Abstract
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) are frequently used lipid-lowering drugs in type 2 diabetes. Recent emerging evidence suggests that statins protect cardiovascular function via lipid-independent mechanisms. However, the potential role of statins in diabetic retinopathy in type 2 diabetes is largely unclear. In present study we have investigated the effect of lovastatin on blood-retinal barrier and inflammatory status in the retina of db/db mice and in cultured retinal cells. Male C57BL/KsJ db/db mice were randomly chosen to receive gastric gavage of lovastatin (10 mg/kg/day) or vehicle control for 6 weeks. Retinal vascular permeability, the tight junction and inflammation were determined. The results showed that db/db mice at age of 19 weeks exhibited significantly increased retinal vascular leakage and decreased tight junction protein level in the retina. Moreover, the expression of pro-inflammatory factors, e.g. ICAM-1 and TNF-α, were drastically up-regulated in diabetic retina. Lovastatin treatment normalized all of these changes. In cultured bovine retinal capillary endothelial cells (RCEC) and human ARPE-19 cells, lovastatin attenuated the decrease of tight junction protein (occludin) and adherens junction protein (VE-cadherin) expression induced by TNF-α, a major pro-inflammatory cytokine in diabetic retinopathy. Lovastatin also attenuated TNF-α expression in RCEC. Towards the mechanism, we showed that lovastatin ameliorated ICAM-1 expression induced by hypoxia and TNF-α in both RCEC and ARPE-19 cells, in part through inhibition of NF-κB activation. Taken together, these findings indicate that lovastatin protects blood-retinal barrier in diabetic retinopathy, which is likely via its anti-inflammatory effects.
Keywords: statin, diabetic retinopathy, blood-retina barrier, tight junction, inflammation
Diabetic retinopathy is a leading cause of blindness in the working-age population in the US (Fong et al., 2003; The Eye Diseases Prevalence Research Group, 2004). Vision loss in diabetic patients is attributed to two major retinal manifestations: diabetic macular edema (DME) resulting from breakdown of the blood-retinal barrier, and to a less extent, proliferative retinopathy caused by aberrant retinal neovascularization (Cunha-Vaz, 1998). As the pathogenic mechanisms of DME are not fully understood, to date the only universally accepted treatment for DME is laser photocoagulation (Girach and Lund-Andersen, 2007). Despite the success of laser treatment on reducing retinal edema, only a small number of patients were able to obtain moderate visual improvement (Early Treatment Diabetic Retinopathy Study Research Group, 1985). Moreover, side effects, such as worsening of visual acuity, thickening of central retina and reduction of visual field, also limited the application of laser therapy (Early Treatment Diabetic Retinopathy Study Research Group, 1991 ). Thus, non-invasive and effective therapeutics for DME are desperately needed.
Blood-retinal barrier breakdown is a hallmark characteristic of DME (Lopes de Faria et al., 1999; Pelzek and Lim, 2002). Growing evidence suggests that over-expression of pro-inflammatory factors is a crucial step in the initiation of this process (Funatsu et al., 2002; Joussen et al., 2002a). In diabetic patients and diabetes animal models, expressions of tumor necrosis factor-alpha (TNF-α) and intercellular adhesion molecule-1 (ICAM-1) are increased in the retina and vitreous, associated with retinal vascular hyper-permeability (Funatsu et al., 2002; Joussen et al., 2002a; Zhang et al., 2006). TNF-α is a potent pro-permeability factors, inducing ICAM-1 expression and subsequent vascular leakage in vitro and in vivo (Bamforth et al., 1996; Kim et al., 2001). Inhibition TNF-α or ICAM-1 expression or activity significantly reduced retinal leukostasis and vascular leakage in animal models of diabetes and uveitis, indicating a causative role of pro-inflammatory factors in blood-retinal barrier breakdown in diabetic retinopathy (Joussen et al., 2002a; Joussen et al., 2002b; Koizumi et al., 2003; Qaum et al., 2001).
