Abstract
Microtubule inhibitors, such as vinblastine, are widely used in cancer chemotherapy. Vinblastine exerts its antitumor effect by inducing apoptosis. In KB-3 cells, we have shown previously that vinblastine activates c-Jun NH2-terminal protein kinase (JNK) and causes Bax mitochondrial translocation and activation. In this study, we sought to test the hypothesis that JNK and Bcl-xL act as positive and negative regulators, respectively, of Bax translocation. The JNK inhibitor SP600125 inhibited vinblastine-induced JNK activation and in concert inhibited Bax mitochondrial translocation, Bax oligomerization, and Bax activation. Furthermore, the JNK inhibitor blocked vinblastine-induced apoptosis. The ability of vinblastine to induce Bax translocation and the inhibitory effect of SP600125 were confirmed in cells stably expressing GFP-Bax. However, if transiently overexpressed, Bax localized to the mitochondria, and this was associated with loss of viability and subsequent cell death. If Bcl-xL was co-expressed with Bax, the cells readily tolerated Bax overexpression. Indeed, physical interaction between Bcl-xL and Bax but not Bak was demonstrated by co-immunoprecipitation. These findings provide novel insight into the role of Bax and its regulation in vinblastine-induced apoptosis.
Keywords: JNK, Bax, vinblastine, apoptosis, Bcl-xL
1. Introduction
Vinblastine is a microtubule inhibitor which is used in treating several types of cancers including Hodgkin's lymphoma and testicular and ovarian cancers (1). Vinblastine inhibits microtubule polymerization by binding to the β-subunit of tubulin dimers. The binding of vinblastine to the plus end of microtubules suppresses microtubule dynamics and causes the arrest of cells at the G2/M transition and cell death by apoptosis (2). Bcl-2 proteins play a critical role in the regulation of apoptosis (3-4). The Bcl-2 family includes anti-apoptotic proteins, such as Bcl-2, Bcl-xL, and Mcl-1, and pro-apoptotic proteins which can be divided into two subgroups, multidomain and BH3-only proteins. The two subgroups function in different ways. BH3-only proteins act as sensors of cellular damage and initiate the death process while multi-BH domain apoptotic proteins act downstream of the BH3-only proteins and as essential mediators of mitochondrial apoptosis. Most antitumor agents trigger apoptotic signaling via the mitochondrial pathway that causes release of cytochrome c and activation of a caspase cascade (5).
In healthy cells, Bax resides in an inactive state in the cytosol of cells. In response to apoptotic signals, Bax undergoes conformational changes that expose membrane-targeting domains, resulting in its translocation to mitochondrial membranes. Mitochondrial membrane-inserted Bax then undergoes further conformational changes which permits its oligomerization and channel-forming activity in the mitochondrial membrane to trigger cytochrome c release (3). Bak, which typically resides in the mitochondrial membrane, undergoes similar changes and exhibits similar functional properties, and cells deficient in both Bax and Bak are highly resistant to most apoptotic stimuli (3-4).
Recent evidence has suggested that c-Jun NH2-terminal protein kinase (JNK) may play a role in apoptosis by regulating the function of Bcl-2 proteins. For example, JNK has been suggested to regulate Bax translocation through phosphorylation of Bim (6), and in another study, JNK was reported to promote Bax translocation through phosphorylation of 14-3-3 proteins (7). JNK and other kinases have also been reported to mediate the phosphorylation of Bax and promote its mitochondrial translocation (8). We have previously shown that vinblastine treatment of KB-3 cells results in activation of JNK (9) and activation of Bax (10). In this paper, we have examined the relationship between these parameters, and present evidence that JNK acts as a positive regulator of Bax translocation and activation and that Bcl-xL acts as a key antagonist of Bax in this system.
2. Materials and methods
2.1 Materials
Antibodies to phospho-(Ser-63) c-Jun (catalog # 2772), GAPDH (catalog # 2772), Bcl-xL (catalog # 2772), and rabbit polyclonal anti-Bax (catalog # 2772) for immunoblotting were obtained from Cell Signaling Technology (Beverly, MA). Caspase 3 antibody (catalog # sc-7148) was obtained from Santa Cruz (Santa Cruz, CA); mouse monoclonal anti-Bak antibody (catalog # AM03) was from Calbiochem (San Diego, CA); antibody to PARP was from BD Biosciences (San Jose, CA); and 6A7 active Bax antibody was from Axxora (San Diego, CA). Lipofectamine (catalog # 18324012), lipofectamine PLUS reagent (catalog # 11514015) and MitoTracker Red CMXRos (catalog # M-7512) were obtained from Invitrogen (Carlsbad, CA). COX II (Complex IV) antibody was from an OXPHOS complex detection kit (catalog # MS601) from MitoSciences (Eugene, OR). Anti-mouse goat IgG agarose beads were from eBiosciences (San Diego, CA). SP600125 was obtained from Biomol (Plymouth Meeting, PA). Vinblastine and other chemicals, unless otherwise stated, were obtained from Sigma Chemical Co. (St. Louis, MO).
