Abstract
Previous studies have suggested that physical activity may lower lung cancer risk. The association of physical activity with reduced chronic inflammation provides a potential mechanism, yet few studies have directly related inflammatory markers to cancer incidence. The relation between physical activity, inflammation, and lung cancer risk was evaluated in a prospective cohort of 4,831 subjects, 43–86 years of age, in Beaver Dam, Wisconsin. A total physical activity index was created by summing kilocalories per week from sweat-inducing physical activities, city blocks walked, and flights of stairs climbed. Two inflammatory markers, white blood cell count and serum albumin, were measured at the baseline examination. During an average of 12.8 years of follow-up, 134 incident cases of lung cancer were diagnosed. After multivariable adjustment, participants in the highest tertile of total physical activity index had a 45% reduction in lung cancer risk compared to those in the lowest tertile (OR=0.55; 95% CI: 0.35–0.86). Participants with white blood cell counts in the upper tertile (≥8×103/μL) were 2.81 (95% CI: 1.58–5.01) times as likely to develop lung cancer as those with counts in the lowest tertile (<6.4×103/μL). Serum albumin was not related to lung cancer risk. There was no evidence that inflammation mediated the association between physical activity and lung cancer risk, as the physical activity risk estimates were essentially unchanged after adjustment for white blood cell count. While the potential for residual confounding by smoking could not be eliminated, these data suggest that physical activity and white blood cell count are independent risk factors for lung cancer.
Keywords: exercise, motor activity, inflammation mediators, leukocytes, lung neoplasms
INTRODUCTION
Lung cancer is the leading cause of cancer death among men and women in the United States (1). Strategies to reduce lung cancer risk besides smoking prevention and cessation are poorly understood. A number of epidemiologic studies have suggested that physical activity may reduce the risk of lung cancer (2–13), with a recent meta-analysis concluding that higher levels of leisure-time physical activity protect against lung cancer (14). However, the International Agency for Research on Cancer concluded in 2002 that the evidence for an association between physical activity and lung cancer remained inconclusive, and two large studies recently found no consistent association between physical activity and lung cancer risk (15, 16).
The value of molecular biomarkers in discerning the relation between physical activity and cancer has recently been recognized (17, 18). The incorporation of biomarkers can be particularly helpful in clarifying inconclusive epidemiologic evidence and investigating potential mechanisms by which physical activity exerts its effects (17). A number of potential mechanisms through which physical activity may offer protection from lung cancer have been proposed. Physical activity and physical fitness are consistently observed to be associated with reduced chronic inflammation, reflected in lower levels of the inflammatory markers serum C-reactive protein, fibrinogen and white blood cell count, and increased levels of serum albumin (a negative acute phase protein) (19–23). Chronic inflammation has been hypothesized to be a risk factor for a wide range of cancers (24–26). Thus, physical activity could reduce lung cancer risk by reducing chronic inflammation. Yet few studies have directly evaluated markers of inflammation in relation to lung cancer incidence (27–30).
We investigated the relation between self-reported physical activity and lung cancer in an established cohort of older adults. Additionally, we measured two inflammatory markers, white blood cell count and serum albumin, in baseline blood samples to evaluate whether inflammation mediates the relation between physical activity and lung cancer.
MATERIAL AND METHODS
Study population
Descriptions of the population and the methods used to identify the population have been previously published (31–33). Briefly, a private census of the population living in Beaver Dam, Wisconsin, was performed by the University of Wisconsin Extension-Survey Research Laboratory between September 15, 1987 and May 4, 1988. Eligibility requirements for entry into the study included living in the city or township of Beaver Dam and being 43 to 84 years of age at the time of the census. A total of 5,925 eligible individuals were identified who met the criteria.
Of the 5,925 eligible individuals, 4,926 (83.1%) participated in the study examination including 2,166 men and 2,760 women. Reasons for non-participation included 225 deaths (3.8%) before the examination, 91 people (1.5%) moved out of the area, 23 people (0.4%) could not be located, and 391 (6.6%) refused to participate. Eligible participants who completed telephone interviews but were not examined (N=269; 4.5%) are not included in this analysis, so that data were available for 4,926 participants who consented to examinations.
Case identification
Incident cases of lung cancer (International Classification of Diseases for Oncology codes C34.0-34.9; (34)) diagnosed in study participants through July 2004 were identified through linkages with the Wisconsin Cancer Reporting System (the statewide mandatory tumor registry), Wisconsin death certificates, and the National Death Index. Deaths due to lung cancer identified through death records that were not also identified by the tumor registry (N=7) were assigned a date of diagnosis equal to the average length of time from diagnosis to death for lung cancer cases in the Wisconsin tumor registry subtracted from their date of death (13 months).
Data collection
All participants provided signed informed consent at the time of the examination. Study questionnaires elicited information on comorbidities, reproductive and menstrual histories (for females), lifestyle factors, health history, medication histories, and demographics. Lifestyle factors on the questionnaires included physical activity, alcohol and caffeinated beverage consumption, smoking history, vitamin and mineral supplement use, and occupational history. Participants reported histories of diagnosis with major chronic medical conditions and surgical history. Collected demographic information included race/ethnicity and education, and participants were asked to report their marital status and income category.
To assess smoking history, subjects were asked if they had smoked more than 100 cigarettes in their lifetime, how many years they have smoked cigarettes, whether they smoke now, how long ago they stopped, and how many cigarettes they smoked per day (currently, or “usually” during smoking history for former smokers).