3-Hydroxy-3-methylglutaryl CoA reductase inhibitors (statins) are inhibitors of cholesterol biosynthesis commonly used in the treatment of dyslipidemia (Danesh and Kanwar, 2004; Ludwig and Shen, 2006). Landmark clinical trials indicate that statins reduce cardiac death and cerebrovascular events in the diabetic populations (Colhoun et al., 2005; Collins et al., 2004; Ludwig and Shen, 2006). It is recommended that statins be considered as the drugs of choice for lowering LDL cholesterol in type 2 diabetes, particularly in those of age over 40 and with other cardiovascular risk factors (American Diabetes Association, 2007). In contrast to the extensively studied beneficial effects of statins on cardiovascular system, there are few studies regarding the efficacy of statins on diabetic retinopathy, although regression of hard exudates in the retina has been reported in a small number of diabetic patients (Cusick et al., 2003; Gupta et al., 2004 ; Sen et al., 2002).
In the present study, we aimed to evaluate the effects of systemic administration of lovastatin on the blood-retinal barrier and inflammation in db/db mice, a rodent model of type 2 diabetes. Moreover, we attempted to elucidate the mechanisms of lovastatin on tight junction and regulation of inflammation in cultured retinal capillary endothelial cells (RCEC) and retinal pigment epithelial (RPE) cells.
Materials and Methods
Animals
Male db/db mice (BKS.Cg-m +/+ Leprdb) and their non-diabetic littermates were purchased from the Jackson Laboratory (Bar Harbor, MI). All experiments were conducted according to the guidelines in the care and use of laboratory animals set forth by the University of Oklahoma. Blood glucose and body weight were monitored every week. At 13 weeks of age, db/db mice were randomly assigned into two groups (n=8). Lovastatin were dissolved in vehicle (vegetable oil) at the concentration of 5 mg/ml. One group of db/db mice received daily gastric gavage of lovastatin at 10mg/kg and the other group were given same amount of vehicle gavage. Non-diabetic littermates received same vehicle treatment. After 6 weeks of treatment, the mice were euthanized, eyes enucleated and retinas dissected as described previously (Zhang et al., 2006).
Cell Culture
Primary bovine RCEC were isolated and cultured as described previously (Zhang et al., 2006). ARPE-19 cells were obtained from ATCC (Manassas, VA) and grown in DMEM with 10% FBS. Confluent monolayer cells were exposed to a medium with 1% FBS for 12 hrs followed by treatment with desired reagents. To study the effect of hypoxia on pro-inflammatory factor expression, cells were exposed to hypoxia (2% oxygen) or normoxia (21% oxygen) with the oxygen concentration being correctly regulated using ProOxC system (BioSpherix, Ltd., Lacona, NY). To ensure the hypoxic condition (≤ 2% oxygen) in the medium, medium was purged with nitrogen before applying to the cells and the oxygen content in the medium was measured using an oxygen electrode.
Measurement of blood lipid concentration
Serum concentrations of cholesterol E, free cholesterol C and triglyceride were measured using commercially available kits (Wako Chemicals USA Inc., Richmond, VA) according to manufacturer’s protocol.
Determination of the blood-retinal barrier breakdown
Blood-retinal barrier breakdown in db/db mice was evaluated by extravascular leakage of albumin into the retina. Briefly, animals were deeply anesthetized with ketamine and xylazine. After opening the chest, a catheter was inserted into the left ventricle and a small incision was made on the right atrium. One hundred ml of 1X PBS was infused until blood run clear. Then mice were sacrificed and eyes enucleated. Retinas were carefully dissected and the extravascular content of albumin in the retina was quantified by Western blot analysis.
Western blot analysis
Retinas and cells were lysed in RIPA buffer with protease inhibitor cocktail, PMSF and sodium orthovanadate (Santa Cruz Biotechnology, Santa Cruz, CA). Protein concentration was quantified by BCA protein assay (Pierce Biotechnology, Inc., Rockford, IL). Fifty μg of protein were resolved by SDS-PAGE and then blotted with specific antibodies: anti-albumin (Bethyl Laboratories, Montgomery, TX), anti-occludin (Zymed Laboratory, San Francisco, CA), anti-ICAM-1, anti-VE-cadherin (Santa Cruz Biotechnology) and anti-TNF-α (Abcam, Cambridge, MA). The same membrane was reblotted with an anti-β-actin antibody (Abcam) as loading control.
