Abstract
Purpose
Carcinoma of the nasal cavity and septum has historically been associated with poor prognosis. This report updates long-term outcomes for radiation therapy (RT) of this disease site at the University of Texas M. D. Anderson Cancer Center.
Methods
Retrospective analysis was performed on 68 patients diagnosed with histologically proven carcinoma of the nasal cavity or septum treated with RT for curative intent between 1969 and 2000. Disease histology was distributed as follows: 45 (66%) squamous cell carcinoma, 12 (18%) adenoid cystic carcinoma, 8 (12%) adenocarcinoma, and 3 (4%) poorly/undifferentiated carcinoma. Thirty-two (47%) patients received definitive RT, 23 of whom received external beam radiation treatment (EBRT), while 9 received brachytherapy. Three (4%) patients received preoperative EBRT, and 33 (49%) patients received postoperative EBRT. Thirteen (19%) patients received neck irradiation. Median dose for patients receiving definitive and postoperative RT was 65 and 58.2 Gy, respectively. Median follow-up for the entire cohort was 11 years (range 2.4–30.1 years).
Results
Nineteen (28%) patients suffered locoregional relapse, 14 (21%) locally and 5 (7%) regionally. Local control at 5 and 10 years was 86% and 76%. Disease specific survival was 86% and 78%, and overall survival was 82% and 62% at 5 years and 10 years, respectively.
Conclusions
This extended follow-up of our institutional experience demonstrates that radiation therapy can provide durable long-term locoregional control and survival outcomes for carcinoma of the nasal cavity and septum.
Keywords: Nasal Cavity, Nasal Septum, Radiotherapy, Carcinoma, Staging
Introduction
Cancers of the nasal cavity are uncommon, accounting for less than 1% of all head and neck malignancies. Staging nomenclature for this disease site has evolved gradually over the past four decades. The American Joint Committee on Cancer (AJCC) did not introduce formal staging criteria for nasal cavity disease until 2001, bringing together cancers originating from the nasal vestibule, septum, and cavity proper under a single moniker. Disease of the vestibule was grouped with cancers of the skin prior to this. Cancers of the septum and cavity were originally cataloged with paranasal sinus disease, but then subsequently grouped separately and staged according to competing ad hoc institutional systems1–3. This has complicated comparison of treatment outcomes across institutions and time.
Nasal cavity disease is frequently treated with multi-modality therapy in the tertiary setting. Radiotherapy is used post-operatively for advanced disease, but can provide definitive treatment in the case of anterior, limited volume disease that would require potentially disfiguring resection. We have previously presented outcomes in 1992 for treating non-vestibular nasal cavity neoplasms with radiotherapy in either setting4. At that time, we used the University of Florida (“Parsons’ System”) staging criteria3 to treat and catalog our study cohort. This report provides a 15-year update to this experience and retrospectively converts original Parsons’ clinical stage for each case to AJCC T stage to assist future comparisons across series.
Methods
We retrospectively reviewed the medical records of 191 patients who presented to our department for cancer of the nasal cavity region between January 1969 and December 2000, after obtaining permission from our Investigational Review Board. One-hundred twenty-three patients were excluded from analysis for the following: disease originating from the skin, nasal vestibule, olfactory region, or paranasal sinuses; recurrent disease; neuroendocrine or melanoma histology; or final treatment at an outside facility. This yielded a study cohort of 68 patients treated for newly diagnosed disease of the nasal septum or cavity.