2.2 Cell culture and transfection
KB-3 human carcinoma cells and MCF-7 breast carcinoma cells were maintained in monolayer culture at 37°C and 5% CO2 in Dulbecco's modified Eagles' medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. The HCT116 cell line and the Bax-deficient HCT116 cell line stably transfected with GFP-Bax (11) were maintained in McCoy's 5A medium with 10% FBS. KB-3 cells transiently overexpressing GFP-Bax, HA-Bcl-xL, myc-Bax, HA-CMV, or myc-CMV were prepared by transfecting cells with 2 μg of plasmid DNA using Lipofectamine Plus reagent in serum-free DMEM. After 3 h, the transfection medium was replaced with complete medium.
2.3 Preparation of cell extracts and subcellular fractions
For whole cell extracts, cells were lysed for 30 min on ice in lysis buffer (40 mM HEPES, pH 7.4, 120 mM NaCl, 1% Triton-X-100, 1 mM EDTA, 5 mM dithiothreitol, 20 mM βglycerophosphate, 1 mM Na3VO4, 50 mM NaF, 20 βg/ml aprotinin, 50 μg/ml leupeptin, 10 μM pepstatin, 1 μM okadaic acid, and 1 mM PMSF), insoluble material was removed by centrifugation at 9,200 × g for 20 min, and protein concentration in the supernatant was determined using Bradford reagent (BioRad). Cytosolic and mitochondrial extracts were prepared using the fractionation kit from GBiosciences. Briefly, 107 cells were washed with PBS twice and then incubated with 0.5 ml of SubCell Buffer-I on ice for 10 min. The cells were lysed using a Dounce homogenizer by 20 strokes of the pestle. The lysate was transferred to a centrifuge tube, the volume adjusted to 0.7 ml, and then 0.35 ml of 3X-concentrated SubCell Buffer II was added. The sample was centrifuged at 700 × g for 10 min to pellet the nuclei and the supernatant was centrifuged at 12,000 × g for 15 min to obtain cytosol in the supernatant and a mitochondrial pellet which was resuspended in storage buffer.
To examine oligomeric forms of Bax, washed cell pellets were incubated in buffer A (10 mM HEPES, pH 7.4, 0.15 M NaCl, 1 mM EGTA, plus phosphatase and protease inhibitors, as above) containing 0.01% digitonin for 2 min at 4°C, then the crosslinking agent dithiobis(succinimidyl propionate) was added to a final concentration of 1 mM after cell permeabilization. After 10 min at 4 °C, crosslinking was quenched with 0.1 volumes of 2 M Tris-HCl (pH 7.4) and cells collected by centrifugation at 15,000 × g for 15 min. The pellet was extracted with buffer A containing 3% CHAPS for 45 min at 4°C to release membrane- and organelle-bound proteins, which were isolated in the supernatant after centrifugation at 15,000 × g for 15 min. The samples were prepared for SDS-PAGE under non-reduced conditions (without βmercaptoethanol) with heating to 70°C for 5 min.
2.4 Immunoprecipitation
To examine the activation status of Bax, cells were lysed in 0.5 ml of lysis buffer (40 mM HEPES, pH 7.4, 120 mM NaCl, 1% CHAPS, 1 mM EDTA, supplemented with protease and phosphatase inhibitors) by incubating on ice for 30 min and centrifugation at 9,200 × g for 20 min. The extracts (1 mg) were precleared with anti-mouse goat IgG agarose beads, according to the manufacturers' directions (eBiosciences), and to the supernatants were added 5 °g of mouse monoclonal antibody (6A7) to active Bax. After mixing for 1 h, anti-mouse IgG agarose beads were added for an additional 3 h. The immunoprecipitates were then washed as described previously (10) and resuspended in SDS sample buffer for 1 h at 37°C and resolved by 12.5% acrylamide SDS-PAGE and analyzed by immunoblotting.
To examine interactions of Bcl-2 protein by co-immunoprecipitation, the cells were lysed in 0.3 ml of 40 mM HEPES (pH 7.5), 0.12 M NaCl, 1% Triton X-100, 1 mM EDTA, containing phosphatase and protease inhibitors as above, and after 30 min on ice centrifuged at 9,200 × g for 20 min. The extract (1 mg) was precleared with anti-rabbit goat IgG agarose beads, according to the manufacturer's directions (eBiosciences), and to the supernatant was added 10 μg of rabbit polyclonal antibody to Bcl-xL. After mixing for 2 h, anti-rabbit goat IgG-agarose beads were added and mixed overnight. The immunoprecipitate was then washed as described previously (10), resolved by SDS-PAGE and analyzed by immunoblotting with the respective antibody.
