Abstract
The basal ganglia of newborns are extremely vulnerable to hypoxic ischemia (HI). Striatal neurons undergo prominent necrosis after HI. The mechanisms for this degeneration are not well understood. Post-asphyxic hypothermia ameliorates the striatal necrosis, but the mechanisms of hypothermia-induced neuroprotection are not known. We used a newborn piglet model of hypoxic-asphyxic cardiac arrest to test the hypotheses that N-methyl-D-aspartate receptor activation and free radical damage coexist, prior to neurodegeneration, early after resuscitation, and that these changes are attenuated with hypothermia. Piglets were subjected to 30 minutes of hypoxia followed by 7 minutes of airway occlusion, causing asphyxia cardiac arrest, and then were resuscitated and survived normothermically for 5 minutes, 3 hours, or 6 hours, or hypothermically for 3 hours. By 6 hours of normothermic recovery, 50% of neurons in putamen showed ischemic cytopathology. Striatal tissue was fractionated into membrane or soluble proteins and was assayed by immunoblotting for carbonyl modification, phosphorylation of the N-methyl-D-aspartate receptor subunit NR1, and neuronal nitric oxide synthase. Significant accumulation of soluble protein carbonyls was present at 3 hours (196% of control) and 6 hours (142% of control). Phosphorylation of serine-897 of NR1 was increased significantly at 5 minutes (161% of control) and 3 hours (226% of control) after HI. Phosphorylation of serine-890 of NR1 was also increased after HI. Membrane associated neuronal nitric oxide synthase was increased by 35% at 5 minutes. Hypothermia attenuated the oxidative damage and the NR1 phosphorylation in striatum. We conclude that neuronal death signaling in newborn striatum after HI is engaged rapidly through N-methyl-D-aspartate receptor activation, neuronal nitric oxide synthase recruitment, and oxidative stress. Postasphyxic, mild whole body hypothermia provides neuroprotection by suppressing N-methyl-D-aspartate receptor phosphorylation and protein oxidation.
Keywords: asphyxia, cardiac arrest, neonatal brain ischemia, pediatric brain damage, protein carbonyl, nitric oxide
1. Introduction
Asphyxia in infants and children, resulting from difficulties during delivery, airway obstruction, asthma, and drowning, can cause cardiac arrest. The survivors of pediatric cardiac arrest can have hypoxic-ischemic encephalopathy (HIE) and long-term neurological disability, including disorders in movement, learning and cognition, and epilepsy (Sunshine, 2003). Term infants that experienced episodes of hypoxia-ischemia (HI) have damage in forebrain and brainstem, with basal ganglia, particularly the striatum, and somatosensory systems showing selective vulnerability (Johnston, 1998). Research on experimental animal models has shown that brain damage caused by perinatal HI is partly mediated by excitotoxic mechanisms resulting from excessive activation of glutamate receptors and oxidative stress (Volpe, 1995; Johnston et al., 2002; Martin, 2003; McQuillen and Ferriero, 2004). The ion channel N-methyl-D-aspartate (NMDA) receptor and intracellular signaling pathways involving nitric oxide (NO) appear to have particularly prominent roles in perinatal brain damage (Johnston 2001; Martin, 2003; McQuillen and Ferriero, 2004). In newborn rats the NMDA receptor antagonist MK-801 ameliorates brain damage following HI (McDonald et al., 1987; Ford et al., 1989), and neonatal mice without an isoform of neuronal nitric oxide synthase (nNOS) have less brain damage than wildtype mice after HI (Ferriero et al., 1996); yet, in a larger pediatric animal model, such as piglet, MK-801 did not protect against brain damage after HI (LeBlanc et al., 1991).
Despite much progress in the epidemiological and pathophysiological understanding of perinatal HI, treatments to prevent successfully the HIE and to restore neurological function in human infants and children that are victims of HI have been limited to supportive care (Johnston et al., 2002; Hamrick and Ferriero, 2003). However, mild hypothermia has gained American Heart Association endorsement as a neuroprotective intervention after HI caused by cardiopulmonary arrest in adult humans (Hypothermia after Cardiac Arrest Study Group, 2002), but the application of this maneuver on infants and children has uncertain efficacy, in part due to variations in the severity of injury and the timing of implementation (Shankaran et al., 2005; Gluckmam et al., 2005). Moreover, the mechanisms of hypothermic neuroprotection are unresolved.
We have developed a piglet model of HI that simulates the brain damage and some of the clinical deficits found in human newborns that are victims of asphyxia (Martin et al., 1997; Johnston, 1998; Brambrink et al., 1999). The one-week-old piglet is equivalent developmentally to human term infants (Dobbing and Sands, 1979), and the injury model is most relevant to profound asphyxia in the full-term neonate (Maller et al., 1998). Importantly, the basal ganglia are selectively vulnerable in this model. The striatum is the most vulnerable. Damage in newborn piglet striatum after cerebral HI induced by asphyxic cardiac arrest evolves rapidly over 24 hours and resembles closely excitotoxic neuronal damage caused by NMDA receptor activation (Martin et al., 2000). Thus, to protect this region early interventions are required. We have hypothesized that the mechanisms for the profound degeneration of striatal neurons after HI involve NMDA receptor-mediated excitotoxicity (Martin et al., 1998; Martin et al., 2000). The NMDA receptor is a tetrameric ion channel comprised of individual protein subunits designated as NR1, NR2A-D and NR3A-B (Prybylowski and Wenthold, 2004). The functional channel must contain at least one NR1 subunit of which there are several variants generated by alternate splicing of the C-terminus (Prybylowski and Wenthold, 2004). The physiological properties of the NMDA receptor are determined by the subunit composition of the heteromeric complex and are modulated by phosphorylation of the NR1 and NR2 subunits (Prybylowski and Wenthold, 2004). Protein kinase C (PKC) phosphorylates NR1 serine residues 890 and 896, and cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) phosphorylates NR1 serine 897 (Tingley et al., 1997). We have shown previously that NMDA receptor subunit proteins are enriched in newborn piglet striatum; moreover, the levels of the different subunits as well as the phosphorylation of NR1 change differentially in the striatum of HI piglets during the period of active cell death after HI (Guerguerian et al., 2002) at which time there is oxidative damage and striatal neuron necrosis (Martin et al., 2000). We have also shown that 24 hours of mild, whole body hypothermia with sedation and paralysis has profound, sustained neuroprotective effects of on the HI piglet striatum (Agnew et al., 2003), consistent with other studies in newborn piglet (Haaland et al., 1997) and rat (Tresher et al., 1997; Bona et al.,1998). However, it is not known when the NMDA receptor alterations indicative of activation and oxidative damage emerge initially in relation to neuronal damage and whether hypothermia impacts on these early perturbations as a possible mechanism for neuroprotection. Thus, we examined the phosphorylation state of NR1 and oxidative stress in the striatum early after asphyxic cardiac arrest and determined if post-HI hypothermia decreases NMDA receptor phosphorylation and oxidative damage in the striatum.