Prior to 1975, patients were originally staged clinically according to the University of Florida system via physical exam, plain X-rays, and polytomograms. After 1975, patients were radiographically staged by CT imaging. Patient characteristics are detailed in Table 1. The majority of patients were male (62%), active smokers (59%), who presented with nasal obstruction (46%) or epistaxis (31%). Site of disease origin was nasal septum in 31 (46%) cases and nasal floor/lateral wall in 37 (54%) cases. Most cases (66%) had squamous cell histology, followed by adenoid cystic carcinoma (18%), adenocarcinoma (12%), and poorly/undifferentiated carcinoma (4%). The vast majority (84%) of nasal septal cases had squamous cell histology. As shown in Tables 1 and 2, the majority (82%) of cases had Parsons’ clinical stage 1 or 2 disease, and most cases originating from the nasal septum (67%) had clinical stage 1 disease. Parsons’ clinical stage and AJCC T stage roughly correlated to one another, although a tendency towards recategorization as AJCC stage T3 or T4 was seen in 14/23 (61%) cases originally staged as Parsons’ clinical stage 2. Stage distribution by treatment time period (before vs. after 1985) is shown in Table 3. More recently treated patients trended towards having more advanced Parsons’ clinical stage at presentation (24% vs. 8% stage 3 cases for after vs. before 1985, respectively, p = 0.07 by Chi-square testing). Any difference lost further significance (42% vs. 27% T3-4 cases for after vs. before 1985, respectively, p = 0.20) after cases were restaged according to AJCC criteria.
Table 1.
Patient characteristics
| All | Nasal Cavity | Nasal Septum | p value | |
|---|---|---|---|---|
| N=68 (%) | N=37 (54) | N=31 (46) | ||
| Gender | ||||
| Male | 42 (62) | 20 (54) | 22 (71) | 0.15 |
| Female | 26 (38) | 17 (46) | 9 (29) | |
| Age | ||||
| Median | 58.5 yrs | 57 yrs | 63 yrs | 0.02 |
| Range | 19–93 yrs | 19–93 yrs | 43–78 yrs | |
| Histology | ||||
| SCC* | 45 (66) | 19 (51) | 26 (84) | 0.004 |
| Others** | 23 (34) | 18 (49) | 5 (16) | |
| Parsons’ clinical stage | ||||
| 1 | 33 (48) | 13 (36) | 20 (67) | 0.031 |
| 2 | 23 (34) | 15 (42) | 8 (27) | |
| 3 | 10 (15) | 8 (22) | 2 (7) | |
| N/A | 2 (3) | |||
| AJCC T stage | ||||
| T1 | 20 (29) | 6 (16) | 14 (45) | 0.063 |
| T2 | 21 (31) | 13 (35) | 8 (26) | |
| T3 | 9 (13) | 5 (14) | 4 (13) | |
| T4 | 14 (21) | 11 (30) | 3 (10) | |
| Tx | 4 (6) | 2 (5) | 2 (6) | |
| Treatment | ||||
| RT Alone | 32 (47) | 11 (30) | 21(68) | |
| Pre-Op RT | 3 (4) | 3 (8) | -- | |
| Post-Op RT | 33 (49) | 23 (62) | 10 (32) | 0.01 |
| Smoking | ||||
| Yes | 41 (60) | 22 (60) | 19 (61) | 0.93 |
| No | 10 (15) | 6 (16) | 4 (13) | |
| Unknown | 17 (25) | 9 (24) | 8 (26) | |
| Neck nodes | ||||
| Positive | 6 (9) | 3 (8) | 3 (10) | 0.13 |
| Negative | 62 (91) | 34 (92) | 28 (90) | |
| Tumor grade | ||||
| Well diff. | 8 (12) | 5 (13) | 3 (10) | 0.47 |
| Mod diff. | 9 (13) | 3 (8) | 6 (19) | |
| Poor diff. | 18 (26) | 9 (24) | 9 (29) | |
| Not specified | 33 (49) | 20 (54) | 13 (42) | |
SCC: Squamous cell carcinoma
Others include patients with salivary gland histology, adenoid cystic carcinoma (n=12), adenocarcinoma (n=8), and undifferentiated/poorly differentiated carcinoma (n=3).
Table 2.
Correlations between Parsons’ stage and AJCC T stage distribution
| T1 | T2 | T3 | T4 | Tx | Total | |
|---|---|---|---|---|---|---|
| 1 | 20 | 12 | 0 | 0 | 1 | 33 |
| 2 | 0 | 9 | 9 | 5 | 0 | 23 |
| 3 | 0 | 0 | 0 | 9 | 1 | 10 |
| N/A | 2 | |||||
| Total | 20 | 21 | 9 | 14 | 2 | 68 |
Table 3.
