Abstract
The objective of this work was to control orientation of bioactive molecules immobilized on a biodegradable substrate to improve their accessibility for binding to cell surface receptors and, therefore, to increase bioactivity. The osteotropic peptide, parathyroid hormone (1-34) (PTH(1-34)), was used to demonstrate the approach. To this end, the intrinsic N-terminal serine residue was oxidized to create an aldehyde group that specifically bound to hydrazide-derivatized poly(lactide-co-glycolide) under neutral conditions to form a hydrazone bond. Use of dihydrazide spacers significantly increased the amount of peptide immobilized compared to simple adsorption or direct, random attachment. In probing accessibility of immobilized PTH(1-34), attachment using longer dihydrazide spacers enhanced binding of an antibody against an epitope in the N-terminal region of the peptide. The longest spacer also increased binding of a C-terminal antibody. Furthermore, substrates with peptide tethered via spacers stimulated intracellular synthesis of cAMP, with activity increasing with dihydrazide length. PTH(1-34) immobilized using the longest spacer was significantly more effective than both random binding and adsorption. Site-directed binding of bioactive peptides to surfaces presents biomolecules for binding with cells so as to enhance interaction with receptors, and therefore the approach may be useful for obtaining preferred localized tissue responses.
Keywords: Cell activation, Osteoblast, Peptide, Surface grafting, Surface modification
INTRODUCTION
Materials and devices for enhanced regeneration of bone are the focus of intensive investigations. Whether being used in a defect site resulting from a congenital condition, trauma, or cancer, or at the interface between a joint or dental implant and the surrounding tissues, the objective is to obtain a more rapid and predictable osteogenic response. In addition to the nature of the defect and the anatomic location, formation of bone in these sites is further confounded by several factors, such as age and health status of the patient.
With the ultimate aim of enhancing osteogenesis, a variety of approaches are being investigated for controlling tissue-biomaterial interactions. For example, proteins and bioactive peptides can be attached to material surfaces to affect the initial adhesion and/or subsequent responses of cells, as recently reviewed in [1,2]. Although physical adsorption is the simplest method, control of the amount and/or orientation of immobilized molecules is difficult to obtain. Using more sophisticated methodology, covalent techniques enable better control of the amount immobilized, prolonged retention of the biomolecules, and ability to dictate the orientation/presentation of molecules. By predetermining the orientation of a biomolecule on a surface, it can be presented in such as way as to make it available for optimal binding to its ligand, e.g., cell surface receptor. Methods for directing the orientation of immobilized molecules have been investigated for chromatographic and biosensor applications [3], but they have not been adequately explored for biomaterials usage.
Parathyroid hormone (PTH) is an 84 residue peptide hormone having a significant role in regulating extracellular calcium homeostasis by acting on kidney, bone, and intestine [4]. Essentially complete biological activity can be found in an N-terminal fragment comprising the first 34 amino acids (PTH(1-34)) [5]. Interestingly, PTH(1-34) can have either anabolic or catabolic effects, depending on the concentration and mode of administration. Whereas high, sustained doses lead to bone resorption, intermittent treatment with higher doses or infusion of low doses result in enhanced formation of bone [6–8]. Immobilization of PTH(1-34) on silk scaffolds has been reported to enhance proliferation of osteoblastic cells [9]. In that study, an attempt to enable immobilization via only the N-terminus was also attempted by replacing lysine residues in the peptide with arginines to leave a reactive amino group at only the terminus, but it did not improve cell responses.
The objective of this study was to apply a versatile strategy for the controlled immobilization of bioactive molecules. Specifically, PTH(1-34) was attached via its N-terminus to a biodegradable polymer, and its biological activity was measured.
MATERIALS AND METHODS
Substrates
Coverslips coated with poly(lactic-co-glycolic acid) (PLGA) were used for experimentation because of their uniform surface area and ease of preparation. Approximately 35 μL of 12% (w/v) acid-terminated PLGA (50:50, MW~12,000; Alkermes, Cincinnati, OH) dissolved in methylene chloride was allowed to air dry on 12 mm glass coverslips for about 30 minutes and then vacuum-dried overnight or until ready for use.
