Abstract
Objective
To quantify the energy efficiency of locomotion and free-living physical activity energy expenditure of transfemoral amputees using a mechanical and microprocessor-controlled prosthetic knee.
Design
Repeated-measures design to evaluate comparative functional outcomes.
Setting
Exercise physiology laboratory and community free-living environment.
Participants
Subjects (N=15; 12 men, 3 women; age, 42±9y; range, 26 –57y) with transfemoral amputation.
Intervention
Research participants were long-term users of a mechanical prosthesis (20±10y as an amputee; range, 3–36y). They were fitted with a microprocessor-controlled knee prosthesis and allowed to acclimate (mean time, 18±8wk) before being retested.
Main Outcome Measures
Objective measurements of energy efficiency and total daily energy expenditure were obtained. The Prosthetic Evaluation Questionnaire was used to gather subjective feedback from the participants.
Results
Subjects demonstrated significantly increased physical activity–related energy expenditure levels in the participant’s free-living environment (P=.04) after wearing the microprocessor-controlled prosthetic knee joint. There was no significant difference in the energy efficiency of walking (P=.34). When using the microprocessor-controlled knee, the subjects expressed increased satisfaction in their daily lives (P=.02).
Conclusions
People ambulating with a microprocessor-controlled knee significantly increased their physical activity during daily life, outside the laboratory setting, and expressed an increased quality of life.
Keywords: Artificial limbs, Energy metabolism, Physical effort, Rehabilitation
Limb loss affects approximately 1.9 million people in the United States,1 of whom 400,000 have amputations above the knee.2 Amputations because of dysvascular conditions accounted for most (82%) limb loss and increased at a rate of 27% from 1988 to 1996.3 A sharp rise in diabetes mellitus is a secondary consequence of obesity that is becoming an epidemic in high-income countries.4 Once a person develops diabetes, there is a 9.9%, twenty-year cumulative incidence of a lower-extremity amputation.5 Further, once a person undergoes a lower-limb amputation, 26% will require a subsequent amputation within 12 months.6 War-related amputations are also on the rise. In the military sector, Kevlar vests have proven dramatically effective in preventing mortal injuries.7 However, exposed limbs are subjected to blast injuries, resulting in increased amputation rates.
After lower-extremity amputation, a person is routinely prescribed a prosthesis that may include a prosthetic foot, pylon, knee, and socket, depending on the level of amputation. There are a number of presently available cost-effective mechanical prosthetic knee components for transfemoral amputees. 8 For the transfemoral amputee, the proper selection of the prosthetic knee is critical because this joint requires the highest degree of control for safe ambulation.9
According to Medicare, the most prescribed mechanical prosthesis for above-knee amputees with a medium to high activity level is the Mauch SNS hydraulic knee (or its equivalent),10 which was first marketed in 1968.11 Over the last 10 years, a new generation of knees with microprocessor control over swing and stance phases of gait has been used by more than 10,000 American transfemoral amputees (K. Walsh, Otto Bock Healthcare, written communication, July 2007). Comparative studies have documented improved gait symmetry,12,13 lower energy consumption,13–22 decreased cognitive demand,9,19,20,23 improved performance on stair and hill descent,9 reduction in stumbles and falls,9 and increased satisfaction9 when using a microprocessor-controlled knee.
Only 2 studies have evaluated the comparative effectiveness of microprocessor-controlled knees by investigating patients in their free-living environment. Hafner et al9 asked subjects to ambulate down a 19° grade and on an uneven terrain obstacle course. Klute et al24 quantified step counts and minutes of activity of amputees in the community environment. However, no study has quantified energy expenditure in the free-living environment. Therefore, the purpose of this study was to quantify the comparative functional outcome of active transfemoral amputees using a microprocessor-controlled knee. We hypothesized that a microprocessor-controlled knee would give the patient improved prosthetic control that would result in increased free-living activity.