2. Experimental procedures
2.1. Piglet model of asphyxic cardiac arrest and hypothermia
Five to seven-day-old male piglets, weighing 3.0–4.5 kg, were used. All procedures on piglets were approved by the institutional Animal Care and Use Committee. Piglets were subjected to asphyxic cardiac arrest with resuscitation (Martin et al., 1997; Brambrink et al., 1999), followed by survival with normothermia for 5 minutes (n=4), 3 hours (n=4), or 6 hours (n=4), or with hypothermia for 3 hours (n=4). Controls were age- and time-matched sham piglets survived under normothermic conditions for 5 minutes, 3 hours, or 6 hours (n = 2 per group) or hypothermic conditions (n =4). Piglets were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneal), intubated and mechanically ventilated with a pressure-controlled ventilator to maintain normoxia (arterial partial pressure of oxygen [PaO2] of 85–100 mmHg), normocarbia (arterial partial pressure of carbon dioxide [PaCO2] of 35–45 mmHg), and acid-base balance (arterial pH 7.35–7.45). Animals were kept normothermic (~ 38.5°C, rectal) throughout the surgical procedure via heated blankets and overhead lamps. Using aseptic surgical techniques, a sterile femoral catheter was inserted into the descending aorta and inferior vena cava through the right groin, tunneled subcutaneously and secured to a right flank exit incision, where long-term access was obtainable. Surgical sites were closed with sterile sutures. Intravenous fluids (0.45% saline or 5% dextrose in 0.45% saline) were administered continuously at 10 ml/hour, adjusting the rate to maintain euvolemia (stable urine output) and normoglycemia (arterial glucose 45–90 mg/dl). The animals received intravenous fentanyl (10 μg/kg) and pancuronium (0.3 mg/kg) during surgical preparation. Fentanyl was repeated every 2 hours as needed for evidence of pain (increased blood pressure or heart rate, or hoof withdrawal), and pancuronium was given as needed for adequate ventilation. Mean arterial blood pressure, heart rate and electrocardiogram were monitored continuously. Ventilation was provided by a neonatal positive pressure ventilator and monitored continuously via clinical assessment and end tidal CO2 values. Arterial blood samples were obtained for measurements of PaCO2, pH, arterial oxygen saturation, hemoglobin concentration, and glucose concentration.
Hypoxia was induced by decreasing the inspired oxygen concentration to 10% for 30 minutes. Hypoxia was confirmed by an arterial blood sample with a PaO2 of ~20–25 mmHg, and an O2 saturation of ~35%. Subsequently, piglets received 5 minutes of room air ventilation (O2 saturation ~80–90%), followed by 7 minutes of airway occlusion (clamping the endotrachael tube, O2 saturation of ~5–15%). At ~1 minute of asphyxia, piglets had a diving reflex (heart rate drops to at least half of baseline value and arterial pressure briefly increases) followed by isoelectric electroencephalographic recording. As airway occlusion is prolonged to 7 minutes, circulatory arrest occurs (arterial blood pressure gradually falls, bradycardia becomes severe, and arterial pulse pressure decreases and sometimes disappears). Piglets were resuscitated by restoring mechanical ventilation with 100% O2, manual chest compressions, and intravenous epinephrine (0.1 mg/kg) and sodium bicarbonate (1 mEq/kg) that were administered until return of spontaneous circulation (ROSC), usually within 2–3 minutes, defined as mean arterial blood pressure ≥ 60 mmHg. Defibrillation (2–5 joules/kg) was performed if ventricular fibrillation occurred. Previous work showed that 7 minutes of asphyxia without the preceding 30 minutes of hypoxia did not produce neuronal injury. The intervening period of 5 minutes of room air ventilation between hypoxia and asphyxia was found to be essential for achieving a reasonable rate of successful cardiac resuscitation. For those piglets surviving longer than 2 hours after resuscitation, they were maintained on continuous intravenous infusions of fentanyl (15–40 μg/kg/hr) and pancuronium (0.3–0.6 mg/kg/hr) throughout the period of intubation.
Some piglets were made hypothermic for 3 hours after ROSC. At 5 minutes of ROSC, cooling was commenced by applying water-circulating blankets (temperature 10°C) underneath the supine piglet. Ice packs were placed around the head and torso. As rectal temperature reached 36°C and 35°C, the ice was removed from the head and torso, respectively. Target temperature was achieved in approximately 30 minutes and maintained by adjusting the temperature of the circulating water blanket. Previous work demonstrated that brain temperature tracked rectal temperature within 0.2°C (Agnew et al., 2003). Mild hypothermia (34°C; normal = 38.5–39.5°C) was chosen rather than more severe hypothermia for a variety of reasons. This degree of hypothermia is easier to achieve and maintain in an intensive care unit setting and can provide significant neuroprotection in this model (Agnew et al., 2003). Moreover, studies in piglet show that cerebral oxygen consumption is very sensitive to temperature because cerebral oxygen consumption can decrease 50% when body temperature was reduced to only 33°C (Busija and Leffler, 1987). Thus, mild hypothermia should be sufficient to reduce demand for ATP in the brain without requiring deep hypothermia that could compromise other organ systems. Piglets undergoing the normothermic recovery protocol had their rectal temperature controlled at 38.5°C – 39.5°C with the use of a warming blanket and overhead heating lamps as necessary. Normothermic piglets were treated in the same manner as the hypothermic piglets with regard to the use of sedation, paralytics, ventilatory control and postoperative care.
2.2. Tissue harvesting and fractionation
To obtain fresh brain tissue for assays, piglets were anesthetized deeply with sodium pentobarbital (65 mg/kg, intraperitoneal) and exsanguinated with ice-cold phosphate-buffered saline (PBS). Brains were removed rapidly and placed on wet ice. The cerebrum was transected midsagittally, and the hemispheres were subdissected to obtain samples from the striatum (including putamen, caudate, internal capsule, nucleus accumbens). These samples were flash-frozen in cold isopentane and stored at −70°C until used for extractions. From the right hemisphere of each animal, a 5 mm brain slab containing the striatum was immersion fixed in 5% acrolein and processed for paraffin histology and quantification of neuronal damage. Brain samples from sham controls and HI piglets were excised and fixed identically. The fixation of thin brain slabs immersed in acrolein is rapid and yields histological preparations with minimal artifact seen by light microscopy (Fig. 1A, sham; Martin et al., 1991). The number of neurons in the putamen with ischemic damage was counted in 10 μm-thick hematoxylin & eosin (H&E) stained sections. Profile counting, rather than stereological methods, was used because we did not fulfill a systematic random sampling design at the inception of the study. In sections that were matched neuroanatomically (one at a level anterior to the decussation of the anterior commissure with the descending fornical columns, and a second anterior to the fornical columns at the level of the septum), ischemic and nondamaged neuronal profiles were counted in six nonoverlapping microscipic fields of the putamen at ×1000 magnification. The fields were selected randomly in a raster pattern starting in the dorsolateral region of the putamen and ending in the ventral putamen. Only cells with discernible nuclei (either normal or pyknotic), identified by careful up-and-down focusing in the z-axis), were counted. The twelve values in each piglet were averaged to obtain a single values fro each animal.