Immobilization Scheme
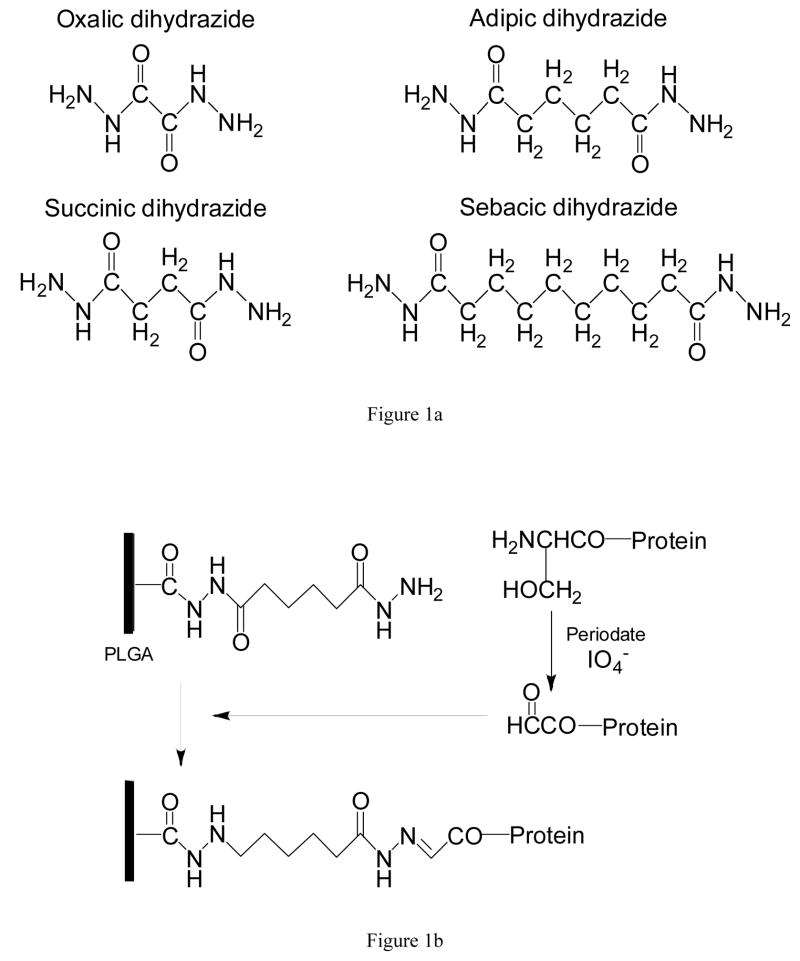
The approach to controlling orientation of PTH(1-34) involved attachment via its N-terminus to hydrazide-derivatized PLGA. Four dihydrazides were investigated as spacers between biomolecule and surface: oxalic (Aldrich, Milwaukee, WI), succinic (Aldrich), adipic (Sigma, St. Louis, MO), and sebacic dihydrazide (TCI America, Portland, OR). Although each has the same backbone structure, they have 2, 4, 6, and 10 carbons, respectively, between the two terminal hydrazide moieties (Figure 1a). To allow focus on spacer length, PLGA was derivatized to have a similar surface density of the different dihydrazides. Based on pilot studies, concentrations of 0.018, 0.057, 0.018, and 0.011 mM were used for oxalic, succinic, adipic, and sebacic dihydrazide, respectively. These spacers were attached to carboxylic acid groups of PLGA using carbodiimide chemistry [10]. Coated coverslips were placed into 24-well plates, and one half volumes of the desired hydrazide solution and of a solution containing a 5:2 molar ratio of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (ECAC; Sigma) and N-hydroxysuccinimide (NHS: Fluka, Buchs, Switzerland) in 0.1 M MES (Sigma) buffer solution, pH 4.5, were added. After reaction for two hours at room temperature, samples were thoroughly washed. The number of available hydrazide groups was determined by treatment with 2,4,6-trinitrobenzene sulfonic acid, as described elsewhere [10,11].
Figure 1.
A) Dihydrazide molecules of increasing length used as spacers between peptide and surface. B) Scheme for controlled attachment of PTH(1-34) to dihydrazide-modified PLGA. Oxidation of the N-terminal serine creates an aldehyde that forms a hydrazone bond with the derivatized substrate.
To enable oriented attachment of PTH(1-34), its N-terminal serine residue was selectively oxidized to an aldehyde moiety [12,13]. PTH(1-34) (Bachem, Torrance, CA) was reconstituted in phosphate-buffered saline (PBS), pH 7.4, at a concentration of 40 μg/mL. Oxidation was carried out by reacting equal volumes of the peptide solution with 10 mM periodic acid (Aldrich) in the dark at room temperature for 45 minutes [14,15]. The reaction was quenched by adding glycerol. After washing and concentrating the peptide using centrifugal filter units (Microcon, 3 kDa NMWL; Millipore, Billerica, MA), oxidized PTH(1-34) was diluted in PBS to a final concentration of 20 μg/mL. Approximately 10 μg of oxidized peptide was added to each PLGA sample, allowing the aldehyde groups to react with the hydrazides at neutral pH to form stable hydrazone bonds [16]. The reaction scheme is shown in Figure 1b. Two additional surfaces were used for comparison. For the first, the same amount of PTH(1-34) was also randomly and directly bound to PLGA surfaces following carbodiimide activation as described above. For the second, peptide was simply adsorbed on PLGA at room temperature for 45 min. Because preliminary studies indicated similar adsorption on derivatized and control PLGA surfaces, untreated PLGA was used for the present work to simplify the number of experimental groups.
