Summary
Interactions between developmental signaling pathways govern the formation and function of stem cells. Prostaglandin (PG) E2 regulates vertebrate hematopoietic stem cells (HSC). Similarly, the Wnt signaling pathway controls HSC self-renewal and bone marrow repopulation. Here, we show that wnt reporter activity in zebrafish HSCs is responsive to PGE2 modulation, demonstrating a direct interaction in vivo. Inhibition of PGE2 synthesis blocked wnt-induced alterations in HSC formation. PGE2 modified the wnt signaling cascade at the level of β-catenin degradation through cAMP/PKA-mediated stabilizing phosphorylation events. The PGE2/Wnt interaction regulated murine stem and progenitor populations in vitro in hematopoietic ES cell assays and in vivo following transplantation. The relationship between PGE2 and Wnt was also conserved during regeneration of other organ systems. Our work provides the first in vivo evidence that Wnt activation in stem cells requires PGE2, and suggests the PGE2/Wnt interaction is a master regulator of vertebrate regeneration and recovery.
Introduction
Stem cells are characterized by their unique abilities to both self-renew and differentiate to produce all mature cell lineages of a given tissue type. In the adult vertebrate HSCs reside in the bone marrow (BM), while during embryonic development several sites successively become competent to produce HSCs (Orkin and Zon, 2008). An understanding of the complex network of inductive signals regulating HSC development is of significant therapeutic interest for HSC maintenance in the adult. The aorta-gonad-mesonephros (AGM) region contains the first adult-type long-term repopulating (LTR-) HSCs in the vertebrate embryo; murine transplantation studies revealed that LTR-HSCs can be found on the ventral wall of the dorsal aorta by e10.5. The Runx1 protein, widely known for its involvement in leukemia, is specifically expressed in the AGM and is required for the formation of functional HSCs (North et al., 2002). The expression of runx1 is highly conserved across vertebrate species (Orkin and Zon, 2008).
We recently showed that PGE2 regulates vertebrate HSC induction and engraftment (North et al., 2007). PGE2 was identified through a chemical genetic screen for modifiers of runx1 expression within the zebrafish AGM. A stabilized derivative, 16,16-dimethyl-PGE2 (dmPGE2), enhanced the formation of stem cells and zebrafish marrow recovery following irradiation injury. dmPGE2 significantly increased ES cell hematopoietic colony formation and the frequency of both short (ST-) and LTR-HSCs in the mouse BM. The exact mechanism by which PGE2 exerts its effects on HSCs remains unknown. PGE2 has a regulatory role during myeloid differentiation, erythropoiesis and stromal cell homeostasis in murine BM (Fisher and Hagiwara, 1984; Nocka et al., 1989; Williams and Jackson, 1980). Additionally, hematopoietic lineage regeneration is impaired in cyclooxygenase (Cox) 2-deficient mice (Lorenz et al., 1999). Together, these data indicate that PGE2 plays a critical role in HSC induction as well as maintenance and function in the adult organism.
Wnt signaling has been similarly implicated in HSC regulation in the adult BM (Reya et al., 2003; Trowbridge et al., 2006). To date, however, a role for wnt in HSC development has not been described. Wnt signaling regulates several aspects of vertebrate embryogenesis, including gastrulation, somitogenesis and organogenesis (Goessling et al., 2008; Weidinger et al., 2005). Wnt activation is required for liver and fin regeneration (Goessling et al., 2008; Stoick-Cooper et al., 2007), as well as the maintenance of hematopoietic, skin, and intestinal stem cells (Congdon et al., 2008; Fevr et al., 2007; Nguyen et al., 2006; Reya et al., 2003). We hypothesized that wnt likely functions as a major regulator of stem cell induction during embryogenesis, and may work in conjunction with PGE2 in HSC formation and hematopoietic regeneration.
Clinical evidence supported the purported interaction between PGE2 and Wnt signaling in vivo. Patients with mutations in the Adenomatous Polyposis Coli (APC) gene, a critical intracellular regulator of wnt signaling, typically develop innumerable colonic polyps and ultimately colon cancer. Treatment with COX inhibitors significantly reduces polyp formation (Giardiello et al., 1993). This observation was confirmed by chemical Cox inhibition in APCMin mice (Boolbol et al., 1996) and genetic deletion of Cox2 and PG synthase (Nakanishi et al., 2008; Oshima et al., 1996). The connection between Wnt and PGE2 has been mechanistically described in cellular proliferation and oncogenesis (Castellone et al., 2005; Shao et al., 2005). However, these studies are limited to in vitro analyses using immortalized cell lines, which often harbor mutations in the wnt pathway itself. As such, they cannot address whether this interaction is functionally relevant in vivo or if it is solely an aberrant regulatory mechanism utilized in carcinogenesis (Buchanan and DuBois, 2006; Clevers, 2006).
Here we show that PGE2 can directly regulate wnt activity in vivo during vertebrate development and organ regeneration. This interaction occurs within HSCs and the hematopoietic niche during embryogenesis and functions to regulate HSC induction. PGE2 was required to mediate the effects of wnt activation and can act to further amplify wnt activity through cAMP/PKA-mediated regulation of β-catenin protein stability in vivo. Both in vitro, in murine ES cell hematopoietic assays, and in vivo following BM transplantation, PGE2 modified wnt-mediated regulation of hematopoietic stem and progenitor populations. Significantly, this role of PGE2 was conserved during regeneration in several organ systems, indicating that the PGE2/wnt interaction serves as a master regulator of vertebrate organ regeneration.
Results
Prostaglandin levels directly affect wnt/β-catenin activity in HSCs in vivo
Clinical evidence from colon cancer patients and cancer cell lines suggested a close association between the wnt and PGE2 pathways (Castellone et al., 2005; Giardiello et al., 2002; Shao et al., 2005). To examine whether PGE2 regulates wnt activity in vivo, we utilized TOP:dGFP β-catenin-responsive reporter zebrafish embryos. 10μM dmPGE2 caused a striking increase in reporter activity throughout the embryo at 36 hours post fertilization (hpf; 99 increased (inc)/111 scored). Specifically, it quadrupled the number of GFP+ cells in the AGM (12±3.4 (dmPGE2) vs 3±1.8 (control (con)) cells, p<0.05; Fig. 1A,B,D). We previously showed by mass spectroscopy that the non-selective cox inhibitor indomethacin (indo) suppressed PGE2 production in zebrafish embryos (North et al., 2007). 10μM indo decreased GFP expression globally and abolished wnt activity within the AGM (72 decreased (dec)/87, 0.7±0.5 cells, p<0.05; Fig. 1C,D). qPCR analysis for GFP confirmed that PGE2 significantly modulates wnt activity in vivo (p<0.05; Fig. 1E).
Figure 1. Prostaglandin levels directly affect wnt activity in zebrafish embryos.
