Abstract
Recent findings indicate that progesterone can attenuate beneficial neural effects of oestrogen. Here, we investigate the hypothesis that progesterone can modulate oestrogen actions by regulating expression and activity of oestrogen receptors, ERα and ERβ. Our studies in cultured neurones demonstrate that progesterone decreases the expression of both ERα and ERβ and, as a consequence, also reduces both ER-dependent transcriptional activity and neuroprotection. These results identify a potential mechanism by which progesterone antagonises neural oestrogen actions, a finding that may have important implications for hormone therapy in postmenopausal women.
Keywords: oestrogen, oestrogen receptors, hormone therapy, neuroprotection, progesterone
The depletion of oestrogen and progesterone in postmenopausal women is associated with increased risk for several disorders in the cardiovascular, skeletal and nervous systems (1). For example, the Women’s Health Initiative clinical trial showed that HT use was associated with reduced incidence of hip fractures but, unexpectedly, increased incidences of both stroke and dementia (2). The disparities between basic research studies that demonstrate neuroprotective effects of oestrogen and recent clinical findings that report adverse neural effects of HT indicate the need for a more complete understanding of oestrogen and progesterone interactions in brain and other tissues. To gain some mechanistic insight into this issue, we studied the effects of progesterone on oestrogen actions in cultured neurones.
One important issue that is not well understood is how neural effects of oestrogen are affected by progestagens. Recent experimental evidence in rodent models shows that prolonged progesterone (P4) exposure often represses beneficial 17β-oestradiol (E2) function in the brain (3-9). The mechanism(s) by which P4 inhibits E2 action in the brain is unclear. Here, we investigate the possibility that P4 modulates E2 action by regulating expression of oestrogen receptors (ERs). We demonstrate that P4 treatment reduces the expression of both ERα and ERβ in cultured neurones in a concentration- and time-dependent manner. We also show that this decrease in ER expression leads to attenuation of E2 activity in the neurones.
We investigated P4 regulation of ER expression and E2 neuroprotection using an established neurone culture paradigm. Experimental animal procedures were conducted in accordance with the University of Southern California guidelines based on National Institute of Health standards. Primary rat cerebrocortical cultures (∼95% neuronal) were generated from gestational day 16-17 rat pups using a previously described protocol (10) with some modifications. Cultures were seeded in multiwell plates at final densities of approximately 2.5 × 104 cells/cm2 (cell viability and luciferase assays) or 8 × 105 cells/cm2 (RNA isolation) and experiments were started 1-2 days after plating. In all experiments, both E2 (Steraloids Inc.; Newport, RI) and P4 (Acros Organics USA; Morris Plains, NJ) were dissolved in ethanol and diluted to required concentrations in culture medium. Cells were harvested for RNA isolation using TRIzol reagent (Invitrogen Corporation; Carlsbad, CA) according to manufacturer’s protocol. ER mRNA levels were analysed using qualitative and quantitative PCR. Following real-time PCR, the relative quantification of mRNA levels from various treated samples was determined by the comparative Ct method (also known as ΔΔCt method) (11).
To assess E transcriptional activity, cultures were co-transfected with a luciferase reporter plasmid containing an upstream oestrogen response element (ERE-luc) in the promoter region (12) (kind gift from Dr. Donald McDonnell, Duke University) and an internal control renilla luciferase expression plasmid (pRL) (Promega; Madison, WI) using Amaxa Nucleofector system (Amaxa; Gaithersburg, MD) and treated with different hormone conditions starting at 4-6 h post-transfection. To assess E2 neuroprotection, cultures were pretreated with different hormone conditions and then exposed to Apoptosis Activator II (AAII), a cell-permeable cytochrome c-dependent caspase activator (13) (Calbiochem; San Diego, CA). Cell viability was determined by counts of viable cells stained with the vital dye calcein AM (Invitrogen) as previously described (10). Raw data were statistically assessed by ANOVA followed by between group comparisons using the Fisher LSD test.