Amount and Accessibility of Immobilized PTH(1-34)
The amount of peptide bound to the PLGA surfaces was measured using the microBCA Protein Assay (Pierce Chemical Co., Rockford, IL). Using a slight modification of the manufacturer’s protocol, biomaterials samples were incubated directly in the working reagent at 37ºC for two hours, after which absorbance of the supernatant was read at 570 nm. PTH(1-34) contents were determined using a standard curve constructed from known dilutions of the peptide.
To quantitatively evaluate accessibility of immobilized PTH(1-34) for potential binding to cell surface receptors, an immunoassay technique was applied. Two goat polyclonal antibodies were used: one had a binding epitope at the N-terminus of the peptide (sc-9676), and the other bound at the C-terminus (sc-9677) (Santa Cruz Biotechnology, Santa Cruz, CA). First, all surfaces were blocked with a solution of PBS containing 0.5% BSA and 0.05% Tween 20 to prevent nonspecific binding. Primary anti-PTH(1-34) antibodies were diluted with PBS at a ratio of 1:500 and then allowed to react with parallel samples for 30 minutes at 37°C. After thorough washing, a secondary antibody, alkaline phosphatase-conjugated mouse anti-goat IgG (Sigma), was diluted in PBS at a ratio of 1:500 and incubated with the samples for 45 minutes at 37°C. Color was developed by reacting with a solution of 0.1% Sigma 104 phosphatase (Sigma) in 10% diethanolamine (Chempure, Houston, TX) at pH 9.8 and measuring absorbance at 410 nm.
Bioactivity of Immobilized PTH(1-34)
Activity of immobilized peptide was determined by measuring cAMP contents in cells cultured on the surface-modified samples. For these experiments, osteoblastic MC3T3-E1 cells (CRL-2593; ATCC, Rockville, MD) were cultured in phenol red-free MEM (GIBCO/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 50 μg/mL ascorbic acid. Following pretreatment with a phosphodiesterase inhibitor, 0.5 mM isobutylmethylxanthine (Sigma), cells were seeded onto samples in 24-well plates at a density of 50,000 cells per well. After 10 min of incubation on the surfaces, cultures were lysed directly in the medium by sonication and freeze-thaw cycles. Intracellular cAMP was measured using a commercially available immunoassay kit (Endogen/Pierce, Rockford, IL) following the manufacturer’s instructions. The cAMP data were normalized by the amount of DNA in each well, as determined using a Hoechst assay [17,18]. In brief, lysates and serial dilutions of calf thymus DNA were reacted with Hoechst 33258 (final concentration, 0.5 μg/mL; Sigma) in the dark for 10 min before measuring fluorescence (λex=356 nm, λem=458 nm). Data were corrected for basal cAMP/DNA levels in unstimulated (no PTH) cultures. Finally, to account for differences in the amounts of PTH(1-34) attached to the various surfaces, bioactivity was expressed as units (nmol cAMP/μg DNA) per μg of peptide.
Statistical Analysis
Data are presented as mean ± standard deviation. A minimum of six replicates was used for each experiment. One-way analysis of variance (ANOVA) was conducted using InStat 3.05 (Graphpad Software, San Diego, CA). Post-hoc comparisons were made using the Tukey-Kramer Multiple Comparison test when the p-value was significant (p<0.05).
RESULTS
Surface Modification
Results from the TNBS assay used to determine the surface density of hydrazide groups available for subsequently binding peptide are presented in Figure 2. As desired to enable focus on effects of spacer length, similar numbers of moieties were available on surfaces derivatized with the different dihydrazide molecules. The average density was approximately 25 hydrazides per nm2 of nominal surface area.
Figure 2.