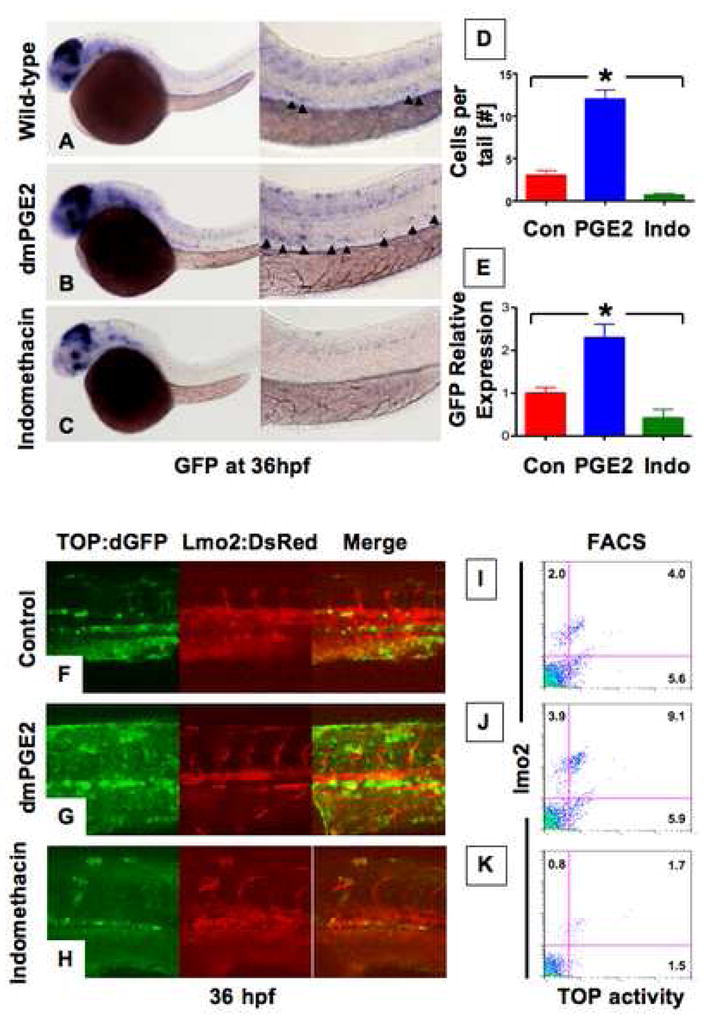
(A–C )In situ hybridization for GFP in TOP:dGFP wnt reporter embryos at 36hpf shows widespread wnt activity; inset, close-up of GFP+ (black arrowheads) cells in the AGM. 10μM dmPGE2 enhanced GFP expression throughout the embryo, while 10μM indo decreased global wnt activity, and in the AGM. (D) Quantification of total GFP+ cells in the major trunk vessels and (E) qPCR analysis for GFP in whole embryo lysates following exposure to dmPGE2 or indo versus vehicle con reveals significant alterations in wnt activity (*significant (sig) across treatment groups, ANOVA, p<0.05, n=10/treatment). (F–H) Representative confocal microscopy images of the AGM region of TOP:dGFP; lmo2:DsRed embryos following exposure to con, dmPGE2, or indo are shown; differences can be seen in the wnt-active (green, left column), HSC/endothelial (red, middle), and colocalized (merged, right) populations. (I–K) Representative FL1 (green)/FL2 (red) FACS plots of individual TOP:dGFP; lmo2:DsRed embryos after exposure to con, dmPGE2 or indo confirm the confocal analyses (summarized in Sup. Fig. 2).
To determine whether wnt activity was localized within the HSCs, we examined TOP:dGFP embryos co-expressing lmo2:dsRed, which label both HSCs and endothelial cells. Wnt activity in wild-type (WT) embryos was found in the endothelial and subendothelial layers of the ventral wall of the dorsal aorta (Fig. 1F), which possess HSC potential in murine transplantation assays (North et al., 2002). dmPGE2 enhanced wnt activity within the HSC/endothelial population and in the subendothelial layer (Fig. 1G). Indo decreased wnt-active cells in the AGM (Fig. 1H). FACS analysis of bigenic fish showed that dmPGE2 significantly enhanced lmo2+ (p<0.05; Sup. Fig. 2A,C) and GFP+ wnt-active cells (Fig. 1I,J, Sup. Fig. 2B); wnt activation was most pronounced within the lmo2+ fraction (Sup. Fig. 2D). Indo significantly diminished wnt-active and lmo2+ cells (p<0.05; Fig. 1K, Sup. Fig. 2A–E). Together, these data demonstrate that PGE2 regulates wnt activity within the HSC/endothelial cell population as well as the hematopoietic niche during embryonic development.
PGE2 modifies wnt-mediated regulation of HSC formation through alterations in β-catenin availability
The role of wnt signaling in regulating embryonic HSC formation has not been determined. Using inducible wnt8 transgenic zebrafish, we previously studied the time-dependent effects of wnt activation on organ formation (Goessling et al., 2008). Here, we examined alterations in the expression of the conserved HSC markers runx1/cmyb at 36hpf (North et al., 2007). Transplantation of FACS-fractionated murine AGM cells demonstrated that all HSCs express Runx1 (North et al., 2002); similarly, in zebrafish, AGM cells from subdissected tail regions, encompassing the runx1+ population, possess LTR-HSC activity, indicating HSC function with the AGM is conserved (North and Zon, unpublished observation). We found that induction of wnt8 during mid-somitogenesis enhanced runx1/cmyb+ cells (47inc/54; Fig. 2A,C). To examine whether PGE2 was required to mediate the effects of wnt activation on HSCs, wnt8 induction was followed by indo exposure; this reduced HSC number to WT levels (43 normal (norm)/46; Fig. 2B,D). These findings were confirmed by qPCR for runx1 and cmyb ( Sup. Fig. 3A,B) and demonstrate that PGE2 is required for wnt-mediated regulation of HSC formation in vivo. Significantly, wnt activation synergized with PGE2 in the majority of embryos (25inc/41; Sup. Fig. 3F–J) examined, demonstrating that PGE2 can function to enhance wnt activity in vivo.
Figure 2. PGE2 regulates Wnt-mediated effects on HSC formation at the level of β-catenin.
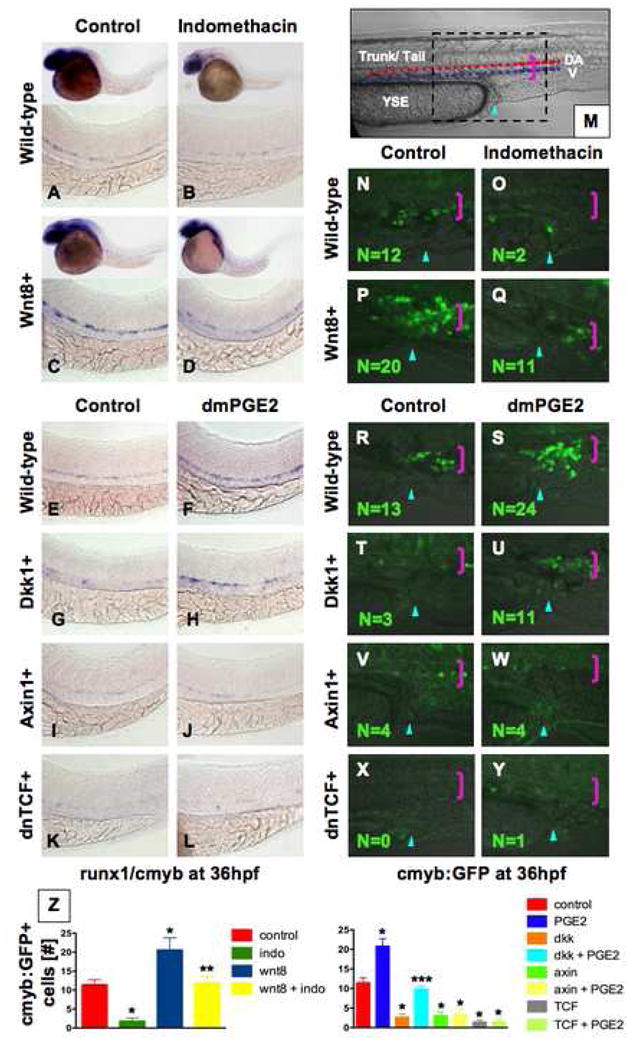
(A–D) wnt8 induction in hs:wnt8-GFP transgenic embryos increased runx1/cmyb+ HSCs compared to WT; indo decreased HSCs in WT and wnt8 embryos. (E–L) Induction of dkk diminished runx1/cmyb expression compared to WT. dmPGE2 enhanced HSCs in WT embryos, and rescued runx1/cmyb expression to approximately WT levels in dkk embryos. Axin (hs:axin-GFP) and dnTCF (hs:dnTCF-GFP) reduced runx1/cmyb expression, and dmPGE2 could not rescue those effects. (M) Schematic of confocal microscopy. Imaging was performed in the trunk/tail region of the embryo, centered around the tip of the yolk sac extension (YSE, blue arrowhead), encompassing the dorsal aorta (red dots) and vein (blue dots), as indicated by the pink bracket. (N–Y) In vivo confocal microscopy of wnt pathway inducible embryos crossed into the cmyb:GFP HSC reporter line confirmed the in situ hybridization analysis and demonstrated quantifiable effects on cell number. (Z) Cell counts (5 embryos/treatment, data represented as mean ± SD) were conducted in the major vessels (pink bracket) in a 40x field centered at the YSE; *sig vs con; **sig vs wnt8; *** sig vs dkk; ANOVA, p<0.001.
