Abstract
Defects causing severe combined immunodeficiency (SCID) have been reported in pathways mediating antigen receptor rearrangement, antigen receptor and cytokine signaling, and purine metabolism. Recognizing that the actin regulator Coronin-1A is essential for development of a normal peripheral T cell compartment in mouse models, we identified absence of Coronin-1A in a girl with T-B+NK+ SCID who suffered recurrent infections including severe post-vaccination varicella at age 13 months. Murine Coronin-1A is essential for release of T cells from the thymus, consistent with the paradoxically detectable thymus in our patient. Molecular analysis revealed a 2 bp deletion in the paternal CORO1A coding sequence paired with a 600kb de novo deletion encompassing CORO1A on the maternal allele. This genomic region at 16p11.2 is subject to recurrent copy number variations associated with autism spectrum disorders, including attention deficit and hyperactivity, present in our patient. This case highlights the first link between actin cytoskeleton regulation and SCID.
Keywords: SCID, Coronin-1A, actin cytoskeleton, chromosome 16p11.2, autism spectrum, attention deficit and hyperactivity
INTRODUCTION
Patients with severe combined immunodeficiency (SCID) exhibit T lymphocytopenia and profound impairments in cellular and humoral immunity. Previously defined molecular defects causing SCID affect pathways mediating antigen receptor rearrangement, antigen receptor signaling, cytokine signaling, and purine metabolism. While mutations in actin and actin regulatory genes have been linked to defects in phagocytosis[1], and the Wiskott-Aldrich syndrome protein WASP is involved in actin polymerization at immunologic synapses[2], an association between actin regulation and SCID has not previously been reported.
Coronins are a highly conserved family of actin regulatory genes[3]. Mice with mutations in Coronin-1A, expressed predominantly in hematopoeitic cells, are T-lymphocytopenic in part due to inability of mature T cells to be released from the thymus into the peripheral circulation[4; 5]. This mouse phenotype prompted an assessment of the CORO1A gene in a cohort of SCID cases of unknown cause. One patient, described below, lacked Coronin-1A expression[5]. This girl had developed chickenpox after receiving live attenuated varicella vaccine and had a T-B+NK+ SCID phenotype, though lymphocyte function was not totally absent. She also had attention deficit hyperactivity disorder (ADHD). Her findings are explained by a mutation in one copy of the CORO1A coding region plus a heterozygous deletion of chromosome 16p11.2 encompassing 25 genes, including CORO1A.
MATERIALS AND METHODS
Patients with SCID and their parents, when available, were enrolled with informed content in IRB-approved protocols to determine genotype and genotype/phenotype correlations. Medical records were reviewed, including results of relevant neurodevelopmental assessments (Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III); Young Children’s Achievement Test (YCAT); and Behavior Assessment System for Children (BASC-2) Parent Version).
Blood samples were transformed with Epstein-Barr virus (EBV) to create B cell lines[6], which in turn were used for flow cytometry. Intracellular immunoflourescence staining was performed using a CytoFix/CytoPerm kit (BD) as directed with polyclonal rabbit anti-Coronin1A serum (Millipore/Upstate cat. no. 07-493) followed by FITC-labeled goat anti-rabbit antibody (Jackson ImmunoResearch cat. no. 111-096-144). DNA was extracted from buccal, blood and EBV cell samples using a Puregene Tissue Extraction kit (Gentra Systems). DNA samples were digested, end-labeled and hybridized to oligonucleotide arrays (Affymetrix Human Mapping SNP 6.0 GeneChip) using the SNP6.0 assay kit and following the manufacturer’s protocol. Copy number was determined using the Affymetrix Genotyping Console and HapMap270 reference model.
CASE HISTORY
The female patient was born at term weighing 5.5 lbs. Her parents were not consanguinous, and there was no history of early death, miscarriage, immunodeficiency, or neurocognitive disorder in an older sister or other relatives. The patient’s first year was marked by two episodes of otitis, upper respiratory tract infections, gasteroesophageal reflux and pneumonia. She had oral thrush requiring treatment with oral fluconazole. She received routine immunizations including live attenuated varicella vaccine at 13 months of age. Three weeks after receiving this vaccine, cutaneous chickenpox vesicles developed, rapidly covering skin and mucosal surfaces despite administration of oral acyclovir. Hospitalization for intravenous acyclovir brought about eventual recovery.