2.5 Immunofluorescent localization of Bax
For transient transfection, the cells (1.5 × 105 in 1 ml of medium) were grown on 25-mm glass coverslips placed in each well of a 6-well tissue culture plate. Following transfection with GFP-Bax plasmid, mitochondria were stained for 30 min at 37 °C with MitoTracker Red CMXRos diluted 1:1000 in growth media. The cells were then fixed and permeabilized for 30 min at room temperature in fixing medium (3.7% paraformaldehyde, 0.18% Triton X-100 in PBS). The cells were then washed with PBS (3 × 5 min) and stained for 3 min with 4'-6-Diamidino-2-phenylindole (DAPI), a fluorescent nuclear stain. The cells were then washed with PBS (3 × 5 min) and the coverslips were removed from the 6-well plates and mounted on glass microscope slides using 25 ul of Vectashield mounting medium (Vector Laboratories, Burlingame, CA). GFP-labeled Bax, mitochondria, and nuclei were visualized using 488- , 561- and 405-nm filters, respectively, of a Zeiss Axio Imager Z1 microscope. For the cells stably transfected with GPF-Bax, the cells (1.5 × 105 in 1 ml of medium) were grown on 10-mm glass coverslips placed in 35 mm tissue culture plates. After treatment with 100 nM vinblastine for 36 h, mitochondria were stained for 30 min at 37 °C with Mitotracker Red diluted 1:1000 in growth media. The cells were then fixed, permeabilized and mounted as described above. Images were taken with a Zeiss LSM510 Meta confocal microscope.
2.6 Apoptosis and viability assays
KB-3 cells were trypsinized following drug treatment and diluted to a concentration of 5 × 104 cells/ml for measurement of apoptosis using a cell death detection enzyme-linked immunosorbent assay kit (Roche Applied Science). This is a quantitative photometric immunoassay for the determination of cytoplasmic histone-associated oligonucleosomes generated during apoptosis. After dilution, the cells were centrifuged at 200 × g for 5 min, and the cell pellet was resuspended in 0.5 ml of incubation buffer and incubated at room temperature for 30 min. After centrifugation at 16,000 × g for 10 min, 0.4 ml of the supernatant was removed and diluted 1:10 in incubation buffer for analysis. The enzyme-linked immunosorbent assay plate was prepared according to the manufacturer's instructions, and 0.1 ml of sample was added to appropriate wells and incubated at room temperature for 90 min. After conjugation and incubation with substrate solution, the plate was shaken on an orbital shaker at 250 rpm for 15 min, and then the absorbance at 405 nm was determined using a Bio-Tek ELx800 microplate reader (Bio-Tek Instruments, Winooski, VT). As a further assessment of cell viability, adherent (viable) and non-adherent (non-viable) cells from each transfection condition were collected separately. The adherent cells were recovered by trypsinization after the non-adherent cells were collected in the medium. The cells were centrifuged at 500 × g, resuspended in 200 μl of PBS, and counted using a FACSCalibur (Beckton-Dickinson).
3. Results
3.1 The JNK inhibitor SP600125 inhibits phosphorylation of c-Jun, Bax translocation and activation, and apoptosis, induced by vinblastine
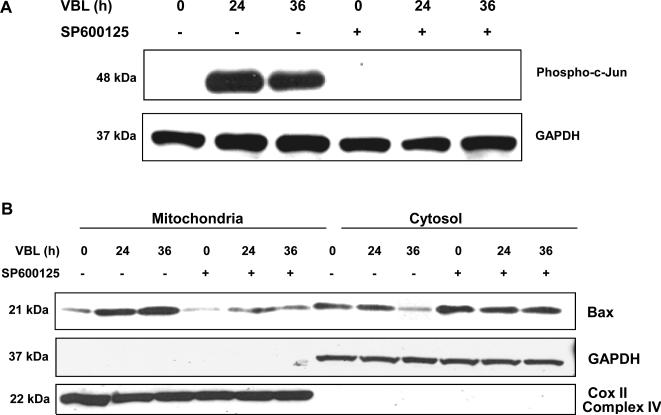
We have previously shown that vinblastine activates JNK in KB-3 cells, a HeLa subline, and promotes mitochondrial translocation, dimerization, and conformational activation of Bax (10). Several studies have suggested that JNK may regulate Bax translocation, either directly or indirectly (6-8). To determine whether JNK regulates Bax during the apoptotic response to vinblastine, we treated cells with vinblastine, in the absence or presence of the JNK inhibitor SP600125. Vinblastine induced robust phosphorylation of c-Jun, which was maximum at 24 h and sustained through 36 h, and SP600125 effectively inhibited this response (Fig. 1A). We next studied Bax mitochondrial translocation. Mitochondrial and cytosolic fractions were prepared from cells treated with vinblastine either without or with SP600125 pre-treatment. The integrity of the fractions was confirmed by immunoblotting for Cox II (complex IV) as a mitochondrial marker and for GAPDH as a cytosolic marker (Fig. 1B). In the absence of SP600125, vinblastine treatment induced an increase in mitochondrial expression of Bax with a concomitant decrease in cytosolic Bax (Fig. 1B). Thus vinblastine induced Bax translocation from the cytosol to the mitochondria. However, when cells were pretreated with SP600125, vinblastine-induced Bax translocation was strongly diminished, with only minor changes in mitochondrial and cytosolic expression of Bax relative to untreated cells (Fig. 1B).
