Abstract
Background:
Advanced glycation end products (AGEs) have been linked to pathogenic mechanisms of diabetes mellitus. However, little is known about the contribution of protein glycation to periodontal disease in patients with diabetes. Therefore, this study investigated whether glycation of type I collagen (COLI) and fibronectin (FN) modified the behavior of human gingival fibroblasts (hGF) and periodontal ligament fibroblasts (hPDL).
Methods:
Procedures for rapid in vitro glycation of COLI and FN used methylglyoxal (MG). Formation of AGEs was analyzed by changes in protein migration using SDS-PAGE and Western blotting with antibodies specific for MG-glycated proteins. Experiments then characterized the effects of glycated FN and COLI on the behavior of hGF and hPDL.
Results:
MG glycated COLI and FN in less than 6 hours. Confirming the specificity of the reactions, antibodies specific for MG-induced AGEs reacted with glycated FN and COLI, but not with control proteins. In cell culture experiments, glycated FN was significantly less efficient in supporting the attachment of hGF and hPDL (P<0.05). Moreover, the morphological parameters for cells, including length, area, perimeter, and shape factor, were altered (P<0.001) for cells on both glycated proteins. Finally, cell migration was reduced on both glycated FN and COLI (P<0.001).
Conclusion:
MG treatment efficiently glycated COLI and FN, providing a new tool to study effects of diabetes on periodontal disease. The substantial effects of glycated COLI and FN on hGF and hPDL behavior indicate that protein glycation contributes to the pathogenesis and altered periodontal wound healing observed in patients with diabetes.
Keywords: Diabetes mellitus, periodontal disease, AGEs, methylglyoxal, fibronectin, type I collagen
INTRODUCTION
A critical consequence of poor glucose control in patients with diabetes (DM) is non-enzymatic glycation and oxidation of proteins and lipids.1 In a hyperglycemic environment, a series of complex molecular rearrangements take place until the reaction equilibrium shifts towards the formation of irreversible advanced glycation end products (AGEs).2 AGE formation takes weeks to months and, thus, primarily affects macromolecules with long half-lives, such as extracellular matrix components.3 AGEs are formed under normal human physiological conditions from a wide range of precursor molecules via the Maillard reaction by a non-enzymatic condensation reaction between reducing sugars and ε-amino groups or N-terminal groups of proteins. Lipids and DNA can also form AGEs, but to a lesser extent.4
Immunohistochemical studies using anti-AGE antibodies have demonstrated the presence of AGE-modified proteins in several human tissues under pathological conditions, including kidneys,5 atherosclerotic lesions of arterial walls,6 myloid fibrils in amyloidosis,7 and gingiva.8 A receptor for AGEs (RAGE) has been detected on vascular and monocytic cells in gingiva.9 However, we are unaware of reports describing RAGEs on periodontal ligament or gingival fibroblasts. Among in vitro studies that have analyzed the behavior of cells exposed to glycated products, Bobbink et al., (1997)10 demonstrated that endothelial cells had decreased cell attachment and spreading when exposed to glycated vitronectin suggesting that AGEs contribute to vascular changes seen in diabetes.
AGEs have been synthesized in vitro by incubation of proteins with glucose, but this reaction may take several weeks because glucose reacts weakly with the amino groups. In comparison, other compounds like glucose-6-phosphate, glyceraldehyde-3-phosphate,11 and dicarbonyls, such as 1-, 3-, or 4-deoxyglucosones, glyoxal, and methylglyoxal are highly reactive intermediates that react readily with proteins.12
Methylglyoxal (MG) is a reactive α-oxalaldehyde metabolite and a toxic metabolite of glucose produced by bacterial and eukaryotic cells. Due to its electrophilic character, it reacts with three amino acid residues: cysteine, arginine and lysine in proteins to form AGEs. MG-derived hydroimidazolone is the major AGE found in vivo.13 Another MG-derived AGE found in human tissues is 5-methylimidazolone. This compound was detected in foam cells in human atherosclerotic lesions.14
MG is present in several tissues of diabetic patients at higher concentrations than in patients without diabetes. For instance, Type 1 diabetes patients have about a seven-fold higher concentration of plasma MG than non-diabetic individuals15 and the concentration of MG in the lens is relatively high (∼1–2 μM).16 The action of highly reactive dicarbonyl compounds, including glyoxal and methylglyoxal, is also enhanced in diabetes, leading to AGE crosslinks.17 Therefore, this study employed MG due to its rapid reaction to yield AGEs and its well documented presence in diabetes.
MG is also found at elevated levels in gingival crevicular fluid of chronic periodontitis patients and may contribute to the destructive periodontal tissue damage.18 Tissue destruction may be more severe in uncontrolled diabetic patients since diabetics carry an abundance of blood and tissue glucose, which may break down to produce MG and other reaction products.
During tissue healing, cells are required to migrate rapidly into the wound site to produce and remodel new extracellular matrix. For wound repair to occur, several classes of molecules are required, including integrins, cell adhesion proteins, and proteases.19 Our hypothesis was that the interaction of cells through their integrins with AGE-modified proteins could induce altered cell behavior, thereby delaying the healing process. Because the prevalence and severity of periodontal disease is increased in metabolically poorly controlled patients with both type 1 and type 2 forms of diabetes, AGEs may negatively affect periodontal cell behavior as observed in other cell types. Experimentally, we took advantage of the rapid protein glycation reaction of MG to glycate two essential extracellular molecules of the periodontium, namely, type I collagen (COLI) and fibronectin (FN). To our knowledge, no studies have characterized the direct effects of AGEs on periodontal cells, specifically at sites involved in periodontal wound healing. The purpose of this study was to investigate in vitro effects of MG-modified matrix proteins on periodontal cells including human gingival and periodontal ligament fibroblasts.