Stage distribution according to period of treatment
| AJCC T Stage | Treatment before/on 1985 N (%) | Treatment after 1985 N (%) | All Patients N (%) | Parsons’ Clinical Stage | Treatment before/on 1985 N (%) | Treatment after 1985 N (%) | All Patients N (%) |
|---|---|---|---|---|---|---|---|
| T1 | 13 (35) | 7 (23) | 20 (29) | 1 | 20 (51) | 13 (45) | 33 (48) |
| T2 | 14 (38) | 7 (23) | 21 (31) | 2 | 14 (36) | 9 (31) | 23 (34) |
| T3 | 3 (8) | 6 (19) | 9 (13) | 3 | 3 (8) | 7 (24) | 10 (15) |
| T4 | 7(19) | 7 (23) | 14 (21) | N/A | 2 (5) | 0 (0) | 2 (3) |
| Tx | 0 | 4 (13) | 4 (6) |
Thirty-two (47%) patients received definitive RT, 23 of which received external beam radiation treatment (EBRT, median dose 65 Gy, range 60–70 Gy), while 9 received brachytherapy (median dose 65 Gy, range 58–70 Gy). Brachytherapy was delivered via interstitial radium needles or iridium wires at a dose rate of 0.4–0.5 Gy/hr for early stage lesions of the lower half of the nasal septum. A representative brachytherapy case is demonstrated in Figure 1. Three (4%) patients received preoperative EBRT (median dose 50 Gy, range 50–72 Gy), and 33 (49%) patients received postoperative EBRT (median dose 60 Gy, range 30–68 Gy). Thirteen patients (19%) received elective irradiation to bilateral facial and upper neck nodes, consisting of 50 Gy in 25 fractions at the discretion of the attending physician. All EBRT patients received conventional treatment on a Cobalt irradiator (31 pts treated prior to 1988) or linear accelerator, via an anterior appositional or wedge-pair field arrangement. Surgery consisted of wide total resection (with or without total rhinectomy) in 28 cases and limited resection in 8 cases. A total of 8 patients underwent neck dissection, of which 3 were for staging of clinically negative nodal basins. Statistical analysis was performed using Stata/SE 10.0 (StataCorp, College Station, TX). The endpoints of the study were local control (LC), regional control (RC), locoregional control, (LRC), disease specific survival (DSS), and overall survival (OS). These were defined from the time of index diagnosis and were estimated by the Kaplan-Meier method. Survival outcomes were analyzed for significance by the log rank test for disease and treatment-related variables, corrected by Bonferroni’s method for multiple comparisons (baseline threshold of significance of 0.05 divided by 21 univariate tests, yielding our accepted threshold of p = 0.002). We used multivariate Cox proportional hazard analysis to assess the effect of patient characteristics and other prognostic factors of significance on DSS, with estimated hazards also reported. The Wald test was used to assess the role of covariates in the model. As appropriate, the significance of differences in proportions were calculated with Chi-square testing and the significance of differences in means were calculated with a Student’s t test, using a threshold of 0.05.
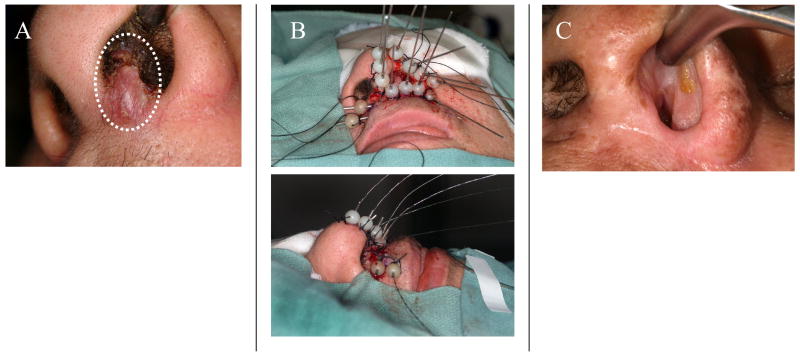
Figure 1.
