Abstract
Voltage gated calcium channels (VGCCs) are well established mediators of pain signals in primary afferent neurons. N-type calcium channels are localized to synaptic nerve terminals in laminae 1 and 2 of the dorsal horn where their opening results in the release of neurotransmitters such as glutamate and substance P. The contribution of N-type channels to the processing of pain signals is regulated by alternate splicing of the N-type channel gene, with unique N-type channel splice variants being expressed in small nociceptive neurons. In contrast, T-type VGCCs of the Cav3.2 subtype are likely localized to nerve endings where they regulate cellular excitability. Consequently, inhibition of N-type and Cav3.2 T-type VGCCs has the propensity to mediate analgesia. T-type channel activity is regulated by redox modulation, and can be inhibited by a novel class of small organic blockers. N-type VGCC activity can be potently inhibited by highly selective peptide toxins that are delivered intrathecally, and the search for small organic blockers with clinical efficacy is ongoing. Here, we provide a brief overview of recent advances in this area, as presented at the Spring Pain Research conference (Grand Cayman, 2008).
Keywords: Dorsal root ganglion, T-type channel, N-type channel, Conotoxin, Burst firing
1. Introduction
Pain is an important physiological response designed to protect us from injury (for review, see Altier and Zamponi, 2004; Smith, 2004). However, numerous pathophysiological conditions, such as diabetes, viral infections, nerve injury and inflammation can give rise to persistent, chronic pain that does not appear to serve a useful purpose and is often refractory to currently available treatment options (Porreca et al., 2002). Upon the occurrence of a painful peripheral stimulus, peripheral nociceptive neurons are activated and a train of action potentials is initiated and propagates along the axons of primary afferent nerve fibers to nerve terminals embedded in laminae 1 and 2 of the dorsal horn of the spinal cord (Krarup, 2003). These nerve terminals release pro-nociceptive neurotransmitters such as glutamate, substance P and CGRP, which then activate postsynaptic receptors on neurons of the spinothalamic tract. These nerve projections to the thalamus allow us to perceive pain (Krarup, 2003). There are also descending pathways from the cortex to the spinal cord that modulate pain responses (Porreca et al., 2002). The propagation and processing of pain signals are dependent on, and modulated by, a host of different ion channels and receptors, among these voltage gated calcium channels (VGCCs; Julius and Basbaum, 2001).
T-type VGCCs are expressed in cell bodies and nerve endings of afferent fibers where they partake in regulating neuronal excitability by contributing to the initiation of action potential trains (Todorovic and Jevtovic-Todorovic, 2006). Specifically, T-type VGCCs can lower the threshold for action potentials, promote bursting activity and synaptic excitation; all actions that favor the development of enhanced pain (Matthews and Dickenson, 2001; Sekizawa et al., 2000). Recently, studies have revealed that Cav3.2 VGCCs facilitate pain signals in peripheral nociceptors (Todorovic and Jevtovic-Todorovic, 2006). Also, in both rat models of diabetic neuropathy and the chronic constriction nerve injury model of neuropathic pain, T-type channel current density is remarkably increased (Jagodic et al., 2007, 2008). Conversely, gene knockout or antisense knockdown of the Cav3.2 isoform of T-type channels, or intrathecal injection of rats with T-type channel inhibitors, results in hyposensitivity to pain due to reduced excitability of the primary afferent fibers (Choi et al., 2007; Bourinet et al., 2005; Todorovic et al., 2002; Flatters and Bennett, 2004, Dogrul et al., 2003; Matthews and Dickenson, 2001; Shin et al., 2008).
On the other hand, high voltage-activated N-type VGCCs are highly expressed at presynaptic nerve terminals where they open in response to incoming action potentials and mediate calcium entry into the synapse. This in turn triggers synaptic vesicle release and the activation of spinothalamic neurons. A critical role of N-type VGCCs in the pain pathway is supported by data obtained from N-type channel null mice (which show an increased threshold for pain) and by the clinical efficacy of intrathecally delivered, selective N-type channel antagonists such as Prialt™ (Saegusa et al., 2001; Hatakeyama et al., 2001; Staats et al., 2004). Furthermore, N-type VGCCs are a key target for inhibition by opioid receptor pathways (Bourinet et al., 1996; Altier and Zamponi, 2004) and descending norepinephrine activation of adrenergic pathways (Pertovaara, 2006). Interestingly, other VGCC subtypes do not appear to play a major role in pain signaling in primary afferent fibers, perhaps with the exception of R-type channels (Saegusa et al., 2000; Matthews et al; 2007). As a result, N-type (Cav2.2) and Cav3.2 T-type VGCCs are considered prime targets for the development of novel analgesics (Fig. 1; Snutch, 2005; Hildebrand and Snutch, 2006).
