Abstract
Inflammatory cytokines are involved in the development of mucus cell metaplasia/hyperplasia (MCM) in otitis media (OM). However, which cytokines play an essential role in MCM OM is not clear at the moment. In this study, we hypothesized that interleukin-10 (IL-10) played an indispensable role in MCM of bacterial OM and used IL-10 knockout mice to test this hypothesis. In wild-type mice, both S. pneumoniae and H. influenzae triggered the development of MCM in the middle ear mucosa. In IL-10 knockout mice, the number of goblet cells and mucin-producing cells in the middle ear was significantly reduced after bacterial middle ear infection compared with that in wild-type mice. We, therefore, concluded that IL-10 plays an essential role in MCM of bacterial OM. IL-10 is a potential target for the treatment of MCM in OM.
Keywords: mucous cell metaplasia, pnumococcal middle ear infection, IL-10 knockout mouse
Introduction
Interleukin-10 (IL-10), produced by a variety of cells including monocytes, macrophages, eosinophils, and bronchial epithelial cells, it is a pleiotrophic cytokine with pro-inflammatory and immunostimulatory effctor functions 1–3, contributing to disease pathogenesis. Over-expression of IL-10 in transgenic mice caused MCM, mucin upregulation, inflammation and tissue remodeling in the lung 4. This prompted us to examine whether IL-10 plays a role in MCM of OM. It has been shown that IL-10 contributed to the changes of mucins and goblet cells in the colon 5 and played a critical role in host resistance and survival to bacterial intestinal infection in the intestine of mice 6.
On the other hand, IL-10 possesses the properties of anti-inflammatory, immunosuppressive, and tissue protective effects 1, 3, 7. This effect is based upon inhibitory effects of IL-10 on many inflammatory cytokines including, interferon-gamma (IFN-γ)8, vascular epithelial growth factor (VEGF) 9, IL-1β, and IL-8 10. During inflammation, macrophages rapidly produce proinflammatory cytokines such as tumor necrotic factor alpha (TNF-α), IL-1, and IL-12 in response to lipopolysaccharide or IFN-γ. Macrophages produce IL-10 following a burst of production of proinflammatory cytokines. Once IL-10 is produced, it suppresses immune responses and inflammatory reactions by suppressing the production of pro-inflammatory cytokines such as IL-1 or by reducing the cell surface expression of immunoreceptors such as major histocompability complex (MHC) class II 11. For this reason, IL-10 is being used in the clinic to treat patients with inflammatory bowel disease, rheumatoid arthritis, psoriasis, cystic fibrosis, and asthma 2, 12, aiming at the suppression of pro-inflammatory cytokine production in these chronic diseases.
The presence of IL-10 in middle ear effusion and mucosa is well documented in both humans 13 and animal models 14, 15. In the middle ear, pneumococcal infection causes upregulation of numerous cytokines 15–17 including IL-10 which is associated with MCM in the middle ear cavity 18. We hypothesized that IL-10 plays a role in MCM of OM.
Materials and Methods
Animals
Approximately 4–6 weeks old C57BL/6 wild type (B6.129P2) mice, IL-10 knockout (B6.129P2-Il10tm1Cgn/JIL10) mice were obtained from the Jackson Laboratory. They were bred and maintained in the animal facility at the University of Minnesota Medical School with the approval of the Institutional Animal Care and Use Committee (IACUC). Mice homozygous for the IL-10tm1Cgn targeted mutation are viable and fertile when housed under Specific Pathogen Free (SPF) conditions. Under conventional housing conditions homozygous mutant mice are small in size, anemic, and have chronic enterocolitis. Intestinal problems are far less severe (local inflammation of the proximal colon) in a pathogen-free environment. Lymphoctye development and antibody responses are normal in homozygous mutant mice. Rats had been shown to be useful for induction of MCM in the middle ear 18, 19. Sprague-Dawley rats (weighing approximately 200 grams) were, therefore, used as positive controls for MCM in this study. Mice were reportedly suitable for acute OM 20 but it is unknown whether mice were suitable for induction of MCM in the middle ear. Mice were selected because of their attractive genetic mutant models and available genome information.