Fig. 1. Effect of the JNK inhibitor, SP600125, on phosphorylation of c-Jun, Bax translocation, Bax oligomerization, Bax activation and PARP cleavage, induced by vinblastine.
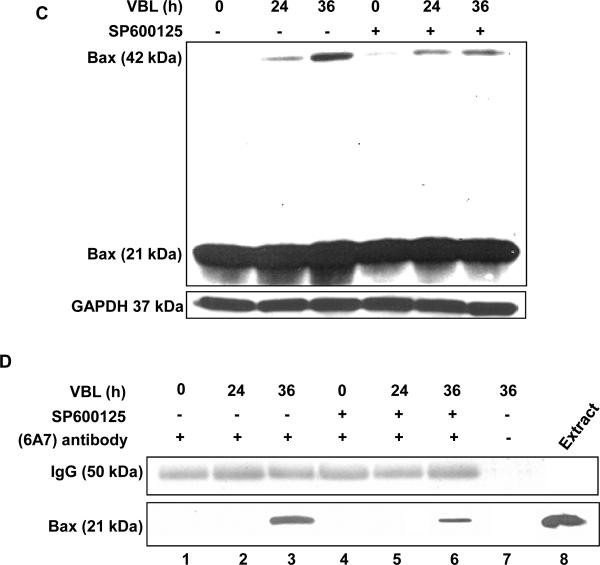
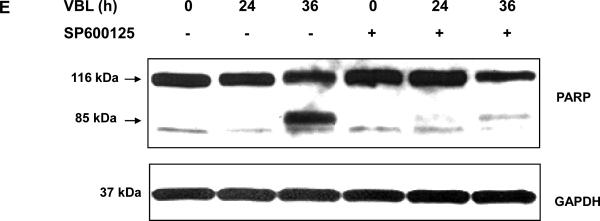
KB-3 cells were pretreated with or without 20 μM SP600125 for 30 min and were then untreated or treated with 30 nM vinblastine for the times indicated. A, whole cell extracts were prepared and subjected to immunoblotting for phospho-(Ser-63) c-Jun (P-c-Jun) and GAPDH as a loading control. B, cytosolic and mitochondrial fractions were prepared and subjected to immunoblotting for Bax, GAPDH and Cox II (Complex IV), respectively. C, cells were permeabilized, treated with digitonin in the presence of 1 mM DSP, and CHAPS-solubilized particulate fractions subjected to SDS-PAGE. Immunoblotting for Bax was performed with the monomeric (21-kDa) and oligomeric (42-kDa) forms shown. D, whole cell extracts were prepared and subjected to immunoprecipitation with anti-Bax 6A7 antibody (lanes 1 ~ 6), followed by immunoblotting for Bax. A precipitates prepared in the absence of 6A7 antibody (lane 7) and a whole cell extract (lane 8) were also examined as controls. E, whole cell extracts were prepared and subjected to immunoblotting for PARP, with uncleaved (116-kDa) and cleaved (85-kDa) species indicated. GAPDH was used as a loading control.
Bax mitochondrial translocation is associated with Bax dimerization and conformational activation (3). As shown in Fig. 1C, vinblastine induced Bax dimerization, and this was strongly diminished after SP600125 pre-treatment. In order to examine Bax activation, we used the 6A7 antibody, which recognizes the conformationally active form of Bax. Extracts were prepared from cells and immunoprecipitation performed with 6A7 antibody, and samples probed for Bax by immunoblotting. In cells treated with vinblastine for 36 h, active Bax was readily observed, and this was strongly diminished in cells pretreated with SP600125 (Fig. 1D). Thus inhibition of JNK causes inhibition of Bax translocation, Bax dimerization, and Bax conformational activation. To determine if this was reflected in a change in apoptosis, PARP cleavage was examined. As shown in Fig. 1E, vinblastine induced PARP cleavage at 36 h, and this was strongly inhibited by pretreatment with SP600125. Collectively, these results provide compelling evidence that JNK promotes Bax-dependent cell death in this system.
