Abstract
Visualization of dendritic spines is an important tool for researches on structural synaptic plasticity. Fluorescent labeling of the dendrites and spines followed by confocal microscopy permits imaging a large population of dendritic spines with a higher resolution. We sought to establish an optimal protocol to label neurons in cortical slices with the carbocyanine dye DiI for confocal microscopic imaging of dendritic spines. DiI finely labeled dendrites and spines in slices prefixed (by cardiac perfusion) with 1.5% paraformaldehyde to the similar extent that could be achieved in live preparation. In contrast, fixation with 4% paraformaldehyde severely compromised dye diffusion. Confocal microscopy showed that structural integrity of dendrites and spines was preserved much better in lightly (1.5%) fixed slices than those prepared without fixation. Quantitative measurement revealed that spine density was lower in live slices than that counted in lightly fixed slices, suggesting that fixation is necessary for an adequate evaluation of spine density. The quality of confocal microscopic images obtained from lightly fixed slices allowed us to observe distinctive morphologies such as branched spines and dendritic filopodium, which may be indicative of structural changes at synapses. This method will thus be useful for studying structural synaptic plasticity.
Keywords: Dendritic spine, Confocal microscopy, Carbocyanine dye, Paraformaldehyde, Synaptic plasticity
1. Introduction
Dendritic spines are the postsynaptic morphological specializations at the excitatory synapse. More than 90% of glutamatergic terminals in mature brain make synaptic contacts at the dendritic spines (Harris and Kater, 1994). It is widely believed that long-term synaptic plasticity is accompanied by structural changes in synapses, particularly at dendritic spines (Bailey and Kandel, 1993; Yuste and Bonhoeffer, 2001; Segal, 2005). Therefore, adequate visualization of dendritic spines is critically important to study synaptic plasticity, a central theme in neurobiology.
Traditionally, Golgi staining or electronmicroscopy has been used to analyze the spine structures. More recent studies have employed fluorescent labeling of neurons followed by confocal microscopy, which can produce spine images of a better resolution with a larger sampling size (Gan et al., 2000; Wallace and Bear, 2004). One of the methods for the fluorescence labeling is using a lipophilic carbocyanine dye. It diffuses along the neuronal membrane labeling dendritic arborization and spine structures (Honig and Hume, 1986; Gan et al., 2000). For a series of experiments to examine changes in synaptic structures in the motor cortex after an injury to the spinal cord (Kim et al., 2006), we attempted to establish an optimal protocol to label and visualize dendritic spines in cortical slices with the carbocyanine dye DiI. We found that fixation of slices with a lower percentage fixative greatly improved quality of neuronal labeling. Here, we introduce our method to label dendrites and spines with the DiI that enabled very fine and detailed images of dendritic spine structures, allowing us a quantitative evaluation of spine density and morphology.
2. Materials and methods
2.1. Animals
Adult female Sprague Dawley rats weighing 200 – 250g (Zivic Inc., Zelienople, PA) were used to visualize dendritic spines in the forelimb motor cortex. They were housed in the Georgetown University Division of Comparative Medicine Facility and all protocols were approved by the Georgetown University Animal Care and Use Committee.
2.2. Preparation of live or fixed cortical slices
Live slices from the sensorimotor cortex (200 μm thickness) were obtained as previously described with a slight modification (Stocca and Vicini, 1998). Rats were decapitated with scissors under a 4% chloral hydrate (400 mg/kg body weight) anesthesia. The brains were rapidly dissected and coronally sectioned into 200 μm slices in ice-cold extracellular medium (NaCl 120 mM; KCl 3.1 mM; K2HPO4 1.25 mM; NaHCO3 26 mM; CaCl2 mM 2.0; glucose 5 mM, sucrose 25mM; and glycine, 1–10 μM) with a vibratome (model Vibratome 1000 classic; Technical Products Int’l. Inc., O’Fallon, MO). The slices were then allowed to recover for about one hour at 37°C in the extracellular medium containing 2 mM MgCl2. This solution was maintained at pH 7.4 by bubbling with 5% CO2-95% O2. To prepare cortical slices fixed with paraformaldehyde (PFA), rats were sacrificed with cardiac perfusion under an overdose of chloral hydrate (1g/kg). Rats were exsanguinated with 200 ml of heparinized saline To compare the effect of depth of fixation on fluorescent labeling of dendritic processes, approximately 300 ml of either 1.5% or 4% PFA solutions (in 0.1 M phosphate buffer) was administered using a peristaltic infusion pump for 10 minutes. After dissection, brains were postfixed in the same fixatives for about one hour and then transferred to phosphate buffered saline (PBS). After washing in PBS, the brains were coronally sectioned into 200 μm slices with the vibratome and collected in the PBS.