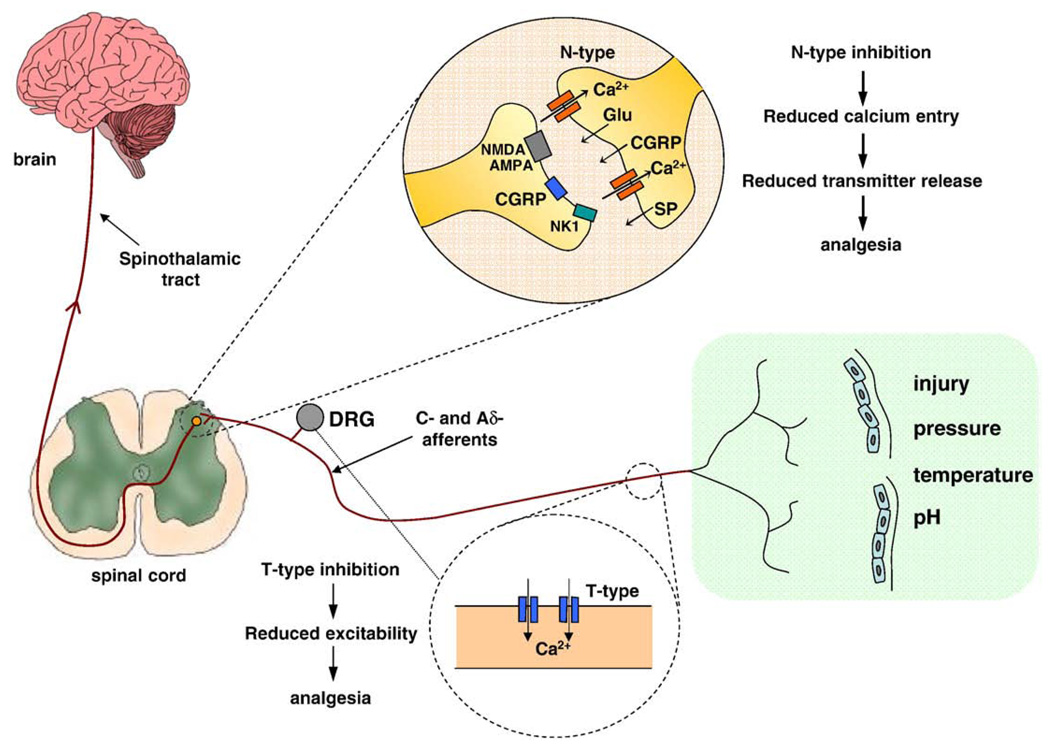
Fig. 1.
N-type and T-type calcium channels in the primary afferent signaling pathway. High voltage-activated N-type channels are highly localized to presynaptic terminals in laminae I and II of the dorsal horn. Action potentials carried along dorsal root ganglion cells (mainly C- and Aδ-afferents) trigger the opening of pre-synaptic N-type calcium channels which in turn initiate the release of nociceptive transmitters such as glutamate, substance P and CGRP onto spinal interneurons and projection neurons. Low voltage-activated T-type calcium channels are primarily localized more upstream in the pathway and are thought to be both involved in generating sensory potentials out near free nerve endings, as well as being present in DRG cell bodies where they likely contribute to the generation and frequency of action potentials. Modified from Hildebrand and Snutch, 2006; Schaible and Richter, 2004.