A representative case of a 59 year-old female diagnosed with a T1N0 squamous cell carcinoma of the left nasal septum and columella treated with definitive interstitial brachytherapy. A) The patient presented with a firm 1.5 cm nodule in this location, without direct extension to the nasal ala. B) A total of 13 interstitial needles were placed intraoperatively; eleven 3.5–4.0 cm needles were placed along the septum, left nasal floor, columella, and left nasal ala, and two 4.0–5.0 cm crossing needles were placed in the moustache region. The needles were loaded with 192-Iridium on post-operative day 1, and 60 Gy was delivered to the tumor volume at a dose rate of 85 cGy/hour. C) Treatment results at four months follow-up. The patient remains disease free after 29 months follow-up.
Results
Survival
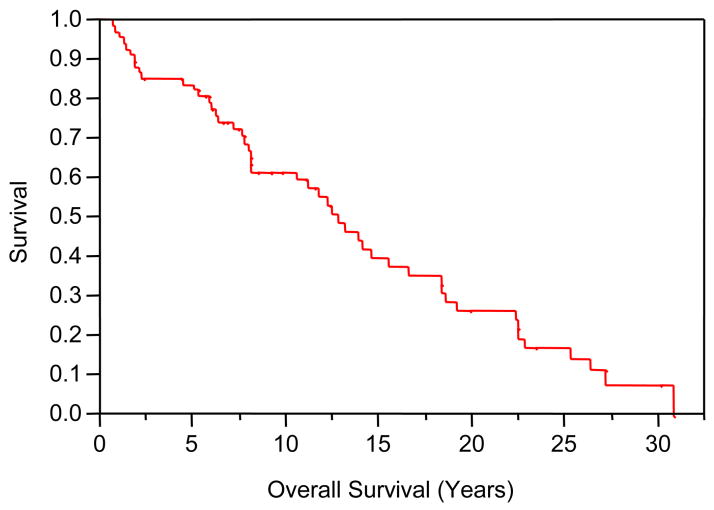
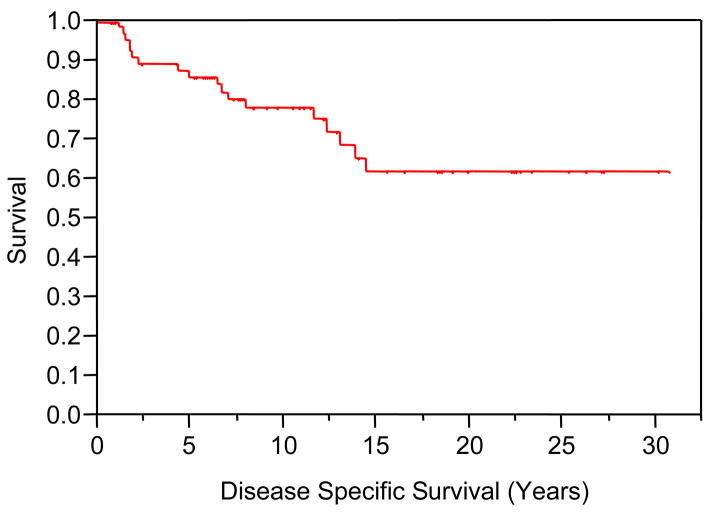
Median follow-up for the study cohort was 11.0 years (mean 8.1 years, range 2.4–30.1 years). At the time of last follow-up, 20 patients (30%) were alive with no evidence of recurrence. DSS at 5, 10 and 15 years post-treatment were 86%, 78%, and 62%, respectively. OS at 5, 10 and 15 years post-treatment were 82%, 62%, and 40%, respectively. Actuarial OS and DSS curves are shown in Figures 2 and 3. As summarized in Table 4, non-squamous histology, Parson’s stage, and AJCC stage were predictive for DSS on univariate log rank analysis. Non-squamous histology (HR = 0.23, 95% CI = 0.08–0.67, p = 0.007) and AJCC stage 3–4 (HR = 3.64, 95% CI = 1.3–10.14, p = 0.01) remained independent predictors for DSS on multivariate Cox regression analysis. Statistically significant univariate predictors were not seen for OS.
