Abstract
Natural killer (NK) cells are innate immune cells that can eliminate their targets through granule release. Here we describe a specialized role for the large GTPase Dynamin 2 (Dyn2) in the regulation of these secretory events leading to cell-mediated cytotoxicity. By modulating the expression of Dyn2 using siRNA or by inhibiting its activity using a pharmacological agent, we determined that Dyn2 does not regulate conjugate formation, proximal signaling, or granule polarization. In contrast, during cell-mediated killing, Dyn2 localizes with lytic granules and polarizes to the NK cell – target interface where it regulates the final fusion of lytic granules with the plasma membrane. These findings identify a novel role for Dyn2 in the exocytic events required for effective NK cell-mediated cytotoxicity.
Keywords: Natural Killer Cells, Signal Transduction, Cytotoxicity, Human
Introduction
Natural killer (NK) cells are critical components of the innate immune system that mediate direct killing of virally-infected or tumorigenic target cells through the release of pre-formed granules. These granules are a form of secretory lysosome and contain enzymes (including granzyme A, granzyme B, and perforin) that are delivered to the target cell and initiate apoptosis (1, 2). During the development of cell-mediated cytotoxicity the microtubule organizing center (MTOC) and granules polarize toward the target cell, and lytic granule release is controlled by specialized subcellular machinery, which facilitates vesicle – plasma membrane fusion (1, 3). The machinery of lysosome secretion in hematopoietic cells is known to utilize the regulator of vesicle docking, Rab27a (4), and the regulator of SNARE-mediated fusion, Munc 13-4 (5). However, the SNAREs and other proteins involved in the regulated secretion of this organelle are not well understood.
Dynamin 2 (Dyn2)3 is the ubiquitously-expressed isoform of the conventional dynamin family of large GTPases (also including Dyn1, found in the brain, and Dyn3, found in the lung, testis, and brain), which has been extensively characterized for its role in receptor-mediated endocytosis and membrane remodeling (6). Structurally, Dyn2 is made up of a GTPase domain, a middle domain involved in oliogmerization, a pleckstrin homology (PH) domain, a GTPase effector domain, and a proline-rich domain (PRD) important for interacting with Src homology 3 (SH3) domains. In addition to its role in endocytosis, Dyn2 has been identified as a regulator of actin assembly, interacting with several components of the actin remodeling machinery including cortactin, Grb2, and Nck (7). We have previously demonstrated that an interaction between Dyn2 and Vav1 regulates actin polymerization in T cells and modulates T cell activation (8). More recently, the yeast homologue of Dyn2, Vps1p, was shown to interact with the t-SNARE, Vam3p, and participate in the fusion of yeast vacuoles (9).
Here we describe a central role for Dyn2 in regulating NK cell-mediated cytotoxicity by controlling the exocytosis of lytic granules. Dyn2 localizes with lytic granules upon NK cell activation and polarizes toward the NK – target cytolytic synapse. Suppression of Dyn2 protein levels using RNA interference (RNAi) or pharmacological inhibition of Dyn2 impairs cell-mediated cytotoxicity. Moreover, the effects of Dyn2 are independent of the proximal signaling events that initiate the generation of a cytotoxic response. Instead, after granule polarization, Dyn2 regulates the terminal phases of granule release. Our results linking Dyn2 to the regulation of secretion in NK cells suggest a novel function for this protein in granule exocytosis as well as a broader role for Dyn2 in cell regulation.
Materials and Methods
Reagents, cells and antibodies
All reagents were obtained from Sigma unless stated otherwise. The P815 murine mastocytoma line was obtained from American Type Culture Collection. The MHC class I-deficient B lymphoblastoid cell line 721.221 was kindly provided by Peter Parham (Stanford University, Palo Alto, CA). Human NK cells were cloned and passaged as described (10). Rabbit polyclonal antibodies to PLC-γ2, SLP-76 and Vav1 were generated and characterized as previously described (11–14). Rabbit polyclonal antibodies to Dyn2 were generated as described (8). Monoclonal anti-NKG2D (R&D Systems), anti–phosphorylated tyrosine (4G10; Upstate), anti-LFA-1 (MHM23), anti-perforin, anti-CD107a-PE (BD Biosciences) and IgG1 (50327; MP Biomedicals) were purchased as indicated. Goat anti-mouse IgG F(ab)2 was obtained from MP Biomedicals.
Plasmids & Recombinant vaccinia
The sequence encoding Dyn2 used to generate vaccinia constructs were amplified, subcloned into the previously described pSP11.FLAG plasmid and recombinant vaccinia were generated by homologous recombination as previously described (8, 14). The cDNA sequence of Dyn2 was verified by PCR. Infections with vaccinia were conducted for 5 hours in serum free media at an MOI of 20:1.
Cytotoxicity assay
The 51Cr-release assays were performed as described previously (10). In some assays, NK cells were pre-treated with the Dyn2 inhibitor dynasore for 30 minutes at the indicated concentrations.
Conjugate formation
NK cells were labeled for 1 h at 37°C with 100 µM sulfofluorescein (Molecular Probes, Eugene, OR), and the K562 target cells were labeled for 1 h at 37°C with 40 µg/ml hydroethidene (Polysciences Inc., Warrington, PA). NK cells were pretreated with a final concentration of 10 µg/µl IgG1 control or anti-LFA-1 antibody for 10 minutes on ice. The cells were then washed and resuspended at a concentration of 5 × 106 cells/ml. The effectors and targets (25 µl of each) were mixed together, and allowed to incubate at 37°C for 10 min before adding 1 ml of ice-cold RPMI 1640 medium. Conjugate formation was assessed using a FACScan (BD Biosciences, San Jose, CA) and is revealed by the simultaneous emission of green and red fluorescence. Results are expressed as the percentage of total NK cells that formed conjugates. In some experiments, NK - target conjugates were labeled with CD107a-PE.