2.3. DiI labeling procedure
Solid DiI crystals (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; catalogue # D-282; Molecular Probe, Eugene, OR) were applied to the slices under a dissecting microscope. DiI crystals were applied using a borosilicate glass micropipette with a sharp and elongated tip, a slight modification of the technique used by Dailey and Smith (1996). DiI crystals adhered on the surface of a pipette tip were delivered to the surface of slices by lightly touching or gently poking the slices with a pipette. Special care was taken to deliver crystals as fine as possible to avoid clumping of the dye. In live slices, the dye diffused along the neuronal membranes immediately after being put on the slices and filled up the full extent of dendritic segments within an hour at the most. Live cortical slices were maintained in the oxygenated extracellular medium for an hour after DiI labeling and then finally fixed with 4% PFA for 30 minutes. Diffusion of DiI was slowed in cortical slices prepared with cardiac perfusion in a concentration-dependent manner (see results section). Therefore, diffusion of DiI was slower in slices fixed with 1.5% PFA than in live slices without fixation. Accordingly, slices fixed with 1.5% PFA were incubated in PBS at room temperature for 4 to 12 hours to allow DiI crystals to diffuse fully along the neuronal membranes. Slices with 4% fixative were bathed in the PBS for 24 hours to allow more time for diffusion. Fixed slices were then fixed again with 4% PFA for 30 minutes. To avoid possible dehydration-induced shrinkage of dendritic structures (Trommald et al., 1995) and dye bleaching, we used glycerol-based mounting medium Mowiol (containing DABCO as an antifade reagent; Calbiochem, La Jolla, CA) instead of xylene. All images were taken within 7 days after coverslipping.
2.4. Laser confocal microscopic imaging of dendritic segments and image analysis
Dendritic spines were imaged using Zeiss 510 Meta confocal laser scanning microscope (LSM 510 META). Dendritic segments that were well separated from neighboring neural processes were randomly sampled and imaged. For basal dendrites, dendritic segments that run tangentially or towards the white matter were imaged. Each dendritic segment we imaged for quantification belonged to a different neuron. All images were taken using the Plan-APOCHROMAT 63× oil-immersion lens (N/A 1.4). We used 2048 × 2048 pixels for frame size without zooming. A 543nm Helium/Neon laser was used to visualize fluorescence emitted by DiI. The configuration parameters were as follows: 1) filters, channel 3 band pass 560–615 nm, 2) pinhole diameter, 108 μm, 3) beam splitters, MBS-HFT UV/488/543, DBS1 mirror, DBS2 NFT, DBS3-plate. After acquisition, brightness and contrast were adjusted to have similar signal intensity in the parent dendrites. Serial stack images with step size ranging from 0.4 to 0.6 μm were collected, and then projected to reconstruct a three dimensional (3D) image.
Dendritic spines were counted using the Zeiss Image Browser software (version 3.5). For an accurate counting, each image was enlarged four times. Both a series of stack images and a 3D projection image were used in a complimentary manner to increase the sensitivity of spine detection. Dendritic spines were identified and counted in the 3D projection image interface. Whenever dendritic spines were too crowded to separate them from each other, we turned to serial stack images to delineate individual spines (Trommald et al., 1995). By scrolling through the stack of different optical sections, individual spine heads could be identified with greater certainty. All dendritic protrusions with a clearly recognizable stalk were counted as spines. When a protrusion was connected to a parent dendrite without a clear stalk, it was counted as a spine only when there was a clear indentation at the either side of junction of the protrusion and dendrite to differentiate a spine from a dendritic kink or swelling. Spine number was divided by the length of dendritic segment to generate dendritic spine density expressed as number/μm.