3.2 Transient transfection of GFP-Bax
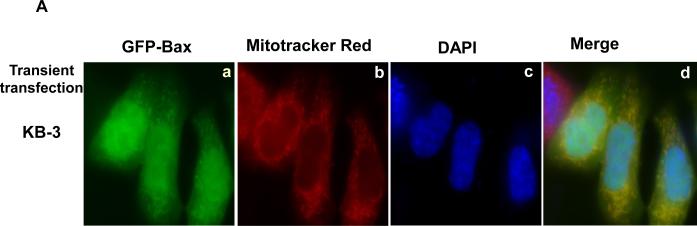
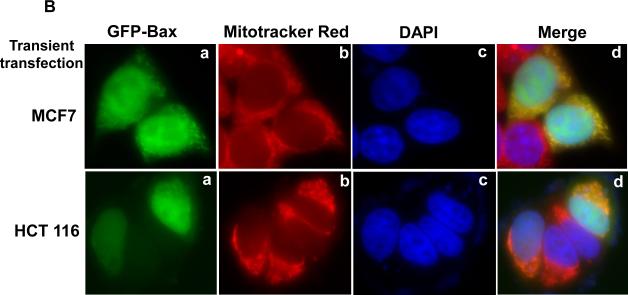
In order to develop a more systematic approach to studying the regulation of Bax translocation in the apoptotic response to vinblastine, we transiently transfected KB-3 cells with a plasmid encoding a GFP-Bax fusion protein. After transfection, mitochondria were stained with Mitotracker Red, and images obtained by fluorescent microscopy. Unexpectedly, in the absence of vinblastine treatment, GFP-Bax localized to the mitochondria (Fig. 2A, panels a-d), in contrast to endogenous Bax which was largely cytosolic (Fig. 1B; Ref. 10). This was not confined to KB-3 cells, as two other human cell lines, HCT-116 colon carcinoma and MCF-7 breast carcinoma, also gave the same result (Fig. 2B, 2C). Therefore, transient transfection of GFP-Bax did not represent a good model system to study endogenous Bax regulation.
Fig. 2. Localization of overexpressed GFP-Bax.
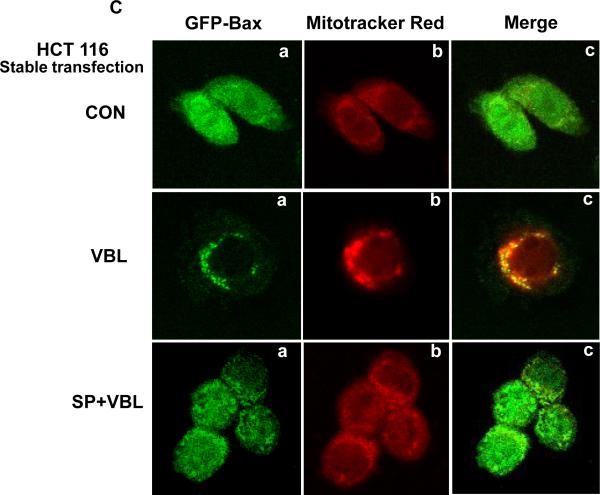
A, KB-3 cells were transiently transfected with GFP-Bax, as described in Materials and methods, and stained for localization of mitochondria and nuclei. a, Bax localization (GFP fluorescence, green signal); b, mitochondria, visualized with MitoTracker Red CMX-Ros (red signal); c, nuclei, visualized with DAPI (blue signal); d, overlay of the three images. The images were taken with a Zeiss Axio Imager Z1 microscope. B, HCT116 or MCF-7 cells were transiently transfected with GFP-Bax. Bax (green, a), mitochondria (red, b), nuclei (blue, c) and an overlay of the merged images (d) were taken with a Zeiss Axio Imager Z1 microscope. C, Bax negative HCT116 cells stably transfected with GFP-Bax were treated with vehicle (0.1 % DMSO), 100 nM vinblastine, or 100 nM vinblastine in the presence of 20 μM SP600125, for 36 h. Bax (green, a), mitochondria (red, b), and overlay of the merged images (c) were taken with a Zeiss LSM510 Meta microscope.
3.3 Bax translocation in Bax-deficient cells stably expressing GFP-Bax
The results presented above suggested that mechanisms normally operant to sequester Bax in the cytosol may be overwhelmed by the transient overexpression of GFP-Bax. In order to confirm that vinblastine causes JNK-dependent Bax translocation, a transfection system was needed where Bax was normally resident in the cytoplasm. Previously, we have shown that in a Bax deficient background in HCT116 cells, stable expression of GFP-Bax results in a diffuse cytosolic pattern of Bax localization (11). This system was therefore tested to determine the effect of vinblastine and the combination of vinblastine and SP600125 on Bax localization. In control GFP-Bax/HCT116 cells, stably expressed Bax showed a diffuse pattern which was distinct from the mitochondrial signal observed through MitoTracker Red staining (Fig. 2C, top panels). After vinblastine treatment, a much more punctate pattern of expression of GFP-Bax was evident, which was coincident to a large degree with that of MitoTracker Red. If cells were pretreated with SP600125 prior to vinblastine, the pattern of expression of GFP-Bax was similar to that of control cells. These results support those of Fig. 1A and confirm that vinblastine induces JNK-dependent Bax mitochondrial translocation in cells stably expressing Bax.