MATERIALS AND METHODS
Advanced Glycation of Matrix Protein
Glycation of type I Collagen
The method applied here to glycate type I collagen with methylglyoxal (MG) was based on previous studies by Morgan et al20 with modifications. Experiments used a 40% aqueous solution of MG†† and rat tail type I collagen (COLI) (4 mg/ml)‡‡. After preliminary testing of different reagent concentrations, reaction times, procedures, and buffers for dialysis, collagen for use in experiments was treated with 257 mM MG in 100 mM sodium phosphate, pH 7.4 for 6 h at 22 °C. Immediately after treatment, samples were dialyzed against 0.02 M acetic acid at 4 °C to maintain the treated collagen in a soluble form. To minimize concentration differences, MG-treated and control COLI samples were subjected to the same reaction times with MG or buffer only, respectively, and dialysis conditions. The dialyzed samples were stored at 4 °C until used in cell behavior experiments.
Final MG-treated and control COLI samples were quantified from digitized images§§ of proteins separated by SDS-PAGE in 7.5% polyacrylamide minislab gels21 after staining with Coomassie brilliant blue. Analyses used imaging software*** and untreated collagen samples of known concentration served as standards. Analyses found minimal protein loss during dialysis against acetic acid and the same final concentrations of glycated and control collagen samples.
Purification of FN from human plasma
FN was purified from human plasma by gelatin-Sepharose affinity chromatography using established procedures22 with the following modifications. After extensive washes with chromatography buffer alone and followed by 1 M NaCl to remove non-specifically bound proteins, bound FN was eluted with 10% (v/v) dimethyl sulfoxide (DMSO) rather than urea in chromatography buffer. To separate FN from gelatin-binding MMPs-2 and -9 that can degrade FN,19 the FN was exchanged into 50 mM sodium phosphate, 150 mM NaCl, pH 7.0 by dialysis, concentrated to ∼1.5 mg/ml using centrifugal filter devices with 10,000 MW cut off†††, and further purified by preparative scale gel filtration‡‡‡. Final fractions were analyzed for purity by SDS-PAGE and for absence of MMP activities by gelatin substrate enzymography as described previously (Steffensen et al 1995).23 After verifying the identity of the final product by Western blot using affinity-purified polyclonal anti-FN antibodies,24 the purified FN was exchanged into phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 4.79 mM Na2HPO4, 0.74 mM NaH2PO4, pH 7.2), quantified by a protein assay§§§, and stored at −80 °C until analyses.
Glycation of human plasma fibronectin
Conditions for glycation of FN by MG were modified from those reported by Shamsi et al. (1998).25 Human plasma FN (∼800 μg/ml) purified as detailed above was incubated with 67 mM MG†† in PBS for 2 h at 22 °C, followed by dialysis against PBS. Extended reaction times resulted in extensive formation of oligomers and protein precipitation. The non-treated FN control sample was diluted to the same concentration in PBS, but was not reacted with MG. After the reaction, MG-treated and control FN samples were immediately dialyzed in an identical manner against PBS at 4 °C.
The BCA protein assay§§§ and densitometric analyses were used to measure FN concentrations. These analyses showed that the protein loss was minor during dialysis for both MG-treated and control samples. To avoid precipitation of the FN-MG sample, which occurred above the physiological concentration of 300 μg/ml, all treated and control FN samples were further diluted with PBS to 140 μg/ml and stored at 4° C until needed for cell behavior experiments. SDS-PAGE analysis of FN and FN-MG samples performed after 23 d indicated that glycated FN was stable over this duration of time.
Verification of glycation reactions
Conjugation of MG to COLI and FN molecules was verified in experiments using an affinity-purified rabbit anti-MG-AGE polyclonal antibody25 kindly donated by Dr Shamsi, Case Western Reserve University, Cleveland, Ohio. This antibody raised against MG-modified ribonuclease A identifies the argpyrimidine epitope and has been found to react with type I collagen prepared from human cornea.25 Glycated COLI and FN samples, as well as untreated control samples, were separated by 7.5% SDS-PAGE gels under reducing conditions (65 mM dithiothreitol) and transferred to Immobilon-P polyvinylidene difluoride membranes (PVDF)**** prior to probing with the MG-specific antibody by previously detailed Western blot procedures, enhanced chemiluminescence reagents,†††† and autoradiography film‡‡‡‡ for detection.23, 26
Cell Behavior Assays
Cell culture
Primary cultures of hGF and hPDL were generated from biopsies of gingiva and from the middle third of periodontal ligaments of root surfaces of extracted teeth with no signs of periodontal disease according to previously detailed methods.27, 28 Cells between passages 3 and 10 were used for all experiments. Generally, established cell cultures were maintained in DMEM-Ham F12 medium (DF)16 supplemented with 10% NCS, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin††.