2.5. Statistical analysis
Statistical analysis was performed with SPSS version 12.0 (Chicago, IL) or GraphPad Prism software version 4.0 (San Diego, CA). Comparison of group means in spine density was performed with unpaired T test. The Kolmogorov-Smirnov test was used to compare the patterns of cumulative frequency plots in the histogram of individual values for spine density.
3. Results
To determine optimal conditions for the fluorescent visualization of dendritic spines with the carbocyanine dye in the rat brain, we compared patterns of DiI labeling between the slices prepared without fixation or with different concentrations of PFA (1.5% and 4%). Fine crystals of DiI were delivered on the surface of cortical slices using a glass micropipette (Fig. 1A). DiI crystals on the slices diffused along the neuronal membranes visualizing somas and dendritic processes studded with spinous protrusions in live slices and those fixed with 1.5% PFA. DiI crystals occasionally clumped together emitting very bright epifluorescence (Fig. 1A, arrows), but neurons adjacent to the bright spot (dye clumps) were finely stained, visualizing dendritic arborization in as much detail as obtained using the traditional Golgi staining. In live slices, dye diffusion was rapidly completed within an hour. The diffusion seemed to be slightly slower in the slices with 1.5% fixative, but we found that complete filling of dendritic processes and spines was accomplished within three hours. Therefore, the pattern of dendritic staining did not progress or change after the initial three or four hours from the dye loading. The final staining pattern was comparable between the slices prepared without fixative and with 1.5% PFA (Fig. 1B, C). However, diffusion of DiI along the dendritic trees was severely compromised by fixation with 4% PFA (Fig. 1D). DiI tended to clump more frequently in the slices fixed with 4% PFA, and the extent of dendritic arborization visualized by the membrane dye was very limited compared to that in live slices and lightly fixed slices. Prolonged incubation (up to seven days) in PBS before second fixation did not improve the dye diffusion along the neuronal membrane (data not shown). These results indicated that a lower concentration (1.5%) of PFA allows the diffusion of lipophilic membrane dye along the neuronal membranes to the similar extent in live slices, whereas fixation with 4% PFA, which is the usual concentration for histopathological analysis, severely restricts staining of the dendritic processes through the dye diffusion.
Figure 1.
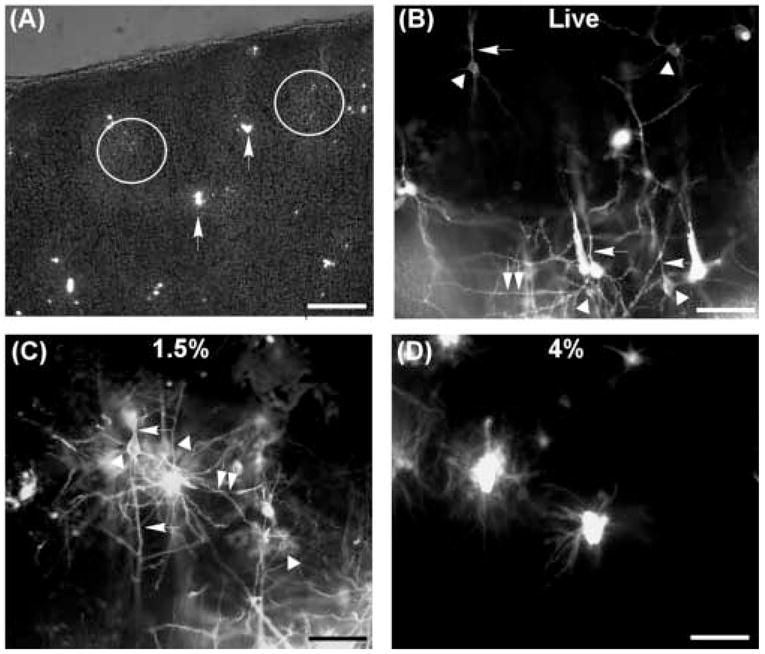
DiI labeling in cortical slices. A, DiI crystals delivered on the surface of slices using a glass micropipette. The image was taken immediately after dye loading. Usually, very fine crystals were delivered onto the surface (fine speckles inside the white circles). DiI crystals sometime clumped together emitting very bright epifluorescence (arrows). The fluorescent image was taken under light illumination to show the edge of the slice. B,C, Labeled neuronal processes in live slices (B) and lightly fixed slices (C). Labeling quality was comparable to each other. Arrow heads indicate neuronal somas, arrows apical dendrites, and double arrows basal dendrites. (D) DiI labeling in slices fixed with 4% paraformaldehyde. Dye diffusion was very limited compared to (B) and (C). Scale bar in (A) = 500 μm, in (B,C,D) = 100 μm.