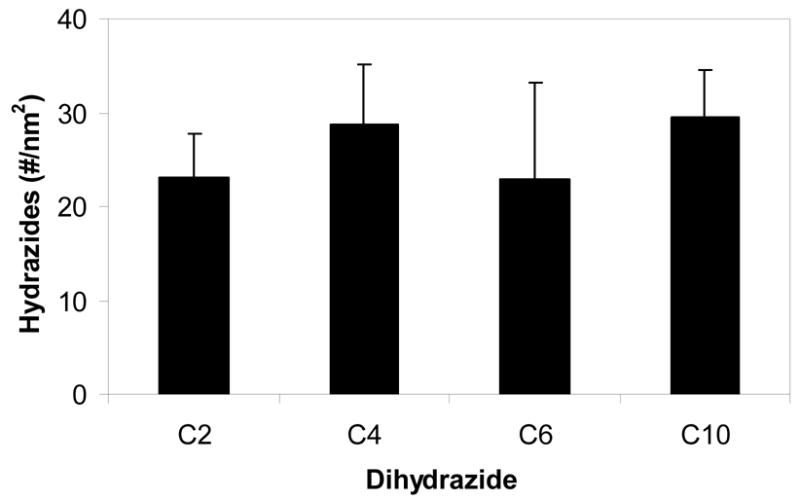
Surface density of hydrazide groups on PLGA following derivatization with dihydrazide spacers of increasing length.
Amount of Immobilized PTH(1-34)
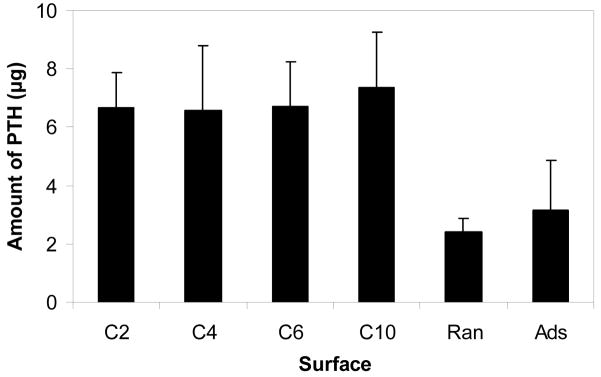
By measuring the quantity of peptide bound to the various surfaces (Figure 3), it was first found that the presence of a spacer significantly increased the amount of PTH(1-34) immobilized (p<0.01). Whereas approximately 6.5 μg of peptide was bound using the four dihydrazide spacers, less than 3 μg was present following simple adsorption or direct, random attachment of the hormone fragment. Interestingly, the length of spacer did not have a significant effect on the amount of peptide attached to PLGA. Adsorption resulted in the greatest variability, about 55% of the mean.
Figure 3.
Amount of PTH(1-34) bound to PLGA. Peptide was immobilized via hydrazide spacers, directly on carbodiimide-activated surfaces (Ran), and by simple adsorption (Ads).
Accessibility of Immobilized PTH(1-34)
Figure 4 shows relative binding of the two antibodies used to probe availability of the immobilized peptide for subsequent interaction with cell surface receptors. Looking first at accessibility of the N-terminus, increasing length of the dihydrazide molecules resulted in a statistically significant trend of enhanced binding of the antibody (p<0.05). Antibody against the N-terminus bound to randomly immobilized PTH(1-34) at a level comparable to the longer spacers, e.g., C6 and C10. Adsorbed peptide was recognized to a noticeably, albeit statistically insignificantly, lesser extent than any other group. Considering availability of the C-terminus of the peptide for binding to an antibody, the C10-derivatized surfaces were recognized significantly more than any of the other surfaces (p<0.001); all the other surfaces bound similar amounts of antibody.
Figure 4.
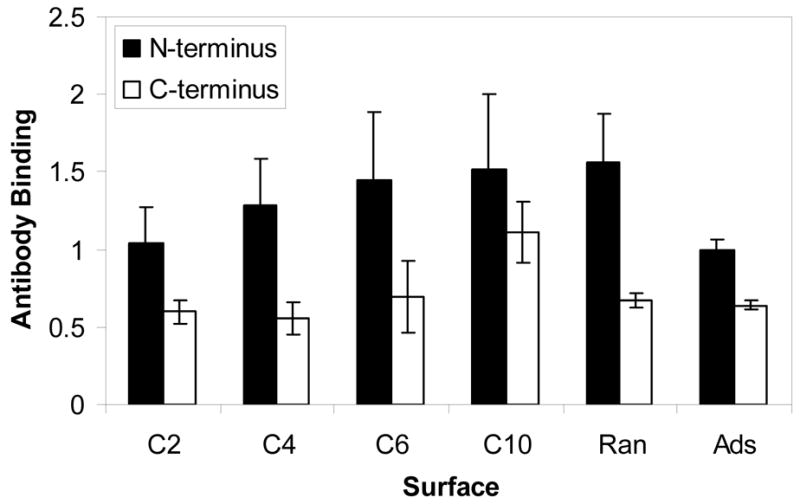
Binding of antibodies to the N- and C-terminal regions of PTH(1-34) attached to PLGA. Peptide was immobilized via hydrazide spacers, directly on carbodiimide-activated surfaces (Ran), and by simple adsorption (Ads).