Figure 2.
Overall Survival
Figure 3.
Disease Specific Survival
Table 4.
Univariate predictors for LRC, DSS, and OS. Kaplan-Meier survival estimates were analyzed for significance by the log rank test for disease and treatment-related variables, corrected by Bonferroni’s method for multiple comparisons (baseline threshold of significance of 0.05/21 univariate tests, yielding an accepted threshold of p = 0.002, designated by *).
| Variable | 5 Yr Local- Regional Control | Log Rank p-value | 5 Yr Disease Specific Survival | Log Rank p-value | 5 Yr Overall Survival | Log Rank p-value |
|---|---|---|---|---|---|---|
| Treatment Time Period | ||||||
| Before/during 1985 | 80.3 | 0.87 | 86.1 | 0.05 | 83.8 | 0.04 |
| After 1985 | 75.5 | 85.9 | 83.8 | |||
| Tumor Site | ||||||
| Nasal Septum | 77.3 | 0.49 | 89.7 | 0.13 | 90.3 | 0.42 |
| Nasal Cavity | 79.2 | 82.9 | 78.4 | |||
| Histology | ||||||
| SCC | 79.8 | 0.17 | 88.6 | 0.0003* | 86.7 | 0.29 |
| Non-SCC | 74.2 | 81.0 | 78.3 | |||
| Dose to Primary | ||||||
| 60Gy Or Less | 72.9 | 0.28 | 78.0 | 0.10 | 75.6 | 0.78 |
| Greater than 60Gy | 82.6 | 93.8 | 91.4 | |||
| Treatment | ||||||
| RT Alone | 81.1 | 0.10 | 93.3 | 0.004 | 93.8 | 0.30 |
| Surgery/RT | 75.4 | 79.4 | 75.0 | |||
| Parsons’ Stage | ||||||
| Stage 1 | 78.7 | 0.15 | 96.9 | 0.002* | 93.9 | 0.77 |
| Stage 2–3 | 76.0 | 77.4 | 72.7 | |||
| AJCC Stage | ||||||
| T1-T2 | 78.0 | 0.94 | 95.0 | 0.001* | 92.7 | 0.01 |
| T3-T4 | 78.0 | 72.0 | 70.4 | |||
Patterns of Failure
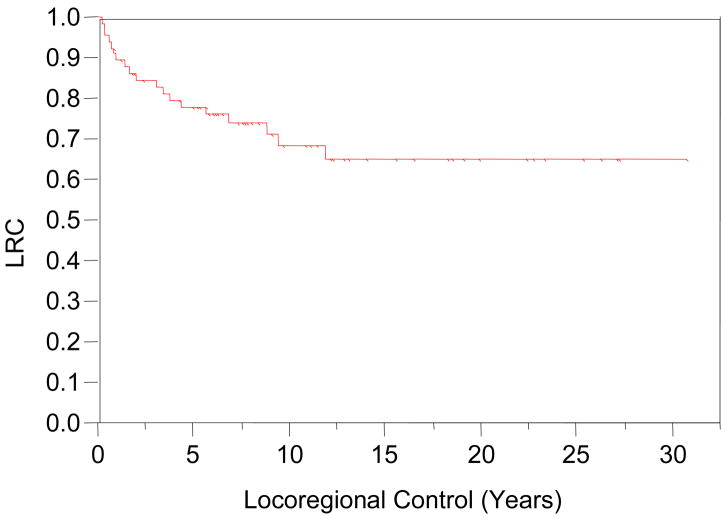
At last follow-up, 43 patients (63%) were without evidence of disease recurrence. LC at 5, 10 and 15 years post-treatment were 86%, 76%, and 72%, respectively. Fourteen (21%) patients suffered local disease relapse. One patient with adenocarcinoma histology recurred more than 10 years from completion of treatment; this recurrence presented in the identical location as the index lesion and was scored as a local failure. RC in the cervical neck at 5, 10 and 15 years post-treatment were 92%, 92%, and 92%, respectively. Neck recurrences occurred in five patients (7%) with a mean post-treatment interval of 16.8 months; range 5–37 months). Two of these five also failed at the primary site. Four cases had squamous histology, while the fifth was an adenoid cystic carcinoma. Three cases had neck dissection surgery at the time of diagnosis; none had prophylactic neck irradiation. Combined locoregional control (LRC) at 5, 10 and 15 years post-treatment were 78%, 69%, and 65%, respectively, as seen in Figure 4. Univariate analysis of clinical covariates did not yield statistically significant predictors for LRC (Table 4).