To assess physical activity, subjects were asked to report the number of city blocks walked per day (12 blocks = 1 mile), flights of stairs climbed per day, and the number of episodes of “regular activity long enough to work up a sweat” each week (35). A summary measure of total physical activity was created by summing the kilocalories (kcal) per week from blocks walked, flights of stairs climbed, and episodes of sweat-inducing activities. For one block walked per day and one flight of stairs climbed per day, we assigned 56 kcal/week and 28 kcal/week, as previously used in analyses of the Harvard Alumni Health Study (5, 6, 36). Duration and intensity of participation in sweat-inducing activities was not ascertained; a typical duration of 30 minutes at a multiple of resting metabolic rate (MET score) of seven was assumed (equivalent to jogging or tennis) (37). Given a resting metabolic rate of 1 kcal/kg/hr and the median subject weight of 76 kg, each sweat-inducing activity episode per week was assigned 266 kcal (= 7 × [1 kcal/kg/hr] × [76 kg] × [0.5 hr]).
Objective measures of comorbidity were collected in addition to self-reported chronic health conditions. Standardized procedures were used to measure height, weight, heart rate, vision, hearing, and blood pressure during the examination (31).
Laboratory analysis
Casual venous blood specimens were obtained at the baseline examination for laboratory analysis. The collection, storage, and laboratory methods for the analysis of serum inflammatory markers have been previously described (38). Immediately after obtaining the baseline blood sample, white blood cell count was determined using a Coulter counter method, and serum albumin levels were determined by Technicon, Inc (Tarrytown, NY).
Statistical analysis
Cox proportional hazards regression was used to estimate the hazard ratio (HR) and 95% confidence intervals (CI) of lung cancer associated with levels of physical activity and inflammatory markers. We tested proportionality assumptions and found no evidence of violation. Regression models were fit according to the number of episodes of sweat-inducing activities, the number of blocks walked, the number of flights of stairs climbed, total physical activity index, heart rate, white blood cell count, and serum albumin level. With the exception of heart rate, the physical activity and inflammatory marker variables were categorized roughly by person-year tertiles, using round numbers as cutpoints. For sweat-inducing activities and city blocks walked per week, more than one-third of person-years had zero activities. All models were adjusted for age (<50, 50–59, 60–69, 70–79, ≥ 80 years), sex, pack-years of smoking (none, tertiles), time since smoking cessation (never smoker, current smoker, quartiles), body mass index (kg/m2, tertiles), alcohol intake (none, <5 drinks per week, ≥ 5 drinks per week), and education (<high school, high school degree, > high school). P-values for trend were evaluated by including categorical variables in the models as continuous linear terms. Age and other covariates were assessed as effect modifiers of the association between physical activity and lung cancer by evaluating the change in the log-likelihood after including their cross-product terms in the regression models. In analyses stratified by smoking history, subjects were considered current smokers if they responded affirmatively to the questionnaire item “Do you smoke now?” and former smokers if they responded negatively but had smoked more than 100 cigarettes in their lifetime. Never and former smokers were combined in the stratified analysis because of insufficient numbers of each separately. Plots of cumulative lung cancer incidence according to the total physical activity index and white blood cell count were produced using the Kaplan-Meier method.
Least squared means and P-values comparing white blood cell count and serum albumin according to tertiles of total physical activity index were calculated using multivariable analysis of variance including covariates for smoking history. Mean levels of white blood cell count and serum albumin at baseline among participants who subsequently developed lung cancer were compared with levels corresponding to participants without lung cancer during the follow-up period using t-tests. Values of albumin and white blood cell count were not transformed for the t-tests since they were approximately normally distributed. P-values using Wilcoxon nonparametric tests were essentially identical to those obtained using t-tests and are not shown.
Study participants reporting a personal history of lung cancer at the baseline examination (N=7), or identified as a case of lung cancer within 12 months of the baseline examination (N=6), were excluded from analysis. An additional 82 people who died within 12 months of their baseline examination are also excluded from this analysis.
RESULTS
During 62,062 person-years of follow-up (an average of 12.8 years per person), 134 cases of lung cancer were diagnosed among the 4,831 subjects without a personal history of lung cancer who survived at least one year after the baseline examination. Among cases, the mean time between baseline examination and diagnosis was 8.0 years (standard deviation: 3.8, range: 1.2–16.3 years). According to tumor registry reports, 51% of the cases were non-smallcell type (23% adenocarcinoma, 14% squamous, 10% large cell, and 4% not otherwise specified), 12% were small cell type, and 37% were unspecified cell types. Of the cases, 23 (17%) were local, 27 (20%) were regional, 45 (34%) were distant and 39 (29%) were unknown stage at diagnosis.
Physical activity variables are summarized according to other selected covariates in Table 1. In general, participants who were more active tended to be younger, have lower body mass, drink more alcohol, and report more years of education than less active participants. The distribution of participants according to smoking status within levels of physical activity depended upon the type of activity: current smokers were less likely to report vigorous activities that caused a sweat but more likely to climb stairs. Participants who were more active had lower heart rates and white blood cell counts than less active participants. After adjusting for smoking status, pack-years, and time since cessation, white blood cell counts declined in successive total physical activity index tertiles (7.6, 7.4, and 7.1 ×103/μL, respectively; P < 0.001). No differences were observed in serum albumin according to physical activity levels.
TABLE 1.