Cell Attachment
Assays to determine concentrations of proteins yielding half-maximal cell attachment were performed as detailed previously.29-31 Of special significance to the present experiments, tissue culture-treated 96-microwell plates were coated with three-fold serially diluted samples of glycated COLI (0 - 24 μg/ml) or glycated FN (0 - 12 μg/ml) or corresponding untreated COLI or FN control proteins for 1 h at 22 °C. After blocking non-specific binding sites with 10 mg/ml heat-denatured BSA for 30 min at 22 °C, 2 × 104 cells were added per well in 100 μl serum-free α-MEM to avoid confounding effects from serum proteins. After incubation for 50 min at 37 °C, non-attached cells were removed by gentle rinses with PBS for cells (137 mM NaCl, 2.68 mM KCl, 4.29 mM Na2HPO4, 1.47 mM kH2PO4, pH 7.4). The attached cells were fixed and stained with crystal violet. Subsequently, the number of cells was quantified by adding 10% acetic acid to the cells and measuring the optical density (590 nm) of the dissolved crystal violet stain using a microplate reader.***** Uncoated wells without BSA blocking served as positive attachment controls, and cell attachment to non-coated but BSA-blocked wells was used to adjust for nonspecific attachment. Protein concentrations required to achieve half-maximal cell attachment were defined from coating concentration versus cell attachment response curves and based on experiments performed in duplicate or triplicate and repeated at least twice.
Cell morphology
Analysis of the effects of glycation on the morphology of cells cultured on COLI and FN were performed as described previously28 with modifications. In brief, a total of 5 × 103 hGF or hPDL cells in serum-free α-MEM§§§§ were seeded on glass cover slips (1 cm2)††††† coated with 12.5 μg/ml glycated forms of either COLI or FN, or corresponding untreated control proteins. This coating concentration corresponded to that resulting in half-maximal attachment in the preceding cell attachment assays. After 12 h in the tissue culture incubator, cells were rinsed with PBS, fixed, and stained. For measuring cell morphologies, images were captured at a 20-fold magnification with a light microscope‡‡‡‡‡ of random fields containing at least 100 cells per condition. Analyses used imaging software§§§§§ and were performed in duplicate for each cell line and condition. The morphological parameters included cell length, area, perimeter, and the cell shape factor was calculated from the formula: ((area/perimeter2) × 4π).32 For rounded cells, the shape factor will approach 1.0, whereas elongated cells will have a smaller shape factor value. One examiner performed all morphology measurements. Repeated measurements (N = 10) demonstrated a variability of less than 2.5% of the mean for cell length and 1.7% for area measurements.
Cell Migration
Cell migration on different substrates was quantified by the migratory expansion of cells after deposition in standard-sized areas formed by identical wells (1.4 mm) in a membrane as described previously.28 In preparation for the present analyses, preliminary experiments determined that the coating concentration associated with the greatest cell migration was 12.5 μg/ml for COLI and FN. This concentration was used subsequently for all experiments. A total of 4 × 103 hGF or hPDL cells in serum-free α-MEM were seeded into each of 6 identical wells on coverslips coated with MG-treated COLI or FN or untreated controls. After cell attachment and removal of well-forming membranes, migration monitored for 3 days in the presence of the minimal concentration of NCS (0.25%) that allowed for cell survival and migration, but reduced most possible cell proliferation and minimized confounding effects from serum proteins. After fixation and staining with crystal violet, cell migration was quantified by the change of cell areas from digital images captured at a 10-fold magnification. Changes in at least three cell areas were expressed as means and standard deviations after subtraction of baseline values for each experimental condition.
Statistical Analyses
Analysis of data used Kruskal-Wallis and Mann-Whitney U tests with Bonferronis correction for multiple comparisons. To reduce measurement errors, one calibrated examiner performed all measurements.
RESULTS
Glycation of Type I Collagen
Reaction conditions
MG efficiently glycated COLI but several methodological issues were encountered and addressed as follows: Due to the unique amino acid composition of COLI and interference with MG, it was inherently difficult to quantify collagen concentration by any of the commonly available methods, including the BCA and the Bradford assays. Therefore, concentrations of treated and control COLI were extrapolated by densitometric analyses of SDS-PAGE gels relative to the known concentration for the native rat tail type I collagen stock solution‡‡, and one stock sample was used for all experiments. Dialysis against 0.02 M acetic acid reliably eliminated residual, unbound MG after the glycation reactions, and resulted in minimal loss of protein.
Verification of COLI glycation
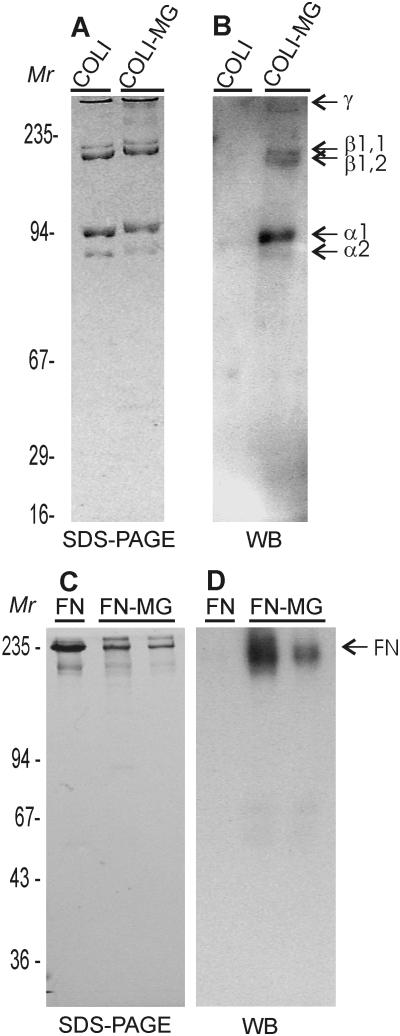
Treatment of COLI in native triple helical conformation resulted in two types of changes in the protein migration as assessed by densitometric analyses of SDS-PAGE gels. First, after MG treatment, an apparent increase in molecular weight was observed. The α1 and α2 bands of COLI showed increases of ∼ 4-7 kDa whereas the β1,1 and β1,2 bands increased by approximately twice that, namely, ∼6-9 kDa (Fig. 1A). The increase in apparent molecular mass indicated that MG had reacted with collagen.