To quantitatively analyze the structure of dendritic spines, we took confocal images of the apical or basal dendritic segments (single or double arrows in Fig. 1B, C, respectively). Since staining of dendritic processes was severely compromised in 4% fixed slices, confocal images were taken in live and lightly (1.5%) fixed slices. Dendritic spines were clearly visualized in both conditions with the imaging parameters used in the current study (Fig. 2). The resolution was high enough to separate individual spines. Dendritic spines were sometimes too crowded to analyze individual spines in a three dimensionally projected image. However, analysis using a series of stack images with a step interval 0.4 to 0.6μm usually allowed identification of individual dendritic spines with a high certainty.
Figure 2.
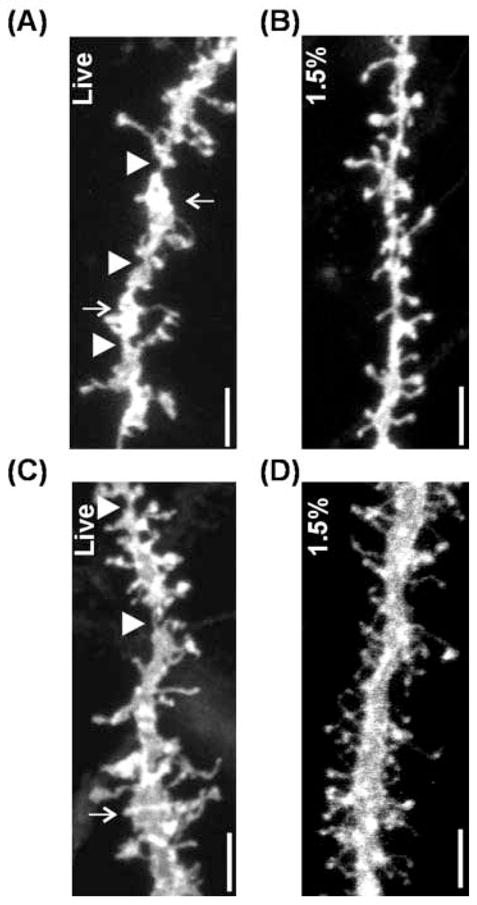
Confocal microscopic images of dendritic segments. A,B, basal dendrites. C,D, apical dendrites. Images of dendritic segment from live slices (A,C) shows aberrant structures such as notching (arrow heads) and swelling (arrows). In contrast, dendritic processes from lightly fixed slices seems to be well preserved (B,D). Scale bar = 5 μm
Although dendritic structures were finely visualized in lower magnification, highly magnified images revealed that structures of dendritic processes seemed to be disintegrated in live slices. They showed frequent notching (arrow heads in Fig. 2A, C) and swelling (arrows in fig. 2A, C) of the dendritic shafts. Although the aberrant morphology was observed in both apical and basal dendrites, basal dendrites tended to be more affected than apical ones often leading to a string-and-beads appearance (Fig. 2A). This suggests that dendrites with smaller diameter are more vulnerable to the structural disintegration. The structural abnormality occurs probably due to a disassembly of cytoskeletal proteins during preparation of slices without fixation (Fiala et al., 2003). Dendritic structures in the slices fixed with 1.5% PFA, in contrast, seemed to be well preserved (Fig. 2B, D). These results indicated that fixation of cortical slices allows a better preservation of the structural integrity of the dendrites.