METHODS
Study Design
The study employed a repeated-measures experimental design whereby only the prosthetic knee joint was changed. The same socket and suspension was used for both studies to eliminate socket fit and suspension variables. Subjects served as their own controls. They were tested with a mechanical fluid-controlled knee prosthesis (11 Mauch SNS,a 2 CaTach,b 1 Black Max,c 1 Century 2000a) and retested with a microprocessor-controlled knee joint (Otto Bock C-Legd). The mechanical prosthesis was tested first because the patients were already acclimated to it. Each subject was then given an acclimation period before testing commenced on the microprocessor-controlled knee. The average acclimation time was 18±8 weeks (range, 10–39wk). The study design was chosen because it reproduced the clinical experience of most transfemoral amputees. An experienced prosthetist, who was certified by the American Board Certification in Orthotics and Prosthetics and had also successfully completed special training in the application of the microprocessor knee, performed all alignment and fitting trials. Prosthetic alignment was confirmed with an L.A.S.A.R. posture system.d
Participants
Fifteen subjects (12 men, 3 women; age, 42±9y; range, 26–57y) with a transfemoral amputation participated in this study. The study was approved by the institutional review board, and informed written consent was obtained. The criteria for enrollment included unilateral transfemoral amputees age 18 and older, amputation for any reason, a minimum of 2 years postamputation, Medicare functional classification level 3 or 4, and current use of a hydraulic control mechanical prosthetic knee. The exclusion criteria were chronic residual limb skin breakdown and secondary medical conditions that would prevent participation in the study. Subjects were also excluded if they had an acute illness or chronic illness or required assistive aids for ambulation. All subjects were required to pass a prosthetic evaluation and functional evaluation conducted by a certified prosthetist. The certified prosthetist checked the prosthetic alignment and observed the gait of each subject to confirm that the subject had obtained optimal gait for each prosthetic knee. Participants in the study were required to be competent with their mechanical knee prostheses before entering the study. These participants were all long-term prosthesis users (20±10y; range, 3–36y). Their body mass index, without the prosthesis, was 25±4kg/m2 (range, 17–28kg/m2). The reasons for amputation were trauma (n=7), cancer (n=6), peripheral vascular disease (n=1), and congenital (n=1). To be considered for this study, stump volume must not have fluctuated significantly within the 6 months before the study. All subjects were unlimited community ambulators. Subjects had no other neuromuscular problems or a partial amputation of the contralateral limb that would preclude them from performing the test protocol.
Experimental Testing
Energy efficiency
An accurate measure of oxygen cost under steady-state conditions was obtained to quantify relative walking efficiency. Subjects walked on an electronically controlled treadmille while breathing into a mouthpiece. Subjects breathed through a disposable pneumotachograph that contained the mass spectrometer gas sampling port. The pneumotachograph registered flow by comparing impact and stagnation pressures in a region of slight narrowing of the flowing gas stream. Breath-by-breath measurements were made using a commercially available automated systemf modified to interface to a respiratory mass spectrometer.g The measurement system was calibrated by using precision-grade gas mixtures. Procedures were applied to ensure appropriate temporal matching between volume (flow) and expired gas fractions.25 Calculation of oxygen and carbon dioxide consumption was performed using the Haldane transformation.26 A steady state was attained in approximately 2 to 3 minutes after exercise was begun. Testing was performed at 3 speeds of 1.6, 3.2, and 4.8km/h. The primary outcome variable was the energy cost a meter (in mL·kg−1·m−1).27 This describes the amount of oxygen needed to walk a unit distance—that is, the energy efficiency. Subjects were also asked to rank their relative effort using the Borg Rating of Perceived Exertion.28
Daily energy expenditure
The TDEE was estimated using the DLW method.29 This is the most accurate and robust method available to estimate energy expenditure in free-living conditions.30 This procedure required that the research volunteers consumed water containing isotopes of oxygen and hydrogen. Accurately weighed doses of deuterium (.978g/kg of BW) and oxygen-18 (.092g/kg of BW) were administered orally. Three urine samples were collected before dosing, and then 3 timed urine samples were collected a day for 10 days. The urine samples were analyzed by using a continuous flow isotope-ratio mass spectrometer.h Analysis of TDEE involved the use of standardized techniques.31,32 Measurement of the difference in clearance of the 2 isotopes from the body represented carbon dioxide production,33,34 which in turn reflected energy expenditure. The TEF was estimated to be 10% of TDEE.35 BMR with respiratory quotient was measured using indirect calorimetry36 before the DLW was consumed. The TDEE was partitioned into the BMR, TEF, and PAEE. The portion of TDEE attributed to physical activity was calculated as the ratio of PAEE to TDEE.
Prosthesis Evaluation Questionnaire
The condition-specific PEQ was used to quantify patient satisfaction. The PEQ is a reliable and valid tool for evaluating persons with lower-limb amputations.37 The questionnaire is composed of 9 validated scales (ambulation, appearance, frustration, perceived response, residual limb health, social burden, sounds, utility, well-being). Scales have been validated for internal consistency and temporal stability.37
Data Analysis
Statistical analysis was performed using a commercial analysis package.i A 2-factor repeated-measures analysis of variance was performed to compare outcomes using the mechanical knee compared with the microprocessor-controlled knee prosthesis. A multivariate approach was used to compare simultaneously the energy efficiency at all walking speeds. Similarly, all PEQ subscales were compared simultaneously. A paired t test was used to compare the PAEE and portion of TDEE attributed to physical activity between the 2 prosthetic knees. Statistical significance was set at P equal to .05.