Bioactivity of Immobilized PTH(1-34)
The ability of the hormone fragment to stimulate production of cAMP in MC3T3-E1 cells is shown in Figure 5. To account for differences in the amount of peptide on the PLGA surfaces, levels of the second messenger were normalized by the mass attached to the substrate (Figure 3). PTH(1-34) tethered to PLGA via dihydrazide spacers showed a trend of increased activity with length of the molecule. Peptide immobilized using the C4 through C10 spacers resulted in significantly greater activity than did the adsorbed peptide (p<0.05). Attachment via the C10 molecule was more effective at stimulating intracellular cAMP than both random, covalent binding and adsorption (p<0.05). Random immobilization enhanced activity, but it was not statistically significantly greater than adsorption.
Figure 5.
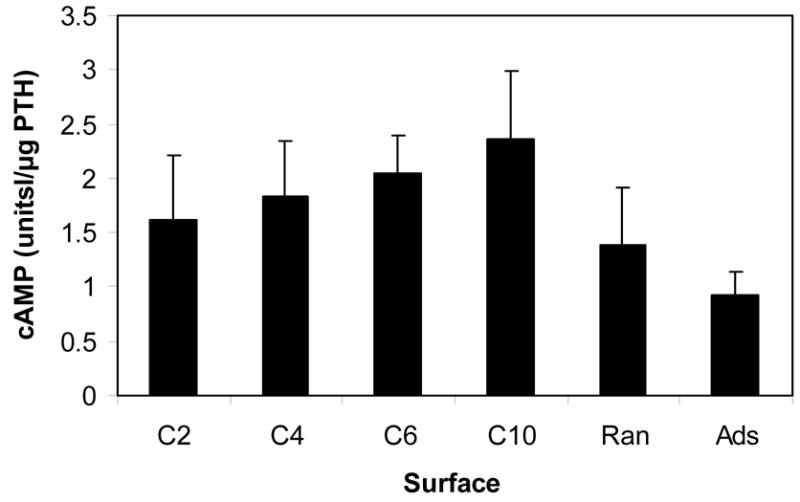
Intracellular cAMP contents in MC3T3-E1 cells cultured on surfaces with attached PTH(1-34). Peptide was immobilized via hydrazide spacers, directly on carbodiimide-activated surfaces (Ran), and by simple adsorption (Ads). The amount of cAMP was normalized by DNA content and the mass of PTH(1-34) present on the surfaces.
DISCUSSION
Immobilization of various biomolecules on biomaterials to alter cell and tissue behavior has been a subject of active investigation. Recent reviews have nicely summarized some of the important developments in this area (e.g., [1,2]). Most methods used for immobilizing proteins and peptides involve formation of covalent bonds between surface-exposed functional groups, such as amino, carboxyl, and thiol groups, of amino acids and suitable substrates [19]. These reactions typically lead to formation of multiple bonds between the surface and each protein molecule, resulting in a heterogeneous population of bound biomolecules. The residues involved in binding to the surface may also be in or near the regions needed for bioactivity of the molecule, e.g., the receptor-binding site of a growth factor or the active site of an enzyme. Thus, reduction in biological activity of the molecules remains a concern following their immobilization. Bioactivity can be further compromised because of altered conformation resulting from the immobilization procedure or from steric hindrance following attachment to the biomaterial surface [3].
Properly designed schemes for attaching biomolecules to surfaces can minimize, or even eliminate, the adverse effects on bioactivity just described. Although not yet widely applied to biomaterials, research has focused on developing well-defined strategies by which proteins can be immobilized in an orderly manner [3]. Directed immobilization requires functionalization of the target molecule, tailoring of the surface, or both. Approaches investigated include: (1) immobilization of glycoproteins through carbohydrate residues [20], (2) immobilization of biotinylated proteins to surfaces containing avidin or streptavidin [21], (3) immobilization of proteins using affinity tags, such as a polyhistidine tail [22], and (4) immobilization of proteins to surfaces through specific cysteine residues [23].
In the present work, the strategy was to bind PTH(1-34) via its N-terminus. For this to occur, a singular functional group able to bind specifically to surfaces must be made available. To this end, the intrinsic amino terminal serine residue was oxidized to yield a single aldehyde moiety that was subsequently bound to hydrazide-derivatized substrates. By conducting the coupling reaction under conditions in which primary amines are protonated, and therefore unreactive to the aldehyde, a stable hydrazone bond was formed. This approach differs from literature reports using the amino terminus of biomolecules for PEGylation or developing solid phase supports, such as by enzymatic ligation [24], reductive alkylation with aldehyde-containing components [25], and acylation for binding to thiol-containing supports [26]. In addition to its relative ease of use, the method did not require significant modification of the biomolecules, and it did not change their charge, because amino groups of PTH(1-34) were not involved. Furthermore, the approach avoided difficulties associated with careful modulation of reaction conditions, e.g., pH, needed to obtain selective binding of just one functional group [15].
