Abstract
Background
Numerous studies have implicated neurogenesis in the hippocampus in animal models of depression, especially those related to controllability and learned helplessness. Here, we tested the hypothesis that uncontrollable, but not controllable stress would reduce cell proliferation in the hippocampus of male and female rats, and would relate to the expression of helplessness behavior.
Methods
To manipulate controllability, groups of male and female rats were trained in one session (acute stress) or over seven sessions (repeated stress) to escape a footshock, while yoked controls could not escape, but were exposed to the same amount of stress. Cell proliferation was assessed with immunohistochemistry of BrdU and immunofluorescence of BrdU and NeuN. Separate groups were exposed to either controllable or uncontrollable stress and their ability to learn to escape during training on a more difficult task was used as a behavioral measure of helplessness.
Results
Acute stress reduced cell proliferation in males, but did not affect proliferation in the female hippocampus. When animals were given the opportunity to learn to control the stress over days, males produced more cells than the yoked males without control. Repeated training with controllable stress did not influence proliferation in females. Under all conditions, males were more likely than females to express helplessness behavior, even males that were not previously stressed.
Conclusions
The modulation of neurogenesis by controllability was evident in males but not in females, as was the expression of helplessness behavior, despite the fact that men are less likely than women to experience depression.
Keywords: dentate gyrus, depression, sex differences, neurogenesis, controllability, stress, learned helplessness
Introduction
Recently, it has been proposed that neurogenesis in the adult brain may play a role in the etiology of major depression (Malberg, Eisch, Nestler, and Duman 2000;Jacobs, Praag, and Gage 2000;Dranovsky and Hen 2006). This idea stems from three lines of evidence. The first is that the production of new neurons in the hippocampus is reduced by stressful experiences, including predator odors, social dominance, maternal deprivation, and mild shocks (Kosorovitskiy and Gould 2004;Tanapat, Hastings, Rydel, Galea, and Gould 2001;Mirescu, Peters, and Gould 2004;Malberg and Duman 2003). The second line of evidence indicates that chronic treatment with most types of antidepressants, including Prozac™ (fluoxetine), increases proliferation in the hippocampus (Malberg, Eisch, Nestler, and Duman 2000;Madsen, Treschow, Bengzon, Bolwig, Lindvall, and Tingstrom 2000;Chen, Rajkowska, Du, Seraji-Bozorgzad, and Manji 2000). Moreover, preventing neurogenesis prevents the ameliorating effects of Prozac™ on aspects of stress-related behavior (Santarelli, Saxe, Gross, Surget, Battaglia, Dulawa, Weisstaub, Lee, Duman, Arancio, Belzung, and Hen 2003). The third line of evidence indicates that the volume of the hippocampus is reduced in depressed patients (Neumeister, Wood, Bonne, Nugent, Luckenbaugh, Young, Bain, Charney, and Drevets 2005). From these lines of evidence, it is suggested that stressful life experience decreases neurogenesis, which ultimately decreases the volume of the hippocampus. The decrease in volume contributes to the symptoms of unipolar depression; the prevention of that decrease with antidepressants explains their efficacy (Malberg, Eisch, Nestler, and Duman 2000).
Learned helplessness is one of the most common animal models of depression (Seligman 1975). With it, animals are exposed to either controllable or uncontrollable stressful events and then later tested on a new task in which all animals are given the opportunity to control the stressor, usually by escape (Overmier and Seligman 1967;Seligman and Maier 1967). Most often, those animals that experienced uncontrollable stressful events do not display the escape response or are retarded in their response. This response has been equated with a sense of “giving up” experienced by humans with major depression. Animals that are initially trained to control the stressor can learn during training on the new task, despite the fact that both groups were exposed to similar amounts of stressful stimuli. Thus, establishing “control” over the stress can ameliorate some of the performance deficits that occur in response to stressful life events (Seligman 1975).
A few studies have used the learned helplessness paradigm to evaluate the putative relationship between depression and neurogenesis. Malberg and Duman (2003) reported that exposure to uncontrollable stress reduces neurogenesis in the hippocampus and induces helplessness behavior, both of which were reversed by treatment with Prozac™ (Malberg and Duman 2003). In contrast, Vollmayr et al., (2003) reported that exposure to uncontrollable stress reduced neurogenesis, but the reduction was not associated with helplessness behavior (Vollmayr, Simonis, Weber, Gass, and Henn 2003). Recently Bland et al. (2006) found that animals with control produced more cells in their hippocampus than those without control (Bland, Schmid, Greenwood, Watkins, and Maier 2006). Most studies relating neurogenesis to depression have focused exclusively on males, despite robust sex differences in the incidence of the disease in humans (Burt and Stein 2002;Kessler, McGonagle, Swartz, Blazer, and Nelson 1993;Martenyi, Dossenback, Mraz, and Metcalfe 2001;Staley, Sanacora, Tamagnan, Maciejewski, Malison, Berman, Vythilingam, Kugaya, Baldwin, Siebyl, Charney, and Innis 2005;Kornstein, Schatzberg, Thase, Yonkers, McCullough J.P,., Keitner, Gelenberg, Davis, Harrison, and Keller 2000;Kessler, McGonagle, Swartz, Blazer, and Nelson 1993). Here, we evaluated the effects of controllable versus uncontrollable acute and repeated stress on hippocampal neurogenesis and learned helplessness behavior in males and females.
Materials and Methods
General Procedures
Subjects and estrous cycle determination
Experiments were approved by the Rutgers University Animal Care and Facilities Committee and are in compliance with the rules and regulations specified by the PHS policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. Adult (2–3 months) male (300–450 grams) and female (250–350 grams) Sprague Dawley rats were individually housed with ad libitum access to food and water and maintained on a 12-hour light/dark cycle. Stages of estrus were determined in female rats with daily vaginal smears, as described (Leuner, Mendolia-Loffredo, and Shors 2004). Female rats without a 4–5 day cycle including proestrus, estrus, diestrus 1, and 2 were excluded.