To genetically localize the interaction between PGE2 and the wnt pathway, transgenic fish expressing inducible negative regulators of wnt signaling were exposed to dmPGE2. Dickkopf (dkk), a membrane-level antagonist, inhibited runx1/cmyb expression (34dec/49; Fig. 2E,G). This effect could be rescued by exposure to dmPGE2 (28norm/51; Fig. 2F,H). Axin, a central component of the β-catenin destruction complex, and a dominant-negative form of the β-catenin transcriptional co-activator T-cell factor (TCF) also severely diminished HSC formation (47dec/52 (axin); 60dec/62 (TCF); Fig. 2I,K). However, these effects could not be overcome by dmPGE2 treatment (40dec/45; 48dec/51; Fig. 2J,L). These results were confirmed by qPCR for runx1 and cmyb (Sup. Fig. 3A,B). Expression of vascular markers flk1 and ephB2 (Sup. Fig. 3C,D) and the wnt target cyclin D1 (Sup. Fig. 3E) were coordinately affected by the PGE2/wnt interaction. These experiments indicate that PGE2 interacts with the wnt pathway at the level of β-catenin destruction to control HSC formation.
To demonstrate a direct effect of the PGE2/wnt interaction on HSC number rather than gene expression alone, inducible wnt transgenics were crossed to cmyb:GFP HSC reporter zebrafish (North et al., 2007). In vivo confocal microscopy (Fig. 2M,N) revealed that indo caused a significant decrease in HSC number (1.8±0.8 vs. 11.4±3.2 (con), p<0.001; Fig. 2O,Z). wnt8 significantly increased HSCs (20.6±3.2, p<0.001; Fig. 2P), while subsequent indo treatment returned HSC numbers to near WT levels (11.8±1.6; Fig. 2Q). dmPGE2 enhanced HSC number, as previously shown (North et al., 2007) (20.8±1.9, p<0.001; Fig. 2R,S). dkk (2.6±0.9, p<0.001; Fig. 2T), axin (3.0±1.0, p<0.001, Fig. 2V), and dnTCF (1.4±0.6, p<0.001; Fig. 2X) diminished HSCs. dmPGE2 could only rescue the effects of dkk, not of axin or dnTCF (9.8±0.8; 3.4±1.3; 1.4±0.9; Fig. 2U,W,Y). These results demonstrate that PGE2 and wnt signaling interact at the level of the β-catenin destruction complex and influence HSC number in the AGM.
The PGE2/wnt interaction affects cell survival and proliferation in the AGM
To further delineate the impact of the PGE2/wnt interaction on developing HSCs, we examined apoptosis and cellular proliferation. During embryonic development only a small fraction of AGM cells appeared TUNEL+ (Fig. 3A,B Sup. Fig. 4A,E); cell counts in a defined anatomical section of the trunk revealed 5.6±1.8 apoptotic cells in untreated WT embryos. wnt8 (3.6±1.1; Sup. Fig. 4C) or dmPGE2 (1.8±1.5; Sup. Fig. 4F) decreased TUNEL+ cells. Indo induced wide-spread apoptosis (40.8±4.6, p<0.001; Sup. Fig. 4B) and counteracted the effect of wnt8 (11.6±2.4; p<0.001; Sup. Fig. 4D). Similarly, dkk, axin, or dnTCF induction increased apoptosis in the AGM (43.2±8.2; 33.2±4.9; 34.6±5.6; p<0.001 vs con; Fig. 3B, Sup. Fig. 4G,I,K), which was improved by dmPGE2 only in dkk embryos (9.2±2.8, p<0.001 vs. dkk; Sup. Fig. 4H,J,L). BrdU incorporation occurred globally during embryonic development, including cells within the AGM region (10.4±2.1; Fig. 3C,D, Sup. Fig. 4M,Q); this effect was significantly enhanced by wnt8 (24.4±5.0; p<0.001; Sup. Fig. 4O) and dmPGE2 (27.8±3.0, p<0.001; Sup. Fig. 4R). Indo suppressed BrdU incorporation (2.8±1.5, p<0.002; Sup. Fig. 4N) and blunted the proliferative effect of wnt activation (7.2±2.6, p<0.001 vs wnt8; Sup. Fig. 4P). dkk, axin, or dnTCF similarly decreased BrdU incorporation (4.6±2.0; 5.8±1.5; 3.6±1.5, p<0.002; Fig. 3D, Sup. Fig. 4S,U,W); dmPGE2 only rescued dkk (11.8±3.4, p<0.001 vs dkk; Sup. Fig. 4T). Together, these results suggest that cellular proliferation and apoptosis may be mechanisms by which PGE2 can modify the effects of wnt signaling on HSCs.
Figure 3. PGE2-mediated modulation of wnt signaling affects cell death and proliferation.
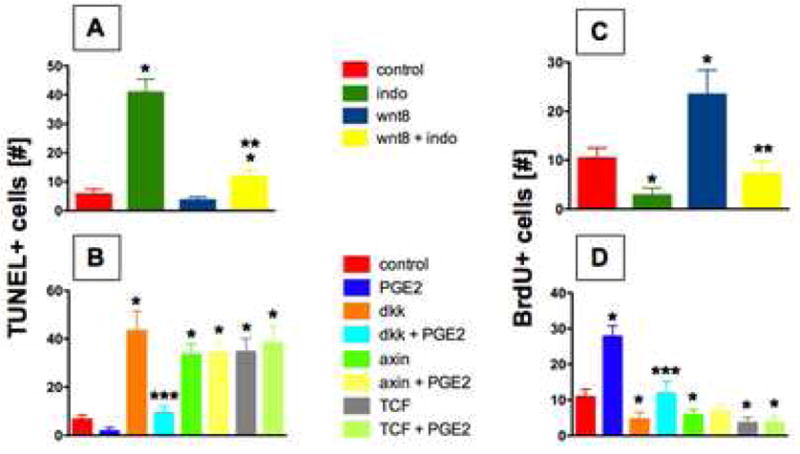
(A,B) Indo treatment enhanced TUNEL+ cells in the AGM in both WT and wnt8 embryos. dkk, axin, or dnTCF enhanced apoptosis; dmPGE2 improved this effect only in dkk transgenics. Cell counts (5 embryos/treatment) in the AGM showed significant effects across treatment groups: *sig vs con; **sig vs wnt8; ***sig vs dkk; ANOVA; p<0.05. (C,D) wnt8 enhanced BrdU incorporation, while indo diminished BrdU in both WT and wnt8 embryos. wnt inhibition by dkk, axin, or dnTCF diminished BrdU incorporation; the effect of dkk could be rescued by dmPGE2. *sig vs con; **sig vs wnt8; ***sig vs dkk; ANOVA; p<0.05.
PGE2 regulates the effects of wnt signaling through cAMP/PKA activity
The genetic interaction studies suggested that PGE2 may directly regulate β-catenin destruction, and subsequent protein available for transcriptional activation. To document whether PGE2 directly enhances β-catenin levels in vivo, Western blot analysis was performed. wnt8 and dmPGE2 increased (Sup. Fig. 3K), whereas indo decreased total β-catenin in WT and wnt8 pooled embryo lysates. qPCR revealed that PGE2 did not affect β-catenin transcription (Sup. Fig. 3L). These data suggest that PGE2 directly influences the stability of β-catenin via non-transcriptional mechanisms.