Pathogens
S. pneumoniae type 6A (Pn6A), originally isolated from clinical OM, has been used for induction of OM in rats with an dose up to 2×109 cfu/mL in 50 μl of bacterial suspension (yielding approximately 107 cfu per bulla) 21. While in mice, inoculation dose was much lower than that in rats, approximately 2×102–3 cfu/mL in 10 μl of bacterial suspension (yielding approximately 10–100 cfu per bulla), as reported recently 20. Pn6A was routinely passed on mice for maintenance of its virulence prior to bullar inoculation. Non-typable H. influenzae (NTHi, strain #12) which is a kind gift of Dr. Stephen Barenkamp at the St. Louis Unversity School of Medicine. Inoculation dose was up to 2×109 cfu/mL in 50 μL of bacterial suspension (yielding approximately107 cfu per bulla) for rats, and up to 108 cfu/mL 20 (i.e., 2×106 cfu per bulla) for mice. All bacteria were suspended in phosphate-buffered saline (PBS) solution.
Induction of OM
Animals were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine (16 mg/kg) and bulli were surgically exposed via a ventral approach as previously described 21. Inoculation volume of bacterial suspension in mice and rats was 10 μL and 50 μL per bulla, respectively, and both bulli of each animal were inoculated. In dose-dependent studies of Pn6A, 3–6 wildtype mice were used for inoculation with doses from 2×102 to 2×109 cfu/mL. In dose-dependent studies of NTHi, 3–6 wildtype mice were used for inoculation with doses from 2×104 to 2×109 cfu/mL. Bulli with inoculation of NTHi at 2×109 cfu/mL, peptidoglycan-polysaccharide (PGPS) of Pn6A at 2 μg/mL served as experimental controls. PGPS of Pn6A was prepared as previously described 22. Additional 5 rats with PBS inoculation into bulli served as blank control. Animals were sacrificed on day 7 for harvest of the bulli and subsequent evaluation of pathological changes by hemotoxylin and eosin (H&E, histology), Alcian blue periodic acid Schiffs (AB-PAS, histochemistry), and mucin immunohistochemistry.
Histology
Bulli were fixed in 10% paraformaldehyde solution overnight, dehydrated in graded alcohol, immersed in xylene for 3 hours, embedded in paraffin and cut at a thickness of 4 microns. Sections were deparaffinized in xylene, dehydrated in graded alcohol and then water, and stained with H&E for histology, AB-PAS for goblet cells, or immunohistochemistry fro mucin-producing cells, as previously described 18.
Goblet cell identification and quantitation
Goblet cells stained with blue or purple in color by AB-PAS were identified as goblet cells. AB-PAS positive cells were viewed under a light microscope. Six areas in each bulla, as indicated in Fig. 2 (panel A), were chosen for measurement of goblet cell numbers. Goblet cells were frequently found in these areas in response to challenge of bacteria in our pilot studies. Areas 1–3 stand for the superior part of the middle ear cavity whereas areas 4–6 for the inferior part of the middle ear cavity.
Fig. 2.
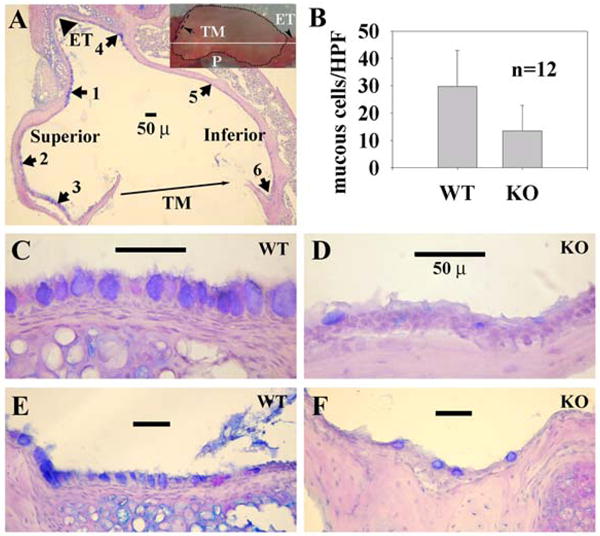
Reduction of goblet cell numbers in the middle ear mucosa of mutant mice by Pn6A. Panel A represents an overview of bullar section with triangle pointing to the Eustachian tube (ET), solid line indicating the tympanic membrane (TM), numbers 1–3 standing for the superior of the middle ear cavity and numbers 4–6 for the inferior of the middle ear cavity. Insert indicates the inferior view of the bulla (dotted line) with arrow to TM and arrowhead to ET (P, promontory; white solid line, cross section orientation). Panel B represents for averaged goblet cell numbers ± standard deviation per high power field (HPF) from the 6 locations indicated in A. Pn6A induced MCM in the middle ear mucosa of wildtype (WT, panels C and E at locations 1 and 4, respectively) mice but less so in the middle ear mucosa of IL-10 knockout (KO, panels D and F at locations 1 and 4, respectively) mice. Bar=50 μ in panels A and C–F.