Cell stimulation, immunoprecipitation and immunoblot analysis
Vaccinia infection of NK cells, cell stimulation, protein immunoprecipitation and detection of tyrosine phosphorylation were done as described (15).
Cell microscopy
Immunofluorescence of fixed NK-containing conjugates was performed similarly to what we have described for T cell-B cell conjugates (16). Briefly, 200µl of NK cells (1×106 cells/ml) were mixed with 200µl of CMAC-stained 721 target cells (1×106 cells/ml) and pelleted together at 500 rpm in serum-free RPMI medium (Invitrogen). These pelleted cells were then incubated at 37°C for up to 30 minutes, gently resuspended, allowed to adhere to poly-L-lysine coated coverslips for 5 minutes at 37°C, fixed, and then stained as previously described (16). For quantification, 50–100 conjugates consisting of one NK cell and one blue CMAC-stained 721 target were chosen randomly and scored for granule polarization. Conjugates were scored positive for polarization if the granules (based on perforin staining) were against the cell-cell interface.
Stimulation and measurement of secretion
Following infection, electroporation or treatment with dynasore, NK cells were resuspended in RPMI+10% FBS and stimulated with TPA and ionomycin or added to high binding plates (Costar) that had been precoated with mAb at the indicated concentration. After 4 hours of treatment at 37°C the supernatant was harvested, and assayed for granzyme A activity. Granzyme A activity was assayed by meauring N-α-carbobenzoxy-l-lysine-thiobenzyl (BLT)-esterase activity. Twenty µl of supernatant, in duplicate, was incubated with 200 µl enzyme substrate, which consisted of 0.2 mM N-benzyloxycarbonyl-l-lysine thiobenzyl ester (Calbiochem, San Diego CA), dissolved in 0.1 M Tris–HCl (pH 8), mixed shortly before the assay with 0.22 mM DNTB (Calbiochem, San Diego CA) in Tris buffer. The assay was run for 5–30 min at room temperature and substrate hydrolysis analyzed at 405 nm by a Beckman Coulter AD340 plate reader. Specific granule exocytosis is expressed as a percentage of the total cellular enzyme content as determined by freeze/thaw after subtracting the spontaneous release for each infection. Hexosaminidase content was determined as previously described (11). Fluorescence was measured with a Molecular Devices Spextra Max M2. Specific granule exocytosis is expressed as a percentage of the total cellular enzyme content determined by triton lysis after subtracting the spontaneous release for each infection.
Dyn2 suppression
The following Dyn2-specific siRNA construct was obtained from Dharmacon: 5’-NNGCACGCAGCUGAACAAGAA-3’. On day 4–5 after passage, actively dividing NK cells were electroporated as previously described (16) with 300 pmol of negative control small interfering RNA (siRNA) (AF488, Qiagen) or Dyn2 siRNA and then were resuspended at a density of 7 × 105 cells/ml in RPMI medium (Invitrogen) plus 10% human serum, 1% l-glutamine (Mediatech), 1% sodium pyruvate (Invitrogen) and 10 U/ml of interleukin 2 (Chiron). Cells were analyzed 48 hours after nucleofection.
Statistical analyses
Statistical analyses were done using either Microsoft Excel or R, and paired Student's t-tests were used to calculate P values.
Results
Dyn2 localizes with lytic granules at the NK cytolytic synapse
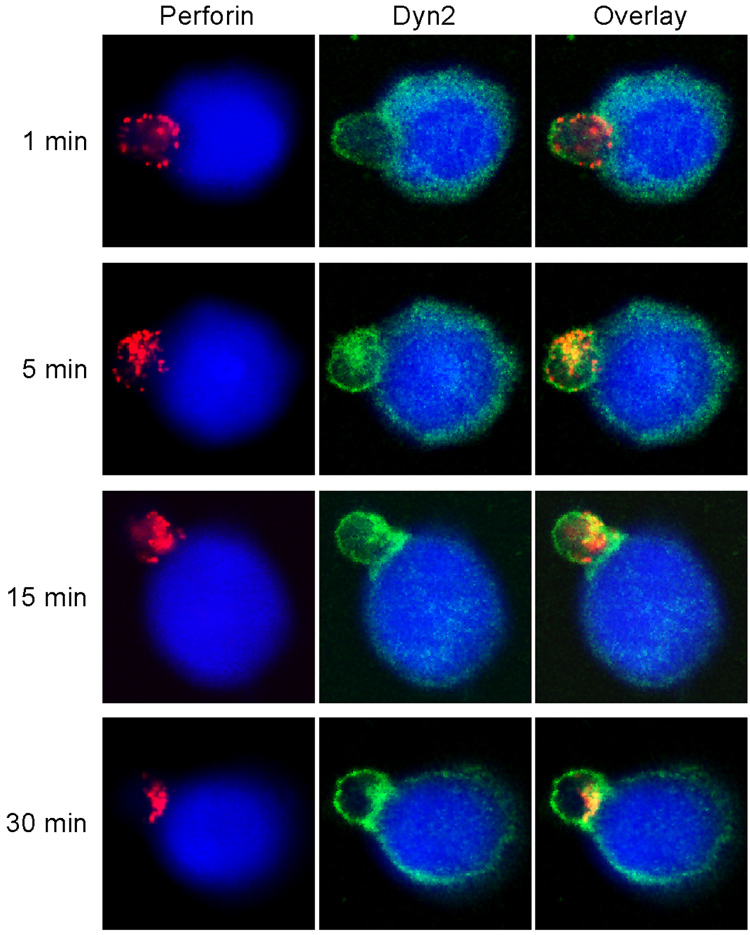
Clonally derived, non-transformed, IL-2 activated human NK cells were used for this and all subsequent assays. We have previously shown that Dyn2 accumulates at the T cell – APC synapse (8) and thus wanted to examine the spatial and temporal localization of Dyn2 during the development of cell-mediated cytotoxicity. To examine this, NK cells were conjugated to 721 target B cells and at different times following conjugation, cells were fixed and stained for endogenous Dyn2 and perforin to label the lytic granules. Dyn2 showed a diffuse staining pattern in both NK cells and 721 target cells. Initially upon target cell recognition Dyn2 did not localize with perforin-containing granules in NK cells (Figure 1, top panel). However, at later timepoints, Dyn2 and the lytic granules colocalized, and subsequently polarized toward the cytolytic synapse (Figure 1, lower three panels). Although we cannot rule out the possibility that Dyn2 from the 721 cells does not contribute to the Dyn2 enrichment at the cytolytic synapse, it is clear that Dyn2 localizes and translocates with the lytic granules upon NK cell activation. These data suggest the possibility that Dyn2 may participate in the generation of cell-mediated killing by NK cells.