Figure 4.
Locoregional Disease Control
Post-Treatment Morbidity
Post-radiotherapy complications are summarized in Table 5. One or more minor late morbid events occurred in 37 patients, yielding a crude rate of 54%. Severe soft tissue complications were uncommon, developing in 3 (4.4%) patients. One patient required corrective surgery for localized bone necrosis. There was also one case of severe trismus and one case of soft tissue necrosis, both managed conservatively. Symptomatic visual impairment occurred in 5 patients (bilaterally in 1 patient), but no cases of blindness were observed. The appearance of severe complications was not associated with disease related factors, or with the timing, dose, or year (pre/post-1985) of radiation treatment. Other mild to moderate complications most frequently consisted of epiphora (10 patients, 15%), epistaxis (7 patients, 10%), or dental problems (5 patients, 7%).
Table 5.
Post-treatment complications
| Complication | N (%) |
|---|---|
| Epiphora | 12 (18) |
| Epistaxis | 7 (10) |
| Dental problems | 5 (7) |
| Visual impairment | 5 (7) |
| Xerostomia | 4 (6) |
| Otitis media | 4 (6) |
| Nasal stenosis | 3 (4) |
| Sinusitis | 2 (3) |
| Ectropion | 2 (3) |
| Loss of smell | 2 (3) |
| Seizure disorder | 1 (<2) |
| Hypothyroidism | 1 (<2) |
| Corneal damage | 1 (<2) |
| Hearing loss | 1 (<2) |
| Facial cellulites | 1 (<2) |
| Bone necrosis | 1 (<2) |
| Soft tissue necrosis | 1 (<2) |
| Trismus | 1 (<2) |
Discussion
Historically, cancer of the nasal cavity has been associated with poor outcomes as a consequence of being grouped with disease of the paranasal sinuses5–7. Our initial analysis4 of institutional results specific to carcinoma of nasal cavity and septum published in 1992 confirmed 83% disease specific survival and 75% overall survival at 5 years, representing significantly better results relative to contemporaneously treated maxillary sinus cancers. Our updated results in this report remain comparable: local relapse free survival were 86% and 76%, disease specific survival were 86% and 78%, and overall survival were 82% and 62% at 5 and 10 years, respectively. Clinical results for septal and cavity cases were not significantly different from one another in this current update.
Prior to the formulation of formal AJCC staging criteria in 2001, nasal cavity cancer was non-uniformly staged across institutions according to several available clinical systems. Our institution utilized the Parsons’/University of Florida system3, which categorizes disease according to degree of local involvement: stage 1—limited to site of origin, stage 2—extension to adjacent sites (e.g. orbit, nasopharynx, paranasal sinuses, skin, pterygomaxillary fossa), and stage 3—invasion of skull base, pterygoid plate destruction, and/or intracranial extension. In contrast, the AJCC T staging system uses more refined criteria to triage cases into 5 categories (T1-T4b) based upon relative degree of surgical resectability. Parsons’ clinical stages 1 and 3 fall strictly within criteria for AJCC T stages 1–2 and 4, respectively However, Parsons’ clinical stage 2 represents a spectrum of intermediate to advanced AJCC T stage presentations, ranging from stage T2 through T4a. The majority of our Parsons’ clinical stage 2 cases migrated towards AJCC T stages T3 and T4 (Table 2). We should note that restaging of cases from our series to the AJCC system did not improve predictive value for treatment outcomes on univariate analysis (Table 4), most likely due to the small sample size. However, back-staging cases according to the AJCC system will assist with future comparisons between modern series and mature longitudinal data from older study cohorts, such as ours.
