Abstract
Objective
Co-occurrence of substance use and bipolar disorders is both common and associated with poor treatment response and greater functional impairment than either disorder alone. The neurophysiological correlates of this co-morbidity however, remain unclear. In this pilot study, we compared brain morphometry between bipolar adolescents with co-occurring cannabis use disorders (CUD) and bipolar adolescents without any substance use disorder.
Methods
Whole-brain structural magnetic resonance imaging (MRI) scans were obtained from 14 bipolar adolescents. Seven study participants were diagnosed with CUD before and/or shortly after their MR scan was obtained, and 7 subjects were free of any substance use disorder at the time of their MR scan as well as during longitudinal follow up. Morphologic differences were calculated using voxel-based morphometry implemented using statistical parametric mapping software (SPM2).
Results
Bipolar adolescents with co-occurring CUD demonstrated decreased gray matter volume (GMV) in the left fusiform gyrus and increased GMV in the right caudate and precentral gyrus, as well as increased gray matter density in the right middle occipital and fusiform gyri and cerebellar vermis.
Conclusions
Bipolar adolescents with CUD demonstrate evidence of greater structural abnormalities than adolescents with bipolar disorder alone in frontal and temporal cortical regions, as well as in subcortical areas linked with emotion and motivational regulation. Although the limited prescan exposure to marijuana in these adolescents tentatively suggests that these findings may reflect underlying differences, the direct effect of cannabis exposure may also be involved.
Introduction
It is well established that bipolar disorder is commonly complicated by high rates of co-occurring substance use disorders, which may reach 40% during adolescence and 60% during adulthood (Regier et al. 1990; Wilens et al. 1999; Strakowski et al. 2000a; Wilens et al. 2004; DelBello et al. 2007). Furthermore, substance use disorders in adolescents are associated with both poor outcome and treatment nonadherence (DelBello et al. 2007). Although the onset of substance use disorders precedes the onset of bipolar disorder in a majority of bipolar adults (Kovasznay et al. 1993; Winokur et al. 1995; Feinman and Dunner 1996; Strakowski et al. 1996; Strakowski et al. 1998; DelBello et al. 1999), relatively few adolescents with bipolar disorder have substance use disorders when first diagnosed (Wilens et al. 1999; Wilens et al. 2004; DelBello et al. 2007). Rates of co-occurring substance use disorders increase, however, to approximately 40% following the onset of bipolar disorder in adolescents, thus providing a “window of opportunity” for early intervention and the implementation of prevention strategies.
Although the underlying causes of the high rate of co-morbidity between bipolar and substance use disorders remain poorly understood, previous investigators have suggested that vulnerability to the development of substance use disorders during the course of bipolar disorder may be related to the presence of specific neurological impairments (Majewska 1996). Structural and functional abnormalities in portions of the prefrontal cortex, as well as the medial temporal and subcortical regions implicated in motivational and emotional processing, have been demonstrated in adolescents with bipolar disorder (Blumberg et al. 2002; Blumberg et al. 2003; Chang et al. 2004; Blumberg et al. 2006; Chang et al. 2006; DelBello et al. 2006) and have also been associated with the development of substance use disorders (Majewska 1996; Pfefferbaum et al. 1998; Franklin et al. 2002; Lingford-Hughes et al. 2003; Kalivas and Volkow 2005; Cahill et al. 2006; Hyman et al. 2006). Consequently, overlapping neurophysiological abnormalities may predispose some patients to developing both bipolar and substance use disorders. Although the cross-sectional design of most morphometric magnetic resonance imaging (MRI) studies of patients with substance use disorders cannot differentiate preexisting structural brain abnormalities from the consequences of repeated exposure to abused substances (Pascual-Leone et al. 1991; Kirsch et al. 2006; Schlaepfer et al. 2006), at least some studies have suggested that cannabis use disorders (CUD) are not associated with neurostructural changes (Delisi et al. 2006; Quickfall and Crockford 2006). This potential confound may be further limited by obtaining brain MRI scans either prior to or shortly after the development of a substance use disorder.