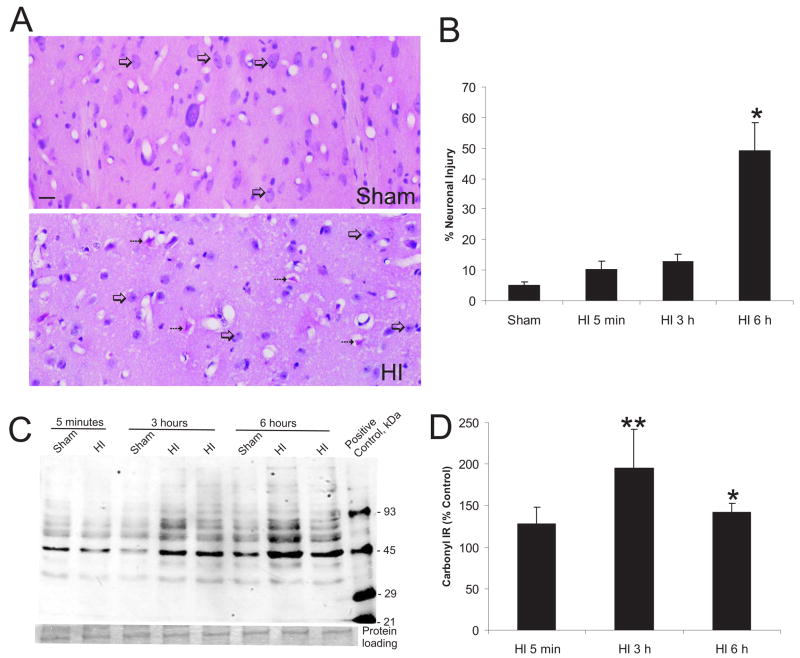
Figure 1.
Ischemic cytopathology in striatum after asphyxic cardiac arrest in piglet develops between 3 and 6 hours but oxidative stress emerges much more rapidly.
A. H&E-staining of piglet forebrain sections shows that ~50% of striatal neurons have ischemic cytopathology (bottom, hatched arrows) at 6 hours after ROSC under normothermic conditions. Neurons appearing normal are round with a non-condensed nucleus and a discrete nucleolus (bottom, open arrows). Time-matched sham controls have mostly normal appearing striatal neurons (top, open arrows). Scale bar = 20 μm. B. Counts (mean ± SEM) of striatal neurons with ischemic cytopathology in piglet at 5 minutes (n=4), 3 hours (n=4), and 6 hours (n=4) after HI compared to anesthetized, instrumented sham controls (n=6). Sham value is derived from piglets survived for 5 minutes, 3 hours, and 6 hours (n=2/group). Asterisk denotes significant difference (p < 0.05) from sham. C. Immunoblot detection of carbonyl-modified soluble proteins in piglets after HI. Representative HI piglets and time-matched sham controls are shown. Striatal soluble fractions (10 μg) were first derivatized, then subjected to SDS-PAGE and electrophoretically transferred to nitrocellulose membranes (see methods for detailed description). Carbonyl-modified molecular weight standards were used as a positive control (far right lane) and the Mr is shown in kDa. Protein loading seen by Ponceau S staining of membrane is shown below. D. Quantification of the optical density of carbonyl immunoreactivity of soluble proteins in the 50–60 kDa range. Values (mean ± sem) presented as percentage of change from sham-time matched controls for each group (5 minute, 3 hour, or 6 hour survivors). Single asterisk denotes significant difference (p < 0.05). Double asterisk denotes significant difference (p < 0.01).
Brain tissue subcellular fractionation was done as described (Martin et al., 2000). Striatal samples (0.4 – 0.6 g from each piglet) were homogenized with a Brinkman Polytron in ice-cold homogenization buffer (20 mM Tris-HCl, pH 7.4, with 10% sucrose, 1 mM EDTA, 5 mM EGTA, 20 U/ml Trayslol, 20 mg/ml leupeptin, 20 mg/ml antipain, 20 mg/ml pepstatin, 20 mg/ml chymostatin, 0.1 mM phenylmethylsulfonyl fluoride, and 10 mM benzamidine). Crude homogenates were centrifuged at 1,000 gav for 10 minutes at 4°C. The supernatant (denoted, S1 fraction) was then centrifuged at 114,000 gav for 20 minutes. The resulting supernatant (S2 soluble, cytosolic-enriched fraction) was collected, and the pellet (P2, plasma membrane- and synapse-enriched fraction) was washed in homogenization buffer (without sucrose) three times by resuspension, each followed by centrifugation at 114,000 gav for 20 minutes. The washed membrane fraction was resuspended fully in this buffer supplemented with 20% (weight/volume) glycerol. This subcellular fractionation method has been validated (Martin et al., 2000). Protein concentrations in soluble and membrane fractions were determined by a Bio-Rad protein assay. Samples were stored at −70°C.
2.3. Immunoblotting
Frozen striatal membrane and soluble fractions were thawed to 4°C, and samples (10–50 μg of total protein) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10–12% gels and transferred electrophoretically to nitrocellulose membranes. To confirm equal loading and to verify uniform protein transfer, gels were stained with Coomassie blue, and nitrocellulose membranes were stained briefly with Ponceau S. Blots were washed with 50 mM Tris-buffered saline (TBS), and subsequently blocked in 2.5% nonfat milk in 50 mM TBS/0.1% Tween-20, or in 1% bovine serum albumin in 50 mM TBS/0.1% Tween-20. Membranes were then incubated overnight at 4°C with primary antibody. To identify activation of the NMDA receptor through changes in the phosphorylation status of NR1, we used anti-phosphorylated serine-890 NR1 and anti-phosphorylated serine-897 NR1 (0.4 μg/ml) rabbit polyclonal affinity-purified antibodies (provided by Dr. Rick Huganir, JHMI). To identify NR1 protein independent of phosphorylation state we used a subunit-specific antibody (Pharmingen) at a concentration of 0.2 μg/ml. The specificities of these NR1 antibodies in piglet brain tissue have been characterized in detail, along with validation of phosphorylation state dependence determined by enzymatic dephosphorylation and western blotting (Guerguerian et al., 2002). nNOS was detected with a mouse monoclonal antibody (Transduction Laboratories) at a concentration of 0.1 μg/ml. After primary antibody incubation, membranes were washed in TBS and incubated for 1 hour at room temperature with Bio-Rad goat anti-mouse IgG or anti-rabbit IgG conjugated to horseradish peroxidase (0.2 μg/ml in blocking buffer). Blots were then washed again in blocking buffer and then TBS; immunoreactive proteins were visualized with an Amersham or Pierce enhanced chemiluminescence (ECL) detection system, and membranes were exposed to radiographic film.
Protein levels were quantified densitometrically using a PC Adobe Photoshop program, and analyzed using Signal Analytics IP Lab Gel software or IMAGE software. Protein levels were expressed as a percentage of control values by comparing the immunodensity of the protein band (average integration of density and area of immunoreactivity) to the immunodensity of the same band in the control lane of the same blot. Normalization of protein immunoreactivity was done by measuring protein bands in either the actual Ponceau S stained nitrocellulose membranes or in the Coomassie stained gels after transfer, whereby individual bands of equivalent molecular weight were scanned and quantified via optical density readings. These values served as denominators for values of immunoreactive proteins of interest from the x-ray film.