An immunologic workup at 15 months of age revealed an absolute lymphocyte count of only 900 with only 110 T cells (normal 10–90% range 2,100–6,200), but near normal numbers of B and NK cells as well as immunoglobulins [7,8] (Table 1). PCR for HIV was negative. Proliferative responses to mitogens were low, and specific antibody titers to tetanus and pneumococcal antigens were low to undetectable despite prior vaccinations. A distinctly atypical feature for SCID was a chest CT scan showing the presence of a thymus (Figure 1A). Adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP) enzyme activities were normal as were DNA coding and splice region sequences of the SCID genes encoding the IL-2 receptor gamma chain (IL2RG), IL-7 receptor α-chain (IL7R), Janus kinase 3 (JAK3), and CD45 (CD45).
Table 1.
Representative immunological studies of blood lymphocytes.
| Patient age (mo) | 15 (after varicella) | 23 | 36–49 |
| WBC/ul | 3,200 | 5,310 | 3.800 |
| Lymphocytes/ul | 9001 | 1,430 | 950 |
| Lymphocyte subsets: number (%) | |||
| CD3+ T cells | 110 (12) | ND2 | 330 (35) |
| CD4+ T helper | 50 (6) | ND | 170 (18) |
| CD8+ T cytotoxic | 30 (3) | ND | 100 (10) |
| CD56/16+, CD3minus; NK cells | 340 (28) | ND | 360 (38) |
| CD19+ B cells | 410 (45) | ND | 250 (26) |
| CD3/45RA %Tcells | 67 (7) | ND | ND |
| CD3/45RO %Tcells | 33 (4) | ND | ND |
| Immunoglobulins (mg/dl) | |||
| IgM | 463 | 55 | 69 |
| IgG | NE4 | 889 | NE |
| IgA | 42 | 88 | 48 |
| IgE | 15 | 10 | 12 |
| Proliferation studies (cpm) | |||
| Phytohemagglutinin | 38,2275 | ND | 10,381 |
| ConcanavalinA | 6,270 | ND | 26,080 |
| Pokeweed mitogen | 4,874 | ND | 10,219 |
| Specific antibody titers | |||
| Tetanus toxoid | 0.02 | ND | ND |
| Hemophilus influenzae type B | 0.7 | ND | ND |
| Varicella, IgM specific | 0.03 | ND | ND |
| Pneumococcal serotype 46 | <0.08 | 1.06 | ND |
| Pneumococcal serotype 6B | <0.08 | 0.28 | ND |
| Pneumococcal serotype 9V | <0.08 | 1.44 | ND |
| Pneumococcal serotype 14 | 0.13 | 3.02 | ND |
| Pneumococcal serotype 18C | 0.08 | 0.6 | ND |
| Pneumococcal serotype 19F | <0.08 | 0.2 | ND |
| Pneumococcal serotype 23F | <0.08 | 0.95 | ND |
| Phage Φ X174 peak titers | |||
| Primary exposure IgM (geom. mean of controls, 8.71) | 2.69 | ||
| Secondary exposure, (geom. mean of controls 550 with 48±25% IgG) | 3.82 7.4% IgG |
Normal child 10–90% ranges[7], 12–24 months (24 months to 6 years, itallics): lymphocytes 3,600–8,900 (2,300–5,400); T cells 2,100–6,200 (1,400–3,700); CD4 1,300–3,400 (700–2,200); CD8 620–2000 (490–1,300); B cells 720–2,600 (390–1,400).
Not done.
Mean immunoglobulin levels±SD[8], 13–24 months (36 months to 5 years, italics): IgM 58 ±23 (61±19); IgG 762±209 (892±183); IgA 50±24 (71±37); IgE 29 (31).
Not evaluable; patient may have received immunoglobulin G prior to 15 mo and did receive it after 24 mo of age.
Reference ranges: PHA, 25,362–241,075; ConA, 21,535–129,556; PWM, 3.665–40,761).
Patient had received 3 doses of 7-valent, protein conjugated pneumococcal vaccine.