With these considerations in mind, we conducted a pilot study comparing the structural neuroanatomy of adolescents with and without a co-occurring CUD, as marijuana is the most commonly used substance of abuse in bipolar adolescents. Patients in the latter group were selected for minimal prescan exposure to substances of abuse to limit potential drug-related neuropathic effects. We hypothesized that there would be structural differences in brain regions involved in emotional regulation between bipolar adolescents with co-morbid CUD and those without CUD.
Methods
Subjects
MRI scans were obtained from 14 adolescents (9 female) diagnosed with bipolar I disorder. At the time of the scan, subjects ranged in age from 12 to 18 years (mean ± standard deviation [SD], 16 ± 2 years). All subjects had a minimum Tanner stage of 3, based on the Tanner Self-Rating Scale (Morris and Udry 1980). Subjects were recruited from the in-patient psychiatric units at Cincinnati Children's Hospital Medical Center and their diagnoses were confirmed using the Washington University Kiddie Schedule for Affective Disorders and Schizophrenia (WASH U K-SADS), by interviewers with established diagnostic reliability (kappa > 0.9) (Geller et al. 2001). No subject had received any psychotropic medication within 72 hours of study participation. All subjects received comprehensive substance abuse evaluations conducted by trained research personnel, supervised by a board-certified child and adolescent psychiatrist (M.P.D.), using the Substance Use Disorders module of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994; First et al. 1995), the Addictions Severity Index (ASI) (McLellan et al. 1992), and the Substance Abuse Course-Modified Life II (Keller et al. 1987). Diagnoses of CUD were based on these assessments conducted at baseline and 4-month intervals over the course of 2 years following the MR scan. Approval for this study was obtained from the Institutional Review Boards for the Cincinnati Children's Hospital Medical Center and the University of Cincinnati College of Medicine. Written informed consent was obtained from a legal guardian and written informed assent was obtained from all study participants.
MR scanning and processing
MR scans were performed on a 3.0 Tesla Bruker Biospec scanner (Bruker Medizintechnik, Karisruhe, Germany). During the study, participants reclined in a supine position on the bed of the scanner and a radio frequency (RF) coil (Bruker NMR Instruments Inc., Fremont, California) was placed over their head. Earplugs and headphones were provided to block background noise. Following a 3-plane gradient echo scan for alignment and localization, a shim procedure was performed to generate a homogeneous, constant magnetic field. A total of 120 contiguous 1-mm axial slices extending superiorly from the inferior aspect of the cerebellum to encompass most of the brain were selected from a sagittal localizer scan. A high-resolution T1-weighted three-dimensional brain scan was then obtained using a modified driven equilibrium Fourier transform (MDEFT) protocol (inversion time [TI] = 550 msec, repetition time [TR] = 16.5, echo time [TE] = 4.3 msec, field of view [FOV] = 25.6 × 19.2 × 14.4, 256 × 128 × 96, flip angle = 20°). Precautions were taken to minimize subject motion during the MRI study by instructing subjects to remain still and packing around their heads with foam padding.
Automated image processing was done using statistical parametrical mapping software (SPM2, Wellcome Department of Cognitive Neurology, University College London, UK) running in MATLAB (MathWorks, Natick, MA). Images were uniformly aligned with regard to head position to provide optimal starting estimates for subsequent spatial normalization. Analysis followed the optimized voxel-based morphometry (VBM) processing strategy of Good and colleagues (2001), as previously described (Adler et al. 2005). Final images were smoothed using a Gaussian kernel with a full width at half-maximum (FWHM) of 12 mm, and processed images from both datasets were analyzed employing the framework of the general linear model within SPM2 (Friston et al. 1995). Contrasts were calculated testing for positive or negative correlations of gray matter volume and density with the parameter of interest. Significance was set at p ≤ 0.001, with a minimum cluster size of 200 voxels (Friston et al. 1995; Wilke et al. 2001; Adler et al. 2005). Points of maximum correlation were converted from Montreal Neurological Institute (MNI) to Talairach coordinates using a nonlinear transformation (Lancaster et al. 2000), and then plotted across individual scans.