This approach also differed from reports in which affinity tags were attached to the termini of vascular endothelial growth factor [27] or epidermal growth factor [28]. In the former, a 15 amino acid sequence ending with a Cys was added to the growth factor’s N-terminus by recombinant DNA techniques. Using thiol chemistry, multiple copies of the modified molecule were conjugated to fibronectin and subsequently adsorbed on tissue culture plastic. In the latter paper, epidermal growth factor was genetically modified to contain a His tag at is C-terminus. It was then bound to Ni(II)-chelated surfaces for expansion of neural stem cells.
Two important parameters affecting bioactivity of immobilized molecules are surface density of functional groups and the length of spacer between protein and surface. Several reports have described the significance of peptide density on cellular responses [29–31], and it is well-documented that the distance between biomolecule and substrate has a significant effect on its activity [32–34]. In the current work, the objective was to investigate effects of spacer length on accessibility and activity following controlled immobilization PTH(1-34). Thus, comparable surface densities of the four dihydrazide molecules were used, and a maximal amount of peptide was bound.
Although comparable amounts of PTH(1-34) were immobilized on hydrazide-derivatized PLGA, significantly less was attached when randomly bound or simply adsorbed. Besides the difference in surface chemistry, orientation of the peptide molecules as they interact with the surface likely played a role in this effect. Both crystal and NMR solution structure analyses of PTH(1-34) show a helical conformation, with the solution structure having some flexibility in the central region [35,36]. Considering this helical structure, binding of the peptide via only its N-terminus would orient it “end on”, and this would be accentuated when the molecules are present at high density. In contrast, use of free, reactive amino groups (during random immobilization via the N-terminus and/or Lys at residues 13, 26, and 27) or other available functional groups (adsorption) could result in both “end on” and “side on” orientations. From the structure deposited in the Protein Data Bank (accession code 1ET1), the helix is approximately 5.1 nm in length and about 0.5 nm in diameter. An over 10-fold difference in projected surface area exists between the two orientations at 0.2 nm2 and 2.5 nm2 for end-on and side-on, respectively. With molecules occupying a much larger area with “side-on” and mixed orientations, steric effects decreased the total amount of peptide bound.
Antibodies are useful for probing the conformation of proteins on surfaces [37,38], which provides information about their availability for potential binding to cells. In the present studies, two antibodies were used to evaluate accessibility of immobilized PTH(1-34). Because the immobilization scheme was intended to tether the peptide by only its N-terminus, using longer dihydrazide spacer molecules would be expected to make the amino terminal epitope more accessible. Indeed, this was observed; antibody binding increased with the length of spacer, which increased distance of the peptide molecules from the surface. With random immobilization, although the peptide molecules were attached directly to the substrate, the N-terminus was available because of abundant “side on” orientations that made the epitope accessible. During adsorption at neutral pH, hydrophobic interactions as well as electrostatic interaction between deprotonated carboxylic acid groups on PLGA and protonated amines, including the N-terminus, of PTH(1-34) may have caused denaturation and greatly reduced accessibility of the peptide. Regarding availability of the C-terminus, peptide attached to the different surfaces was comparably available, with the exception of that bound using the longest spacer. In light of the potential for flexibility in the center of the molecule [35], the greater separation of PTH(1-34) from the surface resulting from immobilization using the C10 dihydrazide enhanced antibody binding to the carboxy-terminal epitope.
To ultimately determine if controlled orientation of PTH(1-34) via the N-terminus improves bioactivity, the ability of the peptide-modified surfaces to increase the intracellular content of cAMP in osteoblastic cells was measured. Effects of PTH are mediated through the PTH/PTHrP (PTH-related protein) receptor, which is a G-protein coupled receptor [4]. Although stimulation of adenylate cyclase is the most common action following binding of PTH to the receptor, phospholipase C mediated events also may occur.
While early studies showed that bioactivity of PTH resides within the first 34 residues of the hormone [5], subsequent investigations further identified the essential segments. The N-terminal region is critical for activating the PTH/PTHrP, but the C-terminal domain is needed for high affinity binding to the receptor [35,36]. For example, removing just the first two amino acids prevents full adenylate cyclase activity, yet the peptide still binds to the PTH/PTHrP receptor.