Quantitative analysis of mucin-producing cells
Goblet cells are specialized in mucin production. To verify their goblet cell identity, immunohistochemistry was performed on middle ear histological sections as previously described 23 with some modifications. Mucin polyclonal antibody used in this study is a custom-made, rabbit anti-rat middle ear mucins, which reacted with multiple mucins and/or mucin-like molecules. Rat middle ear mucins were extracted by the method as previously described 24. Purified middle ear mucins were submitted to Animal Pharm Sevices Inc. for making polyclonal antibodies (anti-serum). Rabbit anti-serum with 1:200 dilution was incubated with middle ear sections for 90 minutes, washed, incubated with secondary antibody conjugated to fluorescein isothiocyanate (FITC-conjugated, mouse anti-rabbit, Millipore). Mucin-producing cells were identified using the above polyclonal mucin antibody and analyzed quantitatively by counting. Results are presented as positive cell numbers per milimeter in length of the middle ear mucosa using the method as previously described 18. Six areas were taken for quantitative analysis of mucin-producing cells after bacterial challenges. Data are presented as mean± standard deviation (SD)
Statistical Analysis
Differences of data between groups were analyzed by non-paired Student’s t-test and/or ANOVA, with p<0.05 considered statistically significant.
Results
In gross examination of the bullar anatomy, there was no deformation in the middle ear of both IL-10 knockout mice compared with that of control wild type mice. There were no goblet cells in the middle ear mucosa but few in the orifice of the Eustachian tube in both wild-type and IL-10 knockout mice before challenge with bacteria (data not shown). H&E was performed for evaluation of inflammatory cell infiltration, oedema, and submucosal collagen deposition. It was demonstrated that inflammatory infiltration, oedema, and submucosal deposition was remarkable in IL-10 knockout mice compared with that in wild type mice. It suggests that inflammation and collagen metabolism in the middle ear mucosa are altered in IL-10 knockout mice.
Establishment of MCM in mouse models
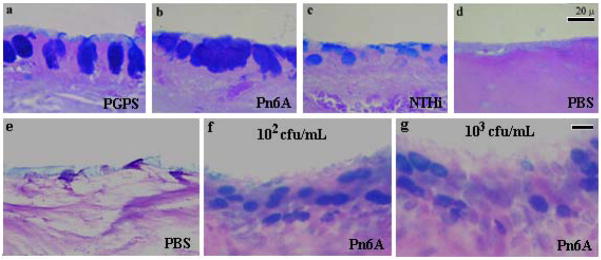
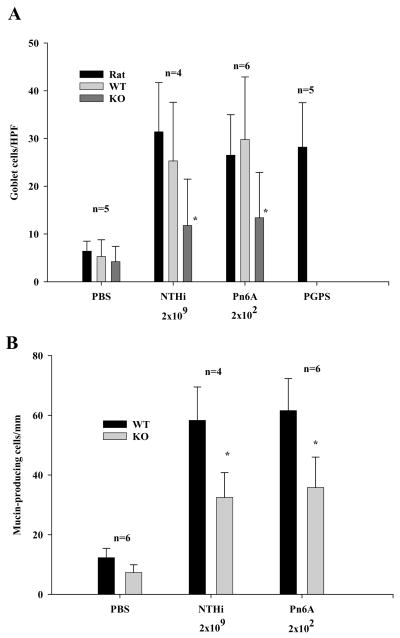
Rats, known to be suitable for induction of MCM with pneumococcus, demonstrated MCM in the middle ear mucosa. The results are presented in Fig. 1 (a–d). Mice were found to be quite susceptible to Pn6A infection. Six mice were inoculated with Pn6A (2×105–9 cfu/mL) bilaterally into the bulli but all animals died on day 2 following the bullar inoculation due to the Pn6A virulence. All mice survived when NTHi was used in the range of 104–9 cfu/mL. Middle ear pathologic examination demonstrated that Pn6A at 107 cfu/mL or higher caused severe OM with partial necrosis of the middle ear epithelium (suggesting the cause of animal death). Animals with bullar inoculation of Pn6A at 104 cfu/mL did not die on day 2 but were sick and died on day 3 or 4. Pn6A at 2×102 cfu/mL in 9 mice and 103 cfu/mL in 9 CBA/J mice were found to be the appropriate dose for mice. Six mice at each