Experiment 1
Acute stress and neurogenesis in male versus female hippocampus
The effect of one session (30 trials) of either controllable or uncontrollable shock on cell proliferation in the dentate gyrus was examined. Rats were injected once with BrdU (200mg/kg), which labels cells in the S phase of mitosis (Miller and Nowakowski 1988;Cameron and McKay 2001). Males were yoked in pairs (n=9) and trained 30 minutes later in an operant conditioning task known as fixed-ratio 1 (FR1) (Shors, Seib, Levine, and Thompson 1989). Females were yoked together according to stage of estrus (n of pairs =11) and trained similarly. Groups of naive controls (Males: n= 11; Females: n=7) were also injected with BrdU, returned to their home cage and sacrificed 2 hours later.
To manipulate controllability, rats were placed in one of two electrically linked shuttleboxes (Med Associates Inc.). Each shuttlebox (46cm × 18cm × 19cm) was located within a sound attenuated illuminated (15W) chamber (69cm × 69cm × 63cm). A scrambled shock generator delivered 1 mA electric pulse through the grid floor and walls of the apparatus. Each shuttlebox consisted of grid flooring, steel walls, a Plexiglas top, and a doorway in the center. During training with an FR-1 task, one rat can escape a 1mA footshock (controllable stress) by traversing the apparatus once, whereas the yoked rat cannot escape (uncontrollable stress), but is exposed to the same amount and duration of shock as the rat that can escape. The animal with control escapes by passing through a doorway and tripping a balance switch, which terminates the shock for both animals simultaneously. Rats were trained for 30 trials with a maximum latency to escape of 20s and an intertrial interval of 60s. Latency to escape was used as a measure of performance in rats exposed to controllable stress. All rats were returned to their home cage and sacrificed by perfusion 2 hours after the BrdU injection.
For sacrifice, rats were deeply anaesthetized with sodium pentobarbital (100 mg/kg) and intracardially perfused with 4% paraformaldehyde in 0.2 M phosphate buffer. Brains were extracted and post-fixed in 4% paraformaldehyde for up to 48 hrs, and were later transferred to 0.1M phosphate buffer (PBS). Subsequently, brains were sliced and stained using immunohistochemistry for BrdU.
Immunohistochemistry methods
Coronal sections (40 μm) were cut through the entire dentate gyrus with an oscillating tissue slicer and then stained using peroxidase methods. Under peroxidase protocol, brain tissue was heated in 0.1 M citric acid, pH 6.0, incubated in trypsin, then incubated in 2N HCl, and incubated overnight in primary mouse anti-BrdU (1:200) and 0.5% Tween 20. The next day, tissue was incubated for 1 hr in biotinylated anti-mouse antibody (1:200), then in avidin-biotin-horseradish peroxidase (1:100), and lastly in diaminobenzidine. After rinsing in PBS, slides were counterstained with cresyl violet and cover-slipped under Permount (Fisher Scientific, Fair Lawn, NJ). Slides were coded, and estimates of total numbers of BrdU-labeled cells were determined using unbiased stereology protocol (West, Slomianka, and Gundersen 1991). BrdU-labeled cells on every twelfth unilateral section throughout the entire rostrocaudal extent of the dentate gyrus (granule cell layer, subgranular zone and hilus) were counted at 1,000X (100x objective with a 10x ocular) on Nikon Eclipse E400 light microscope, avoiding cells in the outermost focal plane. The numbers of BrdU-labeled cells in the dentate gyrus was multiplied by 24 (number of intervening slices and hemispheres) and used as an estimate of total number of newly generated cells.
A subset of male rats was injected with BrdU 30 minutes before being trained with one session of either controllable or uncontrollable stress (FR1). Rats were sacrificed 3 weeks later, along with naïve controls. This time point was chosen since this approximates when newly generated cells express NeuN, a marker of mature neurons (Christie and Cameron 2006). Because we were evaluating a decrease in proliferation, we did not conduct an extensive analysis of differentiation. Slices were incubated in 50% formamide/2x SSC (sodium chloride/sodium citrate buffer) for 2 hours at 65° C, rinsed with 2xSSC, and incubated in 2N hydrochloric acid for 30 min at 37° C. They were then rinsed with borate buffer (pH 8.5) and incubated in 3% Normal Donkey Serum, 0.1% Triton X-100, in tris-buffered saline (TBS+, pH 7.5) for 1 hour at room temperature. Primary antibodies, rat anti-BrdU (1:50, Accurate Chemicals) and mouse anti-NeuN (1:200, Chemicon) were incubated simultaneously in TBS+ overnight at 4° C. They were rinsed with TBS and incubated in secondary antibodies (Jackson Immunoresearch) sequentially: donkey anti-rat biotin-SP (1:200), fluorescin-DTAF-streptavidin (1:200), and donkey anti-mouse rhodamine red X (1:1000). Antibodies were diluted in TBS+ and tissue was rinsed with TBS between incubation periods. Sections were cover-slipped with Polyacquamount (Polysciences, Warrington, PA). BrdU-NeuN co-localization was determined with a Zeiss (Oberkochen, Germany) confocal microscope.
Experiment 2
Repeated stress and neurogenesis in male versus female hippocampus
The effect of seven sessions (30 trials a day) of either controllable or uncontrollable shock on cell proliferation in the dentate gyrus was examined. As before, males were yoked together in pairs (n=15) and females were yoked according to the stage of estrus (n=12). The training procedures were similar to those in the first experiment, except that rats were trained for seven days, instead of one. Thirty minutes before the last session, rats were injected once with BrdU, trained, returned to their home cage, and sacrificed 2 hours later, as before.
