [Google Scholar]
- 10.Hodges RR, Horikawa Y, Rios JD, et al. Effect of protein kinase C and Ca(2+) on p42/p44 MAPK, Pyk2, and Src activation in rat conjunctival goblet cells. Exp Eye Res. 2007;85(6):836–844. doi: 10.1016/j.exer.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin BK. Mucus structure and properties in cystic fibrosis. Paediatr Respir Rev. 2007;8(1):4–7. doi: 10.1016/j.prrv.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 13.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2(2):131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 14.Kunert KS, Keane-Myers AM, Spurr-Michaud S, et al. Alteration in goblet cell numbers and mucin gene expression in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2001;42(11):2483–2489. [PubMed] [Google Scholar]
- 15.Dogru M, Asano-Kato N, Tanaka M, et al. Ocular surface and MUC5AC alterations in atopic patients with corneal shield ulcers. Curr Eye Res. 2005;30(10):897–908. doi: 10.1080/02713680500196715. [DOI] [PubMed] [Google Scholar]
- 16.Rios JD, Zoukhri D, Rawe IM, et al. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40(6):1102–1111. [PubMed] [Google Scholar]
- 17.Ghinelli E, Johansson J, Rios JD, et al. Presence and localization of neurotrophins and neurotrophin receptors in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2003;44(8):3352–3357. doi: 10.1167/iovs.03-0037. [DOI] [PubMed] [Google Scholar]
- 18.Gu J, Chen L, Shatos MA, et al. Presence of EGF growth factor ligands and their effects on cultured rat conjunctival goblet cell proliferation. Exp Eye Res. 2008;86(2):322–334. doi: 10.1016/j.exer.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shatos MA, Gu J, Hodges RR, et al. ERK/p44p42 mitogen-activated protein kinase mediates eGF-stimulated proliferation of conjunctival goblet cells in culture. Invest Ophthalmol Vis Sci. 2008;49:3351–3359. doi: 10.1167/iovs.08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shatos MA, Rios JD, Tepavcevic V, et al. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001;42(7):1455–1464. [PubMed] [Google Scholar]
- 21.Shatos MA, Rios JD, Horikawa Y, et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44(6):2477–2486. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- 22.Horikawa Y, Shatos MA, Hodges RR, et al. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44(6):2535–2544. doi: 10.1167/iovs.02-1117. [DOI] [PubMed] [Google Scholar]
- 23.Takeyama K, Dabbagh K, Lee HM, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999;96(6):3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham MA, Rawe I, Dartt DA, et al. Protein kinase C regulation of corneal endothelial cell proliferation and cell cycle. Invest Ophthalmol Vis Sci. 2000;41(13):4124–4132. [PubMed] [Google Scholar]
- 25.Sharma GD, Ottino P, Bazan NG, et al. Epidermal and hepatocyte growth factors, but not keratinocyte growth factor, modulate protein kinase Cα translocation to the plasma membrane through 15(S)-hydroxyeicosatetraenoic acid synthesis. J Biol Chem. 2005;280(9):7917–7924. doi: 10.1074/jbc.M408852200. [DOI] [PubMed] [Google Scholar]
- 26.Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:D1134–D1147. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- 27.Lee YJ, Keng PC. Studying the effects of actin cytoskeletal destabilization on cell cycle by cofilin overexpression. Mol Biotechnol. 2005;31(1):1–10. doi: 10.1385/MB:31:1:001. [DOI] [PubMed] [Google Scholar]