It has been shown that the number of dendritic spines changes during slice preparation without fixation (Kirov et al., 1999; Kirov et al., 2004a). Therefore, we determined whether a difference in the procedure of slice preparation results in a difference in the number dendritic spines. Gross observation of the confocal images did not show an outstanding difference in spine density between the slices prepared live and lightly fixed slices (Fig. 2). Nonetheless, the aberrant morphology of the dendrites in live slices sometimes hindered an accurate evaluation of dendritic spines. For example, swelling of a portion of dendritic trunk tended to obscure presence of short or medium-length spines even with a detailed analysis with a series of stack images. To quantitatively analyze the effect of fixation procedure on spine density, we counted dendritic spines in the basal dendrites located in the forelimb motor cortical regions (live slices, 11 dendritic segments from two animals; slices fixed with 1.5% PFA, 17 dendritic segments from two animals). Although the mean spine density measured was largely comparable between both slice preparations (Fig. 3A), the mean spine density in live slice preparation was lower by 16% than that in the fixed slices (mean ± SD; 1.38 ± 0.21 and 1.65 ± 0.27, respectively; p < 0.01 by unpaired T test). A cumulative frequency histogram showed a significant left shift of the density distribution in live slices, also indicating a lower spine density measured in live slice preparation (p < 0.05 by Kolmogorov-Smirnov test, Fig. 3B). Our results from the quantitative analysis with confocal microscopic imaging showed that the measurement of spine density in live slices can result in a lower value than that in the fixed slices, suggesting that fixation of cortical slices may be necessary for an adequate evaluation of the spine density.
Figure 3.
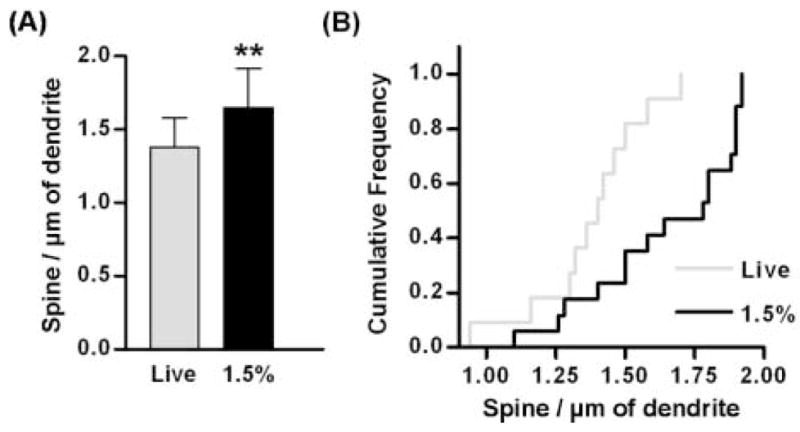
Comparison of spine density measured in live and lightly fixed slices. Spine density measured in lightly fixed slices (black) was higher than that in live slices (gray). Asterisks indicate a statistically significant difference by unpaired T test (p < 0.01). Error bars indicate standard deviation. (B) Cumulative frequency histogram of spine density.
The above results indicated that DiI staining in the slices prepared with a lower percentage fixative produced the best quality of spine images taken with confocal microscope. We were able to obtain high quality images of spine structures with this method, which enabled an accurate and quantitative analysis of spine morphology such as spine length and head diameter in a large population of dendritic spines (Kim et al., 2006). Furthermore, the high resolution of spine images allowed us to observe distinctive synaptic morphologies that had been associated with synaptic remodeling or synaptogenesis. Branched dendritic spines could be readily identified as having two separate spine heads but sharing a common stem (Fig. 4A). In addition, dendritic filopodia, very long and thin protrusions from the dendritic trunk, were observed infrequently in the cortical slices (Fig. 4B). Furthermore, close synaptic appositions between the dendritic spine (postsynaptic) and anterogradely stained axonal process (presynaptic) could be sometimes visualized in this preparation (Fig. 4C). Taken together, the distinctive synaptic morphologies visualized in the slices prepared with the current method could be utilized in the evaluation of structural synaptic plasticity in adult CNS.