Selected participant characteristics at baseline according to physical activity levels.
| Characteristics at baseline | Episodes of sweat-inducing activities per week (%)* | City blocks walked per day (%)* | Flights of stairs climbed per day (%)* | |||
|---|---|---|---|---|---|---|
| None (N = 3,215) | 1 or more (N = 1,614) | None (N = 2,204) | 1 or more (N = 2,610) | 0–2 (N = 2,187) | 3 or more (N = 2,639) | |
| Age | ||||||
| 43–49 | 16% | 20% | 16% | 18% | 12% | 22% |
| 50–59 | 26% | 30% | 25% | 29% | 24% | 30% |
| 60–69 | 27% | 30% | 28% | 28% | 28% | 28% |
| 70–79 | 23% | 17% | 22% | 20% | 27% | 16% |
| 80–86 | 9% | 3% | 9% | 5% | 10% | 4% |
| Sex | ||||||
| Male | 43% | 45% | 37% | 49% | 39% | 47% |
| Female | 57% | 55% | 63% | 51% | 61% | 53% |
| Smoking status | ||||||
| Never | 45% | 45% | 47% | 43% | 46% | 44% |
| Former | 32% | 41% | 33% | 38% | 36% | 35% |
| Current | 23% | 14% | 21% | 19% | 18% | 21% |
| Body mass index tertile (kg/m2) | ||||||
| 1 (<26.2) | 32% | 36% | 32% | 34% | 31% | 35% |
| 2 (26.2–30.3) | 33% | 36% | 31% | 36% | 32% | 35% |
| 3 (>30.3) | 35% | 29% | 36% | 30% | 36% | 30% |
| Alcohol drinks/week | ||||||
| None | 53% | 45% | 56% | 46% | 56% | 46% |
| <5 | 20% | 28% | 20% | 25% | 21% | 24% |
| 5 or more | 26% | 27% | 24% | 29% | 23% | 29% |
| Years of education | ||||||
| <12 | 34% | 19% | 33% | 25% | 36% | 23% |
| 12 | 44% | 43% | 44% | 43% | 42% | 45% |
| >12 | 22% | 38% | 23% | 32% | 22% | 32% |
| Mean (SD) heart rate† | 38.6 (5.9) | 37.5 (5.8) | 38.5 (6.0) | 38.0 (5.8) | 38.5 (6.0) | 38.0 (5.8) |
| Mean (SD) WBC (×103/μL) | 7.5 (2.2) | 7.1 (1.9) | 7.5 (2.2) | 7.3 (2.1) | 7.6 (2.3) | 7.2 (2.0) |
| Mean (SD) albumin (g/dL) | 4.6 (0.4) | 4.7 (0.3) | 4.6 (0.4) | 4.7 (0.4) | 4.6 (0.4) | 4.7 (0.4) |
WBC = white blood cell count.
Information regarding episodes of activity was missing for 2 participants, blocks walked was missing for 17 participants, and stairs climbed was missing for 5 participants.
Thirty second heart rate.
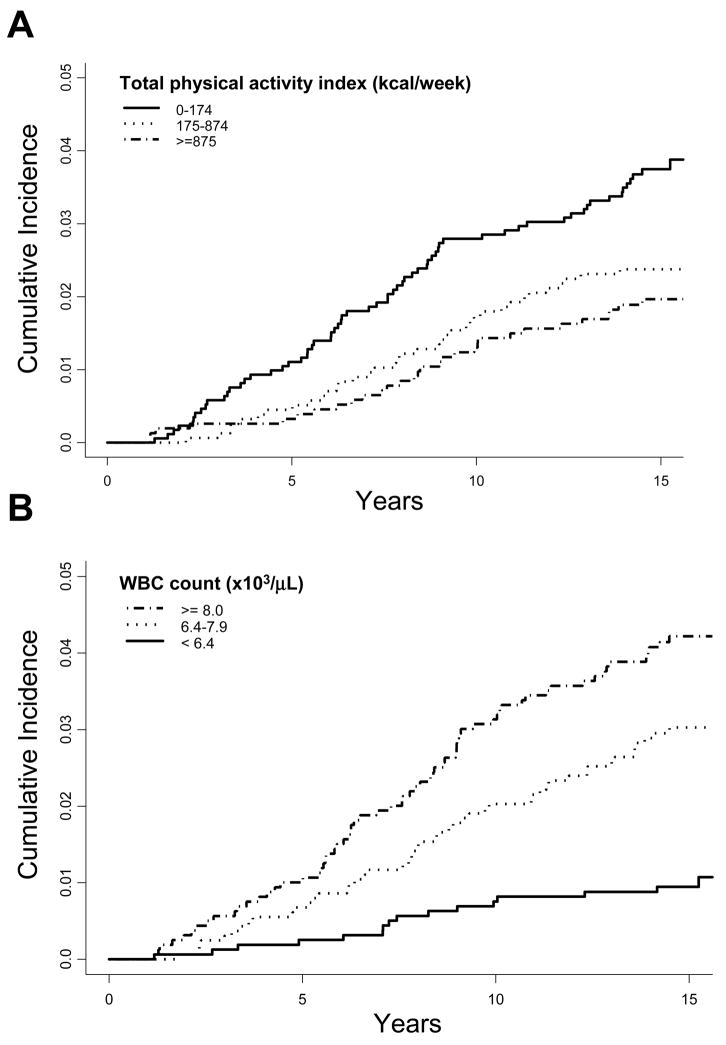
Higher levels of physical activity at baseline were inversely associated with lung cancer incidence (Table 2; Figure 1A). After multivariable adjustment for demographic and lifestyle factors (first column of hazard ratios), risk of lung cancer was reduced by over 40% among participants reporting 12 or more city blocks walked per day (Ptrend = 0.03) and those in the highest tertile of total physical activity index (≥ 875 kcal/week; Ptrend = 0.01). There was a negative association between lung cancer risk and the weekly number of episodes of activity vigorous enough to cause a sweat, although a dose-response pattern was not observed (Ptrend = 0.08). Flights of stairs climbed each day (Ptrend = 0.58) and heart rate (Ptrend = 0.27) were both not associated with lung cancer risk. While power was limited to detect a difference, these associations between physical activity measures and lung cancer did not appear to differ strongly according to sex, age, body mass index, smoking status, or pack-years smoked. Reductions in lung cancer risk were observed with increasing total physical activity index scores in both current and never/former smokers, though the risk reduction was somewhat stronger and statistically significant only in current smokers (Table 3; Pinteraction = 0.99). Similarly, lung cancer risk appeared to decline with increasing total physical activity index scores among both men and women, though the risk reduction was stronger and statistically significant only in men (Table 3, Pinteraction = 0.55). Mean white blood cell count for lung cancer cases was significantly higher at baseline (mean 8.2×103/μL, standard deviation 2.2×103/μL) than for participants who did not develop lung cancer (mean 7.4×103/μL, standard deviation 2.1×103/μL, P < 0.0001). After multivariable adjustment, the hazard rate for lung cancer was 2.8 times as high in participants with white blood cell counts greater than or equal to 8×103/μL compared to those with counts below 6.4×103/μL (Table 2; Figure 1B). Mean levels of albumin at baseline among the lung cancer cases were essentially the same (mean 4.6 g/dL, standard deviation 0.4 g/dL) as for non-cases (mean 4.7 g/dL, standard deviation 0.4 g/dL, P = 0.17), and no association was observed after multivariable adjustment.