Disease failure in the neck was relatively uncommon in our series, occurring in 5 (7%) of cases. All of these patients did not receive elective neck irradiation, although two patients had negative elective neck dissections. These findings are consistent with prior reports of nasal cavity and septal cancer outcomes4, 8–17, which quote crude failure rates in untreated necks ranging from as low as 0–5%, up to approximately 15–25%. Prior series8–10 specifically addressing septal disease suggest that neck failure rates may be as high as 20–54% for this subsite, higher than the 10–15% typically seen for nasal cavity disease. However, these are very small series, ranging from 11 to 15 patients in size, and there remains no definitive data to guide elective nodal treatment for either subsite. Taken together, due to the low inherent risk of occult disease spread to regional lymphatics in early stage cages, our current practice is to reserve elective nodal treatment for locally advanced (T3-4) primary disease involving nasopharynx or skin, as well as for post-surgical recurrences. Fortunately, elective treatment does appear to be effective. None of the 13 (19%) patients our current series treated with elective facial and neck irradiation for such indications suffered a neck failure.
Treatment related morbidity was relatively limited in this series, with most complications requiring only conservative management (Table 5). Severe soft tissue complications developed in only three (4.4%) patients. Visual impairment or loss has been reported as a frequent complication in prior experiences, especially in cases where disease directly encroaches one or both orbits3. In our series, symptomatic visual impairment in one or both eyes occurred in five (7%) patients. We observed no cases of unilateral or bilateral blindness. A contributing factor to this has been our longstanding institutional policy to aggressively pursue surgical resection for Parsons’ clinical stage 2 cases. This permits reduced pre or post-operative doses in many cases, leading to lower incidental doses to critical optic pathway structures.
In summary, this report confirms durable long-term locoregional control and survival outcomes for carcinoma of the nasal cavity and septum with the use of definitive or adjuvant radiotherapy. It is important to note that these results are with the use of conventionally administered EBRT via either a telecobalt unit or linear accelerator. We now treat essentially all patients with intensity modulated radiotherapy (IMRT) technique. This permits aggressive dose delivery of up to 70 Gy while providing markedly improved dose sparing of neighboring structures, including orbit and optic pathways18. Improved disease control and treatment tolerance is therefore expected with IMRT. Our current treatment policy is as follows: small accessible lesions of the nasal septum are managed with either definitive interstitial brachytherapy alone to a dose of 60–65 Gy or with definitive external beam treatment to a dose of 50 Gy, followed by an interstitial brachytherapy boost of 15–20 Gy. Other AJCC T stage 1 and 2 patients can be treated with definitive resection followed by adjuvant external beam radiation for adverse pathologic features to a dose of 56–66 Gy, or with definitive external beam treatment alone to 66–70 Gy. More advanced AJCC T stage 3 and 4 are typically managed surgically, followed by adjuvant external beam radiation for adverse pathologic features to a dose of at least 56 Gy, and up to 70 Gy for residual gross disease. Systemic therapy is not routinely employed, although induction platinum-based chemotherapy is considered on a case-by-case basis in situations of tenuously resectable or unresectable stage 4a or 4b squamous cell disease. As mentioned above, we currently reserve elective nodal treatment for locally advanced (T3-4) primary disease involving nasopharynx or skin, as well as for recurrent disease. Future reports will focus on comparing our long-term conventional treatment outcomes with those obtained with current IMRT techniques.