Figure 1. Analysis of Glycated Type I Collagen and Fibronectin.
Methylglyoxal-treated and untreated control native type I collagen (COLI) and fibronectin (FN) were separated by SDS-PAGE (SDS-PAGE) on 7.5% mini slab gels under reducing conditions (Panel A, C). Migration of glycated COLI (COLI-MG) and glycated FN (FN-MG) demonstrated increased masses and proportions of β and γ collagen components and polymeric FN forms. In Western blot analyses (WB), immunoaffinity-purified polyclonal antibody specific for MG glycation products yielded strong reactions with glycated forms of COLI and FN, but not control proteins confirming that glycation reactions were successful (Panels B and D). Positions of protein standards (Mr), collagen components, and FN are indicated.
Additional measurements of the proportion of the different α-chains suggested that MG treatment induced cross-linkage and formation of oligomers with a concomitant reduction in the single α-chain components (data not shown).
To further verify that glycation of COLI had occurred, MG-treated and control COLI were Western blotted with an affinity purified polyclonal antibody that reacted with MG-treated protein but not with untreated COLI (Fig. 1B).
Glycation of Fibronectin
Reactions of human plasma FN with MG also yielded products that were characterized by altered protein migration in SDS-PAGE gels compared to untreated controls (Fig. 1C). Due to the larger mass of FN (∼220 kDa per chain), little change in the migration of individual FN chains were noted following MG treatment; however, some aggregate formation was again observed by SDS-PAGE (Fig. 1C).
As in the case of COLI, MG treatment caused difficulties in measuring protein concentration with both the BCA and Bradford procedures and, hence, the use of densitometric analysis from SDS-PAGE gel bands was required. By this approach, we detected a protein loss of only ∼7% following MG reaction and dialysis. Finally, Western blotting with the anti-AGE antibody confirmed that FN was glycated by MG treatment (Fig. 1D).
Periodontal Cell Behavior in Response to Glycation of Type I Collagen and Fibronectin
Cell Attachment
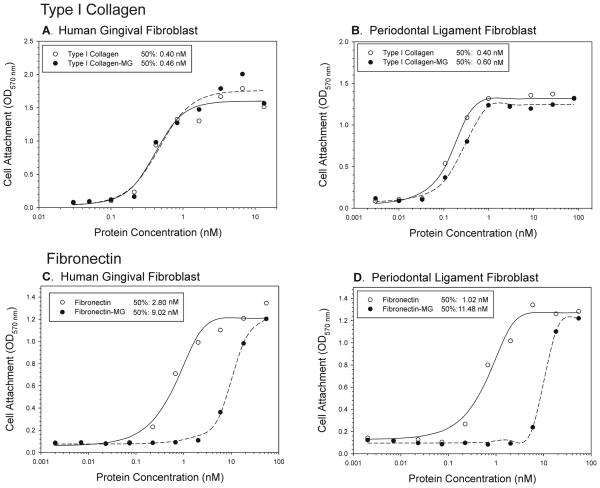
MG treatment of COLI did not significantly change the capacity of COLI to support attachment of human gingival or periodontal ligament fibroblasts (Fig. 2A, B). The half-maximal attachment to MG-treated and control COLI occurred at 0.40 and 0.46 nM for hGF, respectively (Fig 2A), and at 0.40 and 0.60 nM for hPDL, respectively (Fig. 2B).
Figure 2. Cell Attachment to Native and Glycated Type I Collagen and Fibronectin.
The coating concentration of MG-glycated COLI (COLI-MG) and FN (FN-MG), and untreated control proteins (COLI, FN), yielding half-maximal attachment of human gingival (Panels A, C) and periodontal ligament fibroblasts (Panels B, D) were defined. Serial dilutions (0 – 12 μg/ml) of proteins were coated in 96-well microtiter plates. After blocking non-specific binding sites with heat-denatured BSA, 2 × 104 cells per well were added in serum-free medium and allowed to attach in the tissue culture incubator. Attached cells were quantified by spectrophotometric analyses of dissolved cell-bound crystal violet stain at 570 nm with correction for non-specific attachment to BSA only coated wells. Half-maximal attachment concentrations in response to glycation were protein and cell type specific (See data on panels). Glycation of FN strongly reduced the capacity to support attachment for hGF (Panel C) and hPDL cells (Panel D), whereas glycation had minimal effect on cell attachment to COLI (Panels A, B).
In contrast to COLI, attachment of hGF and hPDL to MG-glycated FN was substantially modified (Fig. 2C, D). Whereas the hGF and hPDL had half-maximal attachment at 2.8 and 1.02 nM for native FN, 4 – 10 fold higher concentrations of the glycated FN, 9.02 and 11.48 nM, were required to attain half-maximal attachment for the two cell types, respectively (Fig. 2C, D).
Cell Morphology
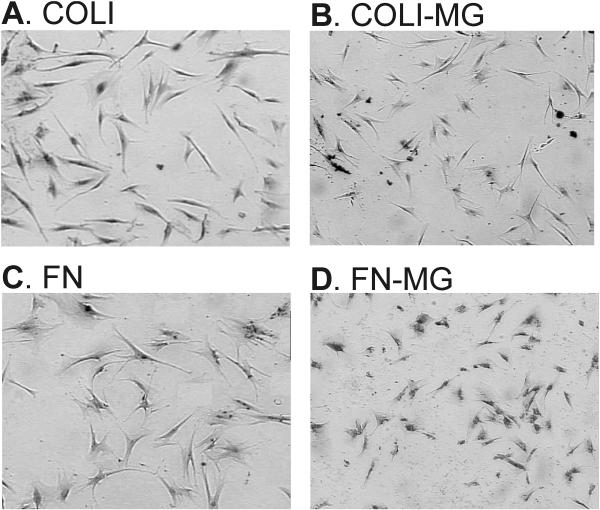
Measurements of cell morphological parameters provide information about the interactions of cells with the substrate. For example, cells that attach and spread well on a coated protein typically will cover a greater surface area and have a higher shape factor. Representative photomicrographs of hGF on MG-treated COLI and FN are presented in Fig. 3 and quantitative data are presented in Table 1.