NF-κB nuclear translocation
ARPE-19 cells were seeded in 96-well plates and grown to 80% confluent. Cells were pre-incubated with or without 1 μM lovastatin for 24 h and exposed to TNF-α (20 ng/ml) for 1 h. Immunocytochemical staining was performed by Hitkit™ HSC Reagent kit (Thermo Fisher Scientific Inc., Waltham, MA) according to manufacturer’s protocol with minor modification. Briefly, cells were fixed and permeabilized for 90 seconds, and then incubated with primary antibody against NF-κB subunit p65 at 4 ºC overnight. After extensive wash, cells were incubated with secondary antibody, and then visualized and photographed under a fluorescent microscope (Olympus, Hamburg, Germany).
NF-κB p65 Transcription Factor Activity Assay
To quantify NF-κB activation in ARPE-19 cells, the activity of p65, a subunit of NF-κB, was assayed by the DNA binding capacity of NF-κB using the TransAM NF-κB kit (Active Motif, Carlsbad, CA) as described previously(Zhang et al., 2008). Briefly, ARPE-19 cells were pre-treated with lovastatin (1 μM) for 24 h and exposed to TNF-α (20 ng/ml) for 1 h. Nuclear proteins were isolated by using the Nuclear Extraction Kit (Active Motif, Carlsbad, CA). Five μg of nuclear extracts were applied to a 96-well plate, which has been immobilized oligonucleotide containing the NFκB consensus site (5′-GGGACTTTCC-3′), and incubated for 1 h at room temperature. After washing, 100 μl of diluted primary antibody was added and incubated for 1 h, followed by incubation with a secondary antibody for another 1 h. The plate was read at 450 nm using a 1420 multilabel counter microplate reader (Perkin Elmer, Waltham, MA). Specificity of the assay was monitored by competition with wild-type NF-κB consensus oligonucleotide or mutated NF-κB consensus oligonucleotide. The experiments were performed in triplicate. The results were normalized by total protein in the samples.
Statistical analysis
The quantitative data were presented as mean±SD. Statistical analyses were performed using one-way analysis of variance (ANOVA) with post hoc test- Bonferroni multiple comparison test. All data from Western blot analysis were subjected to log transformation before statistical analysis. Statistical differences were considered significant at a P value of less than 0.05.
RESULTS
Clinical characteristics of db/db mice after lovastatin treatment
Blood glucose levels and body weight were monitored in db/db mice before and after lovastatin treatment. Serum lipid profile was measured after the lovastatin treatment for 6 weeks. Db/db mice at the age of 13 weeks had significantly elevated blood glucose concentrations (db/db vs. littermates: 405.5 ± 45.3 vs. 132.5 ± 11.4 mg/dL, p<0.001) and increased body weights (db/db vs. littermates: 49.9 ± 2.0 vs. 29.4 ± 1.7 g, p<0.001) when compared with their non-diabetic littermates. Six-week lovastatin treatment had no effect on blood glucose(lovastatin vs. vehicle control: 419.17±48.42 vs 428±18.78 mg/dL, t=0.3235, p>0.05) and body weight (lovastatin vs. vehicle control: 53.23±2.47 vs 52.68±1.70 g, t=0.4136, p>0.05). At the age of 19 weeks, db/db mice had significantly higher levels of serum cholesterol E (db/db vs. littermates: 96.17 ± 2.66 vs 77.75 ± 6.61 mg/Dl), free cholesterol C (db/db vs. littermates: 20.59 ± 3.13 vs 12.88 ± 1.87 mg/dL) and triglycerides (db/db vs. littermates: 96.32 ± 14.81 vs 37.64 ± 14.48 mg/dL) (ANOVA, all p<0.01, Fig. 1). Lovastatin treatment significantly lowered serum cholesterol E (86.24 ± 7.88 mg/dL, t=2.602, p<0.05, Fig. 1) and free cholesterol C levels (16.39 ± 2.11 mg/dL, t=2.667, p<0.05, Fig. 1), but did not alter serum triglyceride level (t=0.7582, p>0.05, Fig. 1).
Figure 1. Serum lipid concentration in db/db mice after lovastatin treatment.