RESULTS
The functional limitation of each subject was evaluated by assessing their energy efficiency. The energy efficiency was 2.3% lower (95% confidence interval, −6.6 to 2.0) when using the microprocessor-controlled knee (fig 1A). However, this difference was not statistically significant (P=.34). Notably, the subject’s perception was that it was easier to walk with the microprocessor-controlled knee than with the mechanical prosthesis (P=.02) (fig 1B).
Fig 1.
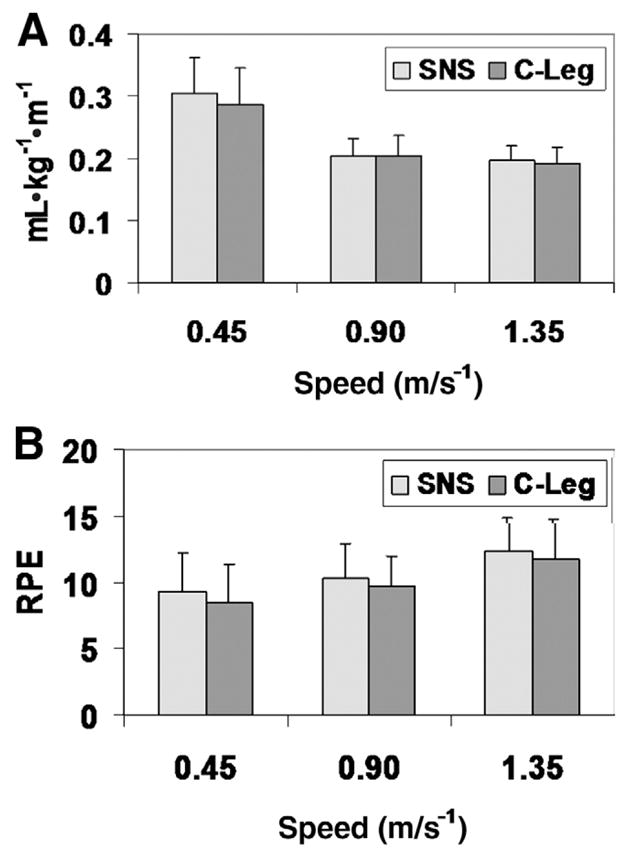
(A) Energy efficiency. (B) Rating of perceived exertion while walking at 3 speeds. There was a nonsignificant (P=.34) improvement in energy efficiency when using the microprocessor-controlled knee. The subject’s perception was that it was easier to walk with the microprocessor-controlled knee (P=.002). Error bars are the SDs of the data. Abbreviation: RPE, rating of perceived exertion.
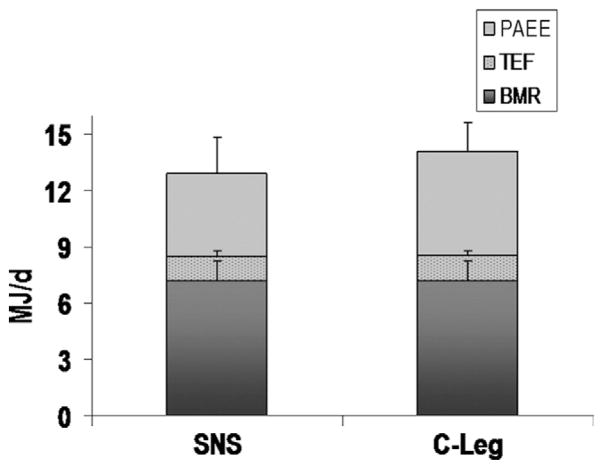
The disability of the research participants was quantified by measuring the TDEE in their free-living environment. The TDEE was higher when the participants wore the microprocessor-controlled knee compared with the mechanical prosthesis (14.1 vs 13.0MJ/d, respectively) (fig 2). The average BMR for the research participants was 7.2±1.0MJ/d. There was a significant increase (P=.04) in PAEE when using the microprocessor-controlled prosthesis compared with the mechanical prosthesis (5.5 vs 4.4MJ/d, respectively). Further, there was a significant 6% increase (P=.02) in the portion of TDEE attributed to physical activity. The amputees expended 33% of their TDEE in physical activity when using the mechanical prosthesis compared with 39% for the microprocessor-controlled prosthesis.