2. N-type calcium channel splicing and pain
Although N-type VGCCs are encoded by a single α1 subunit gene (Cav2.2), structural and functional diversity can be generated though alternate mRNA splicing (Lipscombe, 2005; Gray et al., 2007). Numerous Cav2.2 splice variants have been identified and functionally characterized, but one variant has received particular attention due to its preferential expression in small nociceptive neurons (Bell et al., 2004). In this variant, exon 37b is replaced by exon 37a, leading to a 14 amino acid change in the Cav2.2 C-terminus region without changes in the total numbers of amino acids. The functional consequence of this splicing event included larger whole cell currents, as well as altered responses to the activation of certain G protein coupled receptors (Bell et al., 2004; Castiglioni et al., 2006; Raingo et al., 2007). Altier and colleagues (2007) investigated the physiological role of the exon 37a variant by using siRNA constructs that were designed to selectively knock down channels containing either exons 37a or 37b. Intrathecal injection of these siRNA constructs into rats revealed a critical role of exon 37a containing channels in thermal (assayed by a hot plate test) and mechanical nociception (assayed by von Frey stimulation of the hind paw). Moreover, the exon 37a containing channels were shown to be selectively involved in thermal hyperalgesia in inflammatory and neuropathic pain models, and in mechanical hyperalgesia. In contrast, both splice variants appeared to contribute to mechanical allodynia during neuropathy (Altier et al., 2007). Collectively, these data suggest that channels containing exon 37a are the predominant N-type channel species involved in sensing pain. Interestingly, during spinal nerve ligation, mRNA levels of exon 37a containing channels become downregulated by about 50% whereas those corresponding to exon 37b containing channels remain unaltered (Altier et al., 2007). This may suggest an intrinsic compensatory mechanism by which animals attempt to reduce the levels of the VGCC subtype that mediates pain in neuropathic states. The notion that exon37 containing channels appear to be critical for the transmission of pain signals at the spinal level, yet show only limited expression in other brain regions may provide for an opportunity to develop novel N-type channel blocking molecules that preferentially inhibit the exon37a splice isoform of the channels. This in turn may reduce the potential of side effects seen with global blockade of N-type channels via conotoxin derivatives.
3. Novel peptide toxin inhibitors of N-type channels
It is well established that intrathecal injection of N-type VGCC blocking peptides mediates analgesic behavioral responses in both rats and humans (Altier et al., 2007; Snutch, 2005; Staats et al., 2004). Indeed, Prialt™ is currently used clinically to treat cancer pain, however, its use has been associated with a number of side effects including unruly behavior, hypotension, and memory loss (Staats et al., 2004). The reason for these side effects is unclear, but may include non-selective actions of Prialt™ on targets other than the N-type channels, or perhaps immune reactions to the injected peptides. Hence, the search for peptide inhibitors with a wider therapeutic window continues.
The ω-conotoxins produced by fish hunting cone snails were the first and remain amongst the most selective inhibitors of N-type VGCCs identified. Intrathecal administration of sub-nanomolar doses of the highly N-type selective ω-conotoxins MVIIA or CVID reduced pain behaviours in rats that last for up to 24 h but were accompanied by significant neurological side effects (Malmberg and Yaksh, 1995; Smith et al., 2002). Based on this efficacy, ω-MVIIA (Prialt™) was developed by Neurex and later acquired by Elan. Prialt™ recently gained FDA approval for the treatment of otherwise unmanageable severe chronic pain after extended Phase III trials. ω-CVID (AM336) was also assessed in a small Phase IIa trial in severe cancer pain sufferers and again produced signs of efficacy. Unfortunately, both ω-conotoxins produced unwanted side effects at therapeutic doses, despite having up to 106 fold binding selectivity for N-type over P/Q-type VGCCs (Lewis et al., 2000). While off-target effects on P/Q-calcium channels cannot be discounted, on-target effects at inhibitory descending and interneuron synapses, as well as potential supraspinal effects, are likely to contribute to the dose-limiting side effects produced by these ω-conotoxins. The discovery of new ω-conotoxins and small molecules with selectivity profiles that produce fewer side effects may lead to the development of better N-type VGCC analgesics.