dose survived at least 8 days and three mice died before the experiment ended and were excluded from this study. It was noted that the surviving animals were healthy on the day of sacrifice (day 7). PBS inoculation served as a control. The bullae were harvested for histochemistry. It was found that MCM was obvious in the middle ear mucosa of mice on day 7 following the Pn6A inoculation. Interestingly, there were no obvious differences in goblet cell numbers between different doses of Pn6A inoculation (for 2×102 cfu/mL and 2×103 cfu/mL). Goblet cell numbers for NTHi at 2×109 cfu/mL were 25.3± 12.3 cells per high power field (HPF) but not significantly different from those for NTHi at 2×104 cfu/mL (19.3± 8.1 cells/HPF) and Pn6A at 102 cfu/mL (29.8 ± 13.1 cells/HPF) or Pn6A at 103 cfu/mL (30.9 ± 12.5 cells/HPF). Goblet cell numbers for Pn6A at 104 cfu/mL or above were not applicable because of animal death. Goblet cell numbers for Pn6A at 102 =13.4 ± 9.5 or for Pn6A at 103 =14.9 ± 8.5 (not significantly different).
Fig. 1.
Induction of MCM in the middle ear of mice. In rats (positive controls), induction of MCM by PGPS (panel a) was almost as potent as that by live Pn6A (panel b) but more potent than live NTHi (panel c) in middle ear of rats. Panel d is the control for panels a, b & c (AB-PAS stain). MCM in the middle ear of mice was induced by Pn6A. Mice were inoculated with Pn6A at 102 and 103 cfu/mL, respectively, in the middle ear and sacrificed on day 8 for examination of MCM by AB-PAS stain. There was an obvious increase of AB-PAS positive cells (blue in color) in Pn6A-inoculated middle ear mucosa (f&g) compared with control middle ear mucosa (c). Note that there is no obvious difference in goblet cell numbers between middle ears with different Pn6A inoculum doses (f & g), suggesting that live Pn6A grows exponentially in the middle ear. bar=20 μm applying to each row.
Reduction of goblet cell numbers in mutant mouse middle ear mucosa following bacterial challenges
To determine if IL-10 plays a role in MCM, both bullae of 4–6 knockout mice and 4–6 wild-type mice were inoculated with NTHi (2×109 cfu/mL) and Pn6A (2×102 cfu/mL). H&E and AB-PAS were performed for evaluation of inflammatory cell infiltration and MCM, respectively. AB-PAS staining demonstrated significantly reduced goblet cell counts in the middle ear of IL-10 knockout mice (Fig. 2, b&d) compared with those in wild-type mice (Fig. 2a&c) after challenging with Pn6A at 2×102 cfu/mL. Similar cell counts were observed in the group challenged with NTHi at 2×109 cfu/mL (Fig. 4).
Fig. 4.
Reduction of MCM in the middle ear mucosa of mutant mice after bacterial challenge. Mice were challenged with NTHi and Pn6A, respectively, for evaluation of goblet cell numbers and mucin-producing cells in the middle ear mucosa of mice. Rats were used as positive controls. A, goblet cell numbers were significantly reduced in the middle ear mucosa of mutant mice (IL-10KO) compared with those of wildtype mice (IL-10WT). B, mucin-producing cells were significantly reduced in the middle ear mucosa of mutant mice (IL-10KO) compared with those of wildtype mice (IL-10WT).