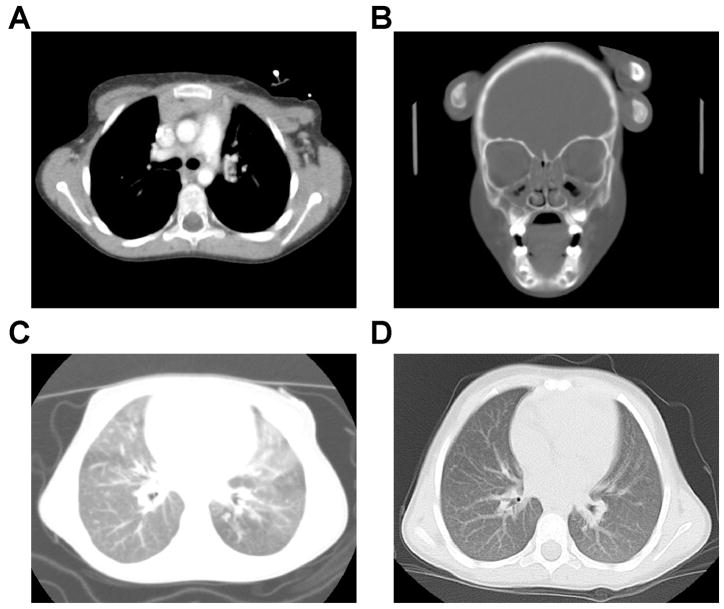
Figure 1.
A. Chest computed tomographic (CT) scan demonstrating presence of thymic tissue in the anterior mediastinum.
B. CT scan of sinuses at age 4, showing complete opacification of the ethmoid air cells with air fluid levels in both maxillary sinuses consistent with pansinusitis. Cultures of sinus aspirates grew Strep. pneumoniae and Moraxella catarrhalis.
C. Chest CT scan at age 4, showing diffuse “ground glass” opacification with superimposed scattered nodules.
D. Chest CT scan at age 5 (12 months post SCT) showing resolution of opacities, normal lung fields and no significant residual pulmonary disease.
Pneumocystis prophylaxis with trimethoprim/sulfamethoxazole was prescribed at age 15 months. Over the following year, the patient suffered from persistent otitis requiring placement of pressure equalization tubes, a hospitalization for rotavirus diarrhea with dehydration, and increasingly severe, recurrent upper and lower respiratory tract infections. At age 2, intravenous immunoglobulin infusions were instituted, but breakthrough infections required nearly continuous additional antibiotics and several hospitalizations. The patient failed to grow and exhibited delays in language and motor development. She suffered from chronic pansinusitis (Figure 1B), and her lung fields developed a ground glass appearance consistent with chronic infection, though no specific organisms were isolated (Figure 1C). At age 33 months, repeat lymphocyte stimulation tests showed poor responses to mitogens and absent responses to tetanus or candida antigens. Antibody response to immunization with bacteriophage φX-174 was markedly impaired, with low primary IgM production and, after a booster injection, little increase in titer and only 8% switching from IgM to IgG (Table 1). This pattern was consistent with lack of helper T cell function for isotype switching, with the possibility of an additional intrinsic B cell defect.
At age 4 years, the patient received a matched unrelated cord blood hematopoietic cell tranplant (HCT) following cytoreduction with fludaribine, melphalan and monoclonal anti-hCD52 antibody. After weathering a post-transplant adenovirus infection and grade I graft vs. host disease (limited to skin and treated with topical steroids), she recovered with complete myeloid and lymphoid donor chimerism. She is currently thriving without immunoglobulin supplementation or antibiotics and has attained normal stature. Pulmonary infiltrates have resolved (Figure 1D).
Neuropsychological evaluation both before and after HCT revealed low average to average cognitive functioning and significant impairment in attention, interaction with others, and functional communication. In school she has exhibited behavioral difficulties, hyperactivity, impulsivity and inattention diagnostic of attention deficit hyperactivity disorder (ADHD). The use of stimulant medication has improved her ability to participate in school, sports and social outlets.