β-catenin levels are tightly controlled within the cell by the destruction complex, consisting of axin, GSK3β, CK1 and APC. In the absence of wnt ligand binding, β-catenin is phosphorylated at N-terminal residues and targeted for ubiquitination/degradation. C-terminal phosphorylation of β-catenin (S675) stabilizes the protein by inhibiting destruction (Hino et al., 2005), and destruction complex assembly can be blocked by phosphorylation of GSK3β at S9 (Fang et al., 2000). PGE2 regulates intracellular phosphorylation through cAMP and downstream effector kinases PKA and PI3K/AKT (Fujino et al., 2002). To demonstrate a functional role for cAMP in modulating wnt activity and HSC development, TOP:dGFP embryos were exposed to forskolin, a cAMP-activator. Forskolin (0.5μM) increased wnt activity (18inc/21; 8.8±1.3 TOP:dGFP+ cells/tail (forskolin) vs 3.8±0.8 (con), p<0.001; Sup. Fig. 5A,C,E) and counteracted the wnt-inhibitory effects of indo (20dec/25), confirming that cAMP functions downstream of PGE2 to regulate wnt signaling (17norm/27; 6.4±1.1 (indo+forskolin) vs 1.1±0.7 (indo), p<0.001; Sup. Fig. 5B,D). We next sought to determine if PKA or PI3K was the secondary effector of cAMP in controlling wnt activity in the AGM in vivo. Exposure to the PKA inhibitor H89 (0.5μM) decreased wnt activity compared to WT (19dec/20; 0.6±0.6; p<0.001; Sup. Fig. 5F,H,L). In contrast, the PI3K inhibitor LY294002 (1μM, LY) did not affect wnt activity appreciably (3dec/27; 3.6±1.1; Sup. Fig. 5J,K). Furthermore, H89, but not LY, blocked PGE2-mediated elevation of wnt activity (23inc/26 (PGE2); 22norm/25 (PGE2+H89); 20inc/26 (PGE2+LY); 11.0±1.6 (PGE2) vs 2.4±1.1 (PGE2+H89), p<0.001; 9.8±1.5 (PGE2+LY); Sup. Fig. 5G,I,K). These results suggest that PGE2 directly enhances wnt activity through cAMP/PKA-signaling.
We next tested whether cAMP and PKA are functionally relevant in PGE2-mediated wnt regulation of HSCs in vivo. Exposure to forskolin increased HSCs (Fig. 4C,G), and rescued the negative effect of indo treatment in WT (21 normal/33; Fig. 4D) and wnt8 embryos (18inc/26, Fig. 4H). PKA inhibition by H89 diminished HSCs in WT embryos and eliminated the effects of dmPGE2 (13norm/19; Fig. 4L). H89 also inhibited the dmPGE2-mediated rescue of dkk embryos (17dec/26; Fig. 4P). The effects of cAMP/PKA modulation were quantitated by qPCR for runx1 (Sup. Fig. 6A,B). The consequence of PKA inhibition was confirmed by exposure to 1μM KT5720 (19dec/31 (KT); 20norm/29 (PGE2+KT); Sup. Fig. 6C–F). Treatment with the PI3K inhibitors LY (4dec/39 (LY); 34inc/42 (PGE2+LY)) and 1μM Wortmannin (5dec/48 (Wort); 34inc/46 (PGE2+Wort)) did not considerably alter HSC development (Sup. Fig. 6G–J). These data indicate PGE2 acts primarily via cAMP/PKA to affect wnt signaling and regulate HSC formation in vivo.
Figure 4. PGE2 regulates the effects of wnt activity on HSCs via cAMP/PKA signaling.
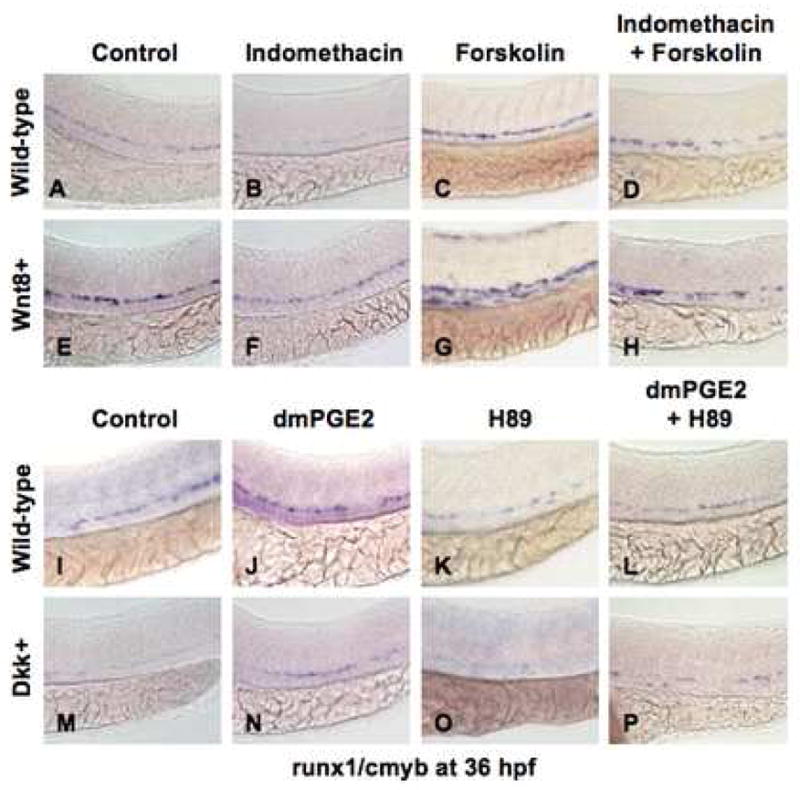
(A,B,E,F; I,J,M,N) The/PGE2/wnt interaction affected runx1/cmyb+ HSC formation, as seen in Fig. 2. (C,G) cAMP enhancement by forskolin increased runx1/cmyb expression in WT embryos and further expanded HSCs in wnt8 embryos. (D,H) Forskolin counteracted the inhibitory effect of indo on HSC formation in WT and wnt8 embryos. (K,O) PKA inhibition by H89 decreased runx1/cmyb expression in WT embryos and eradicated HSC formation in dkk embryos. (L,P) The enhancing effect of dmPGE2 on HSCs was reduced back to baseline levels by PKA inhibition; similarly the dmPGE2-induced rescue of HSCs in dkk embryos was blocked by H89.
The PGE2/wnt interaction is functionally conserved in HSC regeneration
Wnt signaling regulates adult HSC self-renewal, BM recovery and repopulation (Congdon et al., 2008; Reya et al., 2003). To determine whether the PGE2/wnt interaction affects hematopoietic regeneration in adult fish, we examined kidney marrow recovery following irradiation. In TOP:dGFP zebrafish, wnt activity was significantly increased in the recovering marrow compared to unirradiated controls (p<0.001; Fig. 5A). Wnt activity was significantly enhanced by dmPGE2 and markedly diminished by indo (p<0.001; Fig. 5A). FACS analysis of recovering marrow identifies effects on all hematopoietic lineages, including the stem/progenitor population. wnt8 induction significantly increased precursors (18.1±3.5% (wnt8) vs 7.1±3.1% (WT), p<0.001; Fig. 5B); treatment with indo significantly decreased precursor recovery (4.2±2.1%, p<0.02) and diminished the effect of wnt8 (11.6±5.2%, p<0.001), confirming that PGE2 modulates the effects of wnt activation in the adult. In support of this conclusion, the decrease in marrow recovery following induction of dkk (3.4±1.1% (dkk) vs 7.0±1.1% (WT)) was partially corrected by treatment with dmPGE2 (6.3±1.0%, p<0.001; Fig. 5C).
Figure 5. The PGE2/wnt interaction is conserved in HSC regeneration after injury.