Results
Seven patients were diagnosed with CUD either before or subsequent to participating in a structural MRI scan (5 girls/2 boys; mean age ± SD, 15 ± 2 years). Four of these subjects had no history of CUD prior to the scan, and 1 subject reported prescan CUD that exceeded 1 year prior to the MR scan. One subject reported 4 months of heroin dependence that ended 3 months prior to the MR scan, and 1 subject was diagnosed with alcohol dependence in addition to marijuana abuse 1 year after participating in the MRI scan (Table 1). Three of these subjects had been diagnosed with CUD prior to being diagnosed with bipolar disorder. Three of these subjects were diagnosed with co-morbid attention-deficit/hyperactivity disorder (ADHD). Four subjects reported taking psychotropic, antidepressant, or stimulant medications for varying lengths of time prior to the imaging study, including sertraline, clonidine, fluoxetine, trazodone, risperidone, valproate, bupropion, quetiapine, lithium, and methylphenidate.
Table 1.
Substance Use Disorder History
| Prescan | Postscan | |
|---|---|---|
| 1 | One year of cannabis abuse ended 3 years prior to scan | Cannabis abuse |
| 2 | No substance use disorder | Alcohol dependence Cannabis abuse Cannabis dependence |
| 3 | No substance use disorder | Cannabis abuse |
| 4 | Two years of cannabis abuse ended 1 month prior to scan | Cannabis abuse |
| 5 | No substance use disorder | Cannabis abuse |
| 6 | No substance use disorder | Cannabis abuse |
| 7 | Less than 1 year of cannabis abuse/dependence ended 3 months prior to scan; four months of heroin dependence ended 3 months prior to scan | Cannabis abuse |
The other 7 participants reported no substance use disorder prior to participating in the structural MR scan, and remained free of significant substance use during the 2 years following their MR scan (4 girls and 3 boys; mean age ± SD, 16 ± 2 years). Two of these subjects were diagnosed with co-morbid ADHD. Four of these patients reported having been exposed to psychotropic, antidepressant, or stimulant medications prior to the study, including valproate, sertraline, quetiapine, mixed amphetamine salts, and methylphenidate.
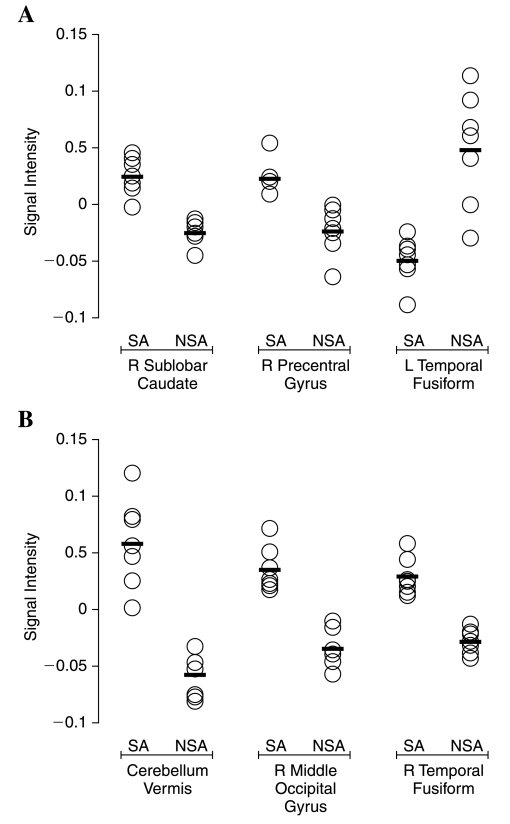
Bipolar subjects with CUD demonstrated decreased gray matter volume in the left fusiform gyrus (Talairach coordinates: −30, −48, −12; Brodmann area 37) and increased gray matter volume in the right caudate (Talairach coordinates: 7, 7, 6) and precentral gyrus (Talairach coordinates: 20, −15, 69; Brodmann area 6) compared with bipolar adolescents without CUD. Additionally, subjects with CUD showed increased gray matter density in the right middle occipital gyrus (Talairach coordinates: 36, −85, 21; Brodmann area 19), right fusiform gyrus (Talairach coordinates: 52, −60, −17; Brodmann area 37) and cerebellar vermis (Talairach coordinates: 3, −79, −20) (Fig. 1). Overlap in gray matter volume and density between groups at the points of maximum correlation was low across structures (Fig. 2).