Figure 3. Cell Morphology in Response to Glycation of Type I Collagen and Fibronectin.
To assess impacts of COLI and FN glycation on periodontal cell morphology, hGF and hPDL were seeded at subconfluent densities in serum-free α-MEM on surfaces coated with 12.5 μg/ml MG-treated COLI (Panel B) or FN (Panel C), or untreated control proteins (Panels A, C). After 12 hours, cells were fixed and stained, and morphologies were quantified from digitized images of the cells (n > 100 per condition). Both hGF (presented here) and hPDL (not shown) displayed markedly different morphologies on both COLI and FN following glycation with MG (magnification presented is 40 ×). Specifically, cells were smaller with a more rounded morphology as also reflected by an increased shape factor. Detailed results are presented for both hGF and hPDL in Table 1.
Table 1.
Effects of glycation by MG of type I collagen and fibronectin on the morphologies of gingival and periodontal ligament fibroblasts after 12 hours
| Collagen* | Collagen-MG† | |||||||
|---|---|---|---|---|---|---|---|---|
| Cell Length‡ | Area | Perimeter | Shape factor§ |
Cell Length | Area | Perimeter | Shape factor |
|
| Human Gingival Fibroblast | ||||||||
| 165.2** | 6631.6 | 453.1 | 0.422 | 128.0‡‡ | 3079.7‡‡ | 362.5‡‡ | 0.321‡‡ | |
| (32.8) | (2392.9) | (97.7) | (0.145) | (42.4) | (1779.9) | (130.3) | (0.135) | |
| Periodontal Ligament Fibroblast | ||||||||
| 215.0 | 12253.5 | 672.1 | 0.364 | 211.1 | 6197.3‡‡ | 510.9‡‡ | 0.320†† | |
| (48.6) | (5422.6) | (163.9) | (0.160) | (59.2) | (2684.2) | (139.9) | (0.132) | |
| Fibronectin* | Fibronectin-MG† | |||||||
| Cell length | Area | Perimeter | Shape factor | Cell length | Area | Perimeter | Shape factor | |
| Human Gingival Fibroblast | ||||||||
| 182.4 | 7963.8 | 527.2 | 0.368 | 134.0‡‡ | 4952.8‡‡ | 346.6‡‡ | 0.520‡‡ | |
| (41.6) | (4470.0) | (142.1) | (0.126) | (31.4) | (1954.4) | (68.5) | (0.138) | |
| Periodontal Ligament Fibroblast | ||||||||
| 200.3 | 13629.0 | 572.4 | 0.527 | 174.9‡‡ | 6587.0‡‡ | 590.6 | 0.275‡‡ | |
| (24.6) | (3614.8) | (76.3) | (0.130) | (56.2) | (3665.0) | (223.6) | (0.141) | |
rat tail type I collagen and human plasma fibronectin
type I collagen and fibronectin were glycated with methylglyoxal as detailed under Materials and Methods
units of measurement presented are cell length and perimeter (μm), area (μm2)
shape factor coefficient reflects (area/perimeter2) × 4π.
presented are means and SD (in parentheses) for 150 measured cells per condition.
Significant (P < 0.01)
Significant (P < 0.001)
(Mann-Whitney U test).
Periodontal fibroblast morphology on glycated type I collagen
After 12 h incubation, hGF displayed markedly different morphologies on control and glycated COLI (Figure 3A, B). Indeed, both cell lengths and areas were significantly greater on native COLI compared to glycated COLI (P<0.001) (Table 1). The perimeter was also reduced (P<0.001) and a lower magnitude of the shape factor indicated that hGF had a more rounded morphology on glycated COLI (P<0.001) (Figure 3A, B; Table 1). Likewise, hPDL on glycated COLI had significantly reduced cell areas (P<0.001), perimeters (P<0.001), and shape factors (P<0.001) than cells on control COLI (Table 1). Overall, the responses to glycated COLI were similar for hGF and hPDL when measured by morphological parameters (Table 1).
Periodontal fibroblast morphology on glycated fibronectin
As was the case for COLI, periodontal fibroblasts had greatly different morphologies when seeded on native compared to glycated FN (Table 1). The 12 h measurements demonstrated that hGF on glycated FN had reduced cell length, area, and perimeter at the P<0.001 significance level (Fig. 3C, D; Table 1). The shape factor was increased reflecting a more rounded cell shape (P<0.001). Similar strong effects of glycation were also detected for hPDL on FN with regards to cell length and area (P<0.001) (Table 1). However, there were no differences in the cell perimeter leading to a significant decrease in the shape factor (P<0.001) indicating a less rounded and more stellate morphology. Thus, the hGF and hPDL responded differently to glycation of FN.
Cell Migration
Glycation of COLI and FN by MG reduced migration of hGF and hPDL (Fig. 4). On COLI, hGF demonstrated greater migration than hPDL cells. However, for both cell types there were significant decreases in migration on glycated relative to control COLI (P<0.001) (Fig. 4A). In comparison, hPDL migrated better on FN than hGF (Fig. 4B). As seen for cells on COLI, glycation of FN also substantially decreased migration of hGF and hPDL fibroblasts (P<0.001) (Fig. 4B).