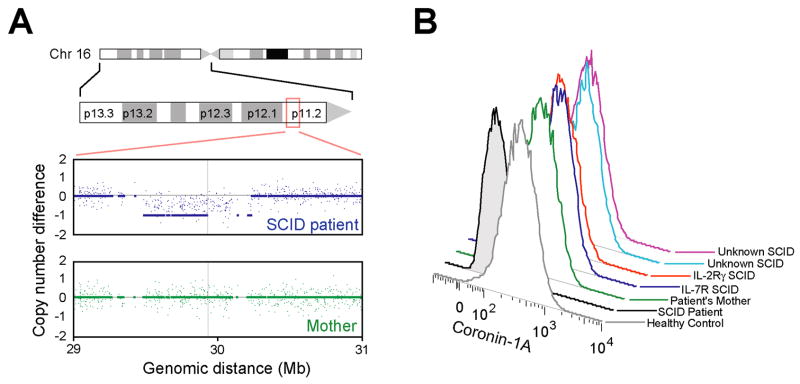
The recent finding that mice with defects of the actin regulatory protein Coronin-1A lack peripheral T lymphocytes in part due to failure of egress from the thymus[4; 5] suggested a defect in the human CORO1A gene as the cause of SCID in this patient. Indeed, her genomic DNA sequence revealed a 2 bp deletion in CORO1A exon 3, c.248-249delCT (not shown), resulting in a frameshift and premature stop codon (p.Pro83ArgfsX10)[5]. This mutation was present in heterozygosity, as expected, in the DNA of the patient’s father, but her mother’s DNA sequence was wild type, suggesting the possibility of a whole gene deletion in the patient, mother or both. Copy number analysis the patient’s and the mother’s genomic DNA on genome-wide oligonucleotide arrays (Figure 2A) revealed that the patient, but not her mother, had hemizygosity throughout a region of 600 kb on chromosome 16p11.2, diagnosing a de novo interstitial deletion encompassing CORO1A and 24 other genes.
Figure 2.
A. Copy number analysis of patient and mother’s genomic DNA across a 2 Mb region of chromosome 16p11.2. Signal intensities from arrays hybridized to DNA from patient (blue) and mother (green) are normalized reference healthy control subjects.
B. Expression of Coronin-1A protein detected by fixation, permeabilizaton and intracellular staining of EBV transformed cells followed by flow cytometry.
The patient’s mutations were predicted to result in absence of Coronin-1A expression, and this was readily confirmed by a flow cytometry assay using B cell lines stained with a polyclonal rabbit anti-Coronin-1A antibody (Figure 2B). Intensity of Coronin-1A staining in the mother’s cells was equivalent to that of control cells. Cells from patients with SCID due to known mutations in IL2RG and IL7R also had normal levels of Coronin-1A (Fig. 2B) as did JAK3 mutant cells (not shown). Using this simple flow cytometric approach as a screen for Coronini-1A deficiency, we tested two additional cell lines from T-B+NK+ SCID patients of unknown etiology, both of which showed normal abundance of Coronin-1A (Fig. 2B).
DISCUSSION
SCID is defined by profound deficiency of T cell numbers and of T and B cell function, leading to opportunistic infections and early death unless treated[9]. We describe the first case of human SCID due to CORO1A deficiency. Our patient developed progressively worsening infections that necessitated HCT, which has been curative. Although the majority of SCID infants are diagnosed in the first year of life, delayed presentations have been described, some of which involve leaky genetic defects, such as hypomorphic mutations allowing residual activity of the ADA enzyme[10; 11]. In contrast, our patient carries null alleles of CORO1A. Thus, her delayed diagnosis reflects either meticulous management of infections, preservation of some functions in a very small pool of peripheral T cells despite Coronin-1A deficiency, or both.
Coronins regulate the actin cytoskeleton through antagonizing actin polymerization and promoting actin severing[3], in contrast to the role of Wiskott Aldrich syndrome protein (WASP) that promotes actin polymerization[2]. While WASP has already shown how actin can be linked to hematopoietic cell dysfunction, CORO1A deficiency now highlights the role of actin cytoskeleton regulation in T cell homeostasis and therefore SCID. Mutations causing SCID have previously been defined in genes that disrupt pathways mediating antigen receptor rearrangement (RAG1/2 and DCLRE1C); the transmission of signals delivered by antigen, cofactor or cytokine binding (CD3 component genes, CD45, IL2RG, JAK3); or purine metabolism (ADA, PNP). Our patient’s mutations in CORO1A now define defective actin regulation as a new pathway in which genetic lesions causing SCID can be found.