TABLE 2.
Hazard ratios and 95% confidence intervals of lung cancer according to physical activity levels and inflammatory markers.
| No. of cases | Person years* | HR (95% CI)† | Ptrend† | HR (95% CI)‡ | Ptrend‡ | |
|---|---|---|---|---|---|---|
| Episodes of sweat-inducing activities per week | ||||||
| 0 | 105 | 36753 | 1 | 1 | ||
| 1–3 | 10 | 10862 | 0.44 (0.23–0.85) | 0.45 (0.23–0.87) | ||
| ≥4 | 19 | 9611 | 0.75 (0.45–1.24) | 0.08 | 0.76 (0.46–1.26) | 0.09 |
| City blocks walked per day | ||||||
| 0 | 73 | 25117 | 1 | 1 | ||
| 1–11 | 44 | 19633 | 0.93 (0.63–1.37) | 0.92 (0.62–1.35) | ||
| ≥12 | 17 | 12292 | 0.53 (0.31–0.90) | 0.03 | 0.52 (0.30–0.89) | 0.02 |
| Flights of stairs climbed per day | ||||||
| 0–1 | 44 | 17715 | 1 | 1 | ||
| 2–5 | 60 | 20224 | 1.53 (1.02–2.29) | 1.53 (1.02–2.29) | ||
| >5 | 30 | 19254 | 0.84 (0.52–1.36) | 0.58 | 0.86 (0.53–1.40) | 0.67 |
| Total physical activity index (kcal/week)§ | ||||||
| 0–174 | 65 | 18531 | 1 | 1 | ||
| 175–874 | 38 | 19120 | 0.72 (0.47–1.09) | 0.72 (0.48–1.09) | ||
| ≥875 | 31 | 19358 | 0.55 (0.35–0.86) | 0.01 | 0.56 (0.35–0.87) | 0.01 |
| Heart rate (30 sec.) | ||||||
| 21–33 | 27 | 12065 | 1 | 1 | ||
| 34–42 | 70 | 33925 | 0.93 (0.59–1.46) | 0.95 (0.60–1.49) | ||
| >42 | 37 | 11235 | 1.30 (0.80–2.16) | 0.27 | 1.25 (0.75–2.09) | 0.35 |
| WBC tertile (×103/μL) | ||||||
| <6.4 | 16 | 19605 | 1 | -- | ||
| 6.4–7.9 | 50 | 19421 | 2.74 (1.53–4.90) | -- | ||
| ≥8 | 68 | 18019 | 2.81 (1.58–5.01) | 0.001 | -- | |
| Albumin tertile (g/dL) | ||||||
| <4.6 | 52 | 19307 | 1 | -- | ||
| 4.6–4.8 | 51 | 20321 | 1.02 (0.69–1.52) | -- | ||
| ≥4.9 | 31 | 17427 | 0.85 (0.54–1.34) | 0.52 | -- | |
WBC = white blood cell count; HR = hazard ratio.
Total person years for cases and noncases in category of activity.
Models are adjusted for age, sex, pack-years of smoking, time since smoking cessation, body mass index, alcohol intake, and education.
Models are adjusted for all variables in †, plus white blood cell count.
Kilocalories per week from city blocks walked, flights of stairs climbed, and sweat-inducing activities (see Methods).
Figure 1.
Lung cancer cumulative incidence according to total physical activity index (A) and white blood cell count (B). Note that lung cancer cases diagnosed within the first year following the baseline exam were excluded.
TABLE 3.
Hazard ratios and 95% confidence intervals of lung cancer according to physical activity by smoking status and gender.
| Total physical activity index* | No. of cases | Person years† | HR (95% CI)‡ | Ptrend‡ |
|---|---|---|---|---|
| Current Smokers | ||||
| 0–174 kcal/week | 36 | 4052 | 1 | |
| 175–874 kcal/week | 15 | 4026 | 0.48 (0.26–0.91) | |
| ≥875 kcal/week | 12 | 3107 | 0.49 (0.25–0.97) | 0.02 |
| Never/Former Smokers | ||||
| 0–174 kcal/week | 29 | 14477 | 1 | |
| 175–874 kcal/week | 23 | 15081 | 0.97 (0.55–1.71) | |
| ≥875 kcal/week | 19 | 16236 | 0.60 (0.33–1.11) | 0.10 |
| Females | ||||
| 0–174 kcal/week | 28 | 11941 | 1 | |
| 175–874 kcal/week | 17 | 10737 | 1.02 (0.54–1.95) | |
| ≥875 kcal/week | 10 | 10166 | 0.66 (0.30–1.44) | 0.35 |
| Males | ||||
| 0–174 kcal/week | 37 | 6590 | 1 | |
| 175–874 kcal/week | 21 | 8383 | 0.56 (0.33–0.97) | |
| ≥875 kcal/week | 21 | 9192 | 0.50 (0.29–0.87) | 0.01 |
HR = hazard ratio.