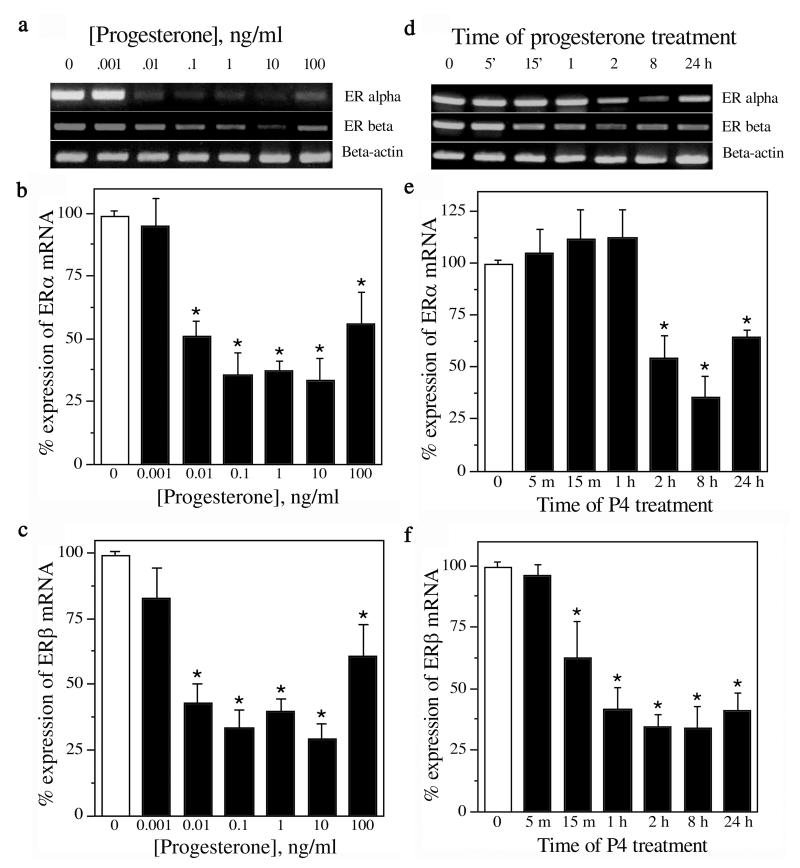
To investigate the effect of P4 on ER expression, cultures were treated with increasing concentrations of P4 (0-100 ng/ml) for 24 h. The cells were harvested for RNA isolation, followed by qualitative and quantitative RT-PCR. Our results show that mRNA expression of both ERα and ERβ was decreased by P4 at 0.01-100 ng/mL (p < 0.0001) (Fig. 1a-c; n=5). The 10 ng/mL (∼30 nM) P4 concentration was chosen for subsequent experiments as it was maximally effective and within the normal physiological range (8 - 50 ng/ml). Next, cultures were treated with 10ng/mL P4 for increasing durations ranging from 5 min to 24 h after which RNA was isolated and quantitatively assessed for ER expression. We found that P4 treatment significantly decreased expression of ERα mRNA by 2 h (p < 0.001) and ERβ mRNA within 1 h (p < 0.0001) (Fig. 1d-f; n=3).
Figure 1.
Progesterone (P4) decreases the expression of ERα and ERβ mRNA in a concentration- and time-dependent manner. Representative agarose gels of RT-PCR products qualitatively show relative changes in mRNA levels of ERα and ERβ induced by 24 h exposure to 0-100 ng/mL (∼0-300 nM) P4 (a) and 0-24 h exposure to 10 ng/ml (∼30 nM) P4 (d); β-actin was used as an internal control. The relative levels of ERα and ERβ mRNA after treatment with various P4 concentrations (b, c; n=5) and for various time points (e, f; n=3) were determined quantitatively using real-time PCR. Data show mean (±SEM) expression levels, relative to vehicle-treated controls, as determined by Ct values (cycle number at which the logarithmic fluorescence crosses the threshold) of ERα and ERβ normalised with corresponding Ct values of β-actin. * Denotes p ≤ 0.01 relative to corresponding vehicle-treated control group. The primer sets used were: ERα- F : 5 ′-CATCGATAAGAACCGGAG-3 ′ a n d R : 5 ′-AAGGTTGGCAGCTCTCAT-3′; ERβ - F: 5′-AAAGTAGCCGGAAGCTGA-3′ and R: 5′-CTCCAGCAGCAGGTCATA-3′; β-actin — F: 5′-AGCCATGTACGTAGCCATCC-3′ and R: 5′-CTCTCAGCTGTGGTGGTGAA-3′.