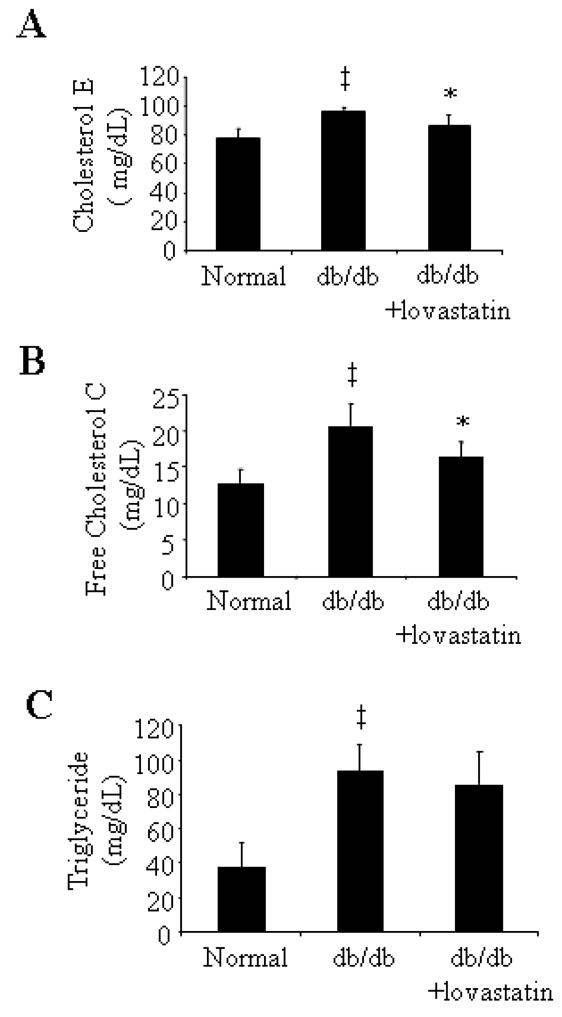
Serum concentrations of cholesterol E (A), free cholesterol C (B) and triglyceride (C) were measured in db/db mice after 6 weeks of lovastatin treatment. Results were obtained from six animals in each group and expressed as mean ± SD. The values statistically different from non-diabetic littermates were indicated by † P<0.05, ‡ P<0.01; from db/db mice treated with vehicle were indicated by *P<0.05, **P<0.01.
Protection of blood-retina barrier by lovastatin in db/db mice
To assess the effects of lovastatin on the blood-retinal barrier, we first examined the integrity of the blood-retinal barrier by determination of vascular leakage of albumin into the retina. After careful and thorough perfusion to remove blood from vascular system, we measured and compared the extravascular albumin content in the retina in db/db mice and their littermates using Western blot analysis. Results showed that the albumin content was 1.9-fold higher in the retinas of db/db mice when compared with non-diabetic littermates (Fig. 2A, p<0.01). Lovastatin treatment significantly decreased extravascular leakage of albumin in db/db mice (Fig. 2A, p<0.01).
Figure 2. Lovastatin protected retinal tight junction and attenuated pro-inflammatory factor expression in the retina in db/db mice.
A: The amount of albumin leaked from blood vessels into the retina in db/db mice was determined by Western blot analysis and semi-quantified by densitometry. The blots were representative of six animals in each group, and the densitometry analysis were presented as mean ± SD. Results showed that the extravascular content of albumin was 1.9-fold higher in db/db mice than their non-diabetic littermates, which was almost completely reversed by lovastatin treatment. B: Occludin expression in the retina was measured by Western blot analysis and quantified by densitometry (mean ± SD). Results were representative of at least four animals in each group. C: Expression of ICAM-1 and TNF-α in the retina of db/db mice was determined by Western blot analysis and semi-quantified by densitometry (mean ± SD). The blots were stripped and reprobed with anti-β-actin antibody. Results are representative images from at least four animals in each group. The values statistically different from non-diabetic littermates are indicated by † P<0.05, ‡ P<0.01; from db/db mice treated with vehicle are indicated by *P<0.05, **P<0.01.
To further determine if the protective effects of lovastatin on the blood-retinal barrier are associated with regulation of the tight junction in diabetic retina, we determined expression of tight junction protein occludin in the retina of db/db mice. The results showed that retinal occludin level decreased in db/db mice to 74.1% of that in non-diabetic littermates (Fig. 2B, p<0.05). Lovastatin treatment almost completely restored retinal occludin expression (Fig. 2B, p<0.05).