Experiment 3
Controllability and helplessness behavior in males versus females
The effect of one session or seven sessions of controllable versus controllable stress on helplessness behavior was examined. Pairs of male (n=9 pairs for acute stress; n=8 pairs for repeated stress) and female rats (n=8 pairs for each) were treated as before, except that they were tested for helplessness rather than cell proliferation. Again, females were yoked according to stage of estrus. One day after the last training session, rats were tested on a fixed-ratio 2 (FR2) task, in which escape was possible for all subjects, but required moving through the doorway twice in order to turn off the shock. Additional groups of male (n=14) and female (n=18) rats, that had not been previously trained or exposed to any footshock, were also tested in the FR2 task. The context for training was altered in the following ways: black and white stripes lined the walls of the chamber, an odor of menthol was placed in each chamber, and white bulbs were replaced with red bulbs. Latency to escape the FR2 was measured over 20 trials of training with a maximum latency of shock of 15s and an intertrial interval of 60s.
Statistical analysis
Cell counts were analyzed using a paired dependent t-test on data collected from yoked pairs of animals. One-way ANOVAs were performed to detect group differences. Performance during training on FR1 and FR2 operant conditioning tasks was analyzed with repeated measures analysis of variance (ANOVA) across sessions or blocks of five trials. Post hoc analyses with Newman-Keuls tests were conducted on significant interactions.
Results
Experiment 1
Acute stress reduced neurogenesis in the male but not in the female hippocampus
Males and females performed differently during training on the FR1 task (Figure 1a, c). Males took longer than females to escape during the first five trials of FR1 training [F(1,18) = 5.01; p<0.05], although the sex difference dissipated by the last trials of training (p>0.05) (Figure 1a, c). Effects of acute stress on proliferation were also different in males versus females. Exposure to the acute stress altered the number of BrdU labeled cells in males [F(2,26)=3.89; p<0.05], with naïve males producing more new cells than either of the stressed groups (p<0.05), irrespective of whether or not they could control the shock [t(8)=−0.65; p>0.05] (Figure 1b). In females, neither type of stressor altered the number of BrdU labeled cells [F(2,26)=2.25; p>0.05] (Figure 1d). Neither was there an effect of controllability on the number of cells produced [t(9)=−0.36; p>0.05] (Figure 1d). Stage of estrus did not alter numbers of the cells produced (p>0.05), although the numbers of animals in each stage were too few to draw conclusions. In neither sex did the number of cells generated in the dentate gyrus correlate with the amount of shocks that the animals received (p>0.05). Three weeks after the BrdU injection, 71% of the cells (n= 4; >10 cells per rat) were labeled with both BrdU and NeuN (Figure 2). Thus, the majority of cells did differentiate into neurons and the percentage is consistent with those reported in our previous studies (Gould, Beylin, Tanapat, Reeves, and Shors 1999) (Leuner, Mendolia-Loffredo, Kozorovitskiy, Samburg, Gould, and Shors 2004). Since the effect of stress was to prevent the production of new cells, the NeuN data simply indicate the percentage of new cells that would have expressed neuronal markers, if they had survived.
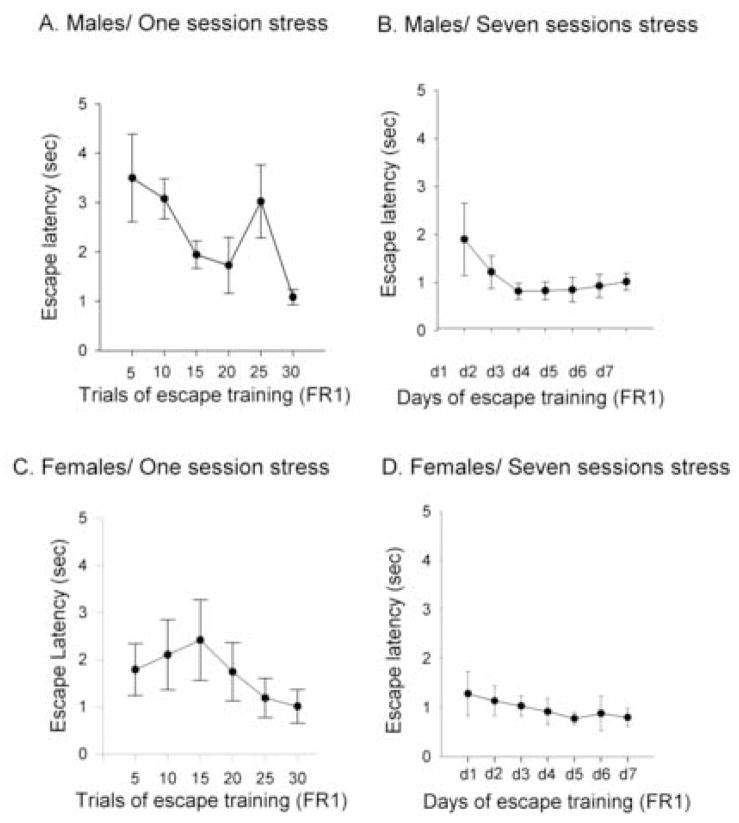
Figure 1. Acute stress decreases cell proliferation in the male but not the female hippocampus.