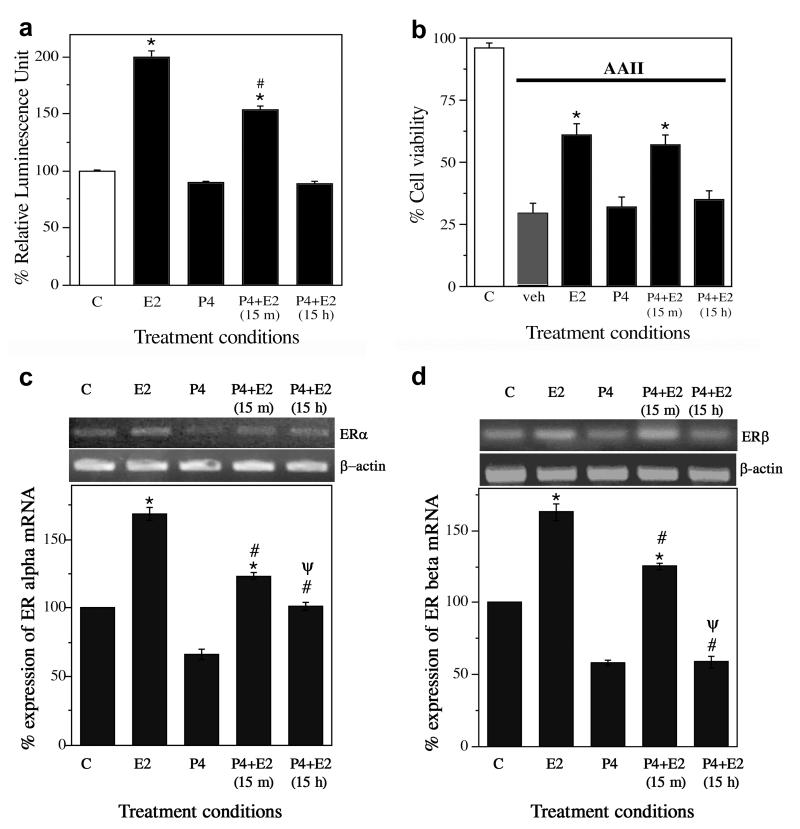
These data show that P4 decreases expression of both ERα and ERβ in a concentration- and time-dependent manner. To determine whether the P4-induced decrease in ER expression reduces ER activity, we examined the ability of E2 to activate ERE-dependent transcription, a measure of classic E2 genomic activity, by transfecting cultures with an ERE-reporter construct (ERE-luc) and measuring how P4 treatment affected E2-induced expression of luciferase. P4 alone treatment was for 15 h followed by 8 h of vehicle treatment and did not have any effect on the luciferase activity. We observed that 8 h exposure to 10 nM E2 resulted in an approximately two-fold increase in luciferase levels (p < 0.0001). This E2-induced increase in luciferase was modestly reduced by short-term 15 min pretreatment with 10 ng/mL P4 and completely blocked by long-term 15 h P4 pretreatment (p < 0.0001) (Fig. 2a; n=3). To investigate whether P4 also attenuates E2-mediated neuroprotection, cultures were pretreated with 10 ng/mL P4 for either 15 min or 15 h, followed by 10 nM E2 for 1 h, and finally 24 h exposure to a toxic concentration (3 μM) of the apoptosis-inducing peptide AAII. For the P4 alone condition, cells were treated with 10 ng/ml P4 for 15 h followed by 1 h vehicle treatment before administering AAII. We observed that E2 alone but not P4 alone significantly reduced neurone loss induced by AAII (p < 0.0001). E2 neuroprotection was not significantly affected by 15 min P4 pretreatment (p = 0.67) but was completely blocked by 15 h P4 pretreatment (p < 0.0001) (Fig. 2b; n=5). Finally, because these experiments were conducted with both P4 and E2, we also considered how P4 affects ER expression in the presence of E2. Thus, we measured mRNA levels of ERα and ERβ under the same treatment parameters used in the E2 genomic activity experiment (Fig. 2a). We found that 8 h treatment with E2 alone significantly increased expression of both ERα (p < 0.0001) and ERβ (p < 0.0001) relative to vehicle control, effects that were reduced by 15 min P4 pretreatment (ERα p < 0.0001; ERβ p < 0.0001) and to a significantly greater extent by 15 h P4 pretreatment (ERα p < 0.001; ERβ p < 0.0001) (Fig. 2c, d; n=3).