Distinctive features of this patient’s immunodeficiency are consistent with findings in mouse models of Coronin-1A deficiency. Unlike other coronin family members, Coronin-1A is expressed primarily in the hematopoeitic system[3], and in Coronin-1A deficient mice, only the T cell compartment was affected[4; 5; 12; 13]. Additional coronin family members, such as Coronin-1B or Coronin-1C, may compensate for Coronin-1A deficiency in other leukocytes. This functional compensation appears to occur similarly in humans because our patient had a T-B+NK+ SCID phenotype. The T cells recoverable in small numbers from Coronin-1A deficient mice have an intrinsic migration defect and diminished, but not absent signaling[4; 5; 12]; similarly our patient’s few T cells had residual function, including proliferative responses to PHA and ConA, and enough B cell helper function to produce normal serum antibody concentrations and low, but measurable antibody titers to pneumococcal protein conjugates (Table 1). Pre-HCT T cells were not available to measure T cell receptor diversity. However T cell function was insufficient to provide normal immune protection from infectious agents such as vaccine strain varicella virus, necessitating eventual HCT therapy. This T cell specific defect suggests that Coronin-1A could be a future therapeutic target for selective modulation of T cell immune responses.
Dysregulation of the actin cytoskeleton impairs survival and egress of mature single positive CD4+CD8− or CD4−CD8+ T cells from the murine thymus[4; 5]. In contrast to most human SCID patients in whom a small or undetectable thymus is a helpful diagnostic feature[14], our Coronin-1A deficient patient had a thymic image on a pre-transplant CT scan, consistent with thymic accumulation of T cells that were unable to exit into the peripheral compartment[5]. Interestingly, an intact thymus has also been reported in SCID patients with CD3δ deficiency, associated with impaired differentiation beyond the somewhat earlier double negative CD4−CD8− stage of thymocyte maturation[15].
The patient’s de novo 600 kb deletion on her maternal copy of chromosome 16p11.2 is flanked by 146 kb segmental duplications with >99% identity (annotated as duplication cluster 3576 [16]). Genomic regions flanked by such duplications are prone to copy number variation (CNV) through non-allelic homologous recombination[17]. Indeed, deletion as well as duplication of this same interval on 16p11.2 is well recognized and has been associated with autism spectrum disorder and neurodevelopmental disorders including ADHD[18; 19; 20]. Thus, in addition to contributing to CORO1A deficiency, our patient’s 16p11.2 deletion also predisposed her to ADHD. CORO1A is expressed in microglia[21], but the particular gene or genes within the deleted region that are related to neurological phenotypes of 16p11.2 CNV are not yet known. Reduced Coroin-1A is not likely to be the basis for neurocognitive difficulties in 16p11.2 CNV cases since its complete deficiency was not accompanied by a severe neurocognitive phenotype in our patient.
CNV is an increasingly appreciated form of genomic diversity with reports estimating that up to 12% of human genes are located in CNV-prone regions[22]. Both de novo and inherited CNVs are recognized in many human conditions[23; 24; 25; 26]. Low copy number repeats flank the DiGeorge syndrome region at 22q11.2[27] and Alu-mediated deletions have resulted in Bruton’s agammaaglobulinemia[16; 28; 29; 30] and ADA deficiency[31; 32]. CNV at 16p11.2 has been estimated to occur in 1.5% of subjects with developmental delay, 1% of individuals with autism, and 0.1% in patients with psychiatric or language disorders, but less than 0.01% in the general population[18]. Given the propensity for CNV at 16p11.2, we anticipate that future cases T-B+NK+ SCID will involve CORO1A defects, including deletions. The flow cytometric assay described here is an efficient means to evaluate patients who have SCID or combined immunodeficiency of unknown genotype, or atypically severe varicella, for Coronin-1A protein expression. Moreover, molecular investigation with high-resolution genomic arrays may reveal additional CNVs that underlie other undefined cases of primary immunodeficiency.