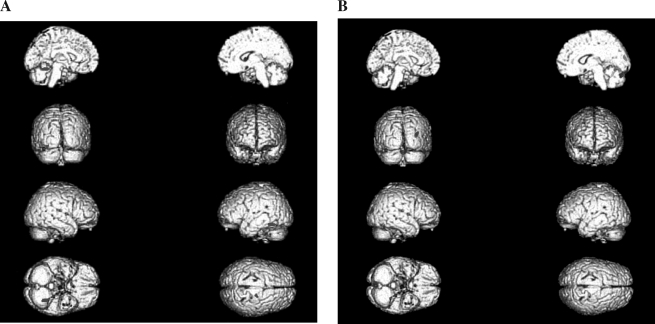
FIG. 1.
Voxel-by-voxel comparison map of gray matter volume (A) and density (B) in bipolar adolescents with and without co-morbid marijuana abuse, overlaid on a T1-weighted three-dimensional anatomic image. Statistically significant differences in gray matter volume were defined as p ≤ 0.001, with a cluster size of 200 (red, abusers > nonabusers; blue, nonabusers > abusers).
FIG. 2.
Comparison of gray matter volume (A) and density (B) values (O) and group means (-) between bipolar adolescents with (SA) and without (NSA) co-morbid marijuana abuse.
Discussion
In this study we found that a CUD in bipolar adolescents was associated with structural differences in frontal and temporal cortical regions, as well as in subcortical areas such as the caudate, that are linked with emotional and motivational regulation. Both functional and structural neuroimaging studies have implicated abnormalities in portions of the frontal cortex, including Brodmann area 11, in substance use disorders (Goldstein and Volkow 2002; Lyoo et al. 2006). These areas are closely networked with portions of the temporal cortex, as well as subcortical structures such as the right caudate that we observed to be enlarged in bipolar adolescents with marijuana abuse. Our findings are consistent with previous suggestions that subcortical dysfunction is associated with impairment in reward mechanisms in patients with substance use disorder. Caudate activity has been linked with craving in functional neuroimaging studies (Breiter et al. 1997; Miller and Goldsmith 2001), and increased activity in the right caudate is associated with alcohol craving (Modell and Mountz 1995; Lingford-Hughes et al. 2003). Similarly, the fusiform gyrus, which we found to be altered in bipolar patients with CUD, has been implicated in craving and drug seeking behavior. For example, Lyoo and colleagues recently demonstrated decreased left fusiform (Brodmann area 37) density in opiate-dependent patients (Lyoo et al. 2006). Functional imaging studies in adolescents suggest the involvement of medial temporal structures as well. Adolescents who used cannabis and tobacco demonstrated decreased right hippocampus activation, compared to controls during an auditory working memory task (Jacobsen et al. 2004).
We observed areas of relative gray matter volume and density differences between bipolar adolescents with and without CUD that broadly overlap with portions of the anterior limbic network, a collection of structures in which we and others have observed morphological abnormalities in patients with bipolar disorder alone (Strakowski et al. 2000b). These areas are involved in elements of mood regulation, suggesting that the development of CUD in bipolar disorder may reflect exaggerated changes in areas also involved in bipolar symptomatology. Our findings may, in part, explain the high rate of co-morbidity between substance abuse and bipolar disorders. Moreover, the relatively low degree of overlap in gray matter volume and density between bipolar adolescents with and without CUD suggests that changes in these regions may be relevant to the development of a CUD in bipolar adolescents. Areas of difference, however, are largely confined to subcortical and posterior parietal brain regions and exclude prefrontal areas previously observed to be affected in adolescents with alcohol and co-morbid axis I conditions (DeBellis et al. 2000; DeBellis et al. 2005). These discrepant findings may be related to underlying differences between alcohol and cannabis use disorders, but they might also reflect the extensive prescan alcohol exposure in the studies reported by DeBellis and colleagues.