Figure 1. Dyn2 localizes with lytic granules at the NK cytolytic synapse.
Clonally derived, non-transformed, human NK cells were conjugated with CMAC-stained 721 target B cells (blue) and at the indicated times following conjugate formation, the cells were fixed and stained with anti-Dyn2 (green) and anti-perforin (red) to label lytic granules.
Dyn2 modulates cell-mediated cytotoxicity
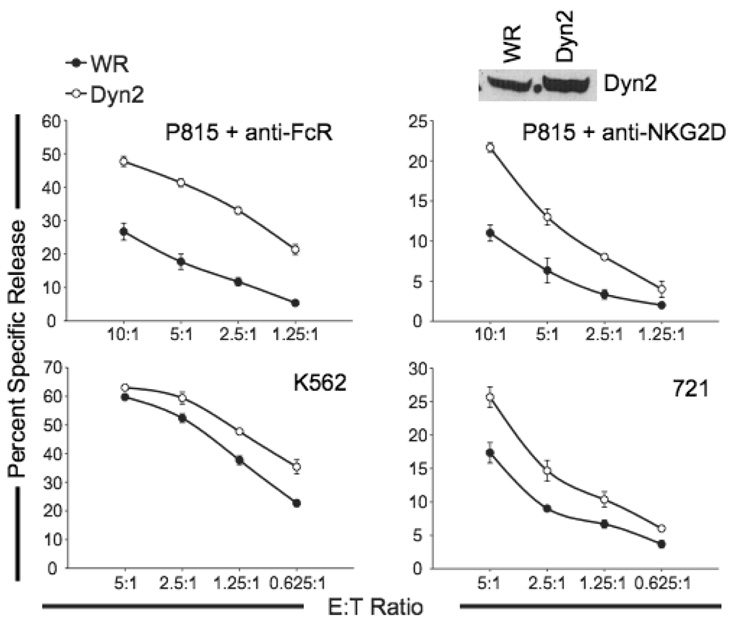
We next examined the role of Dyn2 in NK-mediated cytotoxicity. We infected cells with wild type vaccinia (WR) or FLAG-Dyn2-encoding vaccinia, and examined cytotoxicity using a redirected killing assay against P815 tumor targets that bind anti-FcR mAb or anti-NKG2D mAb. Enhanced expression of Dyn2 consistently increased redirected cytotoxicity through both the FcR and NKG2D receptors (Figure 2, top panels), as well as enhanced natural cytotoxicity of the targets tested (Figure 2, lower panels). These results suggest that Dyn2 positively regulates cell-mediated killing by NK cells.
Figure 2. Enhanced expression of Dyn2 increases NK cell cytotoxicity.
An NK clone expressing control vaccinia (WR) or recombinant FLAG-tagged Dynamin 2 (Dyn2) vaccinia was incubated with the indicated 51Cr-labeled targets and percent specific release was measured over a range of effector:target (E:T) ratios. Lysates were blotted for Dyn2. Data shown are representative of experiments performed with more than10 NK clones.
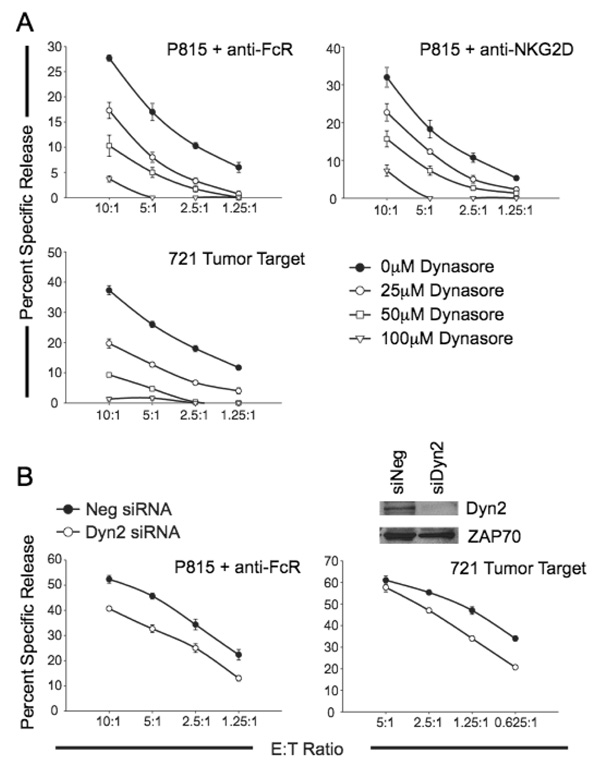
To further substantiate a role for Dyn2 in cell-mediated cytotoxicity, we sought to initially inhibit Dyn2 function using a recently identified pharmacological inhibitor of Dyn1, Dyn2 and the mitochondrial dynamin Drp1 (17). This agent, dynasore, inhibits the GTPase activity of dynamins, an event that is known to be required for the fusion and pinching of vesicles (17). NK clones were pretreated with varying concentrations of dynasore and then their lytic activity was assessed toward 721 and in a redirected killing assay using anti-FcR or anti-NKG2D. Dynasore treatment of NK cells inhibits the development of natural cytotoxicity, as well as FcR- and NKG2D-dependent cytolytic activity in a dose-dependent manner (Figure 3A).