Figure 4.
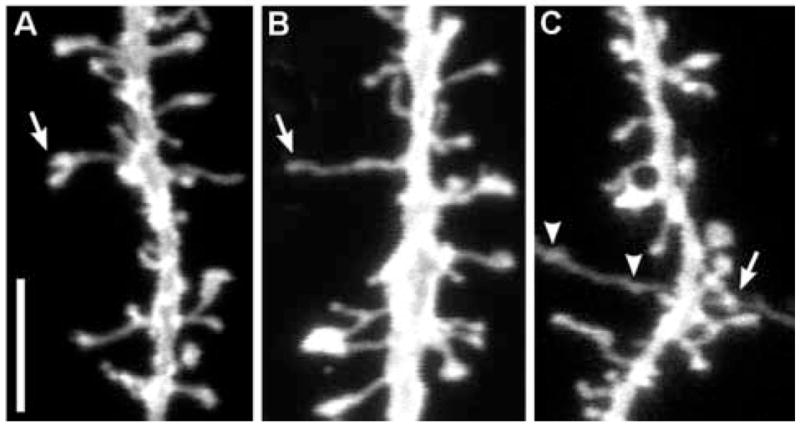
Special morphologies encountered in spine images from lightly fixed slices. A, branched dendritic spine. B, filopodial protrusion. C, A synaptic contact between an axon and a dendritic spine was accidentally imaged (arrow). Arrow heads represent boutons en passant of an accidentally stained axon. Scale bar = 5 μm
4. Discussion
The present study compared different protocols for slice preparation to establish optimal conditions for the fluorescent visualization of dendritic spines in the rat brain. The results indicated that a fixation with the lower percentage PFA results in a superior diffusion of lipophilic dye DiI along the neuronal membranes with adequate preservation of structural integrity. Fixation with 4% PFA, commonly used for intracardial perfusion fixation, led to a severe compromise of dye diffusion through dendritic processes. Carbocyanine dyes have been successfully used to label neuronal structures in formalin-fixed materials (Godement et al., 1987; Kobbert et al., 2000). However, diffusion of the dyes along the axonal membranes was extremely slower in fixed materials than that in live tissues (Wu et al., 2003). DiI delivered by gene gun (DiOlistic method) visualized dendritic architecture in the brain slices fixed with 4% PFA (Gan et al., 2000). The speed of diffusion was several fold slower that that of live slices, however, and the duration of fixation needed to be tightly controlled to get satisfactory staining (Grutzendler et al., 2003; but see also Chen et al., 2006). The current study found that lowering the concentration of PFA dramatically increased the speed and extent of DiI diffusion leading to a complete visualization of the dendrites and spinous protrusions. Thus, it seems that optimization of fixation protocol can greatly improve the quality of DiI staining of dendritic processes and spines.
DiI labeling in live slices resulted in a very rapid and complete filling of dendrites and spines. However, it appeared that structural integrity of the dendrites could not be maintained with the protocol used in this study. The notching and swelling of the dendrites often hindered accurate counting of dendritic spines. A previous electron microscopic study showed that preparation of cortical slices without perfusion-fixation can result in a transient disruption of cellular ultrastructures including abnormalities in cytoskeletal proteins (Fiala et al., 2003). In that study, the ultrastructural abnormalities returned to the state of perfusion-fixed slice in three hours of in vitro incubation. Cortical slices were maintained in vitro for approximately two hours (one hour before and one after DiI labeling in the extracellular medium) in our protocol. Therefore, the structural abnormality might still remain in the slices prepared following our protocol. Drastic changes in temperature usually required during preparation of live slices may be responsible for the structural abnormalities (Fiala et al., 2003; Roelandse and Matus, 2004).