Reduction of mucin-producing cell counts in mutant mouse middle ear mucosa following bacterial challenges
Consistent with goblet cell counts, mucin antibody staining demonstrated that mucin-producing cells decreased in the middle ear mucosa of IL-10 knockout mice compared with those in the control ears following bacterial challenges with NTHi at 2×109 cfu/mL and Pn6A at 2×102 cfu/mL (Fig. 3A–B). Taken 6 locations in the middle ear into account as indicated in Fig. 2A, there was a significant decrease of mucin-producing cells in the middle ear mucosa between mutant and wildtype mice (Fig. 3C, 61.6 ± 10.7 cells/mm in wild mice vs. 35.8±10.2 cells/mm in mutant mice, p<0.01). Similar cell counts from NTHi challenge were observed (see Fig. 4 for comparison of mucin-producing cell numbers between NTHi and Pn6A). In PBS inoculated middle ears, there were few mucin-producing cells identified (Fig. 4B) whereas in non-inoculated middle ears, there were no mucin-producing cells identified (data not shown). Mucin-producing cells for NTHi at 2×109 cfu/mL were 58.3± 11.2 cells/mm but not significantly different 55.6 ± 9.8 cells/mm from NTHi at 2×104 cfu/mL. Mucin-producing cells for Pn6A at 102 were 61.6 ± 10.7 cells/mm or for Pn6A at 103 cfu/mL (65.7 ± 10.5 cells/mm) and not applicable for Pn6A at 104 cfu/mL or above because of animal death. Mucin-producing cells for Pn6A at 102 cfu/mL (35.8±10.2 cells/mm) were not significantly different from Pn6A at 103 cfu/mL (38.9 ± 9.1 cells/mm).
Fig. 3.
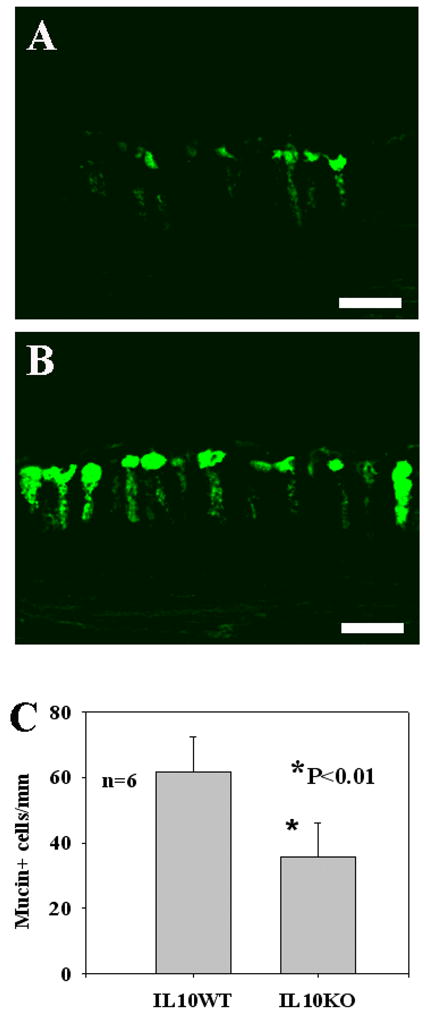
Reduction of mucin-producing cells in mutant mouse middle ear mucosa of mutant mice following bacterial challenges. Panel A represents the density of mucin-producing cells in the middle ear mucosa of mutant mice (referred to as location 2 in Fig. 2) after challenge with Pn6A at 2×102 cfu/mL. Panel B represents the density of mucin producing cells in the middle ear mucosa of wildtype mice after Pn6A challenge at the same concentration. The difference of mucin producing cells in the middle ear mucosa between mutant and wildtype mice is significant (Panel C, p<0.01). Bar=20 μ
Discussion
A hallmark of OM is MCM that is accompanied by effusion rich in mucus (mucins). Infection induces infiltration of inflammatory cells and production of cytokines and chemokines. Cytokines and chemokines, in turn, trigger MCM, a classic pathway for triggering MCM in OM. MCM is induced in acute OM but frequently exacerbated in chronic OM.
However, which cytokines are involved in MCM in the middle ear and which ones are not is not clear at the moment. We and others have shown that bacterial infection or cytokine challenge of middle ear mucosa results in MCM 18, 19, 25 but whether these cytokines are essential for MCM is not tested. Cytokines act as a net in response to bacterial challenges. It is very important to determine which cytokines or chemokines play an indispensable role in MCM in order to design innovative therapeutic interventions toward MCM in OM or other upper respiratory infectious diseases. Mutant mouse models are very useful for screening which cytokines or chemokines can be targeted in terms of controlling MCM in the middle ear under inflammatory conditions. In this study, we demonstrate that IL-10 is a potential target for prevention and treatment of MCM.