Figure 3. Inhibition or depletion of Dyn2 abrogates NK cell cytotoxicity.
A, NK clones were pretreated for 15 minutes with the indicated concentrations of the dynamin GTPase inhibitor dynasore. The cells were subsequently incubated with 51Cr-labeled 721 cells or P815 cells coated with anti-FcR or anti-NKG2D and percent specific release was measured over the indicated E:T ratios. The data shown are representative of experiments performed with 6 NK clones. B, NK clones were electroporated with negative control siRNA or Dyn2 siRNA. 48 hours post-electroporation, cells were incubated with 51Cr-labeled 721 cells or P815 cells coated with anti-FcR and percent specific release was measured over the indicated E:T ratios. Protein lysates are equalized for protein expression and blotted with the indicated antibodies (inset). Data shown are representative of experiments performed with more than10 NK clones.
Previous work has indicated that Dyn2 GTPase activity is required for the endocytosis of granules into the target cell during killing by cytotoxic T lymphocytes (18). Thus, it remained possible that dynasore was not only affecting Dyn2-dependent signaling in NK cells, but also the ability of the target cells to be killed, since dynasore is a reversible inhibitor and is maintained in the killing assay. In order to determine if NK cells require Dyn2 for cellular cytotoxicity, we used siRNA toward Dyn2 to specifically deplete Dyn2 protein levels in NK clones (Figure 3B, inset). As shown in Figure 3B, depletion of Dyn2 resulted in decreased natural cytotoxicity toward 721, as well as FcR-dependent cytolytic activity. Taken together, these data indicate that Dyn2 is involved in the development of cell-mediated killing by NK clones.
Dyn2 does not modulate conjugate formation
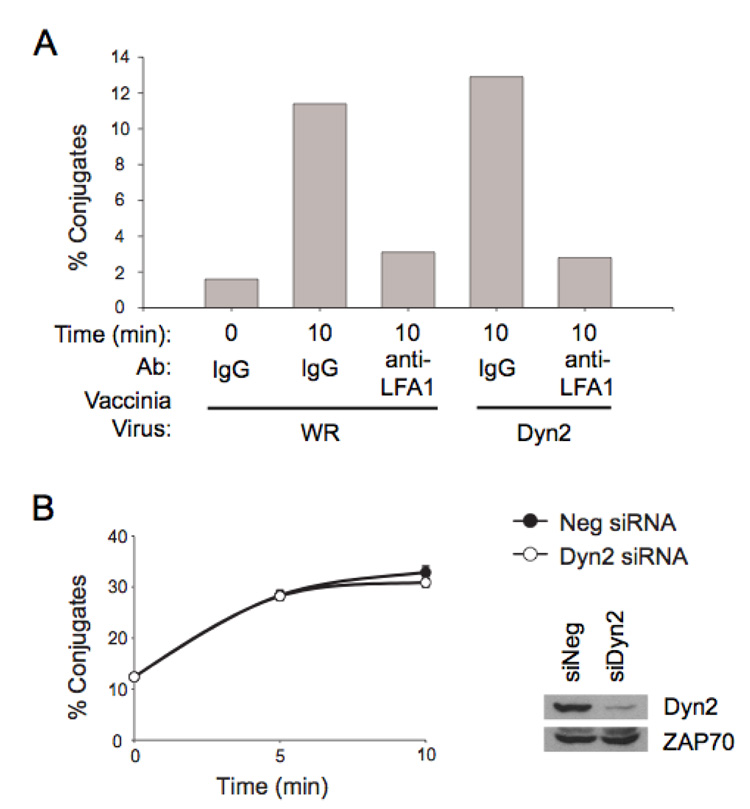
Initiation of cytotoxicity occurs after binding of NK cells to a susceptible target through a variety of adhesion receptors, including LFA-1, and establishment of a stable effector cell–target cell conjugate. No differences in cell surface expression of FcR or LFA-1 were observed when Dyn2 expression was enhanced or suppressed (data not shown). To determine if Dyn2 plays a role in target-effector binding, we assessed the ability of NK cells to bind to the NK-sensitive chronic myelogenous leukemia cell line K562. Conjugate formation was assessed by two-color flow cytometry of NK cells intracellularly stained with sulfofluorescin and K562 target cells stained with hydroethidine; conjugates were determined by simultaneous emission of green fluorescence (NK cells) and red fluorescence (K562). No affect on conjugate formation was observed when the expression of Dyn2 was enhanced, although conjugate formation could be inhibited by the addition of blocking anti-LFA-1 antibodies (Figure 4A). Moreover, siRNA depletion of Dyn2 did not affect the number of NK – 721 conjugates that formed over the indicated timecourse (Figure 4B). Thus, Dyn2 does not affect cytotoxicity by altering the ability of NK cells to form conjugates with target cells.
Figure 4. Dyn2 does not affect NK-target conjugate formation.
A, NK clones infected with the indicated recombinant vaccinia virus were stained intracellularly with sulfofluorescein (green fluorescence). NK cells were treated with IgG1 control or anti-LFA-1 antibody for 10 min on ice and washed once, then incubated for the indicated time at 37°C with K562 target cells which had been stained intracellularly with hydroethidine (red fluorescence). Using flow cytometry, the percentage of NK cells forming conjugates were scored based on the simultaneous emission of both green and red fluorescence. This is a representative example of 3 independent experiments. B, Control siRNA or Dyn2 siRNA electroporated cells were subject to a conjugate assay 48-hrs post electroporation. Conjugate formation was measured at the indicated times post incubation at 37°C. This is a representative example of 3 independent experiments performed in triplicate.