3.4 Bax expression is stabilized and enhanced by co-transfection with Bcl-xL which acts as a key negative regulator
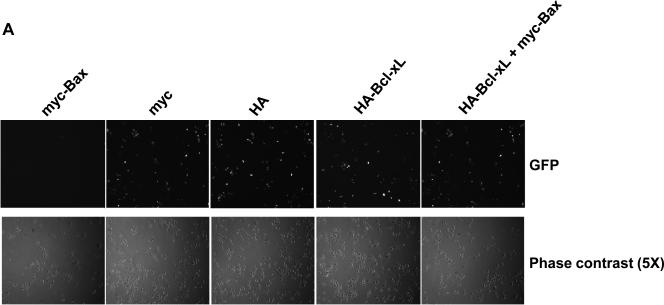
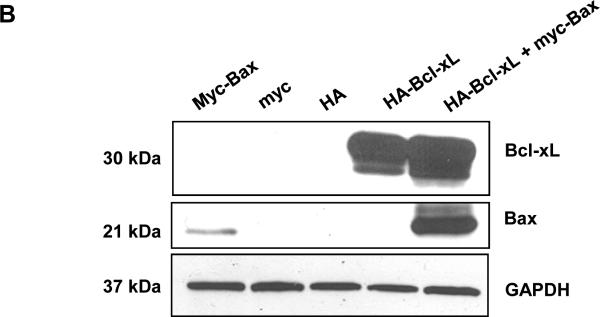
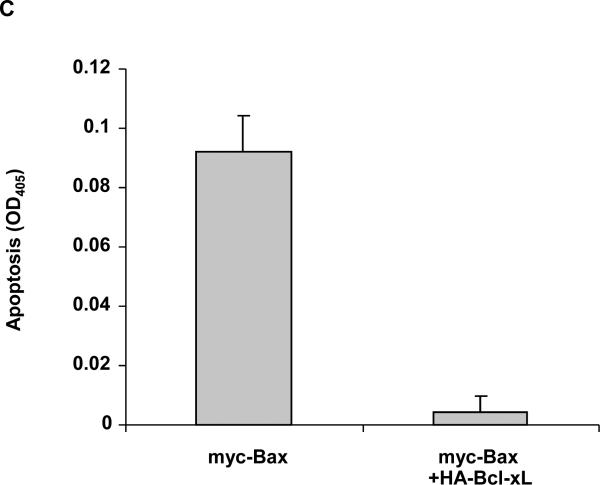
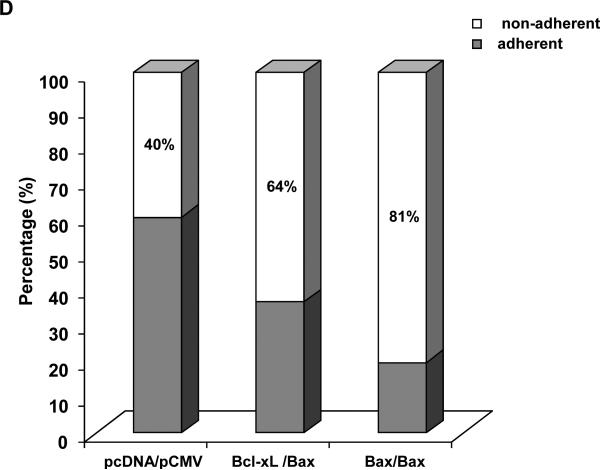
The results presented above demonstrate that JNK is a positive regulator of Bax mitochondrial translocation during vinblastine-induced apoptosis. These findings support results from other studies using different cell lines and apoptotic stimuli, where JNK has also been implicated in the regulation of Bax translocation (6-8). We next sought to identify key negative regulators of Bax translocation. We took advantage of the fact that when GFP-Bax was transiently expressed in KB-3 cells and found to localize to the mitochondria (Fig. 2A), cell viability was quite evidently compromised, based on visual inspection of the cells. To this end, KB-3 cells were transiently transfected with an expression plasmid encoding Myc-tagged Bax together with a plasmid encoding GFP to monitor transfection efficiency. As shown in Fig. 3A, after co-expression of GFP and myc-Bax, only non-fluorescent cells were detectable, indicating a low tolerance for Bax mitochondrial expression. However, when co-expressed with anti-apoptotic HA-Bcl-xL, Bax was readily expressed, as evidenced by the abundance of fluorescently labeled cells (Fig. 3A). In support of these data, immunoblotting revealed much higher expression levels of myc-Bax when myc-Bax and HA-Bcl-xL were co-expressed, relative to the levels seen when myc-Bax was expressed alone (Fig. 3B). Apoptosis assays also demonstrated that anti-apoptotic Bcl-xL co-expression protected the cells from apoptosis induced by Bax expression (Fig. 3C). After transfection with these plasmids, we also monitored cell viability independently by quantitation of non-adherent (non-viable) versus adherent (viable) cells, as described in Materials and methods. Co-expression of Bcl-xL increased the proportion of adherent cells versus expression of Bax alone (Fig. 3D).
Fig. 3. Overexpression of Bcl-xL counters the adverse effects associated with Bax overexpression.