Figure 4. Cell migration on Glycated Type I Collagen and Fibronectin.
To measure the impact of glycation on periodontal cell migration, 5 × 103 hGF (hGF) or hPDL (hPDL) cells were seeded in each of standard sized wells formed on coverslips coated with 12.5 μg/ml glycated COLI or FN, or untreated control proteins as detailed in the Methods section. After attachment, cells were allowed to migrate in medium with minimal serum minimized cell proliferation. After 3 days, cells were fixed, stained with crystal violet, and the migration quantified relative to the baseline from digitized images captured by light microscopy. Both cell lines displayed significantly reduced (P<0.001) migration on surfaces with MG-glycated COLI (Panel A) or FN (Panel B). *, statistically different from non-glycated control protein (P<0.001) by Mann-Whitney U test.
DISCUSSION
It is well recognized that the extracellular matrix (ECM) is important in many phenomena in cell biology.19, 33 Therefore, alterations of key structural components of the ECM, such as type I collagen (COLI) and fibronectin (FN), would be expected to have important consequences. Results from the present investigation demonstrated clearly that glycation of COLI and FN alters several parameters of periodontal cell interaction with the ECM. Due to the potentially negative impact of the modified cellular behavior, this study has added further evidence in support of the concept that modification of the ECM may contribute to the complications of periodontal disease in patients with diabetes.28, 34
Periodontal wound healing requires coordinated and complex cellular activities, including interactions of cells with ECM molecules, as well as with other cells and tissues.19 In vitro analyses of periodontal cells showed that cell type, disruption of the cell layer, and serum concentration influence wound closure in a wound scratch model,35 and that hGF and hPDL behave differently with respect to proliferation, migration, and wound closure.36 Extending those observations of cells on unaltered ECM proteins, we conclude from the present new findings that ECM modifications by glycation induces both periodontal cell type- and protein-specific responses. When translated to the in vivo situation, a consequence of such changes in cell behavior following protein glycation could be a disturbance in the way cells migrate to the wound site and subsequently attach, migrate, and repopulate areas of damaged tissue.
Both hGF and hPDL express α5β1 integrin, the major FN binding receptor, and α2β1 integrin, the central collagen binding receptor 37. The altered cell attachment to glycated FN observed in our studies of full-length matrix molecules could be due to glycation of the arginyl residue in the RGD sequence of FN.38 Likewise, arginine may be susceptible to glycation with MG in the type I collagen cell-binding region (766GTPGPQGIAGQRGVV780).39 However, in our assays, cell attachment to glycated or control COLI did not differ as measured by the concentration of coated protein needed to support half-maximal cell attachment (Fig. 2). In contrast, attachment of cells to glycated FN was significantly reduced compared to the non-glycated FN. We do not have an apparent explanation for the functional difference to glycation for the two proteins at this time.
Analysis of cell morphology in response to glycation demonstrated that cell shape differed after attachment to both glycated COLI and FN. In general, cells on MG-treated proteins were smaller in size after 12 h. Elongated cellular processes were observed on cells attached to glycated collagen and FN, suggesting a decreased ability to spread normally.
While cell attachment was lower only on FN (Fig. 2), cell migration on both COLI and FN was significantly altered by glycation (Fig. 4). These results were obtained by an experimental design using the minimal concentration of serum that allowed for cell survival, but reduced the proliferation to a minimum during the experimental period. Furthermore, cells migrated on surfaces coated with purified preparations of proteins to specifically measure the effects of MG-glycation of COLI and FN. Taken together, our results support our conclusion that there are strong and protein-specific effects of COLI and FN glycation on the migration of hGF and hPDL.
In DM, sustained hyperglycemia results in the formation of AGEs in proportion to the level of glycemic control. Indeed, if glucose control is strictly maintained, there is a reduced risk of diabetic complications.40 Understanding the precise pathogenic mechanisms of AGEs pathogenicity have been the subject of extensive research. Among established effects of glycation of ECM molecules are altered cell-matrix interactions and matrix-matrix interactions, as well as accumulation of collagen molecules resulting from increases in cross-linkages and decreased turnover.3 Such accumulation of glycated proteins in the basement membranes of blood vessels may cause vascular narrowing with decreased blood flow.41
AGEs may also impart their pathogenic effects via interaction with specific cellular receptors. Most well-known among these is the receptor for advanced glycation end- product (RAGE),42 although other less well-known receptors exist.43, 44 RAGE is present at increased levels in diabetes on endothelial cells, monocytes, macrophages, and neurons10, 44, 45 where AGE-RAGE interactions may stimulate increased production of pro-inflammatory cytokines that may negatively impact the pathogenesis of periodontal disease.8, 42 Of interest to the present results, RAGE also contributes to the control of spreading and migration of activated hepatic stellate cells/myofibroblasts.46 Furthermore, accumulation of AGE has been associated with increased oxidative stress, increased tissue breakdown, and altered wound healing in the periodontium and in other tissues and organs.8, 43, 44, 46-48
Lalla et al.9 showed that gingiva from diabetic patients contains higher levels of AGE and that expression of RAGE was increased on the surface of vascular endothelium and monocytes. Moreover, these investigators impeded the progression of periodontal disease in diabetic mice by blocking AGE-RAGE interactions with soluble RAGE.49 It is not certain that such AGE/RAGE interactions governed the MG-induced effects that we discovered for gingival or periodontal ligament fibroblasts.