Fig 2.
TDEE using the mechanical prosthesis (SNS) compared with the microprocessor-controlled knee (C-Leg). The TDEE was partitioned into the BMR, TEF, and PAEE. The PAEE was significantly higher when using the microprocessor-controlled prosthesis (P=.04). Error bars are the SDs of the data.
An important aspect of this study was the patient’s perception of the 2 prosthetic knee joints (fig 3). The microprocessor-controlled knee was rated significantly better than the mechanical prosthesis (P=.02). The microprocessor-controlled knee scored better in 8 of 9 categories on the PEQ. The only category in which the microprocessor-controlled knee scored lower was perceived response. In this category, 8 of 15 subjects rated the microprocessor-controlled knee equal or better than the mechanical prosthesis, whereas the remaining 7 subjects scored the microprocessor-controlled prosthesis sufficiently lower such that the overall mean was less for the microprocessor-controlled knee.
Fig 3.
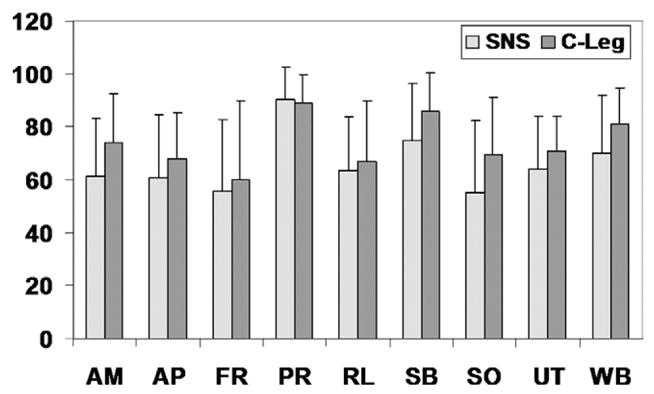
Results of the condition-specific PEQ used to quantify patient satisfaction for the mechanical prosthesis (SNS) and the microprocessor-controlled knee (C-Leg). The microprocessor-controlled knee rated significantly better than the mechanical prosthesis (P=.02). Abbreviations: AM, ambulation; AP, appearance; FR, frustration; PR, perceived response; RL, residual limb health; SB, social burden; SO, sounds; UT, utility; WB, well-being. Error bars are the SDs of the data.
DISCUSSION
To maintain an amputee’s functional status, every effort is made to return the person with a lower-limb amputation to a satisfying locomotor status in their personal living environment. The primary reason expressed by people with lower-extremity amputations for curtailed or limited use of a prosthesis is that walking with the artificial limb is too exhausting.38 The key finding in this study is that transfemoral amputees using a microprocessor-controlled knee spontaneously increased their daily physical activity outside of the laboratory setting. The increase in PAEE with the microprocessor-controlled knee represented more physical movement rather than an increased effort to walk because the energy efficiency of locomotion was statistically equivalent for both prosthetic knees.
The mechanism responsible for the increased activity in patients using a microprocessor-controlled knee is explained by changes in the gait and balance of these patients.39 The gait improved by placing more reliance on the prosthetic limb when using the microprocessor-controlled knee. The participants walked with a more normative gait pattern, which included stance phase knee flexion and an external knee flexion moment during loading response. The improved balance characteristics when using the microprocessor-controlled knee reduced the risk of falling. Falls are a significant problem that affects people with a lower-limb amputation. Among community-living people with a lower-extremity amputation, 52% fell in the past 12 months, 49% were fearful of falling, and 65% had low balance confidence scores.40 Moreover, people with a transfemoral amputation are at significantly greater risk of falling.38,40 Importantly, falling experience and balance confidence are associated with mobility capability and social activity.41 We have determined that after receiving a microprocessor-controlled knee, amputees had improved balance,39 which resulted in greater confidence to increase their personal activity level. Notably, the patients in this study perceived that it was easier for them to walk with the microprocessor-controlled knee. This also contributed to their increased activity levels.
Energy expenditure as measured by oxygen consumption rate is important for amputees. The energy cost of ambulation is greater for amputees than for nonamputees.18 Several studies have compared the energy consumption of the microprocessor-controlled with nonmicroprocessor-controlled knees. These studies generally report lower energy expenditure when using a microprocessor-controlled knee.13–15,19,21,42,43 One study has also demonstrated an improvement in the rate of oxygen consumption for a person with bilateral knee disarticulations.22 There does not appear to be any difference in oxygen consumption rate between different microprocessor-controlled knees.16,21 The results of the present study are similar to reports in the literature. Overall, the users experienced a 2.3% decrease—that is, improvement—in the energy efficiency of walking when using their microprocessor-controlled knees. This difference was not statistically significant. However, the users perceived the microprocessor-controlled knee to require less effort during ambulation. Therefore, the improved energy efficiency could be considered clinically significant.