Kilocalories per week from city blocks walked, flights of stairs climbed, and sweat-inducing activities (see Methods).
Total person years for cases and noncases in category of activity.
Models are adjusted for age, sex, pack-years of smoking, time since smoking cessation, body mass index, alcohol intake, and education.
The variables in Table 2 were similarly associated with lung cancer incidence and lung cancer mortality (data not shown), although for white blood cell count the relation was somewhat stronger for lung cancer mortality (OR = 3.75; 95% CI: 1.89–7.42 for tertile 3 vs. tertile 1).
The results shown in Table 2 were negligibly affected by further adjustment for the presence of diabetes and emphysema at baseline (data not shown). Similarly, further exclusion of 7 cases diagnosed between 12 and 24 months after the baseline examination had a negligible effect on the results. The relations between lung cancer risk, physical activity, and white blood cell count did not appear to be modified by time since the baseline examination. In analyses stratified by the median time between baseline exam and diagnosis (7.9 years), lung cancer risk was associated with physical activity and white blood cell count for both time frames (data not shown). There was limited power to examine these relations by histological subtype. Compared to subjects in the lowest total physical activity index tertile, subjects in the highest tertile were 0.73 (95% CI: 0.40–1.31) times as likely to develop any non-small cell lung cancer and 0.95 (95% CI: 0.41–2.21) times as likely to develop adenocarcinoma. Subjects in the highest tertile of white blood cell count were 3.04 (95% CI: 1.31–7.07) times more likely to develop non-small cell lung cancer and 2.42 (95% CI: 0.89–6.82) times more likely to develop adenocarcinoma than those in the lowest tertile. Too few cases were available to evaluate other specific cell types according to physical activity or white blood cell count.
To assess whether the inverse association between physical activity and lung cancer risk was mediated by inflammation, the regression models evaluating the physical activity/lung cancer association were additionally adjusted for white blood cell count at baseline (Table 2, second column of hazard ratios). This adjustment led to very minimal changes in the lung cancer hazard ratios associated with the various measures of physical activity. Similarly, the lung cancer hazard ratios associated with white blood cell count were not substantially changed after additionally adjusting for total physical activity index (OR=2.76, 95% CI: 1.54, 4.95 and HR=2.76, 95% CI: 1.55–4.91, for 6.4–7.9×103/μL and ≥ 8×103/μL versus <6.4×103/μL, respectively). Finally, white blood cell count did not appear to modify the relation between total physical activity index and lung cancer risk (Pinteraction = 0.86).
DISCUSSION
In this study we found an inverse association between physical activity and lung cancer risk. We also found evidence for a positive association between lung cancer risk and white blood cell count, but not serum albumin. It has been hypothesized that physical activity may lower lung cancer risk by reducing chronic inflammation. However, we found no evidence that the associations of physical activity and white blood cell count with lung cancer risk were mediated through the same biological pathway.
Clearly smoking is a strong causal factor of lung cancer in both men and women, with a population attributable risk of approximately 75–90% in the United States (39, 40). Smoking prevention and cessation are the primary prevention strategies needed to reduce lung cancer incidence. However, the elucidation of other risk factors would aid in lung cancer prevention, particularly in never and former smokers, in whom about 50% of all new lung cancers are diagnosed (41). This study adds additional evidence to the body of literature which suggests that physical activity is a protective factor against the development of lung cancer.
We observed an inverse association between physical activity and lung cancer at the upper end of the 10–40% range of risk reductions observed in the majority of past studies (14). Given the strong relation between smoking and lung cancer risk, residual confounding of the relation between lung cancer risk and both physical activity and white blood cell count remains a concern. In models adjusted for sex, body mass index, alcohol, and education, but not smoking, the relations between lung cancer and physical activity and white blood cell count were stronger (HR = 0.43 and HR = 5.05, for third tertile vs. first tertile of total physical activity index and white blood cell count, respectively) than in models fully adjusted for smoking (HR = 0.55 and HR = 2.81, respectively). Thus it is possible that better measurement of smoking (e.g. more accurate reporting, biomarkers of smoking history) would further attenuate our findings. However, we were able to adjust for a number of prospectively-obtained self-reported smoking parameters, including smoking status, amount of smoking (pack-years) and time since smoking cessation. In analyses stratified by smoking status, physical activity appeared to be associated with reduced lung cancer risk among never and former smokers combined, though this did not reach statistical significance. Too few cases were observed among never smokers (N = 16) to examine this stratum separately. The relation between smoking and adenocarcinoma is weaker than for other cell types (42). In our data, adenocarcinoma was associated with white blood cell count but not total physical activity index score. Though this was based on only 31 events, it suggests additional caution in interpreting the physical activity/lung cancer association.
Exercise is associated with reduced systemic inflammation (particularly C-reactive protein) both between persons in cross-sectional studies and within persons after the initiation of training regimes (21). Inflammation has been proposed to promote carcinogenesis in a wide spectrum of cancers, including lung, through its effects on cell proliferation, survival, and migration (24–26). Inflammatory lung conditions, such as chronic bronchitis and asthma, have previously been linked with increased lung cancer risk (43). Furthermore, the use of aspirin and other non-steroidal anti-inflammatory drugs has been associated with reduced lung cancer risk (44, 45).