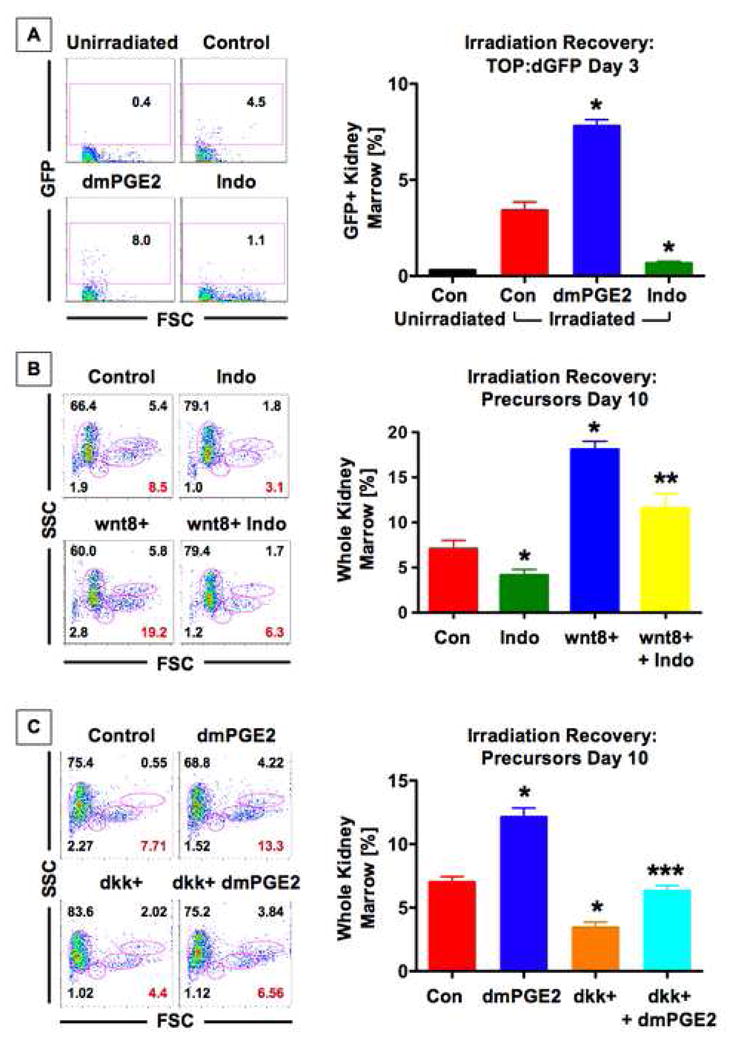
(A) wnt activity in the kidney marrow was enhanced in FACS profiles on day 3 post irradiation (dpi) in TOP:dGFP fish; representative FACS analyses are shown on the left, and summarized (mean ± SD) on the right. dmPGE2 further increased and indo inhibited wnt activity; *sig vs unirradiated con, **sig vs irradiated con, ANOVA, p<0.001, n=5 fish/treatment. (B) wnt8 (bottom left panel) enhanced the precursor population (red), while indo suppressed that effect significantly; *sig vs con, **sig vs wnt8, t-test, p<0.02, n=10–15. (C) dkk reduced progenitor recovery, which was rescued by dmPGE2; *sig vs con, ***sig vs dkk, ANOVA, p<0.05, n=6.
The PGE2/wnt interaction is conserved in mammalian hematopoietic stem and progenitor populations
To determine whether the Wnt and PGE2 pathways interact similarly in the mammalian hematopoietic system, we examined in vitro differentiation of murine embryonic stem cells (mESC). Wnt3A increased the total number of hematopoietic progenitors, while indo led to reduction (p=0.001; Sup. Fig. 7A). Indo diminished the Wnt3A-induced increase (p<0.01), demonstrating a conserved requirement for PGE2 in mediating the effects of Wnt stimulation. Dkk1 exposure reduced total colony number, indicating that Wnt activity is required for hematopoietic progenitor formation and/or expansion (Sup. Fig. 7B). This decrease in hematopoietic progenitors was reverted by dmPGE2 (p=0.046) or forskolin, demonstrating that as in zebrafish, PGE2 modulates Wnt-mediated effects on mammalian hematopoietic stem/progenitor cells via the cAMP/PKA pathway. By Western blot analysis, Wnt3A and dmPGE2 treatment increased total β-catenin levels, while exogenous Dkk1 or indo markedly decreased β-catenin (Sup. Fig. 7C). Indo diminished the effect of Wnt3A, and dmPGE2 improved β-catenin levels after Dkk1 exposure. These results paralleled the effect on colony formation seen in the hematopoietic differentiation assays and correspond to the zebrafish results.
We next tested whether the regulation of Wnt/β-catenin function by PGE2 extended to the mammalian hematopoietic system in vivo. To assess the effects of PGE2/wnt modulation on functional murine stem/progenitor cells, isolated cKit+Sca1+Lineage-(KSL) BM cells were transplanted into lethally irradiated mice. To modulate Wnt activity and PGE2 levels, the GSK3β inhibitor 6-bromoindirubin-3′-oxime (BIO, 50μg/kg) (Meijer et al., 2003), indo (2.5mg/kg), or a combination of both, were administered intraperitoneally to recipient mice every other day. CFU-S12 analysis revealed a significant increase in hematopoietic progenitors after BIO treatment (p=0.015; Sup. Fig. 8A). Concurrent administration of indo reduced CFU-S12 numbers to baseline levels, implying that PGE2 in recipient mice is necessary for the effects of Wnt activation on hematopoietic progenitor cells. To define whether the PGE2/Wnt interaction occurs within hematopoietic progenitors or within the niche, purified KSL cells were treated directly ex vivo prior to transplantation. BIO significantly increased CFU-S12 numbers (p=0.0003), which was further enhanced by combined treatment with dmPGE2 (p<0.05). Indo reduced the effect of BIO to control levels (BIO/indo vs BIO, p<0.0001; Fig. 6A). To confirm that these observations were due to specific interactions with the Wnt pathway and not to off-target effects of BIO, we used KSL cells isolated from APCMin mice with constitutively elevated β-catenin levels (Fig. 6B). APC loss and dmPGE2 synergistically enhanced CFU-S12 number (p<0.0001), while indo inhibited this effect (p=0.0004). Similarly, in KSL cells treated with Dkk1, progenitor number was significantly enhanced by dmPGE2 or forskolin (Sup. Fig. 8B). From these data we conclude that the effect of Wnt activation on adult mouse hematopoietic progenitor populations requires PGE2.
Figure 6. PGE2 influenced wnt-mediated repopulation of murine HSC and progenitors.
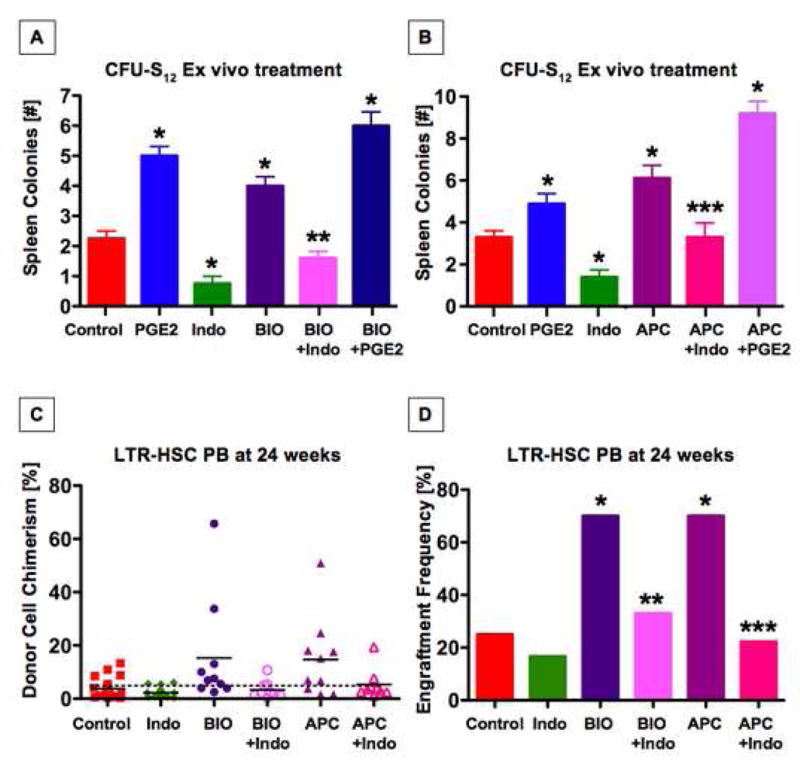
(A) Ex vivo treatment of purified C57Bl/6 KSL cells with indo, BIO, and/or dmPGE2 prior to transplantation into lethally irradiated mice revealed that PGE2 modulation significantly impacts hematopoietic progenitors. BIO significantly enhanced colony formation, which could be suppressed by indo. dmPGE2 further increased the effect of BIO; *sig vs con, ANOVA, p<0.05, n≥7. (B) Effect of ex vivo treatment with dmPGE2 or indo on CFU-S12 colony formation in KSL cells from APCMin mice. Genetic activation of wnt signaling in APCMin hematopoietic progenitor cells has comparable effects on CFU-S12 colony formation to chemical stimulation by BIO, and indo exposure blocks this enhancement; *sig vs con, ANOVA, p<0.05, n≥7. (C,D) Wnt activation through the GSK3β inhibitor BIO or in APCMin marrow enhanced chimerism rates at 24 weeks; each effect could be inhibited by indo. Test cell chimerism of individual mice is shown (C), with the mean/group indicated by a solid black line. The dashed black line demonstrates the 5% cut-off value used to determine engraftment frequencies (D); *sig vs con, Fisher’s exact, p=0.045; n≥8.