This is the first study to measure the TDEE of amputees using the DLW method. This technique is regarded as the criterion standard for measuring all components of energy expenditure including the energy cost for spontaneous and voluntary physical activity. The amputees in this study expended more energy than young able-bodied subjects,44 healthy elderly,44,45 or overweight subjects.46 The total energy expenditure by research participants in this study was comparable to the energy expenditure of elite female runners,47,48 mountain climbers,49 and soldiers.50,51 The portion of TDEE attributed to physical activity for the subjects in this study (33% and 39%, respectively, for the passive mechanical prosthesis and the microprocessor-controlled prosthesis) was higher than for elderly subjects (23%),45 normative weight people (30%),46 and overweight people (32%).46 This high proportion of energy expenditure explains why amputees frequently complain about fatigue.
Few studies have examined the effect of prosthetic intervention on activity level in the daily lives of lower-extremity amputees. Measurements in the free-living environment are important because they document changes that affect net health outcomes. This study demonstrated a spontaneous increase in physical activity when the transfemoral amputees switched from a mechanical to a microprocessor-controlled prosthetic knee component. Our findings differ from those reported by Klute et al,24 who did not find any significant difference in steps a day or minutes a day of activity for the same types of prosthetic components—that is, Mauch SNS versus C-Leg knees. Our findings also differ from those of Hafner et al,9 who reported nonsignificant differences in step frequency and daily distance traveled between mechanical and microprocessor-controlled prosthetic knees. The difference in reported outcomes between these 2 studies and the current study is related to the methodologic difference of these studies. The current study quantified changes at the metabolic level. The studies by Klute24 and Hafner9 used a surrogate measure—that is, step counts—to imply changes at the metabolic level. These 2 methods are not equivalent. For example, a person could walk the same number of steps and expend differing amounts of energy by walking uphill versus downhill. Using the DLW method allowed us to quantify changes in metabolic energy expenditure more accurately.
Study Limitations
There are some limitations to be considered in this study. The knee accommodation time in this study was 4.5 months. This may not have been enough time for all subjects to adapt, but a recent study9 has shown that 3.5 months was the average time required for accommodation to a microprocessor-controlled knee. Further, although this study demonstrates that patients can benefit from a microprocessor-controlled knee, this study was conducted on patients who could be considered unlimited community ambulators. This study does not define the characteristics of patients who can benefit from a microprocessor-controlled knee. People with limited ambulatory ability may not be able to take advantage of advanced technologic features in a microprocessor-controlled knee. However, based on results from Hafner,9 K-level 2 ambulators appear to benefit from reduced stumbles and falls. Additional research needs to be performed to define clearly which patients can benefit from this advanced technology.
CONCLUSIONS
Some insurance companies have been reluctant to provide coverage for microprocessor-controlled knees. These third-party payers acknowledge that microprocessor-controlled lower-limb prostheses represent a promising new technology.52 They consider microprocessor-controlled lower-limb prostheses investigational and experimental because of a lack of sufficient evidence substantiating their effectiveness in reducing disability and improving function over passive mechanical leg prostheses.52 Specifically, they state, “there is a paucity of peer-reviewed, randomized studies evaluating the effectiveness of microprocessor controlled lower limb prostheses. … the evidence supporting the broad effectiveness of microprocessor-controlled prosthetic knees remains inconclusive.”52 Data presented in this study and others9,12–23,39,42,43 demonstrate that microprocessor-controlled knees offer significant efficiency and effectiveness over conventional lower-limb prostheses. This study has demonstrated that when using a microprocessor-controlled knee, patients are more active in their free-living environment and report an improved quality of life.
Acknowledgments
Supported by the National Center for Research Resources (grant no. M01 RR000585); the National Institutes of Diabetes and Digestive and Kidney Diseases (grant nos. R01 DK63226, R01 DK66270); and Otto Bock Healthcare Inc.
We thank Barbara Iverson, AAS, for her role as study coordinator.
List of Abbreviations
- BMR
basal metabolic rate
- BW
body weight
- DLW
doubly labeled water
- PAEE
physical activity–related energy expenditure
- PEQ
Prosthetic Evaluation Questionnaire
- TDEE
total daily energy expenditure
- TEF
thermic effect of food
Footnotes
Össur, Grjothals 5, 110 Reykjavik, Iceland.