A, KB-3 cells were transiently transfected for 48 h with GFP and either myc-Bax-pCMV, myc-pCMV, HA-pCMV, HA-Bcl-xL-pCMV or HA-Bcl-xL-pCMV plus myc-Bax-pCMV, as indicated. Fluorescent images (upper panel) were taken at 488 nm. Phase contrast images (at 5x magnification compared to GFP images) are shown in the lower panel. All images were taken with a Zeiss Axiovert S100 TV microscope. B, KB-3 cells were transiently transfected for 48 h with myc-Bax-pCMV, myc-pCMV, HA-pCMV, HA-Bcl-xL-pCMV, or HA-Bcl-xL-pCMV plus myc-Bax-pCMV, and whole cell extracts were prepared and subjected to immunoblotting for Bcl-xL and Bax. GAPDH was used as a loading control. C, KB-3 cells were transiently transfected with myc-Bax-pCMV only or myc-Bax-pCMV plus HA-Bcl-xL-pCMV for 48 h. The relative extent of apoptosis was assessed quantitatively, as described under “Materials and methods.” Apoptosis induced by control plasmids myc-pCMV and HA-pCMV was averaged and taken as a background and subtracted to obtain the data shown. The results represent mean ± S.D. (n = 6) and are representative of two independent experiments. D, KB-3 cells were transiently transfected with control plasmids (left column) or myc-Bax-pCMV plus HA-Bcl-xL-pCMV or myc-Bax-pCMV only for 48 h, and the adherent and non-adherent cells from each transfection condition shown were collected separately, counted, and expressed as percentage of the total number of cells. Data represent the averages of six independent determinations. The means and standard error for each value, expressed as a percentages, are as follows: control plasmids, adherent 60.0 ± 4 %, non-adherent, 40 ± 6 %; Bcl-xL/Bax plasmids, adherent, 36 ± 4 %, non-adherent, 64 ± 1 %; Bax plasmid, adherent, 19 ± 3 %, non-adherent, 81 ± 6 %.
3.5 Bax-Bcl-xL interaction
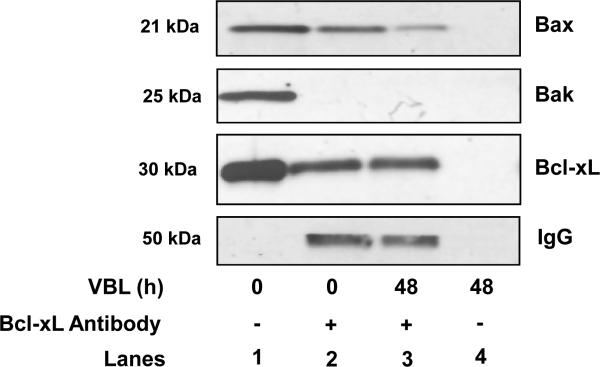
An implication of these findings is that Bcl-xL may physically interact with Bax in this system. In order to test this hypothesis, Bcl-xL was immunoprecipitated from control or vinblastine-treated cells, and samples probed for Bcl-xL, Bax, and Bak. As shown in Fig. 4, Bcl-xL co-precipitated with Bax from control cells, and vinblastine treatment reduced the amount of Bax bound to Bcl-xL. However, we found no evidence of Bcl-xL interaction with Bak. In addition, while Bcl-xL expression blocked vinblastine-induced (10) and Bax-induced (Fig. 3) apoptosis, Bcl-2 expression failed to do so, and Bcl-2 failed to interact with Bax (10). These results suggest that Bcl-xL acts as a selective antagonist of Bax in this system.
Fig. 4. Bcl-xL interacts with Bax but not Bak in KB-3 cells.
KB-3 cells were treated with vehicle or vinblastine (VBL, 30 nM, 48 h), lysates prepared, and immunoprecipitation using the Bcl-xL antibody was performed, as described under “Materials and methods.” Immunoprecipitations were performed with (+Ab, lanes 2 and 3) or without (-Ab, lane 4) the relevant primary antibody as indicated. Immunoprecipitates were subjected to immunoblotting for Bcl-xL, Bax and Bak. A sample of the lysate was also analyzed (lane 4). Immunoblotting for IgG light chain served as a control.
4. Discussion
Many apoptotic stimuli activate the JNK signal transduction cascade and sustained activation of JNK normally signals apoptosis (12). While the mechanisms of pro-apoptotic signaling by JNK are still ill-defined, recent evidence has emerged that Bax is essential for JNK-dependent apoptosis (13,14). Previous work in our laboratory has demonstrated that the microtubule inhibitor vinblastine induces both JNK activation and Bax translocation/activation in KB-3 cells (9,10). In this study we have sought to determine whether these pathways intersect, and in particular to determine whether JNK regulates Bax translocation/activation. The results showed that inhibition of JNK inhibits all of the features associated with Bax activation, including Bax mitochondrial translocation, Bax dimerization, and Bax conformational activation. The mechanisms of JNK regulation of Bax are under investigation. In one study, evidence was presented that JNK and p38 kinase activated by various stressors directly phosphorylate Bax, facilitating Bax mitochondrial translocation (8). In our system, vinblastine does not induce a mobility shift in Bax on SDS-PAGE, but preliminary studies1 have indicated that vinblastine does induce a change in pI of Bax to a more acidic form, which may reflect phosphorylation. In another study, it was proposed that JNK phosphorylates 14-3-3 proteins bound to Bax, causing release of Bax and its relocation from the cytosol to the mitochondria (7). Thus, certain proteins may act to sequester Bax in the cytosol and release Bax upon their phosphorylation. We are currently attempting to identify Bax binding proteins in our system. Based on the results presented here, one of these is clearly Bcl-xL. While Bcl-xL undergoes vinblastine-induced phosphorylation, this is catalyzed by a kinase distinct from JNK (15). Given the key role played by Bax in apoptosis, it is perhaps not surprising that several different signal transduction pathways intersect to regulate Bax activity.