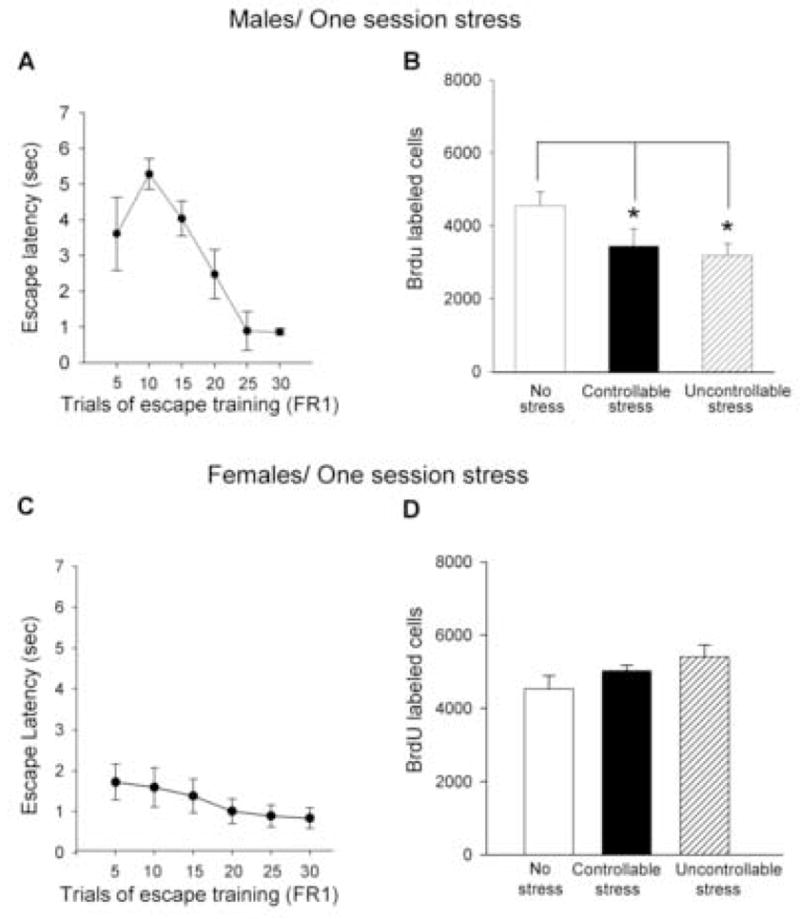
(a) Escape latencies in the FR1 task (means of five trials + SEM) for males exposed to one session (30 trials) of controllable stress. (b) Mean number (means + SEM) of BrdU labeled cells in the dentate gyrus from the same groups, along with a group of naives. (c) Escape latencies in the FR1 task (means of five trials + SEM) for females exposed to one session of controllable stress. (d) Mean number (means + SEM) of BrdU labeled cells in the dentate gyrus from the same groups, along with a group of naives. [* indicates significant differences (p<0.05)].
Figure 2. BrdU and NeuN labeling in the dentate gyrus of hippocampus.
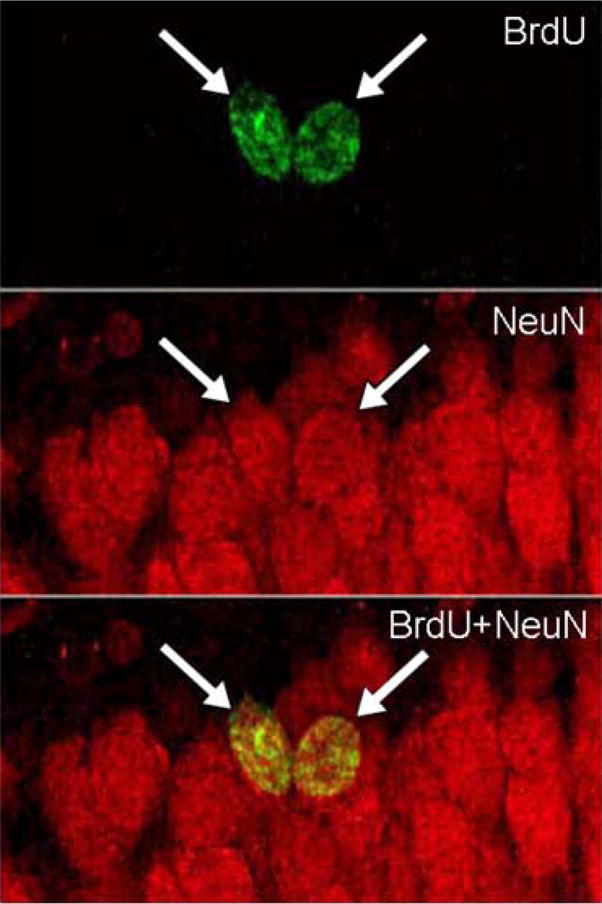
Images illustrate BrdU-labeled cells (green) that expressed NeuN (red), a marker of mature neurons in animals that were sacrificed 3 weeks after the BrdU injection. Arrows indicate cells that were immunoreactive for both BrdU and NeuN.
Experiment 2
Controllability over a repeated stressor modulates neurogenesis in the male but not in the female hippocampus
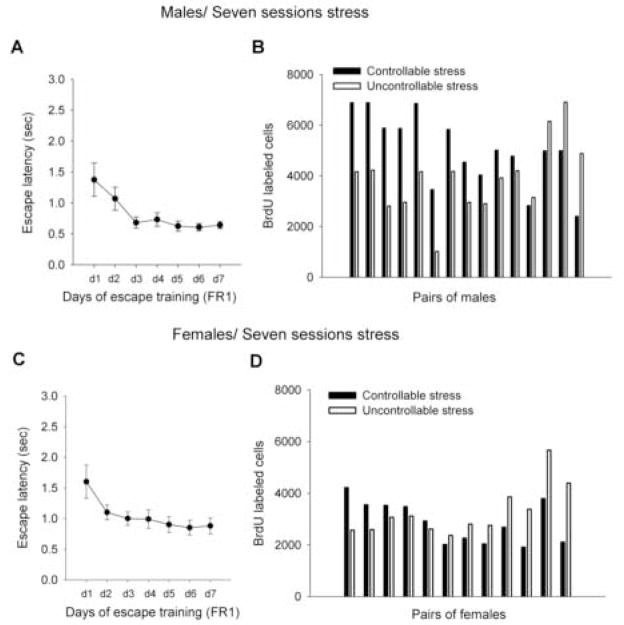
As in Experiment 1, males took longer to escape during the initial trials of training in the first session, but did not differ from females during training on the following days (p>0.05) (Figure 3a, c). A paired dependent t-test revealed that male rats that were trained to escape the shock for 7 days produced more cells than their yoked counterparts, that were exposed to the same amount of shock, but did not have the opportunity to escape [t(14)=2.42; p<0.05] (Figure 3b and Figure 4a). A dependent t-test on similar data in yoked pairs of females was not significant [t (11) =−1.15; p=0.28] (Figure 3d and Figure 4b). Again, there was no effect of stage of estrus on number of BrdU-labeled cells (p>0.05) and the number of BrdU labeled cells did not correlate with the amount of amount of shock that males or females were exposed to during the last session of stress (p>0.05).