An alternative approach to the direct inhibition of N-type calcium channels is indirect inhibition via activation of Gi/Go coupled GPCRs. Whilst μ-opioid receptor agonists (e.g., morphine), α2-adrenergic agonists (e.g., clonidine) and nonselective small molecule norepinephrine transporter (NET) inhibitors (e.g. duloxetine) alleviate many types of pain, dose limiting side effects again also limit their usefulness (Martin and Eisenach, 2001). Since spinal noradrenaline release can reduce transmission by (i) activating inhibitory α2A-adrenoceptors on the central terminals of primary afferent nociceptors (presynaptic inhibition), (ii) direct α2-adrenergic action on pain-relay neurons (postsynaptic inhibition), and (iii) α1-adrenoceptor-mediated activation of inhibitory inter-neurons, spinally administered selective and specific NET inhibitors might be expect to be useful analgesics. The Lewis group isolated several χ-conopeptides from the cone snail Conus marmoreus that proved to be highly selective peptide inhibitors of NET (Sharpe et al., 2001). Interestingly, the binding site for χ-conopeptides on the NET partially overlaps the tricyclic antidepressant binding site but not the NE binding site (Paczkowski et al., 2007). Importantly, MrIA produced a potent anti-allodynic effect without significant side effects when administered intrathecally in rat models of neuropathic pain (Nielsen et al., 2005) and no off-target effects on a range of tissues and targets at <10−5 M. Based on this profile, Xen2174 was developed with improved chemical stability and duration of efficacy in animal models of pain (Nielsen et al., 2005). After extensive preclinical toxicology, Xenome Ltd evaluated Xen2174 intrathecally in a Phase I/IIa safety trial in cancer patients suffering poorly managed pain. Initial results have proved promising both in terms of safety and efficacy. Thus, selective targeting of spinal NET may offer advantages over existing spinal therapies for pain control.
4. Redox modulation of T-type calcium channels
As outlined earlier, Cav3.2 T-type VGCCs are critical mediators of the excitability of primary afferent neurons. The activities of these channels are regulated by a number of cellular mechanisms, including redox modulation. For example, reducing agents such as dithiothreitol (DTT) and L-cysteine (L-cys) selectively enhance low-voltage-activated (T-type) calcium currents in acutely dissociated smaller dorsal root ganglion (DRG) neurons, most of which are nociceptors (Todorovic et al., 2001). Furthermore, L-cys, DTT and other thiol-containing analogues of L-cys produce thermal and mechanical pain sensitization when injected into the peripheral receptive fields of these neurons in vivo (Todorovic et al., 2001; Pathirathna et al., 2006). It has also been reported that reducing agents enhance neuronal excitability in a unique subpopulation of IB4-positive nociceptors, termed “T-rich” cells, that expresses high density of T-type currents and virtually no high-voltage-activated (HVA) Ca2+ currents (Nelson et al., 2005). In current clamp recordings, reducing agents selectively sensitize classical C-type nociceptors with wide action potentials that express T-type currents. This is manifested as a reduced rheobase and increased probability of membrane firing in the presence of physiologically-relevant concentrations of L-cys (Nelson et al., 2007). Furthermore, exogenous (e.g., tricine) and endogenous (e.g., albumin) agents capable of chelating zinc ions mimic and occlude the effects of L-cys and DTT on the amplitude of T-type current and T-channel- dependent cellular excitability of DRG cells. In contrast, cysteine-modifying agents such as N-ethyl-maleimide (NEM) or UV light do not prevent the effects of reducing agents on T-current in DRG cells. Site directed mutagenesis of Cav3.2 revealed that a single-point mutation of Histidine 191 to glutamine completely abolishes sensitivity of Cav3.2 to both reducing and chelating agents, without any adverse effect on channel kinetics per se. Furthermore, the reverse mutation in the Cav3.1 T-type channel subtype was able to confer L-cys and DTT sensitivity to the previously completely redox-insensitive Cav3.1. In addition, H191Q mutation disrupted high-affinity zinc inhibition of Cav3.2, as manifested with a 40-fold increase in IC50 for zinc blockade of Cav3.2 currents, suggesting that the effects of reducing agents may occur indirectly via disinhibition of the channels by endogenous zinc ions. Reducing agents, as well as synthetic and endogenous chelators of zinc, sensitize Cav3.2 T-type current-containing nociceptors isolated from wild-type mice, but not nociceptors from Cav3.2 knockout mice, thus underscoring the potential importance of redox/zinc regulation of T-type channels in the context of pain. Similar mechanism likely exists in vivo since L-cys injected intradermally into hind paws induced profound thermal hyperalgesia in wild-type but not CaV3.2 knockout mice. Because the majority of C-type nociceptors are polymodal, this mechanism of sensitization may be relevant to a variety of pain conditions involving exposure to acute noxious thermal, mechanical, and chemical stimuli. Further, the elucidation of the molecular mechanisms underlying the role of T-type channels in peripheral sensitization of pain responses may offer insight into opportunities for new targets in analgesic pharmacotherapy. Future experiments will address the issue of the pathological conditions that may involve abnormalities of zinc interaction with Cav3.2 in peripheral nociceptors.