There are several limitations to our study that should be considered when interpreting the findings. First, a potential limitation in interpreting the results of any study of morphologic abnormalities associated with substance use disorders is the difficulty in differentiating changes that precede the development of abuse from changes that might result from chronic drug exposure. Whereas this remains a concern for this study, the young age of the subjects as well as their limited substance use prior to the structural MRI scans suggests that the morphological changes we observed are not secondary to the neurotoxic effects of marijuana. In addition, several studies of individuals with a history of greater marijuana consumption failed to identify structural brain deficits (Block et al. 2000; Quickfall and Crockford 2006). Two more recent studies of co-morbid cannabis use in patients with schizophrenia, however, have found evidence of cannabis-related brain changes. Rais and colleagues (2008) found that extensive cannabis use over 5 years was associated with evidence of neuronal atrophy. Bangalore and colleagues (2008) studied first-episode schizophrenic patients with and without frequent cannabis use and found cannabis-related decrements in the volume of the left hippocampus and right posterior cingulate. These findings do suggest that prescan cannabis exposure may be related to some neuroanatomic changes; however, in both of these studies, exposure was greater than allowed here. More importantly, areas of neuronal change observed by Rais and Bangalore do not overlap regions in which we observed CUD-related morphometric differences. The adolescents in this study reported only limited prescan levels of marijuana use; however, their exposure to cannabis at a relatively young age may increase their vulnerability to neuropathic affects (Wilson et al. 2000).
Nicotine abuse or addiction was not an exclusion criterion in this study. At least one study has linked nicotine use with decreased gray matter volume in some brain regions (Brody et al. 2004). An additional limitation of this study is the relatively small sample size, which limits the power to observe group differences. Furthermore, substance use histories were self-reported, and as an illegal substance of abuse, cannabis use data may be unreliable. Patients were assured, however, that reports of substance use would be kept confidential from their parent or legal guardian and that they would not suffer legal or other consequences from divulging cannabis or other substance use to the interviewer. Finally, it is also possible that substance use data acquired postscan may be less subject to recall biases than that acquired prescan. Specifically, prescan data were acquired using a track-back methodology applied over the preceding 4 years. In contrast, postscan substance use was evaluated at 4-month intervals. The limitations of this study necessitate caution in interpreting our results, but the potential importance of early identification of those bipolar adolescents most at risk for developing co-morbid substance use disorders strongly militates for further structural and functional neuroimaging studies across a larger cohort of patients and a broader range of substance abuse disorders.
Footnotes
This study was supported by National Institute of Mental Health (NIMH) grants MH63373, MH64086, and MH58170. A portion of these data was presented at the American Academy of Child & Adolescent Psychiatry Annual Meeting, Boston, Massachusetts, October 27, 2007.
Disclosures
Ms. Jarvis, Dr Elman, and Mr. Mills have no conflicts of interest or financial ties to disclose. Dr. DelBello has received research support from Eli Lilly, Janssen, Pfizer, AstraZeneca, Shire, Somerset Pharmaceuticals, NIDA, NIAAA, NIMH, NARSAD, Thrasher Foundation, Prechter Foundation, and Glaxo-SmithKline. She has served as a consultant for Pfizer, Eli Lilly, AstraZeneca, Glaxo-SmithKline, and the France Foundation (CME company). Dr. DelBello is on the speakers bureaus for AstraZeneca, Bristol Myers Squibb, and the France Foundation (CME company). Dr. Strakowski has received research support from Eli Lilly, Janssen, Pfizer, Forrest, AstraZeneca, Bristol-Myers Squibb, Martek Biosciences, Nutrition 21, Repligen, Johnson and Johnson, Shire, Somerset Pharmaceuticals, NIDA, NIAAA, NARSAD, Thrasher Foundation, and Stanley Medical Research Institute. He has served as a consultant for Pfizer, Eli Lilly, Solvay, and Tikvah. Dr. Strakowski is on the speakers bureaus for the France foundation (CME company), and Dimedix (CME company). Dr. Adler has received research support from Eli Lilly, Janssen, Pfizer, Forrest, Shire, AstraZeneca, Bristol-Myers Squibb, Martek Biosciences, Nutrition 21, Repligen, NIMH, and NARSAD. He has served as a consultant for Lilly and AstraZeneca. Dr. Adler is on the speakers bureaus for AstraZeneca, and the France foundation (CME company).