To directly assess whether the PGE2/wnt interaction had functional significance in HSCs, we performed long-term competitive BM repopulation experiments. ST-repopulation at 6 weeks, and LTR-engraftment at 12 and 24 weeks showed identical results: the average chimerism after BIO treatment was significantly higher (15.3±19.9%) than in untreated animals (3.8±3.8%, p=0.017; Fig 6C), with 7/10 recipients (70%) showing PB chimerism >5% compared to WT (5/20 (25%), Fisher’s exact, p=0.045; Fig. 6C,D). The effect of BIO was reduced towards baseline after indo exposure (3.3±3.3%), with only 3/9 (33.3%) mice showing repopulation from dual-treated test cells. Similar effects were seen in APCMin marrow (7/10 (70%, APC); 2/9 (22%, APC+indo); 14.7±15.0% vs 5.3±5.6%, p=0.004), confirming that the effects were specifically due to alterations in wnt signaling (Fig. 6D). These data indicate that mammalian LTR-HSCs are directly influenced by the PGE2/Wnt interaction.
Several biochemical interactions have been proposed for the relationship between PGE2 and wnt in vitro (Castellone et al., 2005; Fang et al., 2007; Fujino et al., 2002; Hino et al., 2005; Shao et al., 2005). To further characterize the mechanism of PGE2-based wnt regulation in HSCs, we isolated WBM, treated cells ex vivo with PGE2-modifiers, and performed a chemiluminescence assay for cAMP activity. cAMP levels increased in a dose-dependent manner following dmPGE2 exposure, similar to controls exposed to forskolin (Sup. Fig. 8C). Total β-catenin levels in WBM increased following dmPGE2 treatment, while indo diminished β-catenin (Sup. Fig. 8D). These results confirm a direct effect of PGE2 on β-catenin stability in murine hematopoietic cells. Time-course analysis after incubation with either dmPGE2 or indo demonstrated that alterations in total β-catenin were preceded by changes in the phosphorylation status of β-catenin at S675 and GSK3β at S9; these modifications can influence the stability and destruction of β-catenin in vitro (Castellone et al., 2005; Fujino et al., 2002; Hino et al., 2005). These results show that in the hematopoietic compartment PGE2 actively regulates wnt activity through phosphorylation-based modulation of β-catenin protein stability.
The PGE2/wnt interaction is a central regulator of organ regeneration
To explore whether the PGE2/wnt interaction functions as a master regulator of organ regeneration/recovery in non-hematopoietic tissues, we examined the process of liver regeneration, which is known to be wnt dependent (Goessling et al., 2008; Tan et al., 2006). APC+/− fish demonstrated enhanced regenerative capacity compared to WT controls at 3 days post resection (Goessling et al., 2008). As in HSCs, indo significantly diminished organ recovery in both WT and APC+/− fish (p<0.001, Fig. 7A–E). Expression of cyclin D1 was coordinately affected by wnt activity and PGE2 levels (Fig. 7F). Murine liver resections documented conservation of this interaction during mammalian organ regeneration. Following 2/3 partial hepatectomy, nuclear β-catenin accumulation increased in WT and APCMin mice, most strikingly in the periportal region, the location of the presumed hepatic stem cells. Indo diminished β-catenin levels in both WT and APCMin mice, with virtual exclusion of β-catenin from hepatocyte nuclei following indo treatment, confirming that during liver regeneration PGE2 is necessary for β-catenin-mediated wnt signaling (Fig. 7H,J).
Figure 7. The PGE2/wnt interaction is a master regulator of liver regeneration.
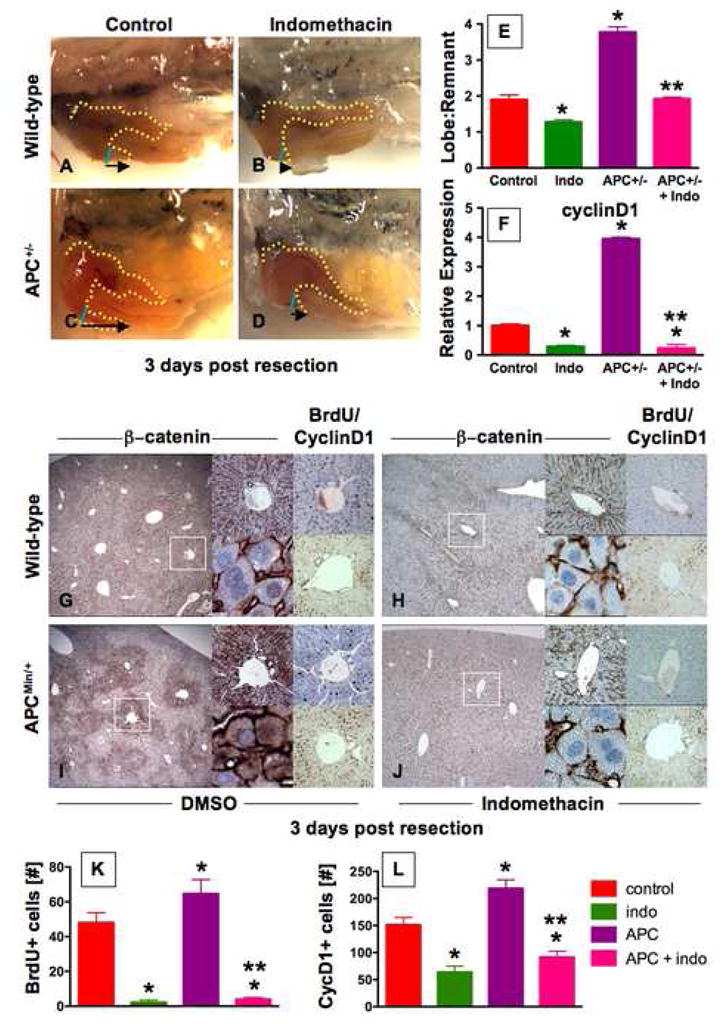
(A–D) Representative photomicrographs of en bloc dissections following liver resections at day 3 are shown; the liver is highlighted by a yellow dotted line, the resection site by a blue line, and the black arrow indicates the amount of liver regrowth. Wnt activation in APC mutant zebrafish enhanced liver regeneration compared to WT. Indo stymied liver regeneration. (E) Quantification of zebrafish liver regeneration showed significant differences across treatment groups; *sig vs con, **sig vs APC+/−, ANOVA, p<0.001, n≥6. (F) qPCR for cyclin D1 gene expression; effects are coordinately regulated by the PGE2/wnt interaction; *sig vs con, **sig vs APC, ANOVA, p<0.001, n=7. (G–J) wnt and PGE2 modulation has significant effects on murine liver regeneration following 2/3 partial hepatectomy. APCMin mice exhibit enhanced β-catenin staining (left panel), particularly in the periportal areas (top middle panel), with noticeable nuclear staining (bottom middle panel). BrdU incorporation (top right panel) and cyclin D1 staining (bottom right panel) indicated enhanced regenerative activity. Indo diminished global β-catenin staining (left and top middle panels), excluded β-catenin from the nuclei (bottom middle panels), and resulted in a corresponding decrease of both BrdU incorporation and cyclin D1. (K,L) Quantification of BrdU incorporation and cyclinD1 staining in corresponding serial sections of regenerating livers; *sig vs con, **sig vs APC, ANOVA, p<0.05, n=10 sections/treatment.