We investigated the relation between two inflammatory markers and lung cancer. White blood cell count is a widely-used nonspecific marker of systemic inflammation (26, 46, 47). We observed reduced white blood cell counts in participants who reported higher physical activity levels, consistent with previous findings (19, 23, 48). Notably, we found that this relation persisted after adjustment for self-reported smoking history. Three studies have reported positive associations between white blood cell count and lung cancer incidence or mortality after adjustment for smoking (30, 46, 47). Similar to our study, Shankar et al. (46) reported increased lung cancer mortality among subjects in the upper quartile of white blood cell count compared to those in the lowest quartile (RR = 2.58, 95% CI: 0.72–9.26 for quartile 4 vs. quartile 1). The results from our study (incidence HR = 2.81; 95% CI: 1.58–5.01; mortality HR = 3.75, 95% CI: 1.89–7.42) and Shankar et al. (46) provide greater risk estimates than those for quartile 4 versus quartile 1 of white blood cell count in Erlinger et al. (47) (mortality HR = 1.79, 95% CI: 0.88–3.62) and the recently reported results of the Women’s Health Initiative (30) (incidence HR = 1.63, 95% CI: 1.35–1.97). The Women’s Health Initiative observed little difference between lung cancer incidence and mortality hazard ratios in relation to white blood cell count.
Serum albumin is a negative acute phase protein - its concentration in the blood is reduced in response to inflammation (49, 50). At least one study has reported an approximate 25% reduction in cancer mortality among middle aged men with a one standard deviation increase in serum albumin (51). We observed little difference in serum albumin among participants according to physical activity level, and no association between serum albumin and lung cancer risk.
To investigate the hypothesis that physical activity lowers lung cancer risk by decreasing systemic inflammation, we further adjusted the regression model of physical activity and lung cancer risk for white blood cell count. In an adequately adjusted model, one would expect the association between physical activity and lung cancer risk to be attenuated if the relation was mediated at least in part by inflammation (represented by white blood cell count) (52). However, we found that the associations between lung cancer risk and both physical activity and white blood cell count were practically unchanged after simultaneous adjustment. Thus the effect of physical activity on lung cancer risk does not appear to be mediated by inflammation, as represented by white blood cell count. Importantly, white cell blood count is only one marker of inflammation; it remains possible that other measures of inflammation may be more relevant to the relation of physical activity and lung cancer.
Physical activity has been proposed to lower lung cancer risk by a variety of other mechanisms. Physical activity might reduce the concentration of carcinogenic agents in the airways, reduce the duration of agent-airway interaction, and reduce particle deposition through increased ventilation and perfusion (53). Physical activity also reduces insulin-like growth factor levels which stimulate cell proliferation (54). Furthermore, physical activity may enhance immune function or endogenous antioxidant defenses (17, 55, 56).
A number of limitations must be considered in the interpretation of this study. We utilized a simple assessment of physical activity. Although an increased heart rate is an objective measure associated with lack of physical activity (57, 58), heart rate is also modified by general health, stress, and other psychosocial factors. Questions regarding the number of blocks walked per week and flights of stairs climbed per day have previously been used in combination with data on recreational physical activity to measure the relation between physical activity and cancer risk in the Harvard Alumni Health Study (5, 6, 59). We did not collect data on specific participation in recreational physical activities, but rather episodes of sweat-inducing activities. A moderate correlation (r = 0.54–0.62) has been reported between episodes of sweat-inducing activities and the Harvard Alumni Activity Survey scores (60, 61), including one study in a population of older women (62). The association between sweat-inducing activities and physical fitness measured on a cycle ergometer, however, has been reported to be stronger in men (r = 0.46) than in women (r = 0.26) (60). Our summary physical activity measure which combined blocks walked, stairs climbed, and sweat-inducing activities was more strongly related to lung cancer risk among men than in women (Table 3), though the test for effect modification did not reach statistical significance (Pinteraction = 0.46).
The limited scope of our physical activity assessment failed to capture variation in intensity and duration of sweat-inducing activities. To create our total physical activity index we assumed a typical duration of 30 minutes for sweat-inducing activities, with an intensity level equivalent to jogging (MET = 7). The results did not appear sensitive to variation in these assumptions: assuming a MET score of 5 for 30 minutes or a MET score of 9 for 1 hour for sweat-inducing activities both resulted in HR = 0.55 for the third tertile of total physical activity index compared to the first tertile.
Notably, our physical activity assessment also failed to capture past history of physical activity. Our failure to capture variation in duration, intensity, and past history of activity would be expected to attenuate the reductions in risk observed in our study. Much more sophisticated assessments of physical activity have been developed since the initiation of our study. Further studies are necessary, in particular, to evaluate lung cancer risk in relation to cumulative lifetime physical activity and to discriminate the effects of physical activity during different time periods in life.
Other unmeasured aspects of a healthy lifestyle may confound the relation between physical activity and lung cancer. A diet high in fruits and vegetables has been associated with reduced lung cancer risk (63). Unfortunately we had limited information on diet and were unable to control for this in our analysis.
Strengths of this study included a population-based cohort of both sexes with excellent follow-up, the prospective assessment of physical activity and inflammatory markers, and the ability to control for a number of prospectively-obtained smoking parameters. It is possible that lower levels of physical activity among future cases might be expected at the baseline exam due to symptoms, such as pain or fatigue, related to undiagnosed lung cancer. To reduce the potential for this bias, we excluded all lung cancer cases who were diagnosed within 12 months of the baseline examination (N= 13). Other diseases, particularly of the lung, may also influence physical activity, inflammation, and lung cancer risk. However, we observed little change in the relations between lung cancer risk, physical activity, and white blood cell count after adjusting for self-reported emphysema and diabetes.