2.4. Protein oxidation assay
To detect oxidative modification of proteins in the piglet striatum after HI we used an OxyBlot protein oxidation detection kit (Intergen, Purchase, NY) for carbonyl groups (aldehydes and ketones). Aliquots of equal amounts of protein (50 μg) were denatured with 10% SDS, then derivatized to 2–4 dinitrophenylhydrazone (DNP) by a reaction with 2–4 dinitrophenylhydrazine (DNPH) for exactly five minutes. This reaction allows for a chemical conjugation of the DNPH to the carbonyl group of the protein side chain to create a hydrazone moiety that can be immunodetected. The reaction was quenched with a neutralizing solution (6% SDS), and a characteristic color change to brown followed. Samples were then placed on ice, and loaded onto 10% gels for SDS-PAGE. Proteins with conjugated DNP residues where detected with polyclonal rabbit antibody DNP used at a concentration of 1:150. Proteins were then visualized goat anti-rabbit HRP-conjugated antibody and ECL.
2.5. Lipid peroxidation assays
Lipid peroxidation is another potent source of oxidative stress, with a detectable byproduct, 4-hydroxy-2-nonenal (4-HNE). Subcellular fractions were evaluated with mouse monoclonal antibodies (at a concentration of 5 μg/ml) to 4-HNE (OXIS, Portland, Oregon) identifying proteins altered via lipid peroxidation. A colorimetric assay for lipid peroxidation (Calbiochem) was used for measurements of lipid peroxidation byproducts, malondialdehyde (MDA) and 4-HNE, in homogenates of control striatum (n =2) and HI piglet (n = 3 per time point) striatum at 5 minutes, 3 hours, or 6 hours after reperfusion. These two byproducts are formed from the peroxidation of polyunsaturated fatty acids and related esters. This assay uses a chromogenic reagent, R1 (10.3 mM N-methyl-2-phenylindole, in acetronitrile), which reacts with MDA and 4-HNE at 45°C. Chemical conjugation of 2 molecules of this reagent with one molecule of either MDA or 4-HNE, produces a stable chromophore with a maximal absorbance of 586 nm. Concentrations of purified MDA and 4-HNE were prepared freshly for the experiment by mixing standard solutions of 100 μM in increments to establish standard curves from 0–5 μM, and 0–200 μM. Samples (500–1000 μg of protein) of membrane and soluble fractions were reacted with R1 at 45°C. Absorbance was measured with a spectrophotometer (Pharmacia) at 586 nm. Levels of lipid peroxidation byproducts, 4-HNE and MDA were based on results of triplicate experiments (increasing sample protein concentrations from 50 to 500 to 1000 μg), and were expressed as a percentage of sham, time-matched control.
2.6. Data and statistical analyses
All data are presented as mean ± SEM. The level of statistical significance was st at p < 0.05. The percent neuronal damage in the striatum at 5 minutes 3 hours and 6 hours after HI, as assessed by H&E staining, was compared to that in controls with the Newman-Keuls test. Statistical analysis for all Western blot data was performed using results normalized as a percentage of a sham-time matched control. Therefore, optical densities from each animal (survived for 5 minutes, 3 hours, or 6 hours) were compared to a time matched sham of the same experiment (from the same gel). Differences between the three survival times (5 minutes, 3 hours, 6 hours) were compared via a one-way analysis of variance (ANOVA). With a significant F value, a post hoc analysis with Neuman-Keuls multiple range test was performed to determine differences between groups. When assessing differences between hypothermic and normothermic three-hour survivor Western blot data, a t test for independent samples was used.
3. Results
3.1. Prominent striatal neuron degeneration emerges between three and six hours after HI in piglets
The evolution of striatal neuron damage was assessed by counting damaged neurons in H&E-stained sections of putamen. By light microscopy, neuronal damage was defined as the presence of eosinophilic staining, cell body shrinkage, and formation of pericellular and perivascular vacuolar spaces (Fig. 1A). Sham controls had no vacuolar changes within the neuropil, and the extracellular matrix was apposed discretely to vessels, neurons, and glia. Staining patterns in sham piglets survived under normothermic conditions for 5 minutes, 3 hours, or 6 hours were similar. Baseline damage in sham piglets due to experimental procedures was ~5% (Fig. 1B). Neuronal damage was not elevated significantly at 5 minutes or at 3 hours after HI, with only 10.5 ± 2.3%, and 13.04 ± 2.1% of striatal neurons showing cytopathology (Fig. 1B). However, piglets had prominent injury to striatal neurons at 6 hours after HI, as 49.2 ± 9.1% of the principal neurons displayed ischemic pathology (Fig. 1).
3.2. Oxidative damage in the form of carbonyl modification of striatal proteins occurs rapidly after HI
Oxidative modification of proteins is a cause of cellular dysfunction. The presence of protein carbonyl groups, a hallmark of the oxidative alteration of proteins, was identified by oxyblot analysis. Significant oxidative damage to striatal soluble proteins was present at 3 hours after HI (Fig. 1C,D). Proteins at the size of 50 kDa and 60–90 kDa appeared to be prominent targets of oxidative stress, although the identities of these proteins are not yet known. Carbonyl formation on ~50 kDa proteins was significantly elevated at 3 hours (196 ± 46% of controls) and at 6 hours (142 ± 11% of controls). Likewise, carbonyl formation on ~60–90 kDa proteins was increased significantly at 6 hours (142 ± 23% of control) after resuscitation.
In contrast lipid peroxidation products did not accumulate in the striatum of HI piglets. Immunodensity for 4-HNE in the soluble striatal protein fractions after HI did not differ from sham time-matched controls at 5 minutes (112% of control), 3 hours (99% of control) and 6 hours (103% of control). Lipid peroxidation, as determined colorimetrically through detection of MDA and 4-HNE, in HI piglet striatum was not different from sham piglets at 5 minutes (98% of control), 3 hours (105% of control), or 6 hours (101% of control).
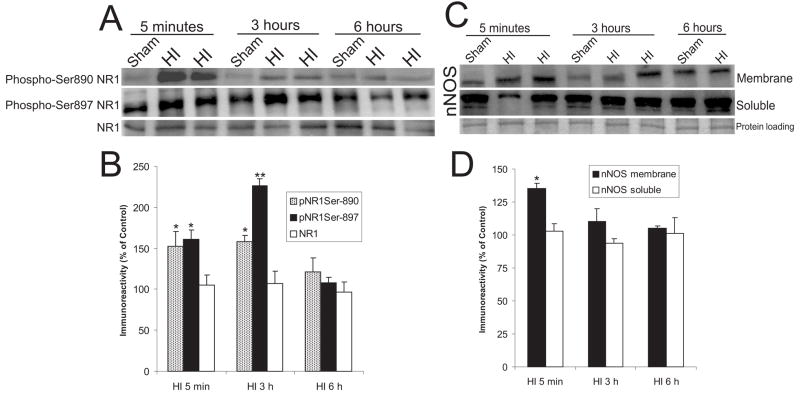
3.3. NMDA receptor phosphorylation is elevated early after HI
The involvement of excitotoxicity in HI piglets was assessed by the presence of NMDA glutamate receptor phosphorylation. Subunit phosphorylation was determined using phosphorylation state-specific antibodies to the NR1 subunit which is required for receptor function (Prybylowski and Wenthold, 2004). Two serine residues of NR1, amino acids 890 and 897, are targets for PKC- and PKA-mediated phosphorylation, respectively (Tingley et al., 1997). In sham control piglet striatum, as in rat forebrain (Cheung et al., 2001), basal phosphorylation of serine-897 was greater than that of serine-890 (Fig. 2A). In HI piglets, compared to their sham, time-matched controls, NR1 phosphorylation at serine890 was increased significantly at 5 minutes (152 ± 18%) and 3 hours (158 ± 8%) after ROSC, but by 6 hours after HI, values were no longer significantly elevated above control (121 ± 17%) (Fig. 2A,B). NR1 phosphorylation of serine-897 after HI was significantly increased by 5 minutes after resuscitation (161 ±10%), and was further increased at three hours (226 ± 9%) (Fig. 2B), but values returned to sham levels by six hours after HI (Fig. 2A, B).