Figure 2.
Progesterone (P4) reduces oestrogen-induced increases in ER activity and neurone survival. (a) ER activity was determined by luciferase assay of neuronal cultures transfected with ERE-luc then treated with vehicle (C), 10 nM E2 (E2), 10 ng/mL (∼30 nM) (P4), or E2 with P4 pretreatment (P4+E2) for either 15 min or 15 h. Data show mean luciferase activity (±SEM) represented as relative luminescence unit and expressed as a percentage of vehicle-treated control condition. * Denotes p < 0.01 relative to vehicle-treated control (C, open bar) and # indicates p < 0.01 relative to E2 group. n=3. (b) Neurone survival was measured in cultures pretreated with 0 or 10 ng/mL P4 for 15 min or 15 h, followed by addition of 0 or 10 nM E2, and finally 24 h exposure to 3 μM apoptosis inducing peptide II (AAII). Cell viability data show average mean cell counts of viable cells (+SEM) expressed as percentage of vehicle-treated control group. * Denotes p < 0.01 relative to vehicle + AAII condition (veh, solid bar). n=5. (c, d) Levels of ERα and ERβ mRNA were determined under the same treatment conditions used in the luciferase assay. Data show representative agarose gels of RT-PCR products (upper panels) and mean (±SEM) mRNA levels determined by quantitative RT-PCR (lower panels). * Denotes increased expression (p < 0.0001) as compared to the vehicle control (C); # Denotes decreased expression (p < 0.0001) as compared to the E2 condition (E2); Ψ Denotes decreased expression (p < 0.001) as compared to the 15 m P4 pretreatment group [P4+E2(15 m)]. n=3.
In this study, we investigated P4 regulation of ER expression and E2 neuroprotection. Our results in neurone-enriched cultures demonstrate that physiologically relevant concentrations of P4 induce a profound and prolonged decrease in expression of both ERα and ERβ transcripts. The potential role of classical PR in mediating this effect is unclear. Although the lowest effective P4 concentration 0.01 ng/mL (∼ 0.03 nM) is below the Kd value of P4-PR interaction (∼1x10-9 M), similarly low P4 concentrations can exert progestagenic actions (14, 15) and high affinity P4 binding sites have been described (16, 17). The observed P4-induced downregulation of ER expression is associated with corresponding decreases in E2-induced transcriptional activity and neuroprotection against apoptosis. Our data demonstrate that one neural consequence of P4 exposure is modulation of E2 activity via regulation of ER levels.