In conclusion, we have demonstrated the feasibility of whole molecule glycation for type I collagen and fibronectin using methylglyoxal. This provides a valuable tool for future experiments with ECM molecules. Secondly, we have demonstrated that glycation of key structural matrix proteins significantly impact the behavior of human gingival and periodontal ligament fibroblasts. Such effects of glycation could fundamentally impact the pathogenesis and wound healing in periodontal tissues of patients with diabetes.
Acknowledgments
We gratefully appreciate the contribution of antibodies by Dr Farrukh A. Shamsi from the Case Western Reserve University, Cleveland, Ohio. Supported by grants DE14236, DE17139, DE016312 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: The views expressed in this article are those of the authors and are not to be construed as official nor as reflecting the views of the United States Air Force or Department of Defense
Summary: Results from cell culture experiments demonstrated that human gingival and periodontal ligament fibroblasts had significantly modified behavior when exposed to type I collagen or fibronectin that had been glycated with methylglyoxal indicating that protein glycation may impact pathogenesis as well as wound healing of periodontal diseases in patients with diabetes.
Sigma Chemical Co., St. Louis, MO
Collaborative Biomedical Products (Bedford, MA
Kodak DC 120 camera; Kodak, Rochester, NY
Kodak 1D, Kodak, Rochester, NY
Centriprep, Millipore, Bedford, MA
HiLoad 16/60 Superdex 200, Amersham-Pharmacia, Piscataway, NJ
BCA assay, Pierce, Rockford, IL
Millipore Corp., Bedford, MA
Supersignal, Pierce, Rockford, IL
BioMax, Kodak, Rochester, NY
Gibco, Rockville, MA
Opsys MR, Dynex Technologies, Chantilly, VA
Corning Inc., Acton, MA
Vanox-T Olympus microscope Olympus, Melville, NY
Image Pro Plus, Media Cybernetics, Silver Springs, MD
Conflict of interests: None of the investigators report any financial relationships to any product or sponsor involved in this study
BIBLIOGRAPHY
- 1.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 2.Means G, Chang M. Nonenzymatic glycosylation of proteins: structure and function changes. Diabetes. 1982;31:1–4. [Google Scholar]
- 3.Monnier VM, Sell DR, Nagaraj RH, et al. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992;41(Suppl 2):36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: A review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 5.Makino H, Shikata K, Hironaka K, et al. Ultrastructure of nonenzymatically glycated mesangial matrix in diabetic nephropathy. Kidney Int. 1995;48:517–526. doi: 10.1038/ki.1995.322. [DOI] [PubMed] [Google Scholar]
- 6.Kume S, Takeya M, Mori T, et al. Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am J Pathol. 1995;147:654–667. [PMC free article] [PubMed] [Google Scholar]
- 7.Miyata T, Taneda S, Kawai R, et al. Identification of pentosidine as a native structure for advanced glycation end products in beta-2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc Natl Acad Sci U S A. 1996;93:2353–2358. doi: 10.1073/pnas.93.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt AM, Weidman E, Lalla E, et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996;31:508–515. doi: 10.1111/j.1600-0765.1996.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 9.Lalla E, Lamster IB, Feit M, Huang L, Schmidt AM. A murine model of accelerated periodontal disease in diabetes. J Periodontal Res. 1998;33:387–399. doi: 10.1111/j.1600-0765.1998.tb02335.x. [DOI] [PubMed] [Google Scholar]
- 10.Bobbink IW, de Boer HC, Tekelenburg WL, Banga JD, de Groot PG. Effect of extracellular matrix glycation on endothelial cell adhesion and spreading: involvement of vitronectin. Diabetes. 1997;46:87–93. doi: 10.2337/diab.46.1.87. [DOI] [PubMed] [Google Scholar]
- 11.Takagi Y, Kashiwagi A, Tanaka Y, Asahina T, Kikkawa R, Shigeta Y. Significance of fructose-induced protein oxidation and formation of advanced glycation end product. J Diabetes Complications. 1995;9:87–91. doi: 10.1016/1056-8727(94)00022-g. [DOI] [PubMed] [Google Scholar]
- 12.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(Pt 1):109–116. [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed N, Thornalley PJ, Dawczynski J, et al. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 14.Uchida K, Khor OT, Oya T, Osawa T, Yasuda Y, Miyata T. Protein modification by a Maillard reaction intermediate methylglyoxal. Immunochemical detection of fluorescent 5-methylimidazolone derivatives in vivo. FEBS Lett. 1997;410:313–318. doi: 10.1016/s0014-5793(97)00610-8. [DOI] [PubMed] [Google Scholar]
- 15.McLellan AC, Thornalley PJ, Benn J, Sonksen PH. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 1994;87:21–29. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- 16.Haik GM, Jr., Lo TW, Thornalley PJ. Methylglyoxal concentration and glyoxalase activities in the human lens. Exp Eye Res. 1994;59:497–500. doi: 10.1006/exer.1994.1135. [DOI] [PubMed] [Google Scholar]
- 17.Chellan P, Nagaraj RH. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys. 1999;368:98–104. doi: 10.1006/abbi.1999.1291. [DOI] [PubMed] [Google Scholar]