5. T-type calcium channels as targets for small molecule analgesics
Interest in developing clinical T-type VGCC inhibitors as a therapy for neuropathic and chronic pain conditions arises from a common theme found in different pain states: the persistent, spontaneous and repetitive activation of the primary afferent neurons (Devor et al., 1994; Kajander and Bennett, 1992; Petersen et al., 1996; Song et al., 1999; Zhang et al., 1999). As outlined above, several lines of evidence implicate the Cav3.2 subtype of the T-type VGCC family in the pathophysiology of neuropathic and inflammatory pain. While there is no selective pharmacologic inhibitor of Cav3.2 channels currently available, prototypical non-selective VGCC inhibitors with T-type channel blockade (e.g., ethosuximide) attenuate dorsal horn neuronal responses in rats (Matthews and Dickenson, 2001). Compounds with modest peripheral T-type channel inhibition (e.g., mibefradil) reverse experimental neuropathic pain (Dogrul et al., 2003), are anti-hyperalgesic (Barton et al., 2005) and effective in models of paclitaxel and vincristine-induced peripheral neuropathy (Flatters and Bennett, 2004). Neuromed’s discovery program has developed a series of small organic compounds that support the hypothesis that Cav3.2 selective VGCC blockers are sufficient to mediate pain relief, including thermal hyperalgesia produced by the inflammogen carrageenan, with efficacy closely paralleling systemic exposure levels.
Based on the physicochemical properties of existing T-type channel inhibitors, we would predict that that compounds that do not readily enter the brain (i.e., ‘peripherally acting’) could have broad spectrum effects on acute and inflammatory pain. It also appears likely that compounds with mixed T-/N-type VGCC blocking activity should be highly efficacious in mediating full spectrum pain relief across a wide range of neuropathic pain dimensions. Finally, compounds with N-type and T-type blocking actions show some interesting potential as abuse deterrents for alcohol abuse (Newton et al., 2004, 2008). If extended to other mechanisms of substance abuse (such as the opioids), centrally acting T-/N-type VGCC blockers may prove to be powerful new mechanism to treat moderate to severe pain states without the abuse potential known for opioids.
6. Summary
Voltage gated calcium channels continue to be major areas of focus in the development of new therapeutic approaches for the treatment of chronic pain. This may not only include the identification of novel channel blocking molecules, but also an exploitation of intrinsic regulatory mechanisms that control the activities of both N- and T-type calcium channels to increase efficacy and limit side effects.
Acknowledgments
GWZ and TPS hold Canada Research Chairs and are supported by grants from the Canadian Institutes of Health Research. GWZ is a Scientist of the Alberta Heritage Foundation for Medical Research. TPS is also the founder and Chief Scientific Officer of Neuromed Pharmaceuticals Inc. SMT is supported by NIGMS grant R01 GM075299. RJL is an NHMRC Senior Research Fellow supported by an NHMRC Program Grant.