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang CC. Treatment of carcinoma of the nasal vestibule by irradiation. Cancer. 1976;38:100–106. doi: 10.1002/1097-0142(197607)38:1<100::aid-cncr2820380118>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins RB, Wynstra JH, Pilepich MV, et al. Carcinoma of the nasal cavity--results of primary and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1988;15:1129–1133. doi: 10.1016/0360-3016(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JT, Mendenhall WM, Mancuso AA, et al. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys. 1988;14:11–22. doi: 10.1016/0360-3016(88)90044-2. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Jiang GL, Frankenthaler RA, et al. Carcinomas of the nasal cavity. Radiother Oncol. 1992;24:163–168. doi: 10.1016/0167-8140(92)90375-5. [DOI] [PubMed] [Google Scholar]
- 5.Frazell EL, Lewis JS. Cancer of the Nasal Cavity and Accessory Sinuses. A Report of the Management of 416 Patients. Cancer. 1963;16:1293–1301. doi: 10.1002/1097-0142(196310)16:10<1293::aid-cncr2820161010>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Leffall LD, Jr, White JE. Cancer of the nasal cavity and paranasal sinuses. Am J Surg. 1966;112:436–438. doi: 10.1016/0002-9610(66)90217-0. [DOI] [PubMed] [Google Scholar]
- 7.Oliver P. Cancer of the nose and paranasal sinuses. Surg Clin North Am. 1967;47:595–600. doi: 10.1016/s0039-6109(16)38234-2. [DOI] [PubMed] [Google Scholar]
- 8.DiLeo MD, Miller RH, Rice JC, et al. Nasal septal squamous cell carcinoma: a chart review and meta-analysis. Laryngoscope. 1996;106:1218–1222. doi: 10.1097/00005537-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Fradis M, Podoshin L, Gertner R, et al. Squamous cell carcinoma of the nasal septum mucosa. Ear Nose Throat J. 1993;72:217–221. [PubMed] [Google Scholar]
- 10.LeLiever WC, Bailey BJ, Griffiths C. Carcinoma of the nasal septum. Arch Otolaryngol. 1984;110:748–751. doi: 10.1001/archotol.1984.00800370050012. [DOI] [PubMed] [Google Scholar]
- 11.Shidnia H, Hartsough AB, Weisberger E, et al. Epithelial carcinoma of the nasal fossa. Laryngoscope. 1987;97:717–723. doi: 10.1288/00005537-198706000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Dulguerov P, Jacobsen MS, Allal AS, et al. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92:3012–3029. doi: 10.1002/1097-0142(20011215)92:12<3012::aid-cncr10131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Logue JP, Slevin NJ. Carcinoma of the nasal cavity and paranasal sinuses: an analysis of radical radiotherapy. Clin Oncol (R Coll Radiol) 1991;3:84–89. doi: 10.1016/s0936-6555(05)81169-0. [DOI] [PubMed] [Google Scholar]
- 14.Katz TS, Mendenhall WM, Morris CG, et al. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24:821–829. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]
- 15.Harbo G, Grau C, Bundgaard T, et al. Cancer of the nasal cavity and paranasal sinuses. A clinico-pathological study of 277 patients. Acta Oncol. 1997;36:45–50. doi: 10.3109/02841869709100731. [DOI] [PubMed] [Google Scholar]
- 16.Olmi P, Cellai E, Chiavacci A, et al. Paranasal sinuses and nasal cavity cancer: different radiotherapeutic options, results and late damages. Tumori. 1986;72:589–595. doi: 10.1177/030089168607200609. [DOI] [PubMed] [Google Scholar]
- 17.Chung CT, Rabuzzi DD, Sagerman RH, et al. Radiotherapy for carcinoma of the nasal cavity. Arch Otolaryngol. 1980;106:763–766. doi: 10.1001/archotol.1980.00790360041011. [DOI] [PubMed] [Google Scholar]
- 18.Pacholke HD, Amdur RJ, Louis DA, et al. The role of intensity modulated radiation therapy for favorable stage tumor of the nasal cavity or ethmoid sinus. Am J Clin Oncol. 2005;28:474–478. doi: 10.1097/01.coc.0000182600.51019.de. [DOI] [PubMed] [Google Scholar]