Figure 2.
NMDA receptor phosphorylation and nNOS recruitment to the synaptic membrane occurs within 5 minutes of recovery from asphyxic cardiac arrest in piglets.
A. Immunoblot detection of NR1 phospho-serine-890, NR1 phospho-serine-897, and NR1 (independent of phosphorylation state) in piglet striatum after HI. Striatal plasma/synaptic membrane protein fractions (50 μg) were subjected to SDS-PAGE and electrophoretically transferred to nitrocellulose membranes, and immunoblotted with the site-specific antibodies to phosphorylated serine-890 NR1, serine-897, and NR1. Representative HI piglets and time-matched sham controls are shown. B. Graph of the optical density of immunoreactivities for phosphorylated serine-890 NR1, serine-897, and NR1 on immunoblots. Data are presented as percentage of change from sham-time matched controls for each group (5 minute, 3 hour, or 6 hour survivors). Values are mean ± sem. Single asterisk denotes significant difference (p < 0.01). Double asterisk denotes significant difference (p < 0.001). C. Immunoblots of nNOS in plasma/synaptic membrane and soluble protein fractions of piglet striatum after HI. Representative HI piglets and time-matched sham controls are shown. Typical verification of equivalent protein loading as seen by Ponceau S staining of nitrocellulose membrane is shown soluble protein blot. Similar results were seen for the membrane proteins. D. Graph of the optical density of nNOS immunoreactivity on immunoblots. Data are presented as percentage of change from sham-time matched controls for each group (5 minute, 3 hour, or 6 hour survivors). Values are mean ± sem. Single asterisk denotes significant difference (p < 0.05).
3.4. NOS is recruited to the plasma membrane early after HI
nNOS can be recruited to the membrane via postsynaptic density 95 and, through the interaction of calcium dependent calmodulin, results in NO production (Sattler et al., 1999). Soluble and membrane piglet striatal protein (50 μg) was probed with an antibody to nNOS (Fig. 2C). nNOS in the membrane fraction at 5 minutes after HI was significantly increased by 35 ± 4% of control (Fig. 2D). Values returned to sham levels by 3 and 6 hours (Fig. 2D). No significant changes in nNOS levels were seen in the soluble protein fraction where nNOS is constitutively enriched (Fig. 2C,D). The recruitment of nNOS to the membrane is a transient early event after HI.
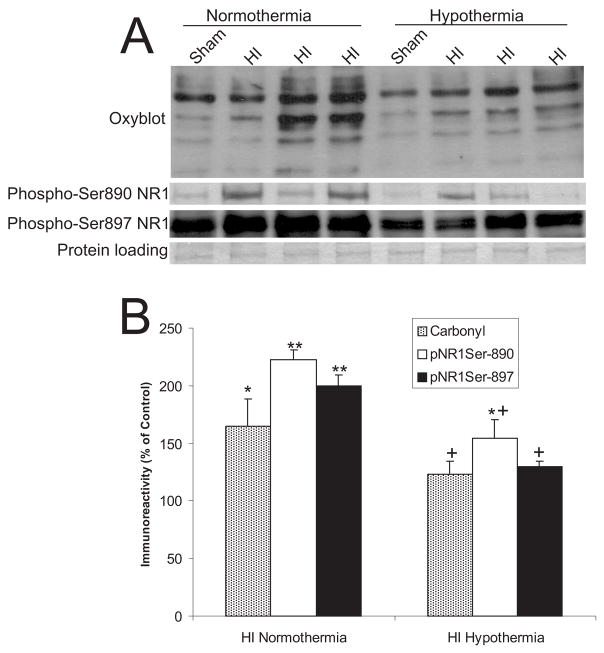
3.5. Postasphyxic hypothermia decreases oxidative stress and NR1 phosphorylation in HI piglets
We examined the effects of hypothermia on brain oxidative stress and NR1 phosphorylation in sham piglets and examined if hypothermia after resuscitation prevents increases in protein carbonyl formation and NMDA phosphorylation seen at 3 hours of normothermic reperfusion. In sham piglets, mild whole body hypothermia lowered baseline oxidative damage and NR1 phosphorylation in striatum (Fig. 3A). Postasphyxic hypothermia significantly attenuated the increases in protein carbonyls and NR1 phosphorylation in piglet striatum (Fig. 3A,B).
Figure 3.
Hypothermia attenuates the oxidative damage and the NMDA receptor phosphorylation.
A. Immunoblot detection of carbonyl-modified proteins (Oxyblot), NR1 phospho-Ser890, and NR1 phospho-Ser897 in striatum of piglets subjected to asphyxic cardiac arrest. Representative HI piglets with normothermic or hypothermic (implemented at 5 minutes after ROSC) recovery for 3 hours and time-matched sham controls are shown. Equivalent protein loading was verified by Ponceau S staining of nitrocellulose membranes (shown is the membrane for phospho-Ser897 NR1; membranes for other blots were similar). B. Graph of the optical density of immunoreactivities for carbonyl-modified proteins, NR1 phospho-Ser890 and NR1 phospho-Ser897 on immunoblots. Data are presented as percentage of sham-time matched controls. Values are mean ± sem. Single asterisk denotes significant difference (p < 0.05) from respective normothermic or hypothermic sham control. Double asterisk denotes significant difference (p < 0.01) from respective normothermic or hypothermic sham control. Plus denotes significant difference (p < 0.05) from normothermic HI piglets.
4. Discussion
This study reveals several new findings important to the understanding of the evolution and mechanisms of striatal neuron degeneration and the mechanisms of hypothermic neuroprotection in a clinically relevant animal model of pediatric HIE. We show that pathophysiological mechanisms of striatal injury engage rapidly. Robust ischemic cytopathology in putaminal neurons emerges between 3 and 6 hours after HI in male piglets. Neuropathological analysis of striatum of asphyxic term humans at 1 day of life shows a similar pattern of neuronal necrosis (Meng et al., 1997). Neuroimaging studies of infants at 24 hours of life after perinatal asphyxia have revealed neuronal integrity abnormalities in basal ganglia by magnetic resonance spectroscopy (Barkovich et al., 2001). This striatal neuron injury in piglet is unlikely to be reversible because its timing is consistent with data showing significant accumulation of DNA double-strand breaks by 3 hours of recovery and damage to 80% of putaminal neurons by 24 hours (Martin et al., 2000). Furthermore, elevated NMDA receptor phosphorylation and prominent oxidative stress are present by 5 minutes after ROSC and are sustained through 3 hours recovery. The early NMDA receptor phosphorylation coincides with rapid recruitment of nNOS to the synaptic/plasma membrane. Lastly, mild whole body hypothermia, which confers profound and sustained protection of striatal neurons in this model (Agnew et al., 2003), attenuates the post-HI NMDA receptor phosphorylation and the oxidative damage.