Lung cancer is both the most common cancer diagnosis in the world and the most common cause of death from cancer (64). The global burden of smoking-related disease is overwhelming, with over 1.3 million new cases of lung cancer and approximately 1.2 million deaths in 2002 (64). Smoking prevention and cessation are imperative in reducing the mortality associated with this disease. Continued study of physical activity in relation to lung cancer risk, particularly among never smokers, may further our understanding of this disease and reveal additional strategies for reducing its burden.
Acknowledgments
FINANCIAL SUPPORT: This study was supported in part by faculty startup funds from the University of Wisconsin School of Medicine & Public Health, National Institutes of Health grants U10 EY006594 and R01 AG11099, and grant HS06941 by the Agency for Healthcare Research and Quality.
The authors would like to acknowledge Dr. Dennis Fryback, Scot Moss, Michael Knudtson, Dr. Lisa Colbert, Laura Stephenson and the staff of the Wisconsin Cancer Reporting System, Hazel Nichols, Andy Bersch, Moneen Meuer, and the participants of the Beaver Dam Studies for their invaluable contributions.
ABBREVIATIONS
- kcal
kilocalories
- HR
hazard ratio
- CI
confidence interval
- WBC
white blood cell
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute ; 2007. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Albanes D, Blair A, Taylor PR. Physical activity and risk of cancer in the NHANES I population. Am J Public Health. 1989;79:744–50. doi: 10.2105/ajph.79.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownson RC, Chang JC, Davis JR, Smith CA. Physical activity on the job and cancer in Missouri. Am J Public Health. 1991;81:639–42. doi: 10.2105/ajph.81.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubik A, Zatloukal P, Boyle P, et al. A case-control study of lung cancer among Czech women. Lung Cancer. 2001;31:111–22. doi: 10.1016/s0169-5002(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 5.Lee IM, Paffenbarger RS., Jr Physical activity and its relation to cancer risk: a prospective study of college alumni. Med Sci Sports Exerc. 1994;26:831–7. [PubMed] [Google Scholar]
- 6.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and risk of lung cancer. Int J Epidemiol. 1999;28:620–5. doi: 10.1093/ije/28.4.620. [DOI] [PubMed] [Google Scholar]
- 7.Mao Y, Pan S, Wen SW, Johnson KC. Physical activity and the risk of lung cancer in Canada. Am J Epidemiol. 2003;158:564–75. doi: 10.1093/aje/kwg186. [DOI] [PubMed] [Google Scholar]
- 8.Olson JE, Yang P, Schmitz K, Vierkant RA, Cerhan JR, Sellers TA. Differential association of body mass index and fat distribution with three major histologic types of lung cancer: evidence from a cohort of older women. Am J Epidemiol. 2002;156:606–15. doi: 10.1093/aje/kwf084. [DOI] [PubMed] [Google Scholar]
- 9.Sellers TA, Potter JD, Folsom AR. Association of incident lung cancer with family history of female reproductive cancers: the Iowa Women’s Health Study. Genet Epidemiol. 1991;8:199–208. doi: 10.1002/gepi.1370080306. [DOI] [PubMed] [Google Scholar]
- 10.Thune I, Lund E. The influence of physical activity on lung-cancer risk: A prospective study of 81,516 men and women. Int J Cancer. 1997;70:57–62. doi: 10.1002/(sici)1097-0215(19970106)70:1<57::aid-ijc9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Alfano CM, Klesges RC, Murray DM, et al. Physical activity in relation to all-site and lung cancer incidence and mortality in current and former smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:2233–41. [PubMed] [Google Scholar]
- 12.Sinner P, Folsom AR, Harnack L, Eberly LE, Schmitz KH. The association of physical activity with lung cancer incidence in a cohort of older women: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2006;15:2359–63. doi: 10.1158/1055-9965.EPI-06-0251. [DOI] [PubMed] [Google Scholar]
- 13.Kubik A, Zatloukal P, Tomasek L, Pauk N, Petruzelka L, Plesko I. Lung cancer risk among nonsmoking women in relation to diet and physical activity. Neoplasma. 2004;51:136–43. [PubMed] [Google Scholar]
- 14.Tardon A, Lee WJ, Delgado-Rodriguez M, et al. Leisure-time physical activity and lung cancer: a meta-analysis. Cancer Causes Control. 2005;16:389–97. doi: 10.1007/s10552-004-5026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bak H, Christensen J, Thomsen BL, et al. Physical activity and risk for lung cancer in a Danish cohort. Int J Cancer. 2005;116:439–44. doi: 10.1002/ijc.21085. [DOI] [PubMed] [Google Scholar]
- 16.Steindorf K, Friedenreich C, Linseisen J, et al. Physical activity and lung cancer risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Int J Cancer. 2006;119:2389–97. doi: 10.1002/ijc.22125. [DOI] [PubMed] [Google Scholar]
- 17.Rundle A. Molecular epidemiology of physical activity and cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:227–36. [PubMed] [Google Scholar]
- 18.Campbell KL, McTiernan A. Exercise and biomarkers for cancer prevention studies. J Nutr. 2007;137:161S–169S. doi: 10.1093/jn/137.1.161S. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13:561–8. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78:819–35. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 21.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 22.Hamer M. The relative influences of fitness and fatness on inflammatory factors. Prev Med. 2007;44:3–11. doi: 10.1016/j.ypmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Pitsavos C, Chrysohoou C, Panagiotakos DB, et al. Association of leisure-time physical activity on inflammation markers (C-reactive protein, white cell blood count, serum amyloid A, and fibrinogen) in healthy subjects (from the ATTICA study) Am J Cardiol. 2003;91:368–70. doi: 10.1016/s0002-9149(02)03175-2. [DOI] [PubMed] [Google Scholar]
- 24.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–83. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 27.Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 28.Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Ito Y, Wakai K, et al. Serum heat shock protein 70 levels and lung cancer risk: a case-control study nested in a large cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1733–7. doi: 10.1158/1055-9965.EPI-06-0005. [DOI] [PubMed] [Google Scholar]
- 30.Margolis KL, Rodabough RJ, Thomson CA, Lopez AM, McTiernan A. Prospective study of leukocyte count as a predictor of incident breast, colorectal, endometrial, and lung cancer and mortality in postmenopausal women. Arch Intern Med. 2007;167:1837–44. doi: 10.1001/archinte.167.17.1837. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–5. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study Ophthalmology. 1996;103:1169–78. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 33.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–66. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 34.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 35.Knudtson MD, Klein R, Klein BE. Physical activity and the 15-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Br J Ophthalmol. 2006;90:1461–3. doi: 10.1136/bjo.2006.103796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 37.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine & Science in Sports & Exercise. 2000;32:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 38.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Department of Health and Human Services. Reducing the health consequences of smoking: 25 years of progress. A report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS Publication No. (CDC) 89–8411, 1989.