Lovastatin attenuated ICAM-1 and TNF-α expression in the retina of db/db mice
Inflammation is a major mediator of the blood-retinal barrier breakdown in diabetic retinopathy. To study if lovastatin protected the blood-retinal barrier via regulation of retinal inflammatory status in db/db mice, we measured expression of pro-inflammatory factors (ICAM-1 and TNF-α) in the retina. Results showed that expression of ICAM-1 and TNF-α was drastically increased in the retina of db/db mice (Figure 2C, p<0.05). Lovastatin treatment effectively attenuated the expression of ICAM-1 and TNF-α in the diabetic retinas (Fig. 2C, p<0.05). These results indicate that lovastatin attenuated inflammation by suppression of pro-inflammatory factor and up-regulation of anti-inflammatory factor expression in the retina.
Lovastatin protected the tight junction in RCEC and ARPE-19 cells exposed TNF-α
TNF-α is a pro-inflammation cytokine, which has been shown to be increased in the diabetic retina as early as 1 wk after the induction of diabetes (Joussen et al., 2002a; Joussen et al., 2002b). Moreover, inhibition of TNF-α activity by etanercept, a soluble TNF-α receptor/Fc construct, significantly ameliorated blood-retinal barrier breakdown in diabetic animals(Joussen et al., 2002a; Joussen et al., 2002b), suggesting a causative role of TNF-α in retinal tight junction damage. To determine whether lovastatin has a direct effect on tight junction in retinal endothelial cells and RPE cells, the major components of blood-retinal barrier, bovine RCEC and human ARPE-19 cells were exposed to TNF-α (10 ng/ml) with or without lovastatin for 24 h. Expression of tight junction protein occludin and adherens junction protein (VE-cadherin) were determined by Western blot analysis. In RCEC, TNF-α significantly reduced occludin (Fig. 3A) and VE-cadherin (Fig. 3B) levels, which were restored by lovastatin at 1 μM (all p<0.05, Fig. 3A and 3B), but not by lovastatin at 0.1 μM. The effect of lovastatin (1 μM) on the TNF-α-induced occludin decrease was also observed in ARPE-19 cells (Fig. 3C and 3D, p<0.05). These results indicate that lovastatin exerts its beneficial effects on both the inner and outer blood retina barrier by up-regulating tight junction protein expression.
Figure 3. Lovastatin protected the tight junction in RCEC and ARPE-19 cells exposed to diabetes-associated stimuli.
A–D). RCEC were exposed to TNF-α (10 ng/ml) in the presence or absence of lovastatin (0.1 and 1 μM) for 24 h. Expression of occludin and VE-cadherin was determined by Western blot analysis (A, C) and quantified by densitometry (B, D). Results were expressed as % of control, and presented as mean ± SD of three independent experiments. † p<0.05, vs. control; * p<0.05, vs. TNF-α. E–F). ARPE-19 cells was treated with TNF-α (20 ng/ml) with or without lovastatin for 24 h. Occludin expression was determined by Western blot analysis (E) and quantified by densitometry (F) . β-actin was used as a control to normalize the loading.
Lovastatin ameliorated TNF-α expression in RCEC
To further determine whether lovastatin regulates TNF-α expression, RCEC were exposed to normoxia (21% O2) or hypoxia (2% O2) in the presence or absence of lovastatin for 24 h. The results showed that lovastatin dramatically decreased TNF-α expression (Fig. 4A, p<0.05). In hypoxia condition, TNF-α expression in RCEC was significantly up-regulated, which was blocked by co-incubation with lovastatin (Fig. 4B, p<0.05). It was noticed that lovastatin treatment resulted in a robust reduction of TNF-α expression in hypoxia-exposed cells to a level even lower than non-stimulated control cells, which was in keeping with its potent inhibitory effect on TNF-α expression in normoxic condition. Exposure to hypoxia also drastically increased ICAM-1 expression, which was completely normalized by lovastatin treatment (Fig. 4C, p<0.05).
Figure 4. Lovastatin ameliorated TNF-α expression in RCEC.
RCEC were exposed to normoxia (21% oxygen) (A) or hypoxia (2% oxygen) (B, C) with or without lovastatin (1 μM) for 24 h. Expression of TNF-α (A, B) and ICAM-1 (C) was determined by Western blot analysis and quantified by densitometry. Results were expressed as % of control, and presented as mean ± SD of three independent experiments. † p<0.05, vs. control; * p<0.05, vs. hypoxia.