The neonatal brain is very vulnerable to oxidative stress (McQuillen and Ferriero, 2004). Our study reveals that oxidative stress mechanisms of neuronal death in the newborn striatum are engaged much more rapidly after or during HI episodes than realized previously (Martin et al., 2000). We found that at 5 minutes of ROSC there is already severe oxidative damage to striatal proteins that precedes the frank neurodegeneration seen at 6 hours of recovery and the accumulation of DNA double-strand breaks seen initially at 3 hours of recovery (Martin et al., 2000). The type of oxidative damage was selectively in the form of protein carbonyls. Markers for lipid peroxidation were not elevated early after HI as determined by two different methods for detecting MDA and 4-HNE. Carbonyl groups (aldehydes and ketones) are introduced into proteins by oxidative reactions of amino acid side chains of glutamate, aspartate, lysine, arginine, proline, and threonine with oxides of nitrogen and by metal catalyzed oxidation (Ischiropoulus and Al-Mehdi, 1995; Troncoso et al., 1995). In the HI piglet striatum, soluble cytosolic proteins accumulated high levels of carbonyl damage. The identities of these proteins are not yet known, but soluble proteins are more vulnerable to carbonyl modification than membrane-associated proteins (Mueller and Martin, unpublished observations). Cytoskeletal proteins such as neurofilaments and tau are highly susceptible to carbonyl modification due to the abundance of lysine residues (Smith et al., 1995). The major carbonyl-modified proteins that we find in HI piglet striatum could be these cytoskeletal proteins based on their size. We have shown previously that β-tubulin undergoes nitrative damage in the HI piglet striatum (Martin et al., 2000).
Pinpointing of the sources of oxidative damage induced by HI in the newborn brain could be of key importance for mechanism-based therapies (McQuillen and Ferriero, 2004). Peroxynitrite can cause dose-dependent increases in protein carbonyl formation (Ischiropoulus and Al-Mehdi, 1995). Signatures of peroxynitrite and hydroxyl radical, formed by peroxynitrite decomposition or metal catalyzed oxidation via Fenton reaction (Beckman, 1991), have been found in HI piglet striatum (Martin et al., 2000). In acidotic states there is a favored release of iron from its protein bound molecules. Free heme has the capacity to act as a catalyst to damage intracellular proteins. We have shown that the mitochondrial protein, cytochrome c, a heme containing molecule, is released into the cytosol by 6 hours after HIE in the piglet (Martin et al., 2000). In vivo, the neonatal brain accumulates higher levels of H2O2 than the adult brain after HI (Lafemina et al., 2006). H2O2 can impair mitochondrial function by inactivating complex IV (Musatov et al., 2004). We have seen inactivation of mitochondrial complex IV in piglet striatum at 3 hours after HI (Martin et al., 2000), which can cause elevated superoxide levels (Musatov et al., 2004) and drive the diffusion-limited reaction with NO to form peroxynitrite (Beckman, 1991). NO production is linked to NMDA receptor-dependent excitotoxicity through the coupling of NR2 subunits, postsynaptic density proteins 93 (PSD-93) and 95 (PSD-95), and nNOS (Sattler et al., 1998). Interestingly, the protein levels of nNOS and PSD-95 have been shown to be elevated in neonatal mouse brain at 24 hours after HI (Jiang et al., 2007). Thus, the recruitment of nNOS to the plasma membrane in combination with NMDA receptor phosphorylation occurring by 5 minutes after ROSC in piglet might be meaningful in this context of peroxynitrite formation. Our data here reinforce the concept that the neonatal brain is very vulnerable to oxidative stress (McQuillen and Ferriero, 2004) and reveal new insight by showing that the evolution of striatal neuron degeneration in neonatal HIE should be studied on a time scale of minutes rather than hours.
High levels of NMDA receptors are expressed on the principal striatal neurons (i.e., medium-sized spiny neurons), which receive abundant glutamatergic corticostriatal inputs (Gerfen, 2000). The developing human striatum also has high levels of NMDA receptors (Lee and Choi, 1992). We found that the NMDA receptor has elevated levels of phosphorylation early after HI. Phosphorylation of NR1Ser-897 was increased significantly at 5 minutes and 3 hours after HI, when few damaged striatal neurons were seen by light microscopy, but then values returned to near-baseline by 6 hours after HI, a time when significant neurodegeneration was present. Similarly, phosphorylation of NR1Ser-890 was increased significantly at 5 minutes of ROSC and remained elevated at 3 hours, although not to the same degree as NR1Ser-897. Although these data cannot reveal whether enhanced NR1 phosphorylation begins in the hypoxic period, asphyxic period, or immediately at the time of ROSC, they show that phosphorylation of this obligatory subunit is increased by 5 minutes after ROSC and is sustained for at least a 3 hour period after reoxygenation. Moreover, these changes in NR1 phosphorylation are not due simply to increased levels of NR1 protein as shown here and before over a longer time course (Guerguerian, et al., 2002). Studies on adult rat have also shown increased phosphoylation of NR1 after transient global ischemia (Cheung et al., 2001; Besshoh et al., 2005). However, in postnatal day 7 rats, decreases in NR1 phosphorylation were found after HI using the Rice-Vannucci model (Vij et al., 2005). These major differences in the results of our study and this previous study (Vij et al., 2005) could be explained by differences in the types of neuropathology seen in HI piglets (selective vulnerability) and rat pups (focal infarct), regional differences in dopaminergic innervation or dopamine receptor expression (Yang et al., 2007), and differences in the contributions of neuronal necrosis and apoptosis to the HIE (Martin et al., 2000).
An assumption made here is that NR1 phosphorylation in the HI piglet striatum relates to its ion channel activity. Specifically, increased NR1 phosphorylation in HI piglet striatum may signify increased activation or potentiation of NMDA receptors and that this process is relevant to the pathophysiology to striatal neuron death. Data show that this assumption has a foundation. PKA-mediated phosphorylation of NR1Ser-897 in hippocampal slices is induced by synaptic activation (Tingley et al., 1997). Electrophysiological studies of medium-sized striatal neurons show that phosphorylation of NR1Ser-897 by PKA enhances NMDA receptor currents through a dopamine D1 receptor and cAMP signaling pathway (Flores-Hernandez et al., 2002; Maldve et al., 2002) and promotes Ca2+ influx through a similar pathway (Dudman et al., 2003; Skeberdis et al., 2006). PKC-dependent phosphorylation of NR1Ser-890 enhances the conductance of native NMDA receptors in spinal cord and hippocampal neurons (Chen and Huang, 1992). NMDA channel open probability is decreased by calmodulin binding to NR1, but NR1Ser-890 phosphorylation inhibits calmodulin binding (Ehlers et al., 1995), demonstrating that NR1 phosphorylation modulates Ca2+-dependent inactivation of NMDA receptors. Interestingly, in vitro studies show that hypoxia increases NMDA receptor Ca2+ flux in cortical neurons through a mechanism involving PKC-mediated phosphorylation of NR1Ser-896 (Bickler et al., 2004). Thus, in HI piglet striatum NMDA receptors could be activated excessively in the hypoxia phase and remain in an activated state for a considerable period of time allowing for Ca2+-dependent excitotoxicity to evolve.