REFERENCES
- Altier C, Zamponi GW. Targeting Ca2+ channels to treat pain: T-type versus N-type. Trends Pharmacol. Sci. 2004;25:465–470. doi: 10.1016/j.tips.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, Zamponi GW. Differential role of N-type calcium channel splice isoforms in pain. J. Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ME, Eberle EJ, Shannon HE. The antihyperalgesic effects of the T-type calcium channel blockers ethosuximide, trimethadione and mibefradil. Eur. J. Pharmacol. 2005;521:79–85. doi: 10.1016/j.ejphar.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Stea T, Snutch TP. Determinants of the G-protein-dependent opioid modulation of neuronal calcium channels. Proc. Natl. Acad. Sci. USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Alloui A, Monteil A, Barrère C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the CaV3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005;24:315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni AJ, Raingo J, Lipscombe D. Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels. J. Physiol. 2006;576:119–134. doi: 10.1113/jphysiol.2006.115030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. Attenuated pain responses in mice lacking Cav3.2 T-type channels. Genes Brain Behav. 2007;6:425–431. doi: 10.1111/j.1601-183X.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- Devor M, Janig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve injured rats. J. Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain. 2003;105:159–168. doi: 10.1016/s0304-3959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Gray AC, Raingo J, Lipscombe D. Neuronal calcium channels: splicing for optimal performance. Cell Calcium. 2007;42:409–417. doi: 10.1016/j.ceca.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama S, Wakamori M, Ino M, Miyamoto N, Takahashi E, Yoshinaga T, Sawada K, Imoto K, Tanaka I, Yoshizawa T, Nishizawa Y, Mori Y, Niidome T, Shoji S. Differential nociceptive responses in mice lacking the alpha (1B) subunit of N-type Ca(2+) channels. NeuroReport. 2001;12:2423–2427. doi: 10.1097/00001756-200108080-00027. [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, Snutch TP. Contributions of T-type Ca channels to the pathophysiology of pain signaling. Drug Discovery Today: Disease Mechanisms. 2006;3:335–341. [Google Scholar]
- Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, Jevtovic-Todorovic V, Todorovic SM. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J. Neurosci. 2007;27:3305–3316. doi: 10.1523/JNEUROSCI.4866-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. Up-regulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J. Neurophysiol. 2008;99:3151–3156. doi: 10.1152/jn.01031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferation and spontaneous discharge in Aβ and Aδ primary afferent neurons. J. Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Krarup C. An update on electrophysiological studies in neuropathy. Curr. Opin. Neurol. 2003;16:603–612. doi: 10.1097/01.wco.0000093104.34793.94. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Nielsen KJ, Craik DJ, Loughnan ML, Adams DA, Sharpe IA, Luchian T, Adams DJ, Bond T, Thomas L, Jones A, Matheson JL, Drinkwater R, Andrews PR, Alewood PF. Novel ω-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J. Biol. Chem. 2000;275:35335–35344. doi: 10.1074/jbc.M002252200. [DOI] [PubMed] [Google Scholar]
- Lipscombe D. Neuronal proteins custom designed by alternative splicing. Curr. Opin. Neurobiol. 2005;5:358–363. doi: 10.1016/j.conb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Effect of continuous intrathecal infusion of ω-conopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain. 1995;60:83–90. doi: 10.1016/0304-3959(94)00094-U. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Eisenach JC. Pharmacology of opioid and nonopioid analgesics in chronic pain states. J. Pharmacol. Exp. Ther. 2001;299:811–817. [PubMed] [Google Scholar]
- Matthews EA, Dickenson AH. Effects of ethosuximide, a T-type Ca(2+) channel blocker, on dorsal horn neuronal responses in rats. Eur. J. Pharmacol. 2001;415:141–149. doi: 10.1016/s0014-2999(01)00812-3. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Bee LA, Stephens GJ, Dickenson AH. The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur. J. Neurosci. 2007;25:3561–3569. doi: 10.1111/j.1460-9568.2007.05605.x. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Joksovic PM, Perez-Reyes E, Todorovic SM. The endogenous redox agent L-cysteine induces T-type Ca2+ channel-dependent sensitization of a novel subpopulation of rat peripheral nociceptors. J. Neurosci. 2005;25:8766–8775. doi: 10.1523/JNEUROSCI.2527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Woo J, Kang H-W, Barrett PQ, Vitko J, Perez-Reyes E, Lee J-H, Shin H-S, Todorovic SM. Reducing agents sensitize C-type nociceptors by relieving high-affinity zinc inhibition of T-type calcium channels. J. Neurosci. 