Acknowledgments
We thank the patient and her family; Joseph Roberts, Rebecca Buckley and Hans Ochs for immunological evaluations; Lolie Yu for transplantation expertise and care; Tonya Lebet for mutation analysis of SCID genes; and Stacy L. Musone and Pui Kwok for help with analysis of copy number variation. Support is gratefully acknowledged from the UCSF Medical Scientist Training Program and Genentech Sandler Family Foundation (L.R.S.), the US Immunodeficiency Network and Jeffrey Modell Diagnostic Centers for Primary Immunodeficiency (J.M.P., R.U.S.), the Howard Hughes Medical Institute and the National Institutes of Health (J.G.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geha RS, Notarangelo LD, Casanova JL, Chapel H, Conley ME, Fischer A, Hammarstrom L, Nonoyama S, Ochs HD, Puck JM, Roifman C, Seger R, Wedgwood J. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120:776–94. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochs HD, Notarangelo LD. Structure and function of the Wiskott-Aldrich syndrome protein. Curr Opin Hematol. 2005;12:284–91. doi: 10.1097/01.moh.0000168520.98990.19. [DOI] [PubMed] [Google Scholar]
- 3.Uetrecht AC, Bear JE. Coronins: the return of the crown. Trends Cell Biol. 2006;16:421–6. doi: 10.1016/j.tcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Foger N, Rangell L, Danilenko DM, Chan AC. Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science. 2006;313:839–42. doi: 10.1126/science.1130563. [DOI] [PubMed] [Google Scholar]
- 5.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, Xu Y, Jenne CN, Foger N, Sorensen RU, Goodnow CC, Bear JE, Puck JM, Cyster JG. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9:1307–15. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller G. Immortalization of human lymphocytes by Epstein-Barr virus. Yale J Biol Med. 1982;55:305–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland E, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA. Lymphocyte subsets in healthy children from birth through 18 years of age: The Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Steihm ER, Ochs HD, Winkelstein JA. Immunodeficiency disorders: General considerations. In: Stiehm ER, Ochs HD, Winkelstein JA, editors. Immunologic Disorders in Infants and Children. 5. Elsevier Saunders; Philadelphia: 2004. pp. 307–308. [Google Scholar]
- 9.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 10.Hirschhorn R, Chen AS, Israni A, Yang DR, Huie ML. Two new mutations at the adenosine deaminase (ADA) locus (Q254X and del nt1050–54) unusual for not being missense mutations. Hum Mutat. 1993;2:320–3. doi: 10.1002/humu.1380020415. [DOI] [PubMed] [Google Scholar]
- 11.Arredondo-Vega FX, Santisteban I, Daniels S, Toutain S, Hershfield MS. Adenosine deaminase deficiency: genotype-phenotype correlations based on expressed activity of 29 mutant alleles. Am J Hum Genet. 1998;63:1049–59. doi: 10.1086/302054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haraldsson MK, Louis-Dit-Sully CA, Lawson BR, Sternik G, Santiago-Raber ML, Gascoigne NR, Theofilopoulos AN, Kono DH. The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity. 2008;28:40–51. doi: 10.1016/j.immuni.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller P, Massner J, Jayachandran R, Combaluzier B, Albrecht I, Gatfield J, Blum C, Ceredig R, Rodewald HR, Rolink AG, Pieters J. Regulation of T cell survival through coronin-1-mediated generation of inositol-1,4,5-trisphosphate and calcium mobilization after T cell receptor triggering. Nat Immunol. 2008;9:424–31. doi: 10.1038/ni1570. [DOI] [PubMed] [Google Scholar]
- 14.Buckley RH. Primary immunodeficiency or not? Making the correct diagnosis. J Allergy Clin Immunol. 2006;117:756–8. doi: 10.1016/j.jaci.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Dadi HK, Simon AJ, Roifman CM. Effect of CD3delta deficiency on maturation of alpha/beta and gamma/delta T-cell lineages in severe combined immunodeficiency. N Engl J Med. 2003;349:1821–8. doi: 10.1056/NEJMoa031178. [DOI] [PubMed] [Google Scholar]