CaTach Inc, 80-A Westpark Drive, Centerville, OH 45459.
TruLife LTD, 3 Cookstown Industrial Estate, Tallaght, Dublin, 24 Ireland.
Otto Bock, Max-Näder-Str 15, Duderstadt, 37115 Germany.
Q-Stress TM65; Quinton Cardiology Systems Inc, 2121 Terry Ave, Seattle, WA 98121.
Model CPX-D; Medical Graphics Corp, 350 Oak Grove Pkwy, St. Paul, MN 55127.
Model 1100; PerkinElmer Life and Analytical Sciences Inc, 940 Winter St, Waltham, MA 02451.
ThermoFinnigan Delta; Thermo Fisher Scientific Inc, 81 Wyman St, Waltham, MA 02454.
Version 9.1; SAS Institute, 100 SAS Campus Dr, Cary, NC 27513–2414.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
References
- 1.Amputation Coalition of America. [Accessed March 14, 2006];Amputation statistics by cause: limb loss in the United States. Available at: http://www.amputee-coalition.org.
- 2.Adams PF, Hendershot GE, Marano MA Centers for Disease Control and Prevention/National Center for Health Statistics. Current estimates from the National Health Interview Survey, 1996. Vital Health Stat 10. 1999;200:1–203. [PubMed] [Google Scholar]
- 3.Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95:875–83. doi: 10.1097/00007611-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–17. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 5.Moss SE, Klein R, Klein BE, Wong TY. Retinal vascular changes and 20-year incidence of lower extremity amputations in a cohort with diabetes. Arch Intern Med. 2003;163:2505–10. doi: 10.1001/archinte.163.20.2505. [DOI] [PubMed] [Google Scholar]
- 6.Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86:480–6. doi: 10.1016/j.apmr.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 7.Gawande A. Casualties of war: military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471–5. doi: 10.1056/NEJMp048317. [DOI] [PubMed] [Google Scholar]
- 8.Michael JW. Modern prosthetic knee mechanisms. Clin Orthop Relat Res. 1999 Apr;361:39–47. doi: 10.1097/00003086-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hafner BJ, Willingham LL, Buell NC, Allyn KJ, Smith DG. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil. 2007;88:207–17. doi: 10.1016/j.apmr.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid Services. [Accessed March 14, 2006];Part B Extract Summary System (BESS) data file, 2000–2005. Available at: http://www.cms.hhs.gov/NonIdentifiableDataFiles/01_PartBExtractSummarySystem.asp#TopOfPage.
- 11.Wilson AB. History of amputation surgery and prosthetics. St Louis: CV Mosby; 1981. [Google Scholar]
- 12.Kirker S, Keymer S, Talbot J, Lachmann S. An assessment of the intelligent knee prosthesis. Clin Rehabil. 1996;10:267–73. [Google Scholar]
- 13.Schmalz T, Blumentritt S, Jarasch R. Energy expenditure and biomechanical characteristics of lower limb gait: the influence of prosthetic alignment and different prosthetic components. Gait Posture. 2002;16:255–63. doi: 10.1016/s0966-6362(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 14.Buckley JG, Spence WD, Solomonidis SE. Energy cost of walking: comparison of “intelligent prosthesis” with conventional mechanism. Arch Phys Med Rehabil. 1997;78:330–3. doi: 10.1016/s0003-9993(97)90044-7. [DOI] [PubMed] [Google Scholar]
- 15.Taylor MB, Clark E, Offord EA, Baxter C. A comparison of energy expenditure by a high-level transfemoral amputee using the intelligent prosthesis and conventionally damped prosthetic limbs. Prosthet Orthot Int. 1996;20:116–21. doi: 10.3109/03093649609164428. [DOI] [PubMed] [Google Scholar]
- 16.Chin T, Machida K, Sawamura S, et al. Comparison of different microprocessor controlled knee joints on the energy consumption during walking in trans-femoral amputees: intelligent knee prosthesis (IP) versus C-leg. Prosthet Orthot Int. 2006;30:73–80. doi: 10.1080/03093640500533414. [DOI] [PubMed] [Google Scholar]