Measurement of spine density was also affected by different procedures of slice preparation. Contrary to the previous study that showed an increase in synapse number in live slice preparation (Kirov et al., 2004a; Kirov et al., 2004b), the spine density was slightly lower when measured in live slices than that in lightly fixed slices. It is possible that a relatively short duration of in vitro incubation in our protocol did not allow new growth of dendritic protrusions, although proliferation of dendritic spines can be detected only within 30 minutes after in vitro incubation (Kirov et al., 2004b). The slightly lower spine density found in live slices is probably due to a difficulty in accurately identifying spines at the regions of structural abnormalities such as swelling and notching. Alternatively, submerging the brains and slices into ice-cold extracellular medium during vibratome sectioning with a short duration of recovery (2 hours in hour protocol) may have caused a hypothermia-induced loss of dendritic spines (Roelandse and Matus, 2004). Taken together, our study indicates that live slice preparation is accompanied by various structural aberrations (at least with our protocol), warranting a need of fixation for a detailed analysis of dendritic spine structures.
The method described in this study has advantages in several aspects for analysis of dendritic spine structures. This method can give an opportunity to study a large population of dendritic spines in detail, which can hardly be provided by electron microscopic study. Given an extremely high variability in morphology of dendritic spines (Kim et al., 2006), the sampling size will be especially critical in a quantitative evaluation of spine morphology. As compared to Golgi staining, fluorescent staining followed by confocal microscopic imaging produces a series of stack images covering three dimensional depths of spines and dendrites, allowing a more sensitive detection of spines. Intracellular injection of fluorescent dyes has been performed to obtain confocal microscopic images of dendritic spines (Buhl, 1993; Trommald et al., 1995; Johansson and Belichenko, 2002; Wallace and Bear, 2004). However, the process of intracellular injection is typically very tedious and only a small number of cells can be labeled. Furthermore, incomplete filling or dye leakage may complicate the experiment (Johansson and Belichenko, 2002). The limitation of the current method was that it was seldom possible to have an entire dendritic tree of a single neuron visualized. In some cases, for example, dye diffusion was limited to dendrites close to where the dye was loaded, whereas the other dendrites of the same neuron were not stained. Therefore, this method seems to be more suitable for structural analysis of spines rather than detailed measurement of dendritic branching parameters.
Dendritic spines are thought to be important structural substrates for synaptic plasticity following behavioral experience and/or CNS injuries such as stroke and trauma (Moser et al., 1994; Johansson and Belichenko, 2002; Kim et al., 2006). For behavioral manipulation or modeling CNS injuries, rat is the preferred species to mouse, in which transgenic expression of fluorescent protein is readily available to study structure of dendrites and spines (van den Pol and Ghosh, 1998). Therefore, the method introduced in this study can be suitably utilized for a study of experience- or injury-dependent plasticity of synaptic structures in rats.
As shown in fig. 4, the high quality of confocal images achieved with this method allowed distinctive synaptic morphologies that are implicated in the process of structural synaptic plasticity. Splitting of existing spines or branched spines may be related to synaptic remodeling or a formation of new synapses the adults CNS (Trommald et al., 1996; Dhanrajan et al., 2004). For example, experience-dependent or lesion-associated synaptic plasticity was accompanied by an increase in the incidence of perforated postsynaptic densities or branched spines measured in electronmicroscopic images (Jones et al., 1997; Jones, 1999). Filopodial dendritic protrusions are thought to make initial contacts with presynaptic partners and thus be involved in synaptogenesis (Ziv and Smith, 1996; Fiala et al., 1998). Therefore, confocal images of dendritic spines with the current method will allow a quantitative assessment of incidence of branched spines and dendritic filopodia in a large population of samples, which would be all the more valuable in a study of structural synaptic plasticity. Moreover, combination of anterograde tracing of axon terminals with different colors will be able to visualize synaptic appositions between a specified axonal tract (e.g. commissural fibers) and neuronal populations in a specific region (e.g. motor cortex).