Proximal signaling is unchanged by Dyn2
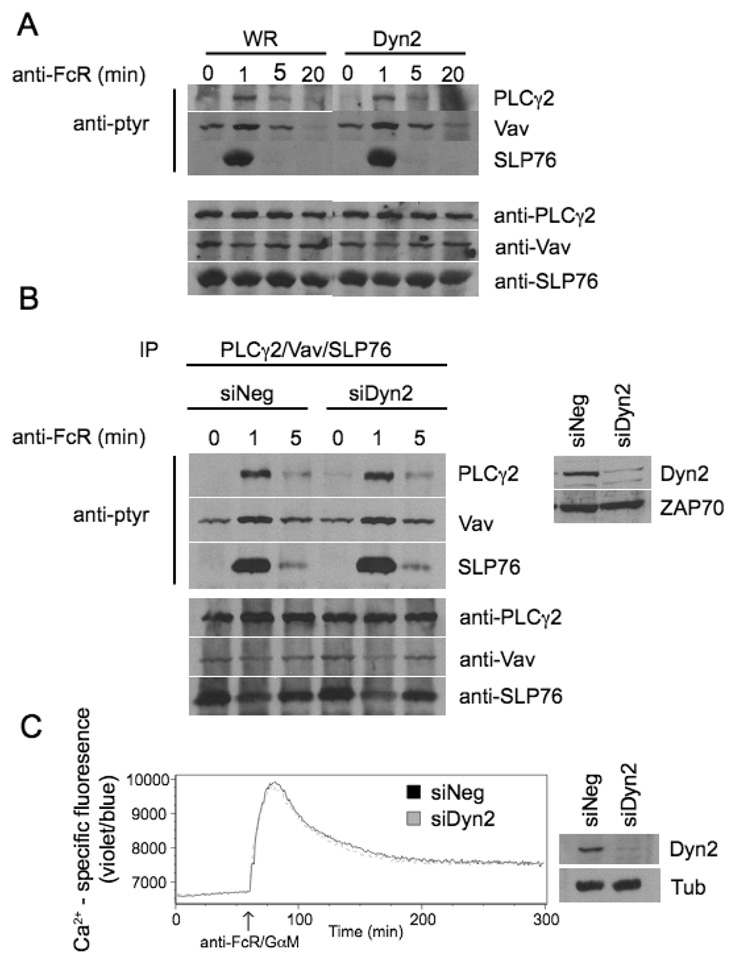
To determine if Dyn2 affects the generation of signaling by activating receptors on NK cells, the proximal tyrosine phosphorylation of PLC-γ2, Vav1, and SLP-76 was examined following the ligation of CD16. Cells were infected with control WR vaccinia or recombinant virus encoding FLAG-Dyn2 and stimulated with anti-FcR followed by goat anti-mouse cross-linking antibody. PLC-γ2, Vav1, and SLP-76 were then immunoprecipitated and no change in tyrosine phosphorylation compared to control was observed (Figure 5A). To further confirm these results, cells were transfected with control siRNA or siRNA against Dyn2 and analyzed for PLC-γ2, Vav1, and SLP-76 phosphorylation in the same manner. No change in proximal signaling from control cells was observed when Dyn2 was suppressed (Figure 5B). Similarly, no differences in calcium signaling were observed upon stimulation of the FcR, with either overexpression or suppression of Dyn2 (Figure 5C and data not shown). These results suggest that Dyn2 is not affecting the most proximal signals initiating NK cell activation, indicating that its role in cytotoxicity is further downstream.
Figure 5. Dyn2 does not affect proximal NK cell signaling events.
A, NK clones infected with WR or Dyn2 recombinant vaccinia virus were stimulated for the indicated times with anti-FcR and then lysed. PLCγ2, Vav, and SLP-76 were then immunoprecipitated from the lysates followed by SDS-PAGE and immunoblot for p-tyr or the indicated proteins. B, NK clones electroporaed with negative control siRNA or Dyn2 suppressing siRNA were stimulated for the indicated times with anti-FcR and then lysed. PLCγ2, Vav, and SLP-76 were then immunoprecipitated and analyzed as in A. The experiments shown are representative of 3 independent experiments. Lysates were equalized for protein expression and blotted for Dyn2 to verify suppression of the protein (inset, B). C, NK clones were electroporated with control or Dyn2 siRNA. Forty-eight hours post electroporation, calcium mobilization in response to anti-FcR/goat anti-mouse was measured using Indo-1 staining and flow cytometry. Data shown are representative of 2 independent experiments.
Dyn2 does not affect granule polarization
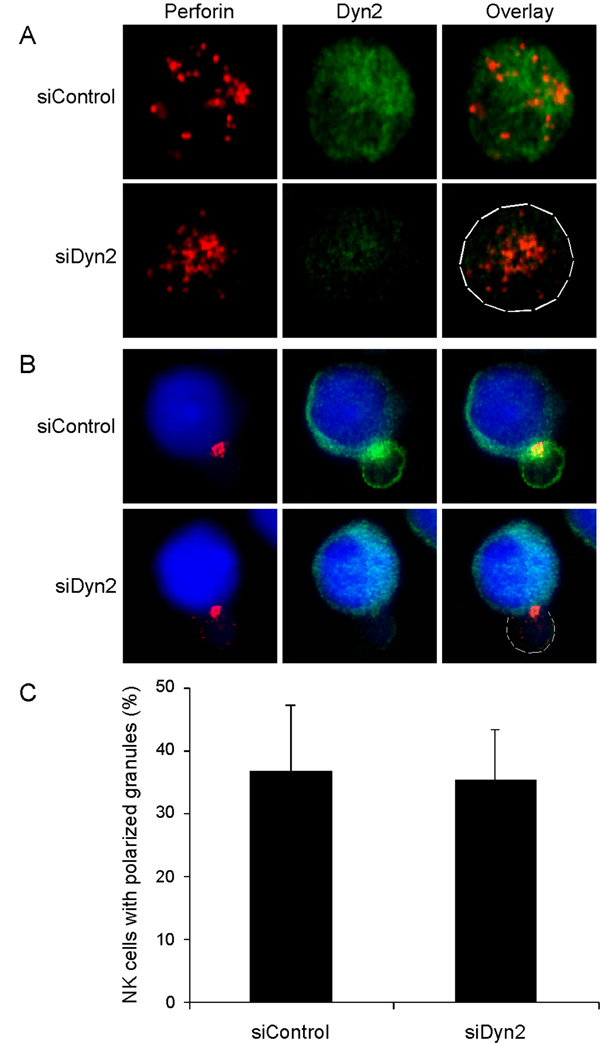
The secretion of lytic granules by cytotoxic cells is preceded by a reorganization of the actin cytoskeleton coupled with reorientation of the MTOC and the lytic granules to a position adjacent to the target cell. Recent work by the Griffiths group (19) demonstrates that in cytotoxic T cells the centrosome is used to deliver the granules to the cell-cell interface. Since Dyn2 appears to accumulate with granules and polarize toward the cytolytic synapse during the generation of cell-mediated killing (Figure 1), we decided to investigate whether depletion of Dyn2 would affect granule polarization. We formed conjugates between CMAC-loaded 721 cells (blue) and NK clones that had been transfected with control siRNA or siRNA toward Dyn2. We subsequently fixed and stained the formed conjugates for perforin and Dyn2. Individual NK cells were also stained and Dyn2-suppressed cells clearly displayed diminished Dyn2 levels compared to control cells, yet contained preformed lytic granules (Figure 6A). Surprisingly, in the NK cell – 721 conjugates, perforin-containing granules localized toward the 721 cells regardless of whether Dyn2 was present (Figure 6B and C). These data indicate that Dyn2 suppression does not affect the ability of lytic granules to polarize to the cytolytic synapse, and thus Dyn2 might be participating in a later step such as granule release.