- 17.Chin T, Sawamura S, Shiba R, Oyabu H, Nagakura Y, Nakagawa A. Energy expenditure during walking in amputees after disarticulation of the hip: a microprocessor-controlled swing-phase control knee versus a mechanical-controlled stance-phase control knee. J Bone Joint Surg Br. 2005;87:117–9. [PubMed] [Google Scholar]
- 18.Chin T, Sawamura S, Shiba R, et al. Effect of an Intelligent Prosthesis (IP) on the walking ability of young transfemoral amputees: comparison of IP users with able-bodied people. Am J Phys Med Rehabil. 2003;82:447–51. [PubMed] [Google Scholar]
- 19.Datta D, Heller B, Howitt J. A comparative evaluation of oxygen consumption and gait pattern in amputees using Intelligent Prostheses and conventionally damped knee swing-phase control. Clin Rehabil. 2005;19:398–403. doi: 10.1191/0269215505cr805oa. [DOI] [PubMed] [Google Scholar]
- 20.Datta D, Howitt J. Conventional versus microchip controlled pneumatic swing phase control for trans-femoral amputees: user’s verdict. Prosthet Orthot Int. 1998;22:129–35. doi: 10.3109/03093649809164474. [DOI] [PubMed] [Google Scholar]
- 21.Johansson JL, Sherrill DM, Riley PO, Bonato P, Herr H. A clinical comparison of variable-damping and mechanically passive prosthetic knee devices. Am J Phys Med Rehabil. 2005;84:563–75. doi: 10.1097/01.phm.0000174665.74933.0b. [DOI] [PubMed] [Google Scholar]
- 22.Perry J, Burnfield JM, Newsam CJ, Conley P. Energy expenditure and gait characteristics of a bilateral amputee walking with C-leg prostheses compared with stubby and conventional articulating prostheses. Arch Phys Med Rehabil. 2004;85:1711–7. doi: 10.1016/j.apmr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Heller BW, Datta D, Howitt J. A pilot study comparing the cognitive demand of walking for transfemoral amputees using the Intelligent Prosthesis with that using conventionally damped knees. Clin Rehabil. 2000;14:518–22. doi: 10.1191/0269215500cr345oa. [DOI] [PubMed] [Google Scholar]
- 24.Klute GK, Berge JS, Orendurff MS, Williams RM, Czerniecki JM. Prosthetic intervention effects on activity of lower-extremity amputees. Arch Phys Med Rehabil. 2006;87:717–22. doi: 10.1016/j.apmr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Proctor DN, Beck KC. Delay time adjustment to minimize errors in breath-by-breath measurement of VO2 during exercise. J Appl Physiol. 1996;81:2495–9. doi: 10.1152/jappl.1996.81.6.2495. [DOI] [PubMed] [Google Scholar]
- 26.Jones NL. Clinical exercise testing. 3. Philadelphia: WB Saunders; 1988. [Google Scholar]
- 27.Kaufman KR, Irby SE, Wirta RW, Sutherland DH. Energy efficient knee-ankle-foot orthosis: a case study. J Prosthet Orthot. 1996;8:79–85. [Google Scholar]
- 28.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 29.Lifson N, Gordon GB, McClintock R. Measurements of total carbon dioxide production by means of D2O18. J Appl Physiol. 1955;7:704–10. doi: 10.1152/jappl.1955.7.6.704. [DOI] [PubMed] [Google Scholar]
- 30.Montoye HJ, Kemper HC, Saris WH, Washburn RA. Measuring physical activity and energy expenditure. Champaign: Human Kinetics; 1995. [Google Scholar]
- 31.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–4. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 32.Starling RD, Toth MJ, Carpenter WH, Matthews DE, Poehlman ET. Energy requirements and physical activity in free-living older women and men: a doubly labeled water study. J Appl Physiol. 1998;85:1063–9. doi: 10.1152/jappl.1998.85.3.1063. [DOI] [PubMed] [Google Scholar]
- 33.Coward WA, Roberts SB, Cole TJ. Theoretical and practical considerations in the doubly-labeled water (2H218O) method for the measurement of carbon dioxide production rate in man. Eur J Clin Nutr. 1988;42:207–12. [PubMed] [Google Scholar]
- 34.Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr. 1992;56:641–55. doi: 10.1093/ajcn/56.4.641. [DOI] [PubMed] [Google Scholar]
- 35.Rising R, Harper IT, Fontvielle AM, Ferraro RT, Spraul M, Ravussin E. Determinants of total daily energy expenditure: variability in physical activity. Am J Clin Nutr. 1994;59:800–4. doi: 10.1093/ajcn/59.4.800. [DOI] [PubMed] [Google Scholar]