Regarding the receptor for bacterium-induced MCM, toll-like receptor 2 (TLR2) may mediate the Pn6A-induced response. It has been shown in the literature that TLR2 is required for recognition of peptidoglycan (PG) and mediates the production of cytokines IL-6 and TNF-α26. In our recent studies, PGPS regulated the expression of TLR2 (data not shown). Generally speaking, bacterial lipoproteins are powerful for induction of many cytokines27. As shown in a recent report, IL-10 production is mainly induced by lipoproteins such as the outer surface A (OspA) and LPS is much less powerful than OspA for induction of IL-10 in macrophages 28. Lipoprotein of Pn6A may stimulate the production of IL-10 via TLR2. Since IL-10 is one of the important cytokines in immune and inflammatory responses and part of the cytokine network, IL-10 is regulated by other inflammatory mediators such as TNF-α 29, 30. This raises a question whether TNF-α induced MCM is via IL-10. This needs to be clarified in future studies.
TLR4 may be involved in the NTHi-induced response because pathogen-associated molecular pattern (PAMP) in gram-negative cell wall component (namely, LPS) interacts with TLR4, yielding signal transduction for immune and inflammatory responses in many cell types 26. However, our pilot study indicated that MCM occurred in TLR4 knockout mice following bacterial challenges with either Pn6A or NTHi (data not shown). This suggests that TLR4 is not essential for MCM in bacterial OM although it may participate in mediating goblet cell metaplasia/hyperplasic in bacterial middle ear infections. Non-cell wall components produced during bacterial infection may play a role in MCM.
At the post-receptor level, many signaling pathways may be involved in the regulation of IL-10 in the middle ear mucosa with bacterial infection. Signals leading to activation of STAT3 (instead of NF-κB) is involved in the regulatory control of IL-10 production in humans 31. Analysis of the human IL-10 promoter sequence revealed the binding site for STAT3 31, verifying that STAT3 regulates the expression of IL-10. Whether bacterial infectin in the middle ear mucosa leads to activation of STAT3 and then IL-10 production warrants future investigations.
Conclusion
We conclude in this study that IL-10 plays an important role in MCM in the middle ear mucosa in response to middle ear bacterial infection through a classic pathway: infection→inflammatory cell infiltration→cytokine release→MCM. Further studies are needed to determine whether IL-10 is a target for the treatment of MCM in OM.
Acknowledgments
This study was performed in accordance with the PHS Policy on Human Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7. U.S.C. et seq.); the animal use protocol was approved by the Instititutional Aniaml Care and Use Committee (IACUC) at the University of Minnesota. This study is supported in part by the NIH grant# R01 DC008165 (to J. L.) and P30 DC04660, and R21 DC009039 (to Q.Y.Z).
References
- 1.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 2.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13(3):257–61. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- 4.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, Graham BS, Johnson TR, Elias JA. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002;277:35466–74. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 5.Makkink MK, Schwerbrock NM, Mahler M, Boshuizen JA, Renes IB, Cornberg M, Hedrich HJ, Einerhand AW, Buller HA, Wagner S, Enss ML, Dekker J. Fate of goblet cells in experimental colitis. Dig Dis Sci. 2002;47(10):2286–97. doi: 10.1023/a:1020147630032. [DOI] [PubMed] [Google Scholar]
- 6.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr, Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168(5):2383–92. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 7.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 8.Borish L. IL-10: evolving concepts. J Allergy Clin Immunol. 1998;101(3):293–7. doi: 10.1016/S0091-6749(98)70238-6. [DOI] [PubMed] [Google Scholar]
- 9.Silvestre JS, Mallat Z, Duriez M, Tamarat R, Bureau MF, Scherman D, Duverger N, Branellec D, Tedgui A, Levy BI. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000;87(6):448–52. doi: 10.1161/01.res.87.6.448. [DOI] [PubMed] [Google Scholar]
- 10.De Beaux AC, Maingay JP, Ross JA, Fearon KC, Carter DC. Interleukin-4 and interleukin-10 increase endotoxin-stimulated human umbilical vein endothelial cell interleukin-8 release. J Interferon Cytokine Res. 1995;15(5):441–5. doi: 10.1089/jir.1995.15.441. [DOI] [PubMed] [Google Scholar]