Acknowledgments
We thank Dr. Bogdan Stoica for technical advice for confocal microscopy, Dr. Zhanyan Fu and Congyi Lu for assistance with DiI labeling. This study was supported by NIH grant NS27054.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Buhl EH. Intracellular injection in fixed slices in combination with neuroanatomical tracing techniques and electron microscopy to determine multisynaptic pathways in the brain. Microsc Res Tech. 1993;24:15–30. doi: 10.1002/jemt.1070240104. [DOI] [PubMed] [Google Scholar]
- Chen BK, Miller SM, Mantilla CB, Gross L, Yaszemski MJ, Windebank AJ. Optimizing conditions and avoiding pitfalls for prolonged axonal tracing with carbocyanine dyes in fixed rat spinal cords. J Neurosci Methods. 2006;154:256–63. doi: 10.1016/j.jneumeth.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanrajan TM, Lynch MA, Kelly A, Popov VI, Rusakov DA, Stewart MG. Expression of long-term potentiation in aged rats involves perforated synapses but dendritic spine branching results from high-frequency stimulation alone. Hippocampus. 2004;14:255–64. doi: 10.1002/hipo.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Kirov SA, Feinberg MD, Petrak LJ, George P, Goddard CA, Harris KM. Timing of neuronal and glial ultrastructure disruption during brain slice preparation and recovery in vitro. J Comp Neurol. 2003;465:90–103. doi: 10.1002/cne.10825. [DOI] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- Godement P, Vanselow J, Thanos S, Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987;101:697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Tsai J, Gan WB. Rapid labeling of neuronal populations by ballistic delivery of fluorescent dyes. Methods. 2003;30:79–85. doi: 10.1016/s1046-2023(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T, Rusakov DA, Bliss TV, Fine A. Repeated confocal imaging of individual dendritic spines in the living hippocampal slice: evidence for changes in length and orientation associated with chemically induced LTP. J Neurosci. 1995;15:5560–73. doi: 10.1523/JNEUROSCI.15-08-05560.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol Learn Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol. 2006;198:401–415. doi: 10.1016/j.expneurol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Harris KM. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nat Neurosci. 1999;2:878–83. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Goddard CA, Harris KM. Age-dependence in the homeostatic upregulation of hippocampal dendritic spine number during blocked synaptic transmission. Neuropharmacology. 2004a;47:640–8. doi: 10.1016/j.neuropharm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience. 2004b;127:69–80. doi: 10.1016/j.neuroscience.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Miller M, Peters A. Maturation of rat visual cortex. II. A combined Golgi-electron microscope study of pyramidal neurons. J Comp Neurol. 1981;203:555–573. doi: 10.1002/cne.902030402. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci U S A. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP. Dendritic differentiation in human cerebral cortex: normal and aberrant developmental patterns. Adv Neurol. 1975;12:91–134. [PubMed] [Google Scholar]
- Roelandse M, Matus A. Hypothermia-associated loss of dendritic spines. J Neurosci. 2004;24:7843–7847. doi: 10.1523/JNEUROSCI.2872-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol (Lond) 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommald M, Jensen V, Andersen P. Analysis of dendritic spines in rat CA1 pyramidal cells intracellularly filled with a fluorescent dye. J Comp Neurol. 1995;353:260–274. doi: 10.1002/cne.903530208. [DOI] [PubMed] [Google Scholar]
- Trommald M, Hulleberg G, Andersen P. Long-term potentiation is associated with new excitatory spine synapses on rat dentate granule cells. Learn Mem. 1996;3:218–28. doi: 10.1101/lm.3.2-3.218. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Ghosh PK. Selective neuronal expression of green fluorescent protein with cytomegalovirus promoter reveals entire neuronal arbor in transgenic mice. J Neurosci. 1998;18:10640–51. doi: 10.1523/JNEUROSCI.18-24-10640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W, Bear MF. A morphological correlate of synaptic scaling in visual cortex. J Neurosci. 2004;24:6928–6938. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Russell RM, Nguyen RT, Karten HJ. Tracing developing pathways in the brain: a comparison of carbocyanine dyes and cholera toxin b subunit. Neuroscience. 2003;117:831–845. doi: 10.1016/s0306-4522(02)00833-3. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]