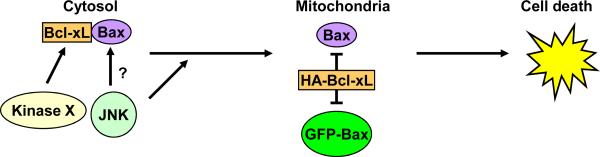
A model summarizing the main findings is shown in Fig. 5. In untreated KB-3 cells, Bcl-xL interacts with Bax in the cytosol, and vinblastine induces JNK-dependent Bax mitochondrial translocation. A kinase distinct from JNK phosphorylates Bcl-xL and this is critical in releasing bound Bax (15). The possibility that JNK may also directly phosphorylate Bax is included in this model. At the mitochondria, Bax induces cell death which is antagonized by Bcl-xL overexpression. GFP-Bax, when transiently expressed in KB-3 cells, localizes directly to the mitochondria and induces cell death, and this too is antagonized by Bcl-xL overexpression.
Fig. 5. Model depicting the main findings.
In control KB-3 cells, Bcl-xL interacts with Bax in the cytosol, and vinblastine induces JNK-dependent Bax mitochondrial translocation. JNK may also directly phosphorylate Bax, and a kinase distinct from JNK phosphorylates Bcl-xL (15). At the mitochondria, Bax induces cell death which is antagonized by Bcl-xL overexpression. GFP-Bax when transiently expressed in KB-3 localizes to the mitochondria and induces cell death, and this too is antagonized by Bcl-xL overexpression.
Acknowledgements
This work was supported by National Institutes of Health Grant CA-109821 (to TCC) and in part by a grant from the University of Arkansas for Medical Sciences Graduate Student Research Fund.
Abbreviations
- JNK
c-Jun NH2-terminal protein kinase
- FBS
fetal bovine serum
- DMEM
Dulbecco's minimal essential medium
- PBS
phosphate-buffered saline
- PARP
poly(ADP-ribose) polymerase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnote: R. Chu and T.C. Chambers, unpublished observation.
References
- [1].Rowinsky EK, Donehower RC. Microtubule-targeting drugs. In: Perry MC, editor. The Chemotherapy Source Book. Lippincott Williams & Wilkins; Baltimore: 1998. pp. 387–423. [Google Scholar]
- [2].Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- [3].Youle RJ, Strasser A. The Bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- [4].Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- [5].Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- [6].Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. PNAS. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, et al. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2003;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim BJ, Ryu SW, Song BJ. JNK-and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- [9].Osborn MT, Chambers TC. Role of the stress-activated/c-Jun NH2-terminal protein kinase pathway in the cellular response to adriamycin and other chemotherapeutic drugs. J Biol Chem. 1996;271:30950–30955. doi: 10.1074/jbc.271.48.30950. [DOI] [PubMed] [Google Scholar]
- [10].Upreti M, Lyle CS, Skaug B, Du L, Chambers TC. Vinblastine-induced apoptosis is mediated by discrete alterations in subcellular location, oligomeric structure, and activation status of specific Bcl-2 family members. J Biol Chem. 2006;281:15941–15950. doi: 10.1074/jbc.M512586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ding WX, Ni HM, Chen XY, Yu J, Zhang L, Yin XM. A coordinated action of Bax, PUMA, and p53 promotes MG132-induced mitochondrial activation and apoptosis in colon cancer cells. Mol Cancer Ther. 2007;6:1062–1069. doi: 10.1158/1535-7163.MCT-06-0541. [DOI] [PubMed] [Google Scholar]
- [12].Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- [13].Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Davis RJ. The bax subfamily of Bcl2-related protein is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang L, Xing D, Liu L, Gao XJ, Chen MJ. TNFα induces apoptosis through JNK/Bax-dependent pathway in differentiated, but not naïve PC12 cells. Cell cycle. 2007;6:1479–1486. [PubMed] [Google Scholar]
- [15].Du L, Lyle CS, Chambers TC. Characterization of vinblastine-induced Bcl-xL and Bcl-2 phosphorylation: evidence for a novel protein kinase and a coordinated phosphorylation/dephosphorylation cycle associated with apoptosis induction. Oncogene. 2005;24:107–117. doi: 10.1038/sj.onc.1208189. [DOI] [PubMed] [Google Scholar]