References
- Adler CM. Levine AD. DelBello MP. Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58:151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- Bangalore SS. Prasad KM. Montrose DM. Goradia DD. Diwadkar VA. Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia—a region of interest, voxel based morphometric study. Schizophr Res. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Block RI. O'Leary DS. Ehrhardt JC. Augustinack JC. Ghoneim MM. Arndt S. Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Blumberg HP. Charney DS. Krystal JH. Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- Blumberg HP. Leung HC. Skudlarski P. Lacadie CM. Fredericks CA. Harris BC. Charney DS. Gore JC. Krystal JH. Peterson BS. A functional magnetic resonance imaging study of bipolar disorder: State- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Blumberg HP. Krystal JH. Bansal R. Martin A. Dziura J. Durkin K. Martin L. Gerard E. Charney DS. Peterson BS. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: A cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Breiter HC. Gollub RL. Weisskoff RM. Kennedy DN. Makris N. Berke JD. Goodman JM. Kantor HL. Gastfriend DR. Riorden JP. Mathew RT. Rosen BR. Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brody AL. Mandelkern MA. Jarvik ME. Lee GS. Smith EC. Huang JC. Bota RG. Bartzokis G. London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Cahill CM. Malhi GS. Ivanovski B. Lagopoulos J. Cohen M. Cognitive compromise in bipolar disorder with chronic cannabis use: Cause or consequence? Expert Rev Neurother. 2006;6:591–598. doi: 10.1586/14737175.6.4.591. [DOI] [PubMed] [Google Scholar]
- Chang K. Adleman NE. Dienes K. Simeonova DI. Menon V. Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chang K. Adleman N. Wagner C. Barnea-Goraly N. Garrett A. Will neuroimaging ever be used to diagnose pediatric bipolar disorder? Dev Psychopathol. 2006;18:1133–1146. doi: 10.1017/S0954579406060548. [DOI] [PubMed] [Google Scholar]
- DeBellis MD. Clark DB. Beers SR. Soloff PH. Boring AM. Hall J. Kersh A. Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- DeBellis MD. Narasimhan A. Thatcher DL. Keshavan MS. Soloff P. Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Strakowski SM. Sax KW. McElroy SL. Keck PE., Jr West SA. Kmetz GF. Familial rates of affective and substance use disorders in patients with first-episode mania. J Affect Disord. 1999;56:55–60. doi: 10.1016/s0165-0327(99)00029-4. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Adler CM. Strakowski SM. The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr. 2006;11:298–311. doi: 10.1017/s1092852900020794. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Hanseman D. Adler CM. Fleck DE. Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- Delisi LE. Bertisch HC. Szulc KU. Majcher M. Brown K. Bappal A. Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman JA. Dunner DL. The effect of alcohol and substance abuse on the course of bipolar affective disorder. J Affect Disord. 1996;37:43–49. doi: 10.1016/0165-0327(95)00080-1. [DOI] [PubMed] [Google Scholar]
- First MB. Spitzer RL. Gibbon M. Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, version 2.0) New York: New York State Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- Franklin TR. Acton PD. Maldjian JA. Gray JD. Croft JR. Dackis CA. O'Brien CP. Childress AR. Decreased gray matter in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Holmes AP. Worsley KJ. Poline JP. Frith CD. Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Geller B. Zimerman B. Williams M. Bolhofner K. Craney JL. Del-Bello MP. Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ. Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD. Johnsrude IS. Ashburner J. Henson RN. Friston KJ. Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Malenka RC. Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK. Mencl WE. Westerveld M. Pugh KR. Impact of cannabis use on brain function in adolescents. Ann NY Acad Sci. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Keller MB. Lavori PW. Friedman B. Nielsen E. Endicott J. McDonald-Scott P. Andreasen NC. The Longitudinal Interval Follow-up Evaluation. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kirsch P. Reuter M. Mier D. Lonsdorf T. Stark R. Gallhofer B. Vaitl D. Hennig J. Imaging gene-substance interactions: The effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Kovasznay B. Bromet E. Schwartz JE. Ram R. Lavelle J. Brandon L. Substance abuse and onset of psychotic illness. Hosp Community Psychiatry. 1993;44:567–571. doi: 10.1176/ps.44.6.567. [DOI] [PubMed] [Google Scholar]