If our data signify an NMDA receptor-dependent mechanism of neuronal degeneration caused by perinatal HI, then pharmacological modulation of NMDA receptor activity should have therapeutic benefit in pediatric HI animal models. This is indeed the case. The NMDA receptor antagonist MK-801 protects against brain injury in neonatal rat following HI (McDonald et al., 1987; Ford et al., 1989), but not in a severe HI model with piglets (LeBlanc et al., 1991). Agmatine (Feng et al., 2002), and endogenous antagonist of the NMDA receptor and a NOS inhibitor, and memantine (Volbracht et al., 2006), a low-affinity uncompetitive NMDA receptor antagonist, also protect against HI injury in neonatal rat. These studies support indirectly the idea that NMDA receptor ion channel activity is increased after HI and that its activation contributes to the neurodegeneration. Our observations are novel because they extend the excitotoxicity theory of HI brain damage by showing that the phosphorylation of NMDA receptor, and recruitment of nNOS to the membrane, precedes light microscopic morphological evidence of brain injury. Thus, this new data and previous data (Guerguerian, et al., 2002) in a large animal model support the idea that the NMDA receptor should still be considered a rational target for mechanism-based neuroprotection in the perinatal brain.
We have not directly determined if the increased NR1 phosphorylation observed early after HI is the result of increased protein kinase activity, decreased phosphatase activity, or both. High levels of dopamine receptors are expressed in the medium-sized spiny neurons, which receive extensive dopaminergic nigrostriatal inputs (Gerfen, 2000). Another substrate for PKA, in addition to NR1Ser-897, is dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa (DARPP-32). PKA-mediated phosphorylation of DARPP-32 on threonine-34 causes its activation as a potent inhibitor of protein phosphatase-1 (Hemmings et al., 1984). DARPP-32 is enriched in the principal medium spiny neurons of the striatum (Walaas et al., 1983). PKA is activated in striatal neurons through increases in cAMP generated by adenyl cyclase that is stimulated by dopamine acting on the D1 dopamine receptor. Thus striatal dopamine receptors could potentiate NMDA receptor activity through DARPP-32-mediated inhibition of protein phosphatase-1. This idea is consistent with the finding that dopamine receptor antagonists, particularly D1 receptor antagonist, attenuate DARPP-32 phosphorylation, Na, K ATPase phosphorylation, and NR1Ser-897 phosphorylation and protect against striatal neuron degeneration in HI piglets (Yang et al., 2007).
We found that mild, whole body hypothermia implemented at 5 minutes after ROSC attenuates both the NMDA receptor phosphorylation (at two serine sites) and the carbonyl formation seen at 3 hours after recovery. This finding suggests that the NMDA receptor phosphorylation is linked mechanistically to the oxidative stress and that the changes are relevant pathophysiologically because hypothermia results in profound and sustained neuroprotection in the HI piglet striatum (Agnew et al., 2003). Our results are important because the mechanisms underlying hypothermic neuroprotection are not understood completely. An understanding of the mechanisms of hypothermic protection of the brain and the effects of hypothermia on brain is needed because induced hypothermia is being used on infants in clinical trials (Gluckman et al., 2005; Shankaran et al., 2005), and there are major knowledge gaps with respect to the application of hypothermia for HIE and its effects on brain (Higgins, 2005). A previous study on piglets analyzed by microdialysis reported that hypothermia reduces release of glutamate and aspartate and the formation of NO in cerebral cortex induced by hypoxia alone (Thoresen et al., 1997). This finding is in keeping with our hypothesis that NMDA receptor-mediated excitotoxicity through NOS activation and peroxynitrite formation has a major role in the neurodegeneration in HI piglet striatum (Martin et al., 2000) and that hypothermia attenuates some or all components of this process. This work is encouraging because its reveals that hypothermia, if implemented soon enough, can protect against the fulminantly occurring neuronal necrosis in piglet basal ganglia (Agnew et al., 2003) possibly through anti-excitotoxic, anti-oxidative stress mechanisms.
Acknowledgments
This work was supported by NINDS grants NS020020, NS034100, and NS052098.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa
- H&E
hematoxylin & eosin
- HI
hypoxia-ischemia
- HIE
hypoxic-ischemic encephalopathy
- 4-HNE
4-hydroxy-2-nonenal
- MDA
malondialdehyde
- NMDA
N-methyl-D-aspartate
- nNOS
nitric oxide synthase
- NO
nitric oxide
- PKA
protein kinase A
- PKC
protein kinase C
- PSD-95
postsynaptic density protein-95
- ROSC
return of spontaneous circulation
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SOD1
superoxide dismutase-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnew DM, Koehler RC, Guerguerian AM, Shaffner DH, Traystman RJ, Martin LJ, Ichord RN. Hypothermia for 24 hours after asphyxic cardiac arrest in piglets provides striatal neuroprotection that is sustained 10 days after rewarming. Pediatr Res. 2003;54:1–10. doi: 10.1203/01.PDR.0000072783.22373.FF. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Westmark KD, Bedi HS, Partridge JC, Ferriero DM, Vigneron DB. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. Am J Neuroradiol. 2001;22:1786–1794. [PMC free article] [PubMed] [Google Scholar]
- Beckman JS. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991;15:53–59. [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS, Ferriero DM. Hypoxia increases calcium flux through cortical neuron glutamate receptors via protein kinase C. J Neurochem. 2004;88:878–884. doi: 10.1046/j.1471-4159.2003.02203.x. [DOI] [PubMed] [Google Scholar]
- Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res. 1998;43:738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Brambrink AM, Martin LJ, Hanley DF, Becker KJ, Koehler RC, Traystman RJ. Effects of the AMPA receptor antagonist NBQX on the outcome of newborn pigs after asphyxic cardiac arrest. J Cereb Blood Flow Metab. 1999;19:927–938. doi: 10.1097/00004647-199908000-00012. [DOI] [PubMed] [Google Scholar]