- 11.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmiel JF, Konstan MW, Knesebeck JE, Hilliard JB, Bonfield TL, Dawson DV, Berger M. IL-10 attenuates excessive inflammation in chronic Pseudomonas infection in mice. Am J Respir Crit Care Med. 1999;160(6):2040–7. doi: 10.1164/ajrccm.160.6.9901043. [DOI] [PubMed] [Google Scholar]
- 13.Skotnicka B, Hassmann E. Cytokines in children with otitis media with effusion. Eur Arch Otorhinolaryngol. 2000;257(6):323–6. doi: 10.1007/s004059900218. [DOI] [PubMed] [Google Scholar]
- 14.Hebda PA, Alper CM, Doyle WJ, Burckart GJ, Diven WF, Zeevi A. Upregulation of messenger RNA for inflammatory cytokines in middle ear mucosa in a rat model of acute otitis media. Ann Otol Rhinol Laryngol. 1998;107(6):501–7. doi: 10.1177/000348949810700608. [DOI] [PubMed] [Google Scholar]
- 15.Melhus A, Ryan AF. Expression of cytokine genes during pneumococcal and nontypeable Haemophilus influenzae acute otitis media in the rat. Infect Immun. 2000;68:4024–31. doi: 10.1128/iai.68.7.4024-4031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yellon R, Marucha LP, Carpenter R, Kreutzer D. In: Sade J, editor. Cytokines and chronic otitis media with effusion; Conference on the Eustachian tube and middle ear disease; Oct26–29, 1989; Kugler Publishers; 1989. [Google Scholar]
- 17.Yellon RF, Leonard G, Marucha PT, et al. Characterization of cytokines present in middle ear effusions. Laryngoscope. 1991;101:165–69. doi: 10.1288/00005537-199102000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Tsuboi Y, Kim Y, Giebink GS, Le C, Paparella MM, Chen N, Schachern PA, Juhn SK, Lin J. Induction of mucous cell metaplasia in middle ear of rats by a three-step method: an improved model for otitis media with mucoid effusion. Acta Otolaryngol. 2002;122:153–60. doi: 10.1080/00016480252814153. [DOI] [PubMed] [Google Scholar]
- 19.Kawano H, Haruta A, Tsuboi Y, Kim Y, Schachern PA, Paparella MM, Lin J. Induction of mucous cell metaplasia by tumor necrosis factor alpha in rat middle ear: the pathologic basis for mucin hyperproduction in mucoid otitis media. Ann Otol Rhinol Laryngol. 2002;111:415–22. doi: 10.1177/000348940211100506. [DOI] [PubMed] [Google Scholar]
- 20.Melhus A, Ryan AF. A mouse model for acute otitis media. Apmis. 2003;111(10):989–94. doi: 10.1034/j.1600-0463.2003.1111012.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Vambutas A, Haruta A, Paparella MM, Giebink GS, Kim Y. Pneumococcus activation of the 5-lipoxygenase pathway and production of glycoproteins in the middle ear of rats. J Infec Dis. 1999;179:1145–51. doi: 10.1086/314714. [DOI] [PubMed] [Google Scholar]
- 22.Tsushiya K, Toyama K, Hamajima Y, Kim Y, Ondrey FG, Lin J. Pneumococcal peptidoglycan-polysaccharide induces the production of interleukin-8 by way of nuclear factor kappa B, nuclear factor interleukin-6, and action protein 1 dependent mechanisms in the airway epithelial cells. Laryngoscope. 2007;117:86–91. doi: 10.1097/01.mlg.0000244182.81768.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Tsuboi Y, Rimell F, Liu G, Toyama K, Kawano H, Paparella MM, Ho SB. Expression of mucins in mucoid otitis media. JARO. 2003 doi: 10.1007/s10162-002-3023-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Haruta A, Kawano H, Ho SB, Adams GL, Juhn SK, Kim Y. Induction of mucin gene expression in middle ear of rats by tumor necrosis factor-a: potential cause for mucoid otitis media. J Infect Dis. 2000;182:882–7. doi: 10.1086/315767. [DOI] [PubMed] [Google Scholar]
- 25.Ueno K, Lim DJ. Heterogeneity of glycoconjugates in the secretory cells of the chinchilla middle ear and eustachian tubal epithelia: a lectin-gold cytochemical study. J Histochem Cytochem. 1991;39:71–80. doi: 10.1177/39.1.1983875. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 27.Wooten RM, Weis JJ. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr Opin Microbiol. 2001;4(3):274–9. doi: 10.1016/s1369-5274(00)00202-2. [DOI] [PubMed] [Google Scholar]
- 28.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168(1):348–55. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 29.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151(12):6853–61. [PubMed] [Google Scholar]
- 30.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol. 1995;7(4):517–23. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 31.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165(3):1612–7. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]