To assess the functional consequences of wnt activity and its suppression by indo, cell proliferation was examined. BrdU incorporation was enhanced in livers of APCMin mice (64.7±8.0 cells/periportal field (APC) vs 48.0±5.7 (con), p<0.001; Fig. 7G,I); indo treatment significantly diminished periportal BrdU incorporation in WT (2.2±1.4, p<0.001) and APCMin mice (4.0±1.1; Fig. 7G–K). Similarly, significant differences were seen in the number of periportal cyclinD1+ cells (151.3±13.6 (con); 218.8±15.7 (APC); 64.0±3.4 (indo); 91.5±10.9 (APC+indo); Fig. 7L). Indo also induced extensive hepatocyte necrosis (Sup. Fig. 8J). Both WT and APCMin mutant livers exhibited β-catenin (S675) (Sup. Fig. 9A,E) and GSK3β (S9) stabilizing phosphorylation (Sup. Fig. 9B,F), which was absent following indo treatment (Sup. Fig. 9C,D,G–I), consistent with our observations in HSCs. These results document that PGE2 regulates Wnt activity through alterations in β-catenin transcriptional availability and subsequent proliferation during solid organ regeneration.
Discussion
This study reveals a previously uncharacterized genetic interaction of PGE2 and the Wnt pathway in hematopoietic stem/progenitor populations in vivo, which is conserved during development and tissue regeneration across vertebrates. While previous studies in cell culture have shown these two pathways interact, our work demonstrates the relevant in vivo mechanisms and functional consequences of this relationship in HSCs. An intriguing finding of our work is that indo treatment significantly suppresses Wnt activity, supporting a model in which PGE2 is required to mediate the full effects of Wnt activation in vivo (Sup. Fig. 1). The conserved interaction of these pathways during wnt-dependent regeneration in other organ systems not only highlights its importance in regulating stem cell/progenitor formation and function, but may expand potential therapeutic interventions to modulate complex signaling networks in regenerative medicine and cancer treatment.
Wnt signaling influences HSC formation
The requirement of wnt activity in HSC self-renewal and marrow repopulation is controversial. Several studies have clearly indicated a positive effect of wnt activation on HSC recovery after injury and in transplantation assays (Congdon et al., 2008; Reya et al., 2003; Trowbridge et al., 2006). Similarly, we found that wnt activation is sufficient to stimulate HSCs. Conversely, β- and γ-catenin deficient animals show no impairment in either hematopoiesis or lymphopoiesis; this was interpreted as evidence that canonical wnt signaling could not play an essential role in HSC regulation (Koch et al., 2008). However, Jeannet et al. found that even in the absence of β- and γ-catenin function, canonical wnt activity was still present (Jeannet et al., 2008). These data imply that the natural complexity and redundancy of the wnt signaling pathway may subvert attempts to render it non-functional, possibly by bypassing components typically considered required. Nevertheless, wnt activation is sufficient to stimulate HSCs. While we did not address the question of a requirement for β-catenin directly, we found that TCF signaling was necessary for optimal HSC formation. These findings are consistent with an emerging role of wnt, not only as a crucial factor affecting body axis and polarity during early development, but as a central regulator of stem and progenitor populations in multiple organs (Goessling et al., 2008; Hirabayashi et al., 2004).
PGE2 regulates wnt activity by direct phosphorylation of β-catenin and GSK3β
Our work shows that PGE2 acts via cAMP/PKA signaling to directly modify β-catenin stability and emphasizes the functional importance and evolutionary conservation of these interactions in organ development and regeneration. PGE2-mediated regulation of Wnt signaling was previously demonstrated in cell lines. The proposed biochemical mechanisms mediating the interaction are remarkably diverse and may depend on the particular cell line studied (Clevers, 2006). Castellone et al. used colon cancer cell lines to demonstrate PGE2/PI3K-mediated activation of Akt, leading to dissociation of Axin1 from the destruction complex (Castellone et al., 2005). While we cannot exclude the existence of this biochemical interaction in vivo, our studies suggest that activation of PKA is the functionally significant effector downstream of PGE2 in HSCs. Likewise, both phosphorylation of GSK3β and β-catenin by PGE2-based activation of PKA have been implicated in Wnt signaling regulation in vitro. Fujino et al. used the transformed HEK293 cell line and showed that both PKA and PI3K could function downstream of PGE2 to phosphorylate GSK3β (Fujino et al., 2002). However, PGE1-induced activation of PKA and phosphorylation of β-catenin at S675 was cell-line dependent (Hino et al., 2005). While we found both phosphorylation events occur in the presence of PGE2 in vivo, it remains to be determined if phosphorylation of β-catenin and GSK3β each play a significant role in the regulation of wnt activity by PGE2 in HSCs, especially given a recently proposed functional redundancy of GSK3β with GSK3α (Doble et al., 2007).
The role of PGE2/wnt interaction in regeneration and carcinogenesis
The role of Wnt in organ regeneration has been described (Goessling et al., 2008; Stoick-Cooper et al., 2007). One universal response to tissue injury is enhanced PGE2 production (Goldstein et al., 1977). PGE2 may have co-evolved with Wnt as a mechanism to rapidly upregulate cellular proliferation to foster organ repair. In this setting, PGE2 - which is produced locally in response to tissue damage - is required for and can enhance the proliferative effects initiated by wnt activitation. Short-term exposure to PGE2 has been shown to increase HSC engraftment and could be useful for regeneration in many organs. In support of this hypothesis, we found that wnt-mediated fin regeneration was similarly regulated by PGE2 levels (Sup. Fig. 9K,L). It is also possible that chronic stimulation of PGE2 may exhaust stem cells, as has been shown for the wnt pathway (Scheller et al., 2006); thus, the therapeutic use of PGE2 to regulate wnt-mediated processes will need to be investigated further.
The genetic interaction between PGE2 and wnt signaling may provide insight into the basis of carcinogenesis occurring in cases of chronic inflammation. Chronic overproduction of PGE2 may lead to constitutive Wnt activation in vivo, resulting in tissue proliferation. Interestingly, it was this central importance given to controlling inflammation during early therapeutic regimens for colon cancer that led to the identification of COX inhibitors as potent regulators of tumor formation, long before the role of the wnt pathway in colonic tumorigenesis had been elucidated (Giardiello et al., 1993). Evolutionarily, what was greatly beneficial for wound healing – the coordinated pro-proliferative, anti-apoptotic effects of wnt and PGE2, may be detrimental in cases of chronic inflammation or constitutive pathway activation and lead to tumor initiation and growth. Targeting the wnt signaling pathway as a cancer treatment option, such as through direct inhibition of β-catenin, has proven problematic because of its ubiquitous importance for tissue homeostasis (Barker and Clevers, 2006). Our results indicate a mechanism whereby chemical manipulation of PGE2 levels or signaling to regulate Wnt activity in stem and progenitor cells may be therapeutically beneficial for the controlled regulation of both tissue repair and cancer treatment.
Experimental Procedures
Also see Supplemental Experimental Procedures.