- Busija DW, Leffler CW. Hypothermia reduces cerebral metabolic rate and cerebral blood flow in newborn pigs. Am J Physiol. 1987;252:H869–H873. doi: 10.1152/ajpheart.1987.253.4.H869. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LYM. Protein kinase C reduces Mg2+ block of NMDA–receptor channels as a mechanism of modulation. Nature. 1992;365:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Cheung HH, Teves L, Wallace MC, Gurd JW. Increased phosphorylation of the NR1 subunit of the NMDA receptor following cerebral ischemia. J Neurochem. 2001;78:1179–1182. doi: 10.1046/j.1471-4159.2001.0780051179.x. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87:922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- Feng Y, Piletz JE, LeBlanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2002;52:606–611. doi: 10.1203/00006450-200210000-00023. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, Holtzman DM, Black SM, Sheldon RA. Neonatal mice lacking neuronal nitric synthase are less vulnerable to hypoxic-ischemic injury. Neurobiol Dis. 1996;3:64–71. doi: 10.1006/nbdi.1996.0006. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Ford LM, Sanberg PR, Norman AB, Fogelson MH. MK-801 prevents hippocampal neurodegeneration in neonatal hypoxic-ischemic rats. Arch Neurol. 1989;46:1090–1096. doi: 10.1001/archneur.1989.00520460072016. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:S64–S70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomized trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Guerguerian AM, Brambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxia-ischemia. Brain Res Mol Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Hamrick SE, Ferriero DM. The injury response in the term newborn brain: can we neuroprotect? Curr Opin Neurol. 2003;16:147–154. doi: 10.1097/01.wco.0000063775.81810.79. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phospatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Higgins RD. Hypoxic ischemic encephalopathy and hypothermia. A critical look Obstet Gynecol. 2005;106:1385–1387. doi: 10.1097/01.AOG.0000190206.70375.b4. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Umemori H, Inoue T, Michikawa T, Kohda K, Mikoshiba K, Yamamoto T. Phosphorylation-dependent regulation of the N-methyl-D-aspartate receptors by calmodulin. J Biol Chem. 1997;272:20805–20810. doi: 10.1074/jbc.272.33.20805. [DOI] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arret Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Ischiropoulus H, Al-Mehidi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364:279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- Jiang X, Mu D, Sheldon A, Glidden DV, Ferriero DM. Neonatal hypoxia-ischemia differentially upregulates MAGUKs and associated proteins in PSD-93-deficeint mouse brain. Stroke. 2003;34:2958–2963. doi: 10.1161/01.STR.0000102560.78524.9D. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Selective vulnerability in the neonatal brain. Ann Neurol. 1998;44:155–156. doi: 10.1002/ana.410440202. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in neonatal hypoxia. Ment Retard Dev Disabil Res Rev. 2001;7:229–234. doi: 10.1002/mrdd.1032. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Nakajima W, Hagberg H. Mechanisms of hypoxic neurodegeneration in the developing brain. Neuroscientist. 2002;8:212–220. doi: 10.1177/1073858402008003007. [DOI] [PubMed] [Google Scholar]
- Lafemina MJ, Sheldon RA, Ferriero DM. Acute hypoxia-ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatr Res. 2006;59:680–683. doi: 10.1203/01.pdr.0000214891.35363.6a. [DOI] [PubMed] [Google Scholar]
- LeBlanc MH, Vig V, Smith B, Parker CC, Evans OB, Smith EE. MK-801 does not protect against hypoxic-ischemic brain injury in piglets. Stroke. 1991;22:1270–1275. doi: 10.1161/01.str.22.10.1270. [DOI] [PubMed] [Google Scholar]
- Lee H, Choi BH. Density and distribution of excitatory amino acid receptors in the developing human brain: a quantitative autoradiographic study. Exp Neurol. 1992;118:284–290. doi: 10.1016/0014-4886(92)90185-s. [DOI] [PubMed] [Google Scholar]
- Maldev RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. DARPP-32 and regulatiobn of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci. 2002;5:641–648. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- Maller AI, Hankins LL, Yeakley JW, Butler IJ. Rolandic type cerebral palsy in children as a pattern of hypoxic-ischemic injury in the full-term neonate. J Child Neurol. 1998;13:313–321. doi: 10.1177/088307389801300702. [DOI] [PubMed] [Google Scholar]
- Martin LJ. Mechanisms of brain damage in animal models of hypoxia-ischemia in newborns. In: Stevenson DK, Benitz WE, Sunshine P, editors. Fetal and Neonatal Brain Injury: Mechanisms, Management, and the Risks of Practice. 2. Oxford University Press; 2003. pp. 30–57. [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol. 1991;309:445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997a;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traystman RJ. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997b;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Price AC, Kaiser A, Agnew DM, Ichord RN, Traystman RJ. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol Dis. 2000;7:169–191. doi: 10.1006/nbdi.2000.0282. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Silverstein FS, Johnston MV. MK-801 protects the neonatal brain from hypoxic-ischemic damage. Eur J Phramacol. 1987;140:359–361. doi: 10.1016/0014-2999(87)90295-0. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Ohyu J, Takashima S. Cganges in AMPA glutamate and dopamine D2 receptors in hypoxic-ischemic basal ganglia necrosis. Pediatr Neurol. 1997;17:139–143. doi: 10.1016/s0887-8994(97)00087-8. [DOI] [PubMed] [Google Scholar]
- Musatov A, Hebert E, Carrol CA, Weintraub ST, Robinson NC. Specific modification of two tryptophans within the nuclear-encoded subunits of bovine cytochrome c oxidase by hydrogen peroxide. Biochemistry. 43:1003–1009. doi: 10.1021/bi0358925. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Wenthold RJ. N-methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J Biol Chem. 2004;279:9673–9676. doi: 10.1074/jbc.R300029200. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Smith MA, Rudnicka-Nawrot M, Richey PL, Praprotnik D, Mulvihill P, Miller CA, Sayre LM, Perry G. Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer’s disease. J Neurochem. 1995;64:2660–2666. doi: 10.1046/j.1471-4159.1995.64062660.x. [DOI] [PubMed] [Google Scholar]
- Sunshine D. Perinatal asphyxia: an overview. In: Stevenson DK, Benitz WE, Sunshine P, editors. Fetal and Neonatal Brain Injury: Mechanisms, Management, and the Risks of Practice. 2. Oxford University Press; 2003. pp. 3–29. [Google Scholar]
- Thoresen M, Satas S, Puka-Sundvall M, Whitelaw A, Hallstrom A, Loberg EM, Ungerstedt U, Hagberg H. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. NeuroReport. 1997;8:3359–3362. doi: 10.1097/00001756-199710200-00033. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Trescher WH, Ishiwa S, Johnston MV. Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev. 1997;19:326–338. doi: 10.1016/s0387-7604(97)00027-2. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Costello AC, Kim JH, Johnson GVW. Metal-catalyzed oxidation of bovine neurofilaments in vitro. Free Radic Biol Med. 1995;18:891–899. doi: 10.1016/0891-5849(94)00224-8. [DOI] [PubMed] [Google Scholar]
- Vij S, Vannucci SJ, Gurd JW. Differential effects of hypoxia-ischemia on phosphorylation of the N-methyl-D-aspartate receptor in one- and three-week-old rats. Dev Neurosci. 2005;27:211–219. doi: 10.1159/000085994. [DOI] [PubMed] [Google Scholar]
- Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23:2611–2622. doi: 10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. Saunders; Philadelphia: 1995. [Google Scholar]
- Walaas SI, Asward DW, Greengard P. A dopamine- and cyclicAMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Torbey M, Li X, Bernardy J, Golden WC, Martin LJ, Koehler RC. Dopamine receptor modulation of hypoxic-ischemic neuronal injury in striatum of newborn piglets. J Cereb Blood Flow Metabol. 2007;1:1–13. doi: 10.1038/sj.jcbfm.9600440. [DOI] [PMC free article] [PubMed] [Google Scholar]