Figure 3. Controllability over a repeated stressor modulates neurogenesis in the male but not the female hippocampus.
(a) Escape latencies in the FR1 task (means of 30 trials + SEM) for males exposed to seven sessions of controllable stress and (b) the number of BrdU labeled cells in the dentate gyrus of 15 yoked pairs of the males. Pairs were sorted into those demonstrating an increase in proliferation with controllable stress, from the left and ending with the opposite response to the right.
(c) Escape latencies in the FR1 task (means of 30 trials + SEM) for females exposed to seven sessions of controllable stress and (d) the number of BrdU labeled cells in the dentate gyrus of 12 female yoked pairs. Pairs were sorted into those demonstrating an increase in proliferation with controllable stress, from the left and ending with the opposite response to the right.
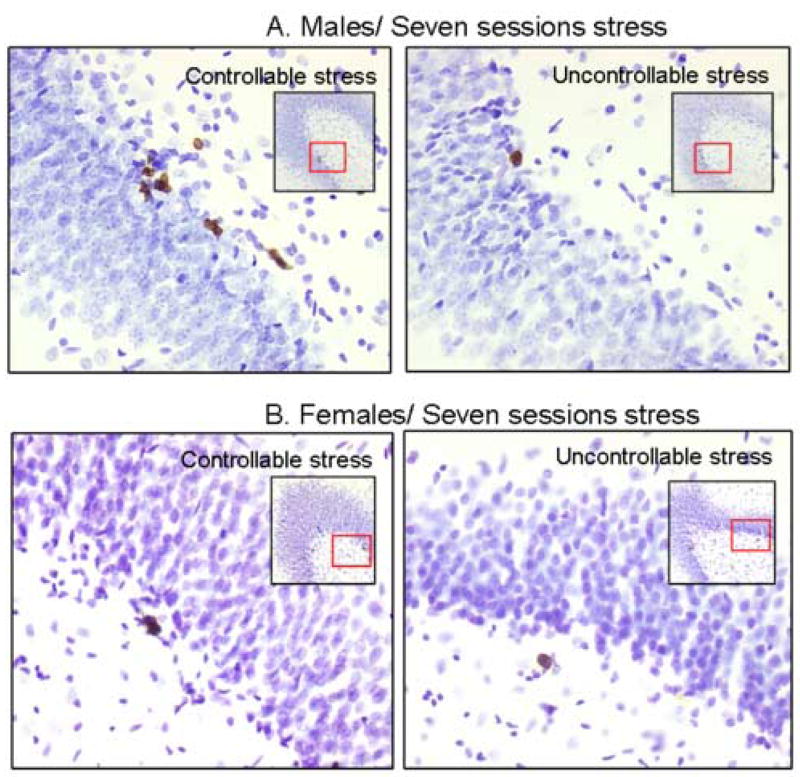
Figure 4. New cells generated in the male and female hippocampus in response to controllable versus uncontrollable stress.
BrdU labeled cells in the dentate gyrus of a (a) male and (female) hippocampus after training with seven sessions of controllable or uncontrollable stress. Larger images were magnified ×400 and the smaller images ×200.
Experiment 3
Sex differences in helplessness behavior
As in the first two experiments, males took longer than females to escape during the initial trials of training on the first session of the FR1 task [F (1,15) = 5.2; p<0.05], but the sex difference dissipated within the 30 trials of training (Figure 5a and b). All groups learned to escape within the 7 sessions, as evidenced by a decrease in escape latency across sessions culminating in very low latencies (Figure 5c and d) [males: [F(6,42)=8.76; p<0.001]; females: [F(6,42)=6.94; p<0.001]. There was no sex difference in performance during training on the FR1 task across 7 days of training (p>0.05) (Figure 5c and d). However, sex differences were evident during training on the FR2 task. Overall, females expressed shorter escape latencies than did males, irrespective of the pretraining condition (p<0.001) (Figure 6). Females that were previously exposed to one session of FR1 training (irrespective of controllability) outperformed males, with performance analyzed in blocks of 5 trials each [first block: F(1,32)= 18.95; p<0.001; second block: F(1,32)= 25.28; p<0.001; third block: F(1,32)= 11.48; p<0.005; fourth block: F(1,32)= 10.34; p<0.005) (Figure 6b, e). Females also outperformed males during training on FR2 task, even after they were exposed to seven sessions of uncontrollable stress (first block: F(1,14)= 8.8; p=0.01; second block: F(1,14)= 5.96; p<tr5; third block: F(1,14)= 6.11; p<0.05) (Figure 6c, f).
Figure 5. Performance during training with the FR1 task in males versus females.
(a) Escape latencies in the FR1 task (means of five trials + SEM) for males trained with one session of controllable stress. (b) Escape latencies in the FR1 task (means of 30 trials + SEM) for males trained with seven sessions of controllable stress. (c) Escape latencies in the FR1 task (means of five trials + SEM) for females trained with one session of controllable stress. (d) Escape latencies in the FR1 test (means of thirty trials + SEM) for females exposed to seven days of controllable stress.