Lovastatin attenuated TNF-α-induced ICAM-1 expression in RCEC and ARPE-19 cells
ICAM-1 is an important adhesion molecule up-regulated by a number of diabetic stimuli, including TNF-α. ICAM-1 is also recognized as a central mediator for leukostasis and endothelial injury in diabetic retinopathy (Clausen et al., 2000; Joussen et al., 2002a; Mitamura et al., 2001; Shestakova et al., 2002; Tashimo et al., 2004). We further determined whether lovastatin down-regulates ICAM-1 in RCEC and ARPE-19 cells exposed to TNF-α. The results showed that exposure of RCEC to TNF-α (10 ng/ml) for 24 h induced a significant increase of ICAM-1 expression, which was decreased by lovastatin at 1 μM, but not 0.1 μM (Fig. 5A). ICAM-1 expression was also induced by TNF-α in ARPE-19 cells, and attenuated by lovastatin treatment (Fig 5B, p<0.05).
Figure 5. Lovastatin attenuated TNF-α-induced ICAM-1 expression in RCEC and ARPE-19 cells.
A). RCEC were exposed to TNF-α (10 ng/ml) in the presence or absence of lovastatin (0.1 and 1 μM) for 24 h. Expression of ICAM-1 was determined by Western blot analysis and quantified by densitometry. B). ARPE-19 cells were treated with TNF-α (20 ng/ml) with or without 1 μM lovastatin for 24 h. Expression of ICAM-1 was determined by Western blot analysis and quantified by densitometry. Results were expressed as % of control, and presented as mean ± SD of three independent experiments. † p<0.05, vs. control; * p<0.05, vs. TNF-α.
Inhibition of TNF-α-induced NF-κB activation by lovastatin in ARPE-19 cells
NF-κB has been shown as a central regulator of pro-inflammatory genes in the retina, such as ICAM-1 and iNOS (Joussen et al., 2002a; Ledebur and Parks, 1995). We next determined whether lovastatin suppresses TNF-α-induced ICAM-1 expression via inhibition of NF-κB activation. As translocation of NF-κB from the cytoplasm to the nucleus is a crucial step for its activation, we examined the effects of lovastatin on NF-κB nuclear translocation using immunocytochemical method. In non-stimulated condition NF-κB was localized exclusively in the cytoplasm of ARPE-19 cells (Fig. 6A-a). Incubation with TNF-α (20 ng/ml) for 1 h, caused translocation of NF-κB from cytoplasm to nucleus in most cells (Fig. 6A-b). Pretreatment with lovastatin (1μM) for 24 h partially blocked the TNF-α-induced NF-κB translocation (Fig. 6A-c).
Figure 6. Lovastatin inhibited TNF-α-induced NF-κB activation in ARPE-19 cells.
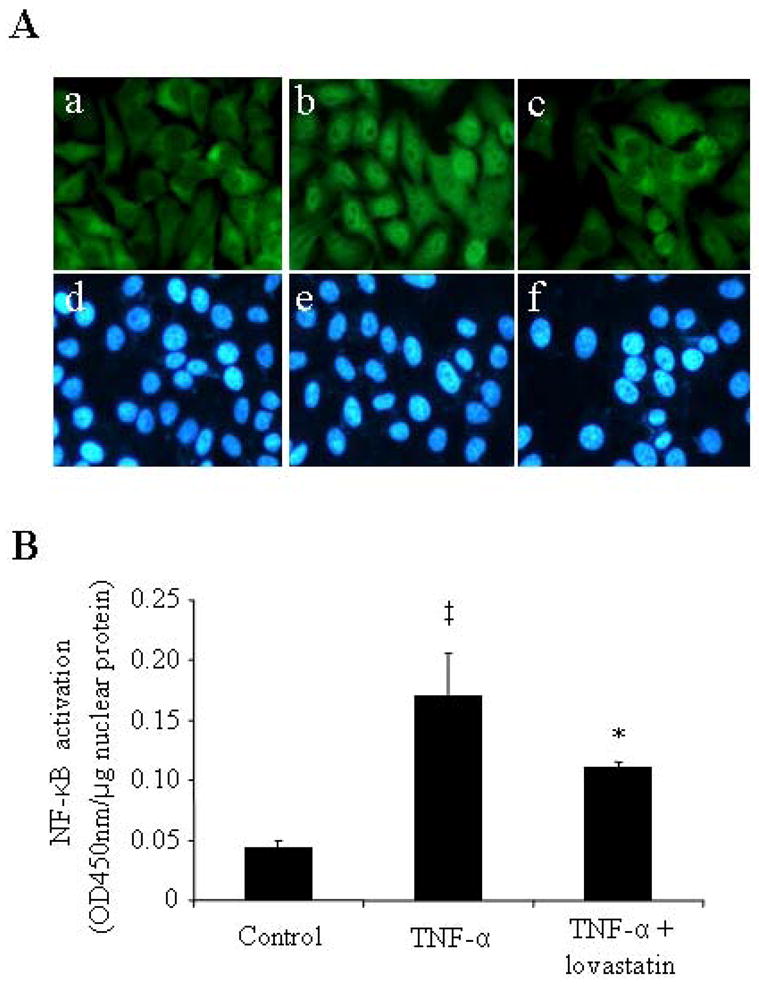