Zebrafish husbandry
Zebrafish were maintained according to IACUC protocols. The zebrafish transgenic lines lmo2:DsRed, TOP:dGFP, hs:wnt8-GFP, hs:dkk-GFP, hs:dnTCF-GFP, hs:axin-GFP, and cmyb:GFP and the APC mutant were described previously (Dorsky et al., 2002; Hurlstone et al., 2003; Lewis et al., 2004; North et al., 2007; Stoick-Cooper et al., 2007; Weidinger et al., 2005; Zhu et al., 2005).
Embryonic zebrafish experiments
All analysis was performed at 36hpf. Embryos were exposed to compounds from 10 somites to examination at the following doses, unless otherwise indicated: dmPGE2, indo 10μM; forskolin, H89 0.5μM; LY294002, KT5720, Wortmannin 1μM; DMSO carrier content was 0.1%. In situ hybridization with standard zebrafish protocols (zfin.org/ZFIN/Methods/ThisseProtocol.html) was performed for runx1, cmyb, and GFP; qualitative changes from WT are reported as the # altered/# scored (median examples shown). Photomicrographs were taken of representative examples with Nomarski optics at 4x and 20x. FACS and confocal microscopy were conducted as described (North et al., 2007). qPCR (primers shown in Supplemental Table 1) and Western blot analysis were performed on pooled whole embryo lysates. BrdU and TUNEL analysis was performed as published (Shepard et al., 2005).
Heatshock modulation of wnt signaling
Embryonic heatshock experiments were conducted as previously described (Goessling et al., 2008); out-crossed embryos at the 10 somite stage were shocked at 38°C for 20 mins, and sorted by genotype on the basis of GFP expression. Heatshock modulation in adult zebrafish was performed as indicated below.
Adult zebrafish experiments
Adult zebrafish were exposed to 23 Gy of γ-irradiation. KM recovery was analyzed by FACS at days 3 or 10 as described (North et al., 2007); heatshock occurred at 38°C from 36–48 hours post irradiation (hpi) and chemical treatment from 48hpi-60hpi. KM from individual fish was manually dissected in 0.9% PBS, dissociated and examined for alterations in GFP expression, or HSC recovery by forward scatter/side scatter. Liver resections and regeneration analysis were conducted as described (Goessling et al., 2008); heatshock occurred from 6–16 hours post resection (hpr) and chemical treatment from 18–30hpr. Liver regrowth was quantified in en bloc dissected specimen by measuring the total length of the inferior liver lobe and the length of the remnant lobe to the original resection site.
cAMP luminescence assay
50,000 WBM cells were exposed to increasing doses of either dmPGE2 or forskolin for 15 minutes. The luminescence assay was performed according to standard manufacturer protocol (Promega, cAMP Glow).
ES cell culture and differentiation
ES cells were cultured and differentiated as embryoid bodies (EBs) as described (Kyba et al., 2002) with modifications: EBs were transferred to differentiation media on day 2 and incubated for 4 days. On day 4, PGE2/Wnt modulators were added alone or in combination: 20μM indo, 5μM forskolin, 10ng/ml Wnt3A, 400ng/ml Dkk1, 10μM dmPGE2. On day 6, EBs were plated (105 cells) into M3434 methylcellulose. CFU-Cs (CFU-E (erythroid), CFU-M (monocyte), CFU-G (granulocyte), CFU-GM (granulocyte-monocyte), and CFU-GEMM (granulocyte-erythroid-monocyte-megakaryocyte)) were scored on day 8–10 by morphology. Averages +/−SEM in the fold changes of total CFU-Cs relative to control were calculated.
Murine transplantation
WBM from 8-week old C57Bl/6 or APCMin mice were used for transplantation studies. For CFU-S12 analysis, KSL cells were FACS-sorted, treated as indicated and transplanted at 500 cells/recipient as previously described (North et al., 2007); spleen colonies were counted at day 12. For LTR low-dose competitive transplantation using CD45.1/CD45.2 mice, 15,000 treated test WBM cells were exposed ex vivo as indicated and injected with 200,000 untreated competitors into recipient mice; PB was obtained at 6, 12 and 24 weeks and multilineage chimerism >5% was determined by FACS analysis.
Murine liver resections
Two-third partial hepatectomy was performed as described (Greene and Puder, 2003); C57Bl/6 or APCMin mice were injected with DMSO or 2.5mg/kg indo i.p. every 12 hours, beginning 12 hours prior to resection. BrdU was injected 2 hours before mice were sacrificed. BrdU and CD1 cell counts were performed on corresponding sections of 20x microscopy fields focused on the periportal regions (n=10 sections).
Statistical Analysis
Pooled data were calculated as mean ± SD, with number of repeats as indicated. Pairwise comparison was performed by t-test, multiple comparisons by ANOVA, unless otherwise noted, using SigmaStat 3.5 software.
Supplementary Material
Acknowledgments
D. Langenau and C. Ceol for critical reading of the manuscript; C.R. Walkley and L. Purton for advice on HSC transplants; and J. Harris and C.C. Cutting for technical assistance. This work was supported by the NIH (WG, GQD, RTM, LIZ), the American Gastroenterological Association (WG), the American Cancer Society (TEN), and the Human Frontier Science Program (SL). GQD, RTM, LIZ are HHMI investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ, et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- Buchanan FG, DuBois RN. Connecting COX-2 and Wnt in cancer. Cancer Cell. 2006;9:6–8. doi: 10.1016/j.ccr.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Clevers H. Colon cancer--understanding how NSAIDs work. N Engl J Med. 2006;354:761–763. doi: 10.1056/NEJMcibr055457. [DOI] [PubMed] [Google Scholar]
- Congdon KL, Voermans C, Ferguson EC, DiMascio LN, Uqoezwa M, Zhao C, Reya T. Activation of Wnt signaling in hematopoietic regeneration. Stem Cells. 2008;26:1202–1210. doi: 10.1634/stemcells.2007-0768. [DOI] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JW, Hagiwara M. Effects of prostaglandins on erythropoiesis. Blood Cells. 1984;10:241–260. [PubMed] [Google Scholar]
- Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, Piantadosi S, Garrett E, Geiman DE, Hubbard W, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis AP, Puder M, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- Goldstein IM, Malmsten CL, Samuelsson B, Weissmann G. Prostaglandins, thromboxanes, and polymorphonuclear leukocytes: mediation and modulation of inflammation. Inflammation. 1977;2:309–317. doi: 10.1007/BF00921010. [DOI] [PubMed] [Google Scholar]
- Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, Raible DW. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- Lorenz M, Slaughter HS, Wescott DM, Carter SI, Schnyder B, Dinchuk JE, Car BD. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp Hematol. 1999;27:1494–1502. doi: 10.1016/s0301-472x(99)00087-9. [DOI] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, Xu D, Rosenberg DW. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Nocka KH, Ottman OG, Pelus LM. The role of marrow accessory cell populations in the augmentation of human erythroid progenitor cell (BFU-E) proliferation by prostaglandin E. Leuk Res. 1989;13:527–534. doi: 10.1016/0145-2126(89)90119-7. [DOI] [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- Shepard JL, Amatruda JF, Stern HM, Subramanian A, Finkelstein D, Ziai J, Finley KR, Pfaff KL, Hersey C, Zhou Y, et al. A zebrafish bmyb mutation causes genome instability and increased cancer susceptibility. Proc Natl Acad Sci U S A. 2005;102:13194–13199. doi: 10.1073/pnas.0506583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med. 2006;12:89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Williams N, Jackson H. Limitation of macrophage production in long-term marrow cultures containing prostaglandin E. J Cell Physiol. 1980;103:239–246. doi: 10.1002/jcp.1041030208. [DOI] [PubMed] [Google Scholar]
- Zhu H, Traver D, Davidson AJ, Dibiase A, Thisse C, Thisse B, Nimer S, Zon LI. Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish. Dev Biol. 2005;281:256–269. doi: 10.1016/j.ydbio.2005.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