- Lancaster JL. Woldorff MG. Parsons LM. Liotti M. Freitas CS. Rainey L. Kochunov PV. Nickerson D. Mikiten SA. Fox PT. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes AR. Davies SJ. McIver S. Williams TM. Daglish MR. Nutt DJ. Addiction. Br Med Bull. 2003;65:209–222. doi: 10.1093/bmb/65.1.209. [DOI] [PubMed] [Google Scholar]
- Lyoo IK. Pollack MH. Silveri MM. Ahn KH. Diaz CI. Hwang J. Kim SJ. Yurgelun-Todd DA. Kaufman MJ. Renshaw PF. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl) 2006;184:139–144. doi: 10.1007/s00213-005-0198-x. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Cocaine addiction as a neurological disorder: Implications for treatment. NIDA Res Monogr. 1996;163:1–26. [PubMed] [Google Scholar]
- McLellan AT. Kushner H. Metzger D. Peters R. Smith I. Grissom G. Pettinati H. Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller NS. Goldsmith RJ. Craving for alcohol and drugs in animals and humans: Biology and behavior. J Addict Dis. 2001;20:87–104. doi: 10.1300/J069v20n03_08. [DOI] [PubMed] [Google Scholar]
- Modell JG. Mountz JM. Focal cerebral blood flow change during craving for alcohol measured by SPECT. J Neuropsychiatry Clin Neurosci. 1995;7:15–22. doi: 10.1176/jnp.7.1.15. [DOI] [PubMed] [Google Scholar]
- Morris NM. Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc Dev. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A. Dhuna A. Anderson DC. Cerebral atrophy in habitual cocaine abusers: A planimetric CT study. Neurology. 1991;41:34–38. doi: 10.1212/wnl.41.1.34. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A. Sullivan EV. Rosenbloom MJ. Mathalon DH. Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Quickfall J. Crockford D. Brain neuroimaging in cannabis use: A review. J Neuropsychiatry Clin Neurosci. 2006;18:318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Rais M. Cahn W. Van Haren N. Schnack H. Caspers E. Hulshoff Pol H. Kahn R. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165:490–496. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- Regier DA. Farmer ME. Rae DS. Locke BZ. Keith SJ. Judd LL. Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Schlaepfer TE. Lancaster E. Heidbreder R. Strain EC. Kosel M. Fisch HU. Pearlson GD. Decreased frontal white-matter volume in chronic substance abuse. Int J Neuropsychopharmacol. 2006;9:147–153. doi: 10.1017/S1461145705005705. [DOI] [PubMed] [Google Scholar]
- Strakowski SM. McElroy SL. Keck PE., Jr West SA. The effects of antecedent substance abuse on the development of first-episode psychotic mania. J Psychiatr Res. 1996;30:59–68. doi: 10.1016/0022-3956(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Strakowski SM. Sax KW. McElroy SL. Keck PE., Jr Hawkins JM. West SA. Course of psychiatric and substance abuse syndromes co-occurring with bipolar disorder after a first psychiatric hospitalization. J Clin Psychiatry. 1998;59:465–471. doi: 10.4088/jcp.v59n0905. [DOI] [PubMed] [Google Scholar]
- Strakowski SM. DelBello MP. Fleck DE. Arndt S. The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry. 2000a;48:477–485. doi: 10.1016/s0006-3223(00)00900-8. [DOI] [PubMed] [Google Scholar]
- Strakowski SM. DelBello MP. Adler C. Cecil DM. Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000b;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Biederman J. Millstein RB. Wozniak J. Hahesy AL. Spencer TJ. Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:680–685. doi: 10.1097/00004583-199906000-00014. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Biederman J. Kwon A. Ditterline J. Forkner P. Moore H. Swezey A. Snyder L. Henin A. Wozniak J. Faraone SV. Risk of substance use disorders in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1380–1386. doi: 10.1097/01.chi.0000140454.89323.99. [DOI] [PubMed] [Google Scholar]
- Wilke M. Kaufmann C. Grabner A. Pütz B. Wetter TC. Auer DP. Gray matter changes and correlates of disease severity in schizophrenia: A statistical parametric mapping study. Neuroimage. 2001;13:814–824. doi: 10.1006/nimg.2001.0751. [DOI] [PubMed] [Google Scholar]
- Wilson W. Mathew R. Turkington T. Hawk T. Coleman RE. Provenzale J. Brain morphological changes and early marijuana use: A magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Winokur G. Coryell W. Akiskal HS. Maser JD. Keller MB. Endicott J. Mueller T. Alcoholism in manic-depressive (bipolar) illness: Familial illness, course of illness, and the primary-secondary distinction. Am J Psychiatry. 1995;152:365–72. doi: 10.1176/ajp.152.3.365. [DOI] [PubMed] [Google Scholar]