Figure 6. Dyn2 does not affect granule polarization.
A, Primary human NK cells were transfected with control siRNA or siRNA directed against Dyn2. After 72 hours, these cells were fixed and stained with anti-Dyn2 (green) to analyze suppression and anti-perforin (red) to label lytic granules. B, Primary human NK cells were tranfected as in A and then conjugated to CMAC-stained 721 target B cells (blue) and incubated for 30 minutes at 37°C. These NK-721 conjugates were then fixed and stained with anti-Dyn2 (green) and anti-perforin (red). C, Conjugates from B were scored for perforin-containing granule polarization to the NK-721 cytolytic synapse. Data shown are representative or 2 experiments performed in triplicate.
Dyn2 regulates granule secretion
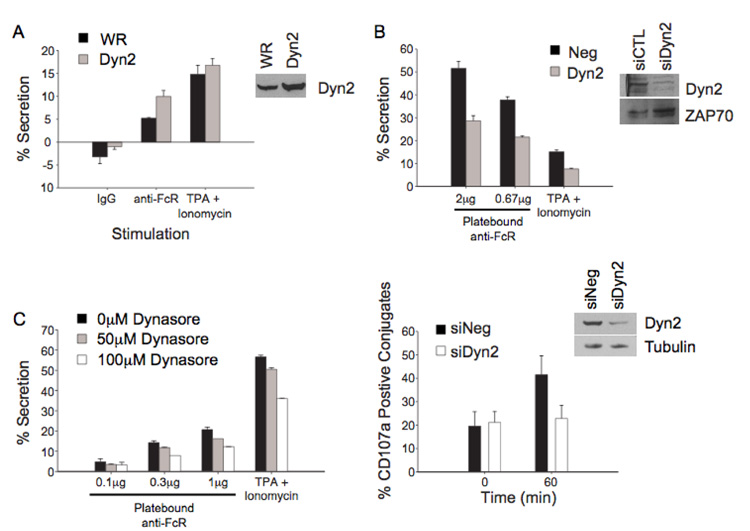
NK cells mediate cytotoxicity by the release of granule contents, including perforin and granzymes, which induce apoptosis of the target cell. In order to look at the regulation of secretion, we stimulated cells with anti-Fc receptor, which results in proximal tyrosine phosphorylation, inducing degranulation. In order to determine if Dyn2 overexpression affected NK cell secretion, we expressed wild type Dyn2 in NK cells and stimulated using plate bound antibody against the FcR, and the amount of granzyme A released was assessed by a BLT esterase assay. Expression of Dyn2 enhanced secretion induced by anti-FcR stimulation over that of the WR infected cells (Figure 7A). In order to determine the effect of Dyn2 suppression on granule release, NK cells were transfected with Dyn2 specific siRNA, and were stimulated and assayed as in Figure 7A. Suppression of Dyn2 reduced the amount of granzyme A released following anti-FcR ligation (Figure 7B) although total cellular granzyme levels remained similar in control and Dyn2 suppressed cells (data not shown). Additionally, treatment of NK cells with concentrations of dynasore that inhibited cellular cytotoxicity also abrogated FcR-mediated exocytosis (Figure 7C). These results demonstrate that the effect of Dyn2 suppression on degranulation correlates with the decrease seen in cell-mediated cytotoxicity. Consistent with this idea, surface expression of the lytic granule membrane protein CD107a in NK cell – target cell conjugates was dramatically reduced in NK cells suppressed for Dyn2 (Figure 7D).
Figure 7. Dyn2 modulates granule secretion by NK clones.
A, NK cells were infected with recombinant vaccinia expressing WR or Dyn2. Cells were stimulated with plate bound anti-FcR or TPA (100 ng/ml) and ionomycin (10 µM). Supernatants were harvested after 4 hours at 37°C and granzyme A content was assessed using a BLT esterase assay. B, NK cells were electroporated with control or Dyn2 suppressing siRNA then stimulated using the indicated concentration of plate bound anti-FcR antibody. Additionally, low-dose PMA (10 ng/ml) and ionomyin (1 µM) were used to stimulate the Dyn2-suppressed cells. Granzyme A secretion was measured as described in A. Lysates were equalized for protein expression and blotted for Dyn2 to verify suppression of the protein. C, NK cells were treated with the indicated concentrations of dynasore and stimulated with plate-bound anti-FcR or TPA and Ionomycin. Granzyme A secretion was measured as described in A. D, NK cells were electroporated with control or Dyn2 suppressing siRNA and the percentage of NK cell – target conjugates expressing CD107a was determined using flow cytometry at the indicated time points.