In the present work, as would be expected because of the greater space requirements for binding of cell surface receptors to immobilized peptide molecules, the presence of the dihydrazide spacer molecules enhanced bioactivity of the attached PTH(1-34). The longest (C10) spacer resulted in the largest intracellular content of cAMP. In comparison, Itoyama et al. [34] reported that bioactivity of the immobilized enzyme papain peaked with a spacer between four and six carbons or increased monotonically through eight carbons. Whereas PTH(1-34) either randomly immobilized via any available amino group or simply adsorbed on PLGA stimulated the MC3T3-E1 cells, with immobilization being marginally better, they were significantly less effective than peptide bound via the C10 molecule. These results are consistent with the need for both the N- and C-terminal regions of PTH(1-34) for full bioactivity.
CONCLUSIONS
Immobilization of osteotropic peptides and proteins on biomaterial surfaces places them precisely where they are needed, i.e., at the tissue-implant interface. If biomolecules are properly presented to cells during the wound healing process, desirable events may be preferentially enhanced. The objective of the present work was to determine if controlled attachment of PTH(1-34) solely by its N-terminus would make it more accessible for subsequent interaction with osteoblastic cells. Results demonstrate that tethering the peptide to PLGA surfaces using dihydrazide spacer molecules made its terminal regions more accessible, which translated to better cellular activity as measured by cAMP production. The approach described is applicable to any biomolecule with an intrinsic N-terminal serine or into which one can be introduced. This directed binding of bioactive molecules to biomaterial surfaces presents the species for binding with cells in a manner that enhances interaction with cell surface receptors.
Acknowledgments
This work was supported by NIH/NIAMS (AR048700).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter. 2008;4:46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- 2.Morra M. Biochemical modification of titanium surfaces: peptides and ECM proteins. Eur Cell Mater. 2006;12:1–15. doi: 10.22203/ecm.v012a01. [DOI] [PubMed] [Google Scholar]
- 3.Rao SV, Anderson KW, Bachas LG. Oriented immobilization of proteins. Mikrochim Acta. 1998;128:127–143. [Google Scholar]
- 4.Brown EM, Juppner H. Parathyroid Hormone: synthesis, secretion, and action . In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, D.C: American Society for Bone and Mineral Research; 2006. pp. 90–99. [Google Scholar]
- 5.Potts JT, Jr, Tregear GW, Keutmann HT, Niall HD, Sauer R, Deftos LJ, et al. Synthesis of a biologically active N-terminal tetratriacontapeptide of parathyroid hormone. Proc Natl Acad Sci USA. 1971;68:63–7. doi: 10.1073/pnas.68.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podbesek R, Edouard C, Meunier PJ, Parsons JA, Reeve J, Stevenson RW, et al. Effects of two treatment regimes with synthetic human parathyroid hormone fragment on bone formation and the tissue balance of trabecular bone in greyhounds. Endocrinology. 1983;112:1000–6. doi: 10.1210/endo-112-3-1000. [DOI] [PubMed] [Google Scholar]
- 7.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 8.Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1-34) on rat bone. Bone. 1995;16:477–84. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 9.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–48. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Puleo DA, Kissling RA, Sheu M-S. Immobilization of bioactive protein, including BMP-4, on plasma-treated Ti-6Al-4V. Biomaterials. 2002;23:2079–2087. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 11.Puleo DA. Retention of enzymatic activity immobilized on silanized Co-Cr-Mo and Ti-6Al-4V. J Biomed Mater Res. 1997;37:222–228. doi: 10.1002/(sici)1097-4636(199711)37:2<222::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Geoghegan KF, Stroh JG. Site-directed conjugation of nonpeptide groups to peptides and proteins via periodate oxidation of a 2-amino alcohol. Application to modification at N-terminal serine. Bioconjug Chem. 1992;3:138–146. doi: 10.1021/bc00014a008. [DOI] [PubMed] [Google Scholar]
- 13.Gaertner HF, Offord RE. Site-specific attachment of functionalized poly(ethylene glycol) to the amino terminus of proteins. Bioconjug Chem. 1996;7:38–44. doi: 10.1021/bc950074d. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe CA, Hage DS. Studies on the rate and control of antibody oxidation by periodate. Anal Biochem. 1995;231:123–130. doi: 10.1006/abio.1995.1511. [DOI] [PubMed] [Google Scholar]
- 15.Hermanson GT. Bioconjugate Techniques. San Diego: Academic Press; 1996. [Google Scholar]