- 36.Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. Am J Clin Nutr. 2000;72:1451–4. doi: 10.1093/ajcn/72.6.1451. [DOI] [PubMed] [Google Scholar]
- 37.Legro MW, Reiber G, del Aguila M, et al. Issues of importance reported by persons with lower limb amputations and prostheses. J Rehabil Res Dev. 1999;36:155–63. [PubMed] [Google Scholar]
- 38.Gauthier-Gagnon C, Grise MC, Potvin D. Predisposing factors related to prosthetic use by people with a transtibial and transfemoral amputation. J Prosthet Orthot. 1998;10:99–109. doi: 10.1016/s0003-9993(99)90177-6. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman KR, Levine JA, Brey RH, et al. Gait and balance of transfemoral amputees using passive mechanical and microprocessor controlled prosthetic knees. Gait Posture. 2007;26:489–93. doi: 10.1016/j.gaitpost.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Miller WC, Speechley M, Deathe B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch Phys Med Rehabil. 2001;82:1031–7. doi: 10.1053/apmr.2001.24295. [DOI] [PubMed] [Google Scholar]
- 41.Miller WC, Deathe AB, Speechley M, Koval J. The influence of falling, fear of falling, and balance confidence on prosthetic mobility and social activity among individuals with a lower extremity amputation. Arch Phys Med Rehabil. 2001;82:1238–44. doi: 10.1053/apmr.2001.25079. [DOI] [PubMed] [Google Scholar]
- 42.Orendurff M, Segal AD, Klute GK, McDowell ML, Pecoraro J, Czerniecki J. Gait efficiency using the C-leg. J Rehabil Res Dev. 2006;43:239–46. doi: 10.1682/jrrd.2005.06.0095. [DOI] [PubMed] [Google Scholar]
- 43.Seymour R, Engebretson B, Kott K, et al. Comparison between the C-leg microprocessor-controlled prosthetic knee and nonmicroprocessor-controlled prosthetic knees: a preliminary study of energy expenditure, obstacle course performance and quality of life survey. Prosthet Orthot Int. 2007;31:51–61. doi: 10.1080/03093640600982255. [DOI] [PubMed] [Google Scholar]
- 44.Harris AM, Lanningham-Foster LM, McCrady SK, Levine JA. Nonexercise movement in elderly compared with young people. Am J Physiol Endocrinol Metab. 2007;292:E1207–12. doi: 10.1152/ajpendo.00509.2006. [DOI] [PubMed] [Google Scholar]
- 45.Goran MI, Poehlman ET. Total energy expenditure and energy requirements in healthy elderly persons. Metab Clin Exp. 1992;41:744–53. doi: 10.1016/0026-0495(92)90315-2. [DOI] [PubMed] [Google Scholar]
- 46.Welle S, Forbes GB, Statt M, Barnard RR, Amatruda JM. Energy expenditure under free-living conditions in normal-weight and overweight women. Am J Clin Nutr. 1992;55:14–21. doi: 10.1093/ajcn/55.1.14. [DOI] [PubMed] [Google Scholar]
- 47.Edwards JE, Lindeman AK, Mikesky AE, Stager JM. Energy balance in highly trained female endurance runners. Med Sci Sports Exerc. 1993;25:1398–404. [PubMed] [Google Scholar]
- 48.Schulz LO, Alger S, Harper I, Wilmore JH, Ravussin E. Energy expenditure of elite female runners measured by respiratory chamber and doubly labeled water. J Appl Physiol. 1992;72:23–8. doi: 10.1152/jappl.1992.72.1.23. [DOI] [PubMed] [Google Scholar]
- 49.Westerterp KR, Kayser B, Brouns F, Herry JP, Saris WH. Energy expenditure climbing Mt. Everest J Appl Physiol. 1992;73:1815–9. doi: 10.1152/jappl.1992.73.5.1815. [DOI] [PubMed] [Google Scholar]
- 50.DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D218O to measure energy expenditure of soldiers at different energy intakes. J Appl Physiol. 1989;67:1922–9. doi: 10.1152/jappl.1989.67.5.1922. [DOI] [PubMed] [Google Scholar]
- 51.Tharion WJ, Baker-Fulco CJ, Bovill ME, et al. Adequacy of garrison feeding for Special Forces soldiers during training. Mil Med. 2004;169:483–90. doi: 10.7205/milmed.169.6.483. [DOI] [PubMed] [Google Scholar]
- 52. [Accessed April 1, 2008];Empire BlueCross BlueShield: Medical policy: microprocessor controlled lower limb prostheses. Policy OR-PR.00003. Aug 11, 2006. Available at: http://www.empireblue.com/provider/noapplication/f2/s5/t9/pw_ad080410.pdf.