Our findings are consistent with an accumulating set of observations that P4 can inhibit beneficial E2 actions in brain. Continuous P4 exposure maintained over weeks to months in ovariectomised female rodents has been observed to attenuate several E2-induced actions, including neuroprotection from kainate lesion (8), reduction in the Alzheimer’s disease-related protein β-amyloid (9), and increased expression of the neurotrophins brain-derived neurotrophic factor, nerve-growth factor, and neurotrophin 3 (3). Our data show that P4 decreases ER expression within hours, suggesting that even relatively short term P4 exposure may attenuate E2 actions in vivo. In agreement with this prediction, acute P4 blocks E2-induced upregulation of the anti-apoptotic protein Bcl-2 in ovariectomised female rats (4). Interestingly, E2-induced increase in hippocampal spine density in female rats is potentiated 4 h following P4 treatment but blocked after 18 h P4 treatment (7). Thus, P4 may exert independent protective effects and or initially potentiate protective E2 actions but with prolonged exposure P4 can attenuate E2 action via ER downregulation. This interpretation is consistent with observations that although P4 can be neuroprotective (18), both P4 (8) and synthetic progestagens (18, 8) can also block E2 neuroprotection. Although P4 was not neuroprotective in our paradigm, we suggest that P4 has neural benefits both directly and interactively with E2 (19) that may be optimised by delivery in a cyclic manner (which parallels natural fluctuations) rather than continuously. In one of the few studies to investigate this important issue, Gibbs found that activity of choline acetyltransferase — the enzyme catalysing biosynthesis of the neurotransmitter acetylcholine — in ovariectomised female rats is modestly increased by E2, significantly elevated by E2 paired with a cyclic P4 regimen but reduced by E2 with continuous P4 exposure (5).
In summary, our findings suggest a novel mechanism by which P4 can affect oestrogen actions: downregulation of ER expression. Additional research is needed not only to further define this progestagen-oestrogen interaction, but also to evaluate its potential involvement in regulating neural hormone actions in intact brain. Should these findings extrapolate to humans, they would have significant implications for the design of hormone therapy in postmenopausal women.
Acknowledgements
This research was funded by NIH grant AG026572.
References
- 1.LaCroix AZ. Estrogen with and without progestin: benefits and risks of short-term use. Am J Med. 2005;118(Suppl 12B):79–87. doi: 10.1016/j.amjmed.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, et al. Novel Perspectives for progesterone in Hormone Replacement Therapy, with special reference to the nervous system. Endocrine Reviews. 2007;28:387–439. doi: 10.1210/er.2006-0050. [DOI] [PubMed] [Google Scholar]
- 3.Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroreport. 1998;9:593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- 6.Murphy DD, Segal M. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology. 2000;72:133–143. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- 7.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 8.Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- 9.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordey M, Gundimeda U, Gopalakrishna R, Pike CJ. Estrogen activates protein kinase C in neurons: role in neuroprotection. J Neurochem. 2003;84:1340–1348. doi: 10.1046/j.1471-4159.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Hall JM, McDonnell DP, Korach KS. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol Endocrinol. 2002;16:469–486. doi: 10.1210/mend.16.3.0814. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen JT, Wells JA. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc Natl Acad Sci U S A. 2003;100:7533–7538. doi: 10.1073/pnas.1031631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallejo G, Ballare C, Baranao JL, Beato M, Saragueta P. Progestin activation of nongenomic pathways via cross talk of progesterone receptor with estrogen receptor beta induces proliferation of endometrial stromal cells. Mol Endocrinol. 2005;19:3023–3037. doi: 10.1210/me.2005-0016. [DOI] [PubMed] [Google Scholar]
- 15.Teves ME, Barbano F, Guidobaldi HA, Sanchez R, Miska W, Giojalas LC. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil Steril. 2006;86:745–749. doi: 10.1016/j.fertnstert.2006.02.080. [DOI] [PubMed] [Google Scholar]
- 16.Luconi M, Bonaccorsi L, Maggi M, Pecchioli P, Krausz C, Forti G, Baldi E. Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane. J Clin Endocrinol Metab. 1998;83:877–885. doi: 10.1210/jcem.83.3.4672. [DOI] [PubMed] [Google Scholar]
- 17.Helguero LA, Lamb C, Molinolo AA, Lanari C. Evidence for two progesterone receptor binding sites in murine mammary carcinomas. J Steroid Biochem Mol Biol. 2003;84:9–14. doi: 10.1016/s0960-0760(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 19.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, et al. Progesterone Receptors: Form and function in Brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]