- 16.O’Shannessy DJ, Wilchek M. Immobilization of glycoconjugates by their oligosaccharides: use of hydrazido-derivatized matrices. Anal Biochem. 1990;191:1–8. doi: 10.1016/0003-2697(90)90377-l. [DOI] [PubMed] [Google Scholar]
- 17.Raiche AT, Puleo DA. Cell responses to BMP-2 and IGF-I released with different time-dependent profiles. J Biomed Mater Res Part A. 2004;69A:342–350. doi: 10.1002/jbm.a.30006. [DOI] [PubMed] [Google Scholar]
- 18.Jeon JH, Thomas MV, Puleo DA. Bioerodible devices for intermittent release of simvastatin acid. Int J Pharm. 2007;340:6–12. doi: 10.1016/j.ijpharm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor RF. Protein Immobilization: Fundamentals and Applications. New York: Marcel Dekker, Inc; 1991. [Google Scholar]
- 20.O’Shannessy DJ, Hoffman WL. Site-directed immobilization of glycoproteins on hydrazide-containing solid supports. Biotechnol Appl Biochem. 1987;9:488–496. doi: 10.1111/j.1470-8744.1987.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilchek M, Bayer EA. Introduction to avidin-biotin technology. Methods Enzymol. 1990;184:5–13. doi: 10.1016/0076-6879(90)84256-g. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson J, Mosbach K, Bülow L. Affinity precipitation and site-specific immobilization of proteins carrying polyhistidine tails. Biotechnol Bioeng. 1996;51:221–228. doi: 10.1002/(SICI)1097-0290(19960720)51:2<221::AID-BIT12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Viswanath S, Wang J, Bachas LG, Butterfield DA, Bhattacharyya D. Site-directed and random immobilization of subtilisin on functionalized membranes: activity determination in aqueous and organic media. Biotechnol Bioeng. 1998;60:608–616. [PubMed] [Google Scholar]
- 24.Tanaka T, Kamiya N, Nagamune T. N-terminal glycine-specific protein conjugation catalyzed by microbial transglutaminase. FEBS Lett. 2005;579:2092–6. doi: 10.1016/j.febslet.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 25.Na DH, Lee KC, DeLuca PP. PEGylation of octreotide: II. Effect of N-terminal mono-PEGylation on biological activity and pharmacokinetics. Pharm Res. 2005;22:743–9. doi: 10.1007/s11095-005-2590-y. [DOI] [PubMed] [Google Scholar]
- 26.Wetzel R, Halualani R, Stults JT, Quan C. A general method for highly selective cross-linking of unprotected polypeptides via pH-controlled modification of N-terminal alpha-amino groups. Bioconjug Chem. 1990;1:114–22. doi: 10.1021/bc00002a005. [DOI] [PubMed] [Google Scholar]
- 27.Backer MV, Patel V, Jehning BT, Claffey KP, Backer JM. Surface immobilization of active vascular endothelial growth factor via a cysteine-containing tag. Biomaterials. 2006;27:5452–8. doi: 10.1016/j.biomaterials.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Nakaji-Hirabayashi T, Kato K, Arima Y, Iwata H. Oriented immobilization of epidermal growth factor onto culture substrates for the selective expansion of neural stem cells. Biomaterials. 2007;28:3517–29. doi: 10.1016/j.biomaterials.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113:1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 30.Griffith LG, Lopina S. Microdistribution of substratum-bound ligands affects cell function: Hepatocyte spreading on PEO-tethered galactose. Biomaterials. 1998;19:979–986. doi: 10.1016/s0142-9612(97)00185-3. [DOI] [PubMed] [Google Scholar]
- 31.Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. J Biol Chem. 2000;275:6813–6818. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- 32.Hou KC, Zaniewski R, Roy S. Protein A immobilized affinity cartridge for immunoglobulin purification. Biotechnol Appl Biochem. 1991;13:257–268. [PubMed] [Google Scholar]
- 33.Naumann M, Reuter R, Metz P, Kopperschlager G. Affinity chromatography of bovine heart lactate dehydrogenase using dye ligands linked directly or spacer-mediated to bead cellulose. J Chromatogr. 1989;466:319–329. doi: 10.1016/s0021-9673(01)84627-6. [DOI] [PubMed] [Google Scholar]
- 34.Itoyama K, Tanibe H, Hayashi T, Ikada Y. Spacer effects on enzymatic activity of papain immobilized onto porous chitosan beads. Biomaterials. 1994;15:107–112. doi: 10.1016/0142-9612(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 35.Barden JA, Kemp BE. NMR solution structure of human parathyroid hormone(1-34) Biochemistry. 1993;32:7126–32. doi: 10.1021/bi00079a008. [DOI] [PubMed] [Google Scholar]
- 36.Jin L, Briggs SL, Chandrasekhar S, Chirgadze NY, Clawson DK, Schevitz RW, et al. Crystal structure of human parathyroid hormone 1-34 at 0.9-A resolution. J Biol Chem. 2000;275:27238–44. doi: 10.1074/jbc.M001134200. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Simonovsky FI, Ratner BD, Horbett TA. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: a comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J Biomed Mater Res A. 2005;74:722–38. doi: 10.1002/jbm.a.30381. [DOI] [PubMed] [Google Scholar]
- 38.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–98. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]