2007;27:8250–8260. doi: 10.1523/JNEUROSCI.1800-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Orr CJ, Wallace MJ, Kim C, Shin HS, Messing RO. Deletion of N-type calcium channels alters ethanol reward and reduces ethanol consumption in mice. J. Neurosci. 2004;24:9862–9869. doi: 10.1523/JNEUROSCI.3446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Zeng L, Wang V, Connolly J, Wallace MJ, Kim CK, Shin HS, Belardetti F, Snutch TP, Messing RO. A blocker of N- and T-type voltage-gated calcium channels attenuates ethanol-induced intoxication, place preference, self-administration, and reinstatement. J Neurosci. 2008;28:11712–11719. doi: 10.1523/JNEUROSCI.3621-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Lewis RJ, Alewood D, Drinkwater R, Palant E, Patterson M, Yaksh TL, McCumber D, Smith MT. Anti-allodynic efficacy of the χ-conopeptide, Xen2174, in rats with neuropathic pain. Pain. 2005;118:112–124. doi: 10.1016/j.pain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Paczkowski FA, Sharpe IA, Dutertre S, Lewis RJ. Conopeptide and tricyclic antidepressant interactions at the norepinephrine transporter define a new transporter model. J. Biol. Chem. 2007;282:17837–17844. doi: 10.1074/jbc.M610813200. [DOI] [PubMed] [Google Scholar]
- Pathirathna S, Todorovic SM, Covey DF, Jevtovic-Todorovic V. Differential effects of endogenous cysteine analogues on thermal nociception in intact rats. Pain. 2006;125:53–64. doi: 10.1016/j.pain.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog. Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Petersen M, Zhang J, Zhang J-M, LaMotte RH. Abnormal spontaneous activity and responses to norepinepherine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–397. doi: 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Raingo J, Castiglioni AJ, Lipscombe D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat. Neurosci. 2007;10:285–292. doi: 10.1038/nn1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proc.Natl.Acad. Sci. U. S. A. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa H, Kurihara T, Zong S, Kazuno A, Matsuda Y, Nonaka T, Han W, Toriyama H, Tanabe T. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001;20:2349–2356. doi: 10.1093/emboj/20.10.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Richter F. Pathophysiology of pain Langenbecks Arch. Surg. 2004;389:237–243. doi: 10.1007/s00423-004-0468-9. [DOI] [PubMed] [Google Scholar]
- Sekizawa S-I, French AS, Torkkeli PH. Low-voltage-activated calcium current does not regulate the firing behaviour in paired mechanosensory neurons with different adaptation properties. J. Neurophysiol. 2000;83:746–753. doi: 10.1152/jn.2000.83.2.746. [DOI] [PubMed] [Google Scholar]
- Sharpe IA, Gehrmann J, Loughnan ML, Thomas L, Adams DA, Atkins A, Palant E, Craik DJ, Adams DF, Alewood PF, Lewis RJ. Two new classes of conopeptides inhibit the a1-adrenoceptor and noradrenaline transporter. Nat. Neurosci. 2001;4:902–907. doi: 10.1038/nn0901-902. [DOI] [PubMed] [Google Scholar]
- Shin HS, Cheong EJ, Choi S, Lee J, Na HS. T-type Ca2+ channels as therapeutic targets in the nervous system. Curr. Opin. Pharmacol. 2008;8:33–41. doi: 10.1016/j.coph.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Smith M, Cabot PJ, Ross FB, Robertson AD, Lewis RJ. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain. 2002;96:119–127. doi: 10.1016/s0304-3959(01)00436-5. [DOI] [PubMed] [Google Scholar]
- Smith PA. Neuropathic pain: drug targets for current and future interventions. Drug News Perspect. 2004;17:5–17. doi: 10.1358/dnp.2004.17.1.829021. [DOI] [PubMed] [Google Scholar]
- Snutch TP. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx. 2005;2:662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X-J, Hu S-J, Greenquist KW, Zhang J-M, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J. Neurophysiol. 1999;82:3347. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR, Mayo M, McGuire D, Ellis D. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V, Meyenburg A, Mennerick S, Perez-Reyes E, Romano C, Olney JW, Zorumski CF. Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron. 2001;31:75–85. doi: 10.1016/s0896-6273(01)00338-5. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Meyenburg A, Jevtovic-Todorovic V. Mechanical and thermal antinociception in rats following systemic administration of mibefradil, a T-type calcium channel blocker. Brain Res. 2002;951:336–340. doi: 10.1016/s0006-8993(02)03350-4. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V. The role of T-type calcium channels in peripheral and central pain processing. CNS Neurol. Disord. Drug Targets. 2006;5:639–653. doi: 10.2174/187152706779025490. [DOI] [PubMed] [Google Scholar]
- Zhang J-M, Song X-J, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesic produced by chronic compression of the dorsal root ganglion. J. Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]