- 40.Shopland DR. Tobacco use and its contribution to early cancer mortality with a special emphasis on cigarette smoking. Environ Health Perspect. 1995;103 (Suppl 8):131–42. doi: 10.1289/ehp.95103s8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong L, Spitz MR, Fueger JJ, Amos CA. Lung carcinoma in former smokers. Cancer. 1996;78:1004–10. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 43.Mayne ST, Buenconsejo J, Janerich DT. Previous lung disease and risk of lung cancer among men and women nonsmokers. Am J Epidemiol. 1999;149:13–20. doi: 10.1093/oxfordjournals.aje.a009722. [DOI] [PubMed] [Google Scholar]
- 44.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–46. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Smith CJ, Perfetti TA, King JA. Perspectives on pulmonary inflammation and lung cancer risk in cigarette smokers. Inhal Toxicol. 2006;18:667–77. doi: 10.1080/08958370600742821. [DOI] [PubMed] [Google Scholar]
- 46.Shankar A, Wang JJ, Rochtchina E, Yu MC, Kefford R, Mitchell P. Association between circulating white blood cell count and cancer mortality: a population-based cohort study. Arch Intern Med. 2006;166:188–94. doi: 10.1001/archinte.166.2.188. [DOI] [PubMed] [Google Scholar]
- 47.Erlinger TP, Muntner P, Helzlsouer KJ. WBC count and the risk of cancer mortality in a national sample of U.S. adults: results from the Second National Health and Nutrition Examination Survey mortality study Cancer. Epidemiol Biomarkers Prev. 2004;13:1052–6. [PubMed] [Google Scholar]
- 48.Church TS, Finley CE, Earnest CP, Kampert JB, Gibbons LW, Blair SN. Relative associations of fitness and fatness to fibrinogen, white blood cell count, uric acid and metabolic syndrome. Int J Obes Relat Metab Disord. 2002;26:805–13. doi: 10.1038/sj.ijo.0802001. [DOI] [PubMed] [Google Scholar]
- 49.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- 50.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 51.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2:1434–6. doi: 10.1016/s0140-6736(89)92042-4. [DOI] [PubMed] [Google Scholar]
- 52.Szklo M, Nieto FJ. Epidemiology: beyond the basics. Gaithersburg, MD: Aspen Publishers, Inc; 1999. [Google Scholar]
- 53.IARC. Weight control and physical activity. Vol. 6. Lyon, France: IARC Press; 2002. IARC handbooks on cancer prevention. [Google Scholar]
- 54.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 55.McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes and Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 56.Rundle AG, Orjuela M, Mooney L, et al. Preliminary studies on the effect of moderate physical activity on blood levels of glutathione. Biomarkers. 2005;10:390–400. doi: 10.1080/13547500500272663. [DOI] [PubMed] [Google Scholar]
- 57.Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999;33:44–52. doi: 10.1161/01.hyp.33.1.44. [DOI] [PubMed] [Google Scholar]
- 58.Wannamethee G, Shaper AG, Macfarlane PW. Heart rate, physical activity, and mortality from cancer and other noncardiovascular diseases. Am J Epidemiol. 1993;137:735–48. doi: 10.1093/oxfordjournals.aje.a116734. [DOI] [PubMed] [Google Scholar]
- 59.Lee IM, Paffenbarger RS, Jr, Hsieh CC. Physical activity and risk of prostatic cancer among college alumni. Am J Epidemiol. 1992;135:169–79. doi: 10.1093/oxfordjournals.aje.a116269. [DOI] [PubMed] [Google Scholar]
- 60.Siconolfi SF, Lasater TM, Snow RC, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. Am J Epidemiol. 1985;122:101–5. doi: 10.1093/oxfordjournals.aje.a114068. [DOI] [PubMed] [Google Scholar]
- 61.Washburn RA, Adams LL, Haile GT. Physical activity assessment for epidemiologic research: the utility of two simplified approaches. Prev Med. 1987;16:636–46. doi: 10.1016/0091-7435(87)90047-8. [DOI] [PubMed] [Google Scholar]
- 62.LaPorte RE, Black-Sandler R, Cauley JA, Link M, Bayles C, Marks B. The assessment of physical activity in older women: analysis of the interrelationship and reliability of activity monitoring, activity surveys, and caloric intake. J Gerontol. 1983;38:394–7. doi: 10.1093/geronj/38.4.394. [DOI] [PubMed] [Google Scholar]
- 63.World Cancer Research Fund. Food, nutrition and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research; 1997. pp. 130–147. [Google Scholar]
- 64.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]