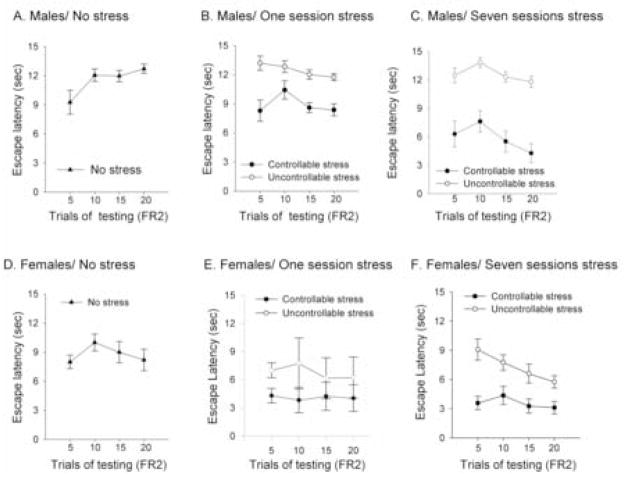
Figure 6. Males express learned helplessness behavior, whereas females do not.
(a) Escape latencies in males that were only trained on the operant task in which they had to cross the shuttle-box apparatus twice to escape (FR2) (means of five trials + SEM). (b) Escape latencies during training on the FR2 task (means of five trials + SEM) after exposure to one session of controllable or uncontrollable stress (30 trials). (c) Escape latencies in males that were trained on the FR2 task (means of five trials + SEM) after exposure to seven sessions of controllable or uncontrollable stress. (d) Escape latencies in females that were only trained on the operant task in which they had to cross the shuttle-box twice to escape (FR2) (means of five trials + SEM). (e) Escape latencies during training on the FR2 task (means of five trials + SEM) in females after exposure to one session of controllable or uncontrollable stress. (f) Escape latencies in the FR2 test (means of five trials + SEM) after exposure to seven sessions of controllable or uncontrollable stress.
Controllability did influence learning to escape during the FR2 task in males and females, but the effect depended on the length of previous training. With one session of FR1 training in males, there was an interaction between the performance across trials and the pretraining condition [F(6,87)=3.52; p<0.005], but post-hoc analysis suggested no difference in their overall performance (p>0.05) (Figure 6b). Males previously exposed to controllable or uncontrollable stress for one session did not alter their latency to escape across trials during testing on the FR2 task, and thus did not learn [F(3,24)=1.56; p>0.05; F(3,24)=1.91; p>0.05, respectively] (Figure 6b). Interestingly, the latency to escape in the FR2 task actually increased in the males that were not previously trained in the FR1 (no stress group) [F (3,39)= 7.33; p<0.001] (Figure 6a). Thus, it would appear that the males became helpless as they were being trained de novo on the more difficult FR2 task.
For males tested on the FR2 task after 7 days of FR1 training, there was an effect of trials [F(3,81)=4.30; p<0.01], the pretraining condition [F(2,27)=10.34; p<0.001] and an interaction between the two factors [F(6,81)=4.00; p<0.001]. Post-hoc analysis revealed that performance of males that had learned to escape the footshock over the 7 sessions of FR1 training (controllable stress) differed from those that could not escape (uncontrollable stress) or those that had no previous stress exposure (p<0.001). Male rats that were trained with seven sessions of FR1 (controllable stress) reduced their escape latencies across blocks of trials of FR2 task, and thus expressed evidence of learning [F(3,21)=3.64; p<0.05] (Figure 6c). Those that could not escape over the seven of FR1 training (uncontrollable stress) did not reduce their latency to escape during FR2 task, did not learn, and thus expressed evidence of helplessness [F(3,21)=1.58; p=0.22] (Figure 6c). However, as noted, male rats that were naïve prior to training on the FR2 task also did not show evidence of learning and therefore also expressed helplessness behavior [F(3,39)= 7.33; p=0.001] (Figure 6a). In fact, only males exposed to 7 sessions of escape training in FR1 (controllable stress) learned to escape during testing in the FR2 task (Figure 6c). Again, these data suggest that if the males are not previously trained to escape, they express helplessness behavior.
In females, the response to repeated training was quite different. After one session of FR1 training, there was an effect of the pretraining condition on performance during training on the FR2 task [F(2,31)=6.97; p=0.003] with no interactions (Figure 6e). Those that were trained to escape an FR1 for one session (controllable stress) expressed a decrease in escape latency and thus learned to escape during training on the FR2 task and did so faster than the females that were exposed to the uncontrollable stress for one session [F(1,14)= 6.11; p<0.05; F(1,14)= 16.9; p<0.001, for the two first blocks of trials, respectively], although the groups were not different when assessed across the 20 trials of training. Both groups (controllable and uncontrollable stress) responded sooner than the naïve females, especially on the first trials of training with the FR2 task [F(2,21)=4.39; p<0.05; F (2,21)=14.9; p<0.001, for the two first blocks of trials respectively]. Escape latencies among the three groups did not differ during the last 10 trials of training on the FR2 task (Figure 6d, e). In contrast to males, females with no previous FR1 training (no stress group) expressed a decrease in latency across trials and thus learned to escape during training on the FR2 task [F(3,51)=4.24; p<0.005] (Figure 6d). Although escape latencies increased between the first 5 and 10 trials (p<0.001), they decreased over the remaining trials (p<0.005).
The escape latencies expressed by females in the FR2 task after seven sessions of FR1 training indicated an effect of trials [F(3,93)=4.97; p<0.005], pretraining condition [F(2,31)=5.93; p<0.01] and an interaction between the two factors [F(6,93)=2.68; p<0.05]. Post-hoc analysis revealed that the escape latencies of females trained for seven sessions of escapable footshock in FR1 (controllable stress) differed from those exposed to the same amount of inescapable footshock (uncontrollable stress) (p<0.05). However, females that were exposed to seven sessions of the uncontrollable stress decreased their escape latencies over trials in the FR2 task [F(3,21)=4.35; p<0.05], and thus learned to escape (Figure 6f), as did the females that were not exposed to any stress before training on the FR2 [F(3,51)=4.24; p<0.005] (Figure 6d). Females that had learned to escape during FR1 training (controllable stress) did not reduce their latency to escape during testing on the FR2 task [F(3,21)= 1.88; p>0.05] (Figure 6f), probably because they performed so well during the early trials of FR2 testing. Although females previously exposed to controllable stress performed differently than those exposed to uncontrollable stress, both groups showed evidence of learning to escape the footshock in the FR2 task.