Exocytosis of lytic granules in NK cells and CTLs requires a transient increase in intracellular Ca2+ concentration. This increase in Ca2+ is required for the activation of the fusion machinery and docking and fusion of the granule with the membrane (2). Using TPA and ionomycin, we artificially stimulated secretion while bypassing the proximal signaling events. Enhanced expression of Dyn2 had only a modest effect on a strong stimulation of exocytosis by TPA and ionomycin (Figure 7A). In addition, when using a weak PMA and ionomycin stimulus, Dyn2-suppressed cells (Figure 7B), as well as dynasore-treated cells demonstrate a decrease in exocytosis (Figure 7C). Taken together, these data indicate that modulation of cytotoxicity by Dyn2 is downstream of the Ca2+ signal.
Discussion
We have shown that Dyn2 is an important modulator of NK cell-mediated cytotoxicity. Using both enhanced expression and suppression of the protein, we demonstrate that Dyn2 affects cell-mediated killing. Recent work in cytotoxic T cells demonstrates polarization of the MTOC delivers the lytic granules to the cell interface (19). However, polarization of granules is not sufficient for granule release. In fact, some receptors on NK cells have the ability to polarize lytic granules to the site of effector target contact without being able to stimulate granule exocytosis (20). Other insights into the distinction between granule polarization and exocytosis have also been gained from the study of human and mouse genetic diseases. In addition to the disease states that have been shown to result in normal granules, which are unable to polarize correctly such as Hermansky-Pudlak syndrome, disease states have also been identified in which lytic granules polarize correctly but are unable to be released. In familial hemophagocytic lymphohistiocytosis (FHL) type 2 defects in Rab27a (4) and Munc 13-4 (5) result in cytotoxic cells with granules that polarize correctly but are unable to fuse with the membrane and deliver their content. Additionally, mutations in the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) synataxin 11 is associated with FHL type 4 and has recently been shown to be involved in NK cell granule exocytosis (21, 22). A role for Dyn2 in secretion following the polarization of the MTOC indicates its modulation of cytotoxicity is similarly in the final steps: granule fusion with the membrane and exocytosis.
An exocytic role for Dyn2 is surprising given its extensive characterization as a GTPase dependent regulator of membrane fission and endocytosis. However, several observations suggest that dynamins may have a broader role in membrane dynamics and can participate in coordinating fission with fusion (23, 24). Dynamins have been shown to play a GTPase-dependent role in coordinating membrane recapture during focal exocytosis of macrophages (3) and in secretory granule exocytosis in chromaffin cells (25) and neuroendocrine cells (24). More recently, Dyn2 has been shown to be involved in the secretion of insulin from preformed granules in response to glucose and other stimuli (26). Dynamins have also been found in complexes with docking SNAREs, such as syntaxin (25), and SNARE-interacting proteins, such as EHSH1 (27) and synaptophysin (28), but if dynamin may regulate exocytosis via any of these interactions it is not yet clear. Most intriguingly, a yeast homologue of dynamin, Vps1p, binds to Vam3p, a t-SNARE, regulating its ability to mediate fusion (9).
Dyn1 has been found to localize to sites of insulin release in pancreatic β-cells, where it is thought to participate in the reuptake of vesicles by cavicapture (32). Intriguingly, this process occurs independently of the recruitment of proteins important for ‘classical’ endocytosis such as clathrin, amphiphysin and epsin (32). Similarly, we find that in isolated NK cell clones, perforin-containing granules and Dyn2 do not colocalize; however, upon interaction with a target cell, Dyn2 co-localizes with the polarizing lytic granules and traffics with these granules to the cytolytic synapse. It is of interest that a similar redistribution of Munc13-4 with lytic granules has been found in CTLs (5). Similar to what we have found for Dyn2, Munc 13-4 is not required for vesicle polarization or docking at the cytolytic synapse, but is required for the final step in membrane fusion (5). In contrast, depletion of Dyn2 does not completely block granule secretion or killing, but overall, there is decreased secretion of lytic granule constituents, which might result in a decrease in cell-mediated killing. It remains possible, that in NK cells, there are limited lytic granule fusion sites within the formed cytolytic synapse, and that dynamin-dependent granule fission of the empty lytic granules, either via a cavicapture or kiss-and-run model, is required for the docking of new lytic granules at the fusion site. In the absence of Dyn2, initial granule release would occur, but subsequent bursts of granule release would be limited due to inefficient removal of empty granules from the cytolytic synapse.
A role for Dyn2 in the modulation of cell-mediated cytotoxicity and granule fusion/fission provides a new understanding of the means by which Dyn2 operates and the processes it regulates. Mutations in Dyn2 have recently been implicated in Charcot-Marie-Tooth (CMT) disease, a form of peripheral neuropathy. Several mutations in Dyn2 were found in patients with CMT, localizing to the PH domain and decreasing membrane association of Dyn2 (33). Mutations in Dyn2 were also recently discovered in patients with centronuclear myopathy (34). A better understanding of the signaling pathways and domains of Dyn2 that regulate its localization with lytic granules, as well as the pathways that are regulated by Dyn2 has potential to illuminate how mutations in this protein cause disease.
Footnotes
Supported by the NIH (CA47752 and AI065474 to D.D.B.)
Abbreviations used in this paper: Dyn2, Dynamin 2; MTOC, microtubule organizing center; PRD, proline rich domain; siRNA, small interfering RNA
Publisher's Disclaimer: "This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org."