- 16.Cheung J, Estivill X, Khaja R, MacDonald JR, Lau K, Tsui LC, Scherer SW. Genome-wide detection of segmental duplications and potential assembly errors in the human genome sequence. Genome Biol. 2003;4:R25. doi: 10.1186/gb-2003-4-4-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper GM, Nickerson DA, Eichler EE. Mutational selective effects on copy-number variants in the human genome. Nat Genet. 2007;39:S22–9. doi: 10.1038/ng2054. [DOI] [PubMed] [Google Scholar]
- 18.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, Platt OS, Ruderfer DM, Walsh CA, Altshuler D, Chakravarti A, Tanzi RE, Stefansson K, Santangelo SL, Gusella JF, Sklar P, Wu BL, Daly MJ. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–75. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 19.Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, Gilliam TC, Nowak NJ, Cook EH, Jr, Dobyns WB, Christian SL. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–38. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 20.Ghebranious N, Giampietro PF, Wesbrook FP, Rezkalla SH. A novel microdeletion at 16p11.2 harbors candidate genes for aortic valve development, seizure disorder, and mild mental retardation. Am J Med Genet A. 2007;143A:1462–71. doi: 10.1002/ajmg.a.31837. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 22.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, Cho EK, Dallaire S, Freeman JL, Gonzalez JR, Gratacos M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang F, Zhang J, Zerjal T, Zhang J, Armengol L, Conrad DF, Estivill X, Tyler-Smith C, Carter NP, Aburatani H, Lee C, Jones KW, Scherer SW, Hurles ME. Global variation in copy number in the human genome. Nature. 2006;444:444–54. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, Nibbs RJ, Freedman BI, Quinones MP, Bamshad MJ, Murthy KK, Rovin BH, Bradley W, Clark RA, Anderson SA, O’Connell JR, Agan BK, Ahuja SS, Bologna R, Sen L, Dolan MJ, Ahuja SK. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–40. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 24.Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature. 2006;439:851–5. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- 25.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Moller HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Muhleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nothen MM, Peltonen L, Collier DA, St Clair D, Stefansson K, Kahn RS, Linszen DH, van Os J, Wiersma D, Bruggeman R, Cahn W, de Haan L, Krabbendam L, Myin-Germeys I. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halford S, Lindsay E, Nayudu M, Carey AH, Baldini A, Scambler PJ. Low-copy-number repeat sequences flank the DiGeorge/velo-cardio-facial syndrome loci at 22q11. Hum Mol Genet. 1993;2:191–6. doi: 10.1093/hmg/2.2.191. [DOI] [PubMed] [Google Scholar]
- 28.Fiorini M, Franceschini R, Soresina A, Schumacher RF, Ugazio AG, Rossi P, Plebani A, Notarangelo LD. BTK: 22 novel and 25 recurrent mutations in European patients with X-linked agammaglobulinemia. Hum Mutat. 2004;23:286. doi: 10.1002/humu.9219. [DOI] [PubMed] [Google Scholar]
- 29.Jyonouchi H, Geng L, Toruner GA, Vinekar K, Feng D, Fitzgerald-Bocarsly P. Monozygous twins with a microdeletion syndrome involving BTK, DDP1, and two other genes; evidence of intact dendritic cell development and TLR responses. Eur J Pediatr. 2008;167:317–21. doi: 10.1007/s00431-007-0493-0. [DOI] [PubMed] [Google Scholar]
- 30.Rohrer J, Minegishi Y, Richter D, Eguiguren J, Conley ME. Unusual mutations in Btk: an insertion, a duplication, an inversion, and four large deletions. Clin Immunol. 1999;90:28–37. doi: 10.1006/clim.1998.4629. [DOI] [PubMed] [Google Scholar]
- 31.Berkvens TM, van Ormondt H, Gerritsen EJ, Khan PM, van der Eb AJ. Identical 3250-bp deletion between two AluI repeats in the ADA genes of unrelated ADA-SCID patients. Genomics. 1990;7:486–90. doi: 10.1016/0888-7543(90)90190-6. [DOI] [PubMed] [Google Scholar]
- 32.Markert ML, Hutton JJ, Wiginton DA, States JC, Kaufman RE. Adenosine deaminase (ADA) deficiency due to deletion of the ADA gene promoter and first exon by homologous recombination between two Alu elements. J Clin Invest. 1988;81:1323–7. doi: 10.1172/JCI113458. [DOI] [PMC free article] [PubMed] [Google Scholar]