- 18.Kashket S, Maiden MF, Haffajee AD, Kashket ER. Accumulation of methylglyoxal in the gingival crevicular fluid of chronic periodontitis patients. J Clin Periodontol. 2003;30:364–367. doi: 10.1034/j.1600-051x.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 19.Steffensen B, Hakkinen L, Larjava H. Proteolytic events of wound healing - coordinated interactions between matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 20.Morgan PE, Dean RT, Davies MJ. Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch Biochem Biophys. 2002;403:259–269. doi: 10.1016/s0003-9861(02)00222-9. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Engvall E, Roushlahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 23.Steffensen B, Wallon UM, Overall CM. Extracellular matrix binding properties of recombinant fibronectin type II-like modules of human 72-kDa gelatinase/type IV collagenase. High affinity binding to native type I collagen but not native type IV collagen. J Biol Chem. 1995;270:11555–11566. doi: 10.1074/jbc.270.19.11555. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. MMP-2 contributes to cancer cell migration on collagen. Cancer Res. 2005;65:130–136. [PubMed] [Google Scholar]
- 25.Shamsi FA, Partal A, Sady C, Glomb MA, Nagaraj RH. Immunological evidence for methylglyoxal-derived modifications in vivo. Determination of antigenic epitopes. J Biol Chem. 1998;273:6928–6936. doi: 10.1074/jbc.273.12.6928. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Morlandt ABP, Xu X, Carnes DL, Chen Z, Steffensen B. Tetracycline at subcytotoxic levels inhibits MMP-2 and -9 but does not remove the smear layer. J Periodontol. 2005;76:1129–1139. doi: 10.1902/jop.2005.76.7.1129. [DOI] [PubMed] [Google Scholar]
- 27.Oates TW, Hoang AM. Periodontal ligaments. In: Koller MR, Palsson BO, Masters JRW, editors. Human cell culture. V. Kluwer Academic Publishers; Amsterdam: 2001. pp. 27–41. [Google Scholar]
- 28.Stanley C, Wang Y, Pal S, et al. Fibronectin-fragmentation is a feature of both periodontal disease sites and diabetic foot and leg wounds and modifies cell behavior. J Periodontol. 2008;79:861–875. doi: 10.1902/jop.2008.070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillies RJ, Didier N, Denton M. Determination of cell number in monolayer cultures. Anal Biochem. 1986;159:109–113. doi: 10.1016/0003-2697(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 30.Kueng W, Silber E, Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989;182:16–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- 31.Steffensen B, Bigg HF, Overall CM. The involvement of the fibronectin type II-like modules of human gelatinase A in cell surface localization and activation. J Biol Chem. 1998;273:20622–20628. doi: 10.1074/jbc.273.32.20622. [DOI] [PubMed] [Google Scholar]
- 32.Shah AK, Sinha RK, Hickok NJ, Tuan RS. High-resolution morphometric analysis of human osteoblastic cell adhesion on clinically relevant orthopedic alloys. Bone. 1999;24:499–506. doi: 10.1016/s8756-3282(99)00077-0. [DOI] [PubMed] [Google Scholar]
- 33.Milam SB, Steffensen B, Haskin C, et al. Cell adhesion proteins in oral biology. Crit Rev Oral Biol Med. 1991;2:451–491. doi: 10.1177/10454411910020040201. [DOI] [PubMed] [Google Scholar]
- 34.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 35.Lackler KP, Cochran DL, Hoang AM, Takacs V, Oates TW. Development of an in vitro wound healing model for periodontal cells. J Periodontol. 2000;71:226–237. doi: 10.1902/jop.2000.71.2.226. [DOI] [PubMed] [Google Scholar]
- 36.Oates TW, Mumford JH, Carnes DL, Cochran DL. Characterization of proliferation and cellular wound fill in periodontal cells using an in vitro wound model. J Periodontol. 2001;72:324–330. doi: 10.1902/jop.2001.72.3.324. [DOI] [PubMed] [Google Scholar]
- 37.Steffensen B, Duong AH, Milam SB, et al. Immunochemical localization of cell adhesion proteins and integrins in the periodontium. J Periodontol. 1992;63:584–592. doi: 10.1902/jop.1992.63.7.584. [DOI] [PubMed] [Google Scholar]
- 38.Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol. 1999;31:539–544. doi: 10.1016/s1357-2725(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 39.Chong SA, Lee W, Arora PD, et al. Methylglyoxal inhibits the binding step of collagen phagocytosis. J Biol Chem. 2007;282:8510–8520. doi: 10.1074/jbc.M609859200. [DOI] [PubMed] [Google Scholar]
- 40.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 41.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- 42.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 43.Li YM, Mitsuhashi T, Wojciechowicz D, et al. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci U S A. 1996;93:11047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horiuchi S, Higashi T, Ikeda K, et al. Advanced glycation end products and their recognition by macrophage and macrophage-derived cells. Diabetes. 1996;45(Suppl 3):S73–S76. doi: 10.2337/diab.45.3.s73. [DOI] [PubMed] [Google Scholar]
- 45.Federoff HJ, Lawrence D, Brownlee M. Nonenzymatic glycosylation of laminin and the laminin peptide CIKVAVS inhibits neurite outgrowth. Diabetes. 1993;42:509–513. doi: 10.2337/diab.42.4.509. [DOI] [PubMed] [Google Scholar]
- 46.Fehrenbach H, Weiskirchen R, Kasper M, Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001;34:943–952. doi: 10.1053/jhep.2001.28788. [DOI] [PubMed] [Google Scholar]
- 47.Oldfield MD, Bach LA, Forbes JM, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Twigg SM, Chen MM, Joly AH, et al. Advanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitus. Endocrinology. 2001;142:1760–1769. doi: 10.1210/endo.142.5.8141. [DOI] [PubMed] [Google Scholar]
- 49.Lalla E, Lamster IB, Feit M, et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. 2000;105:1117–1124. doi: 10.1172/JCI8942. [DOI] [PMC free article] [PubMed] [Google Scholar]