Discussion
The present results indicate that controllability modulates neurogenesis in the adult hippocampus in males, but not in females, at least using the conditions described here. The males that were able to learn to control a stressor presented over seven days produced more cells in their hippocampus than their yoked counterparts, an effect that was observed in 11 out of 15 pairs. In contrast and on average, females that were able to learn to control the same stressor over seven days produced as many cells as their yoked counterparts; only two of the 12 females with control produced more cells than their yoked counterparts. We also found that exposure to just one session of stress, irrespective of controllability, decreased proliferation in males, whereas it did not affect proliferation in females. Because females escaped sooner than males in the first trials of FR1 task, they were possibly exposed to less duration of shock, which could explain the sex differences in proliferation after acute stress. Thus, the behavior of the females may have affected their response to acute stress at the cellular level, something to consider when evaluating animal models of depression. Consistent with this, exposure to controllable stress alleviated the expression of helplessness behavior in both females and males, but females learned to escape more rapidly than did males, irrespective of their opportunity to control the stressor. Thus, females more readily learned the FR2 task and were less likely to become helpless. In contrast, even males that were not previously stressed did not learn to escape in the FR2 task and thus exhibited evidence of helplessness, despite the fact that they had the opportunity to escape. It could also be argued that the males were less likely than females to reenter the chamber in which they had just been shocked and thus learned better than females. In the end, it can only be said that controllability had a more pronounced effect in males, because those that learned to control the stress were “protected” from helplessness behavior and the reduced proliferation effect. Males that were either exposed to uncontrollable stress or could not learn to escape (no stress group) were more likely to “give-up” than were females exposed to the same conditions.
The present study confirms previous findings that uncontrollable stress induces learned helplessness behavior and decreases hippocampal cell proliferation in male rats (Malberg and Duman 2003) and that cell proliferation in the hippocampus is affected by uncontrollable, but not controllable stress (Bland, Schmid, Greenwood, Watkins, and Maier 2006). The means by which uncontrollable stress reduces proliferation are unknown, but serotonin is likely involved. Chronic treatment with serotonergic drugs can reverse or prevent the effects of uncontrollable stress on neurogenesis (Santarelli, Saxe, Gross, Surget, Battaglia, Dulawa, Weisstaub, Lee, Duman, Arancio, Belzung, and Hen 2003;Malberg and Duman 2003). Also, exposure to similar types of stressors as used here, activate neurons in the dorsal raphe nucleus (Maier and Watkins 2005), which send serotonergic projections to the hippocampus (Conrad, Leonard, and Pfaff 1974;Maier and Watkins 2005) and affect neurogenesis (Brezun and Daszuta 1999;Huang and Herbert 2005). The stress hormone corticosterone could also be involved, since it decreases cell proliferation in the adult hippocampus (Cameron and Gould 1994). If it is necessary, it is probably not sufficient, since controllable and uncontrollable stress increase corticosterone to similar levels that remain elevated even in the animals that learn to control the stressor (Shors, Seib, Levine, and Thompson 1989).
The present results suggest that learning to control a stressor over days can alter the production of new cells in the male hippocampus, but they do not necessarily indicate that new cells are involved in learning how to control the stressor. This scenario is unlikely for several reasons. First, the cells would be very immature at the time of the training experience. The cells become incorporated into the granule cell layer more than a week after birth and well after the training experience has ended (Cameron, Woolley, McEwen, and Gould 1993;Zhao, Teng, Summers, Ming, and Gage 2006). When BrdU was injected during training, we observed no increase in cell numbers (Gould, Beylin, Tanapat, Reeves, and Shors 1999). Also, processes of learning that increase neurogenesis have generally been limited to those that depend on the hippocampus (Leuner, Gould, and Shors 2006). Because learning the operant response tested here does not depend on the hippocampus, it is unlikely that such learning would increase neurogenesis. However, males that learned to control the stressor over seven days of training produced more cells than the other groups, including their respective females. Thus, it is possible that the training regimen was a form of environmental enrichment which enhanced proliferation, as it has been shown before (Kempermann, Kuhn, and Gage 1997).
The current data suggest that the new cells in the female hippocampus are less responsive to stress, as suggested by others. For example, neurogenesis in adult females is reportedly not affected by predator odors, whereas it is decreased in males (Falconer and Galea 2003). Similarly, hippocampal cell proliferation in females was not affected by 8 days of uncontrollable footshock stress, and reportedly increased after longer exposure (21 days) (Westenbroek, Den Boer, Veenhuis, and Ter Horst 2004). Since we did not compare the animals trained over seven days to naïve controls, we cannot state with certainty that neurogenesis was reduced in both groups – those exposed to uncontrollable and controllable stress over the seven days. This explanation seems unlikely since one session of stress did not alter proliferation relative to naïve controls. Also, we did not assess the effects of stress on neurogenesis for each stage of the estrous cycle, but did yoke the females according to the stage of estrous at the beginning of training and in most cases, the pairs were in the same stage at the time of sacrifice. With this strategy, there was no effect of controllability on neurogenesis in the female hippocampus.