References
- 1.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annual review of cell and developmental biology. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 2.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annual review of immunology. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 3.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiological reviews. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 4.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nature genetics. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, de Saint Basile G. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 6.Hinshaw JE. Dynamin and its role in membrane fission. Annual review of cell and developmental biology. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer DA. Regulating actin dynamics at membranes: a focus on dynamin. Traffic (Copenhagen, Denmark) 2004;5:463–469. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 8.Gomez TS, Hamann MJ, McCarney S, Savoy DN, Lubking CM, Heldebrant MP, Labno CM, McKean DJ, McNiven MA, Burkhardt JK, Billadeau DD. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nature immunology. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 9.Peters C, Baars TL, Buhler S, Mayer A. Mutual control of membrane fission and fusion proteins. Cell. 2004;119:667–678. doi: 10.1016/j.cell.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Windebank KP, Abraham RT, Powis G, Olsen RA, Barna TJ, Leibson PJ. Signal transduction during human natural killer cell activation: inositol phosphate generation and regulation by cyclic AMP. J Immunol. 1988;141:3951–3957. [PubMed] [Google Scholar]
- 11.Ting AT, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc gamma receptor activation induces the tyrosine phosphorylation of both phospholipase C (PLC)-gamma 1 and PLC-gamma 2 in natural killer cells. The Journal of experimental medicine. 1992;176:1751–1755. doi: 10.1084/jem.176.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binstadt BA, Billadeau DD, Jevremovic D, Williams BL, Fang N, Yi T, Koretzky GA, Abraham RT, Leibson PJ. SLP-76 is a direct substrate of SHP-1 recruited to killer cell inhibitory receptors. The Journal of biological chemistry. 1998;273:27518–27523. doi: 10.1074/jbc.273.42.27518. [DOI] [PubMed] [Google Scholar]
- 13.Billadeau DD, Mackie SM, Schoon RA, Leibson PJ. The Rho family guanine nucleotide exchange factor Vav-2 regulates the development of cell-mediated cytotoxicity. The Journal of experimental medicine. 2000;192:381–392. doi: 10.1084/jem.192.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billadeau DD, Brumbaugh KM, Dick CJ, Schoon RA, Bustelo XR, Leibson PJ. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. The Journal of experimental medicine. 1998;188:549–559. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upshaw JL, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. The isoforms of phospholipase C-gamma are differentially used by distinct human NK activating receptors. J Immunol. 2005;175:213–218. doi: 10.4049/jimmunol.175.1.213. [DOI] [PubMed] [Google Scholar]
- 16.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Developmental cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Veugelers K, Motyka B, Frantz C, Shostak I, Sawchuk T, Bleackley RC. The granzyme B-serglycin complex from cytotoxic granules requires dynamin for endocytosis. Blood. 2004;103:3845–3853. doi: 10.1182/blood-2003-06-2156. [DOI] [PubMed] [Google Scholar]
- 19.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 20.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. The Journal of experimental medicine. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arneson LN, Brickshawana A, Segovis CM, Schoon RA, Dick CJ, Leibson PJ. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol. 2007;179:3397–3401. doi: 10.4049/jimmunol.179.6.3397. [DOI] [PubMed] [Google Scholar]
- 22.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, Hultenby K, Winiarski J, Roche PA, Nordenskjold M, Henter JI, Long EO, Ljunggren HG. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holroyd P, Lang T, Wenzel D, De Camilli P, Jahn R. Imaging direct, dynamin-dependent recapture of fusing secretory granules on plasma membrane lawns from PC12 cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16806–16811. doi: 10.1073/pnas.222677399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galas MC, Chasserot-Golaz S, Dirrig-Grosch S, Bader MF. Presence of dynamin--syntaxin complexes associated with secretory granules in adrenal chromaffin cells. Journal of neurochemistry. 2000;75:1511–1519. doi: 10.1046/j.1471-4159.2000.0751511.x. [DOI] [PubMed] [Google Scholar]
- 26.Min L, Leung YM, Tomas A, Watson RT, Gaisano HY, Halban PA, Pessin JE, Hou JC. Dynamin is functionally coupled to insulin granule exocytosis. The Journal of biological chemistry. 2007;282:33530–33536. doi: 10.1074/jbc.M703402200. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto M, Schoch S, Sudhof TC. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? The Journal of biological chemistry. 1999;274:18446–18454. doi: 10.1074/jbc.274.26.18446. [DOI] [PubMed] [Google Scholar]
- 28.Daly C, Ziff EB. Ca2+-dependent formation of a dynamin-synaptophysin complex: potential role in synaptic vesicle endocytosis. The Journal of biological chemistry. 2002;277:9010–9015. doi: 10.1074/jbc.M110815200. [DOI] [PubMed] [Google Scholar]
- 29.Kwan EP, Gaisano HY. Glucagon-like peptide 1 regulates sequential and compound exocytosis in pancreatic islet beta-cells. Diabetes. 2005;54:2734–2743. doi: 10.2337/diabetes.54.9.2734. [DOI] [PubMed] [Google Scholar]
- 30.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. Journal of immunological methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Palfrey HC, Artalejo CR. Secretion: kiss and run caught on film. Curr Biol. 2003;13:R397–R399. doi: 10.1016/s0960-9822(03)00320-8. [DOI] [PubMed] [Google Scholar]
- 32.Tsuboi T, McMahon HT, Rutter GA. Mechanisms of dense core vesicle recapture following "kiss and run" ("cavicapture") exocytosis in insulin-secreting cells. The Journal of biological chemistry. 2004;279:47115–47124. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- 33.Zuchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De Jonghe P, Merory J, Oliveira SA, Speer MC, Stenger JE, Walizada G, Zhu D, Pericak-Vance MA, Nicholson G, Timmerman V, Vance JM. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nature genetics. 2005;37:289–294. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]
- 34.Bitoun M, Maugenre S, Jeannet PY, Lacene E, Ferrer X, Laforet P, Martin JJ, Laporte J, Lochmuller H, Beggs AH, Fardeau M, Eymard B, Romero NB, Guicheney P. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nature genetics. 2005;37:1207–1209. doi: 10.1038/ng1657. [DOI] [PubMed] [Google Scholar]