In these studies, we attempted to relate sex differences in neurogenesis with the expression of helplessness behavior. First, there were pronounced sex differences in helplessness behavior and to our surprise, sex differences were evident in naïve animals that were simply trained on the two types of operant conditioning tasks. In general, females outperformed males during the early trials of training, irrespective of whether they were previously exposed to a stressor. Sex differences in performance during the FR1 task dissipated quickly, but persisted for animals trained on the FR2 task. In fact, males provided no evidence that they would learn to escape. It is as if they became helpless during the course of training. Sex differences in avoidance behavior have been also noted previously and may reflect sex differences in activity and/or different strategies in response to the footshock (Kirk and Blampied 1985;Steenbergen, Heinsbroek, van Hest, and van de Poll 1990;Beatty and Beatty 1970). Females often respond to the shock with increased activity, whereas males tend to freeze (Shors 1998).
Some of these effects are dependent on the stage of estrus in which the training occurs (Jenkins, Williams, Kramer, Davis, and Petty 2001), although those reported here are probably not. We have recent data showing that these sex differences remain even in ovariectomized females and thus occur in the absence of estrogen and progesterone (Dalla, Edgecomb, Mathew, and Shors 2006).
The data presented suggest that females are resistant to the negative effects of stress on learning an operant response, in which volitional motor activity is required to learn. Similar effects are not observed for example with classical conditioning and are in fact, quite the opposite. Using a classically conditioned eyeblink task, we have found that exposure to uncontrollable stress significantly reduces learning ability in females and actually enhances learning in males (Leuner, Mendolia-Loffredo, and Shors 2004). Thus, it cannot be said that stress does not affect females as much as males; rather the effects of stress on learning in females are often different from those expressed by males. These differences add to the growing body of evidence suggesting that females respond differently to stressful experience than do males (Cahill 2006;Bale 2006;McCarthy and Konkle 2005), differences which may relate to sex differences in mental illness, especially those associated with depression.
Acknowledgments
This work was supported by NIH (NIMH 59970), (NIMH 59740), and NSF (IOB-0444364) to T.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Beatty WW, Beatty PA. Hormonal determinants of sex differences in avoidance behaviors in rodents: organizational and activational influences. J Comp Physiol Psychol. 1970;73:446–455. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- 3.Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress–induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–597. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- 4.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neurosci. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 5.Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. Journal of Clinical Psychiatry. 2002;63:9–15. [PubMed] [Google Scholar]
- 6.Cahill L. Why sex matters for neuroscience. Nature Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 7.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neurosci. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 8.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 9.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neurosci. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. Journal of Neurochemistry. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 11.Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- 12.Conrad LC, Leonard CM, Pfaff DW. Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1974;156:179–205. doi: 10.1002/cne.901560205. [DOI] [PubMed] [Google Scholar]
- 13.Dalla C, Edgecomb C, Mathew J, Shors TJ. Females do not express learned helplessness behavior. Soc Neurosci Abstr. 2006 doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- 14.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falconer EM, Galea LAM. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975:22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- 16.Gould E, Beylin AV, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the adult hippocampal formation. Nature Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 17.Huang GJ, Herbert J. Serotonin modulates the suppressive effects of corticosterone on proliferating progenitor cells in the dentate gyrus of the hippocampus in the adult rat. Neuropsychopharmacology. 2005;30:231–241. doi: 10.1038/sj.npp.1300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Molecular Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins JA, Williams P, Kramer GL, Davis LL, Petty F. The influence of gender and the estrous cycle on learned helplessness in the rat. Biol Psychiatry. 2001;58:147–158. doi: 10.1016/s0301-0511(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 20.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 21.Kessler R, McGonagle K, Swartz M, Blazer D, Nelson C. Sex and depression in the National Comorbidity Survey, I. Life-time prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 22.Kirk RC, Blampied NM. Activity during inescapable shock and subsequent escape avoidance learning: female and male rats compared. New Zealand J Psychol. 1985;14:9–14. [Google Scholar]
- 23.Kornstein SG, Schatzberg AF, Thase mE, Yonkers KA, McCullough JP, et al. Gender differences in the treatment response to sertraline versus imipramine in chronic depression. American Journal of Psychiatry. 2000;158:1531–1533. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 24.Kosorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuner B, Gould E, Shors TJ. Is there a link between neurogenesis and learning? . Hippocampus. 2006 doi: 10.1002/hipo.20153. In press. [DOI] [PubMed] [Google Scholar]
- 26.Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive shock therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 29.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 31.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martenyi F, Dossenback M, Mraz K, Metcalfe S. Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: a double-blind trial of antidepressants with serotonergic and norepinephrinergic reuptake inhibition. European Neuropsychopharmacology. 2001;11:227–232. doi: 10.1016/s0924-977x(01)00089-x. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Frontiers in Neuroendocrinology. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 35.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 36.Neumeister A, Wood S, Bonne O, Nugent aC, Luckenbaugh Da, Young T, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Overmier JB, Seligman MEP. Effects of inescapable shock on subsequent escape and avoidance learning. J Comp Physiol Psychol. 1967;63:23–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- 38.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 39.Seligman MEP. Helplessness. San Francisco: Freeman; 1975. [Google Scholar]
- 40.Seligman MEP, Maier SF. Failure to escape traumatic shock. J Comp Physiol Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- 41.Shors TJ. Stress and sex effects on associative learning: For better or for worse. The Neuroscientist. 1998;4:353–364. [Google Scholar]
- 42.Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation (LTP) in rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 43.Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.06.012. in press. [DOI] [PubMed] [Google Scholar]
- 44.Steenbergen HL, Heinsbroek RPW, van Hest A, van de Poll NE. Sex-dependent effects of inescapable shock administration on shuttle-box escape performance and elevated plus-maze behavior. Physiol Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- 45.Tanapat P, Hastings NB, Rydel TA, Galea LAM, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 46.Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 47.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anatomical Record. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 48.Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Zhao C, Teng eM, Summers RG, Ming G, Gage FH. Distinct morphological stages of dentate gyrus neuron maturation in the adult mouse hippocampus. The Journal of Neuroscience. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]