Sub-confluent ARPE-19 cells were pre-incubated in the absence or presence 1 μM lovastatin for 24 h before the treatment with or without TNF-α (20 ng/ml) for 1 h. A: Cells were fixed and stained by an anti-NF-κB antibody and visualized under a fluorescent microscope. Magnification: 400×. a–c: NF-κB staining; d–e: nuclear staining. Note that the signal of NF-κB was diffusely distributed in the cytoplasm in untreated control cells (A-a), and translocated from the cytoplasm to the nuclei in the cells exposed to TNF-α (A-b), which was effectively blocked by lovastatin (A-c). B: Transcriptional activity of NF-κB in the nuclear extract of ARPE-19 cells was quantified by TransAM™ NF-κB p65 transcription factor assay. Results were presented as mean ± SD (n=3). The values statistically different from control cells were indicated by ‡ P<0.01; from TNF-α-treated cells were indicated by *P<0.05.
To further confirm the inhibitory effect lovastatin on the TNF-α-induced NF-κB activation, we quantified the DNA-binding activity of NF-κB in ARPE-19 cells exposed to TNF-α for 1 h with or without pretreatment of lovastatin. The results showed that TNF-α induced a 3.7-fold increase of NF-κB activity. Pre-treatment with lovastatin significantly attenuated the TNF-α stimulated NF-κB activation (Fig. 6B). These results indicate that lovastatin suppressed the TNF-α-induced ICAM-1 expression at least in part through inhibition of NF-κB activation.
DISCUSSION
Clinical trials demonstrate that statins have significant benefits on reducing cardiovascular risk and diabetic macrovascular complications (Colhoun et al., 2005; Collins et al., 2004; Ludwig and Shen, 2006). However, the effect of statins on diabetic macular edema in type 2 diabetes is undefined. In the present study, we demonstrated, for the first time, protective effect of lovastatin on the blood-retinal barrier and tight junction in db/db mice and cultured retinal cells. In parallel, lovastatin ameliorated retinal inflammation, which is at least in part via the inhibition of NF-κB activation, suggesting a salutary effect of lovastatin on diabetic retinopathy.
The db/db mouse is a commonly used genetic model of type 2 diabetes with hyperlipidemia. Systemic administration of lovastatin for 6 weeks significantly lowered serum cholesterol levels, but did not affect serum triglyceride levels in db/db mice. As LDL extravasation and oxidation correlate with inflammation and pericyte loss in diabetic retinopathy (Wu et al., 2008), lowering serum LDL level may in part contribute to the beneficial effects of lovastatin on retinopathy in db/db mice. To further elucidate if lovastatin has direct effects on the blood-retinal barrier, we determined the effect of lovastatin on the tight junction and inflammation in cultured retinal RCEC and RPE cells, the two major components of blood-retinal barrier (Vinores et al., 1999). The results showed that lovastatin at 1 μM attenuated the decrease of tight junction protein (occludin) and adherens junction (VE-cadherin) induced by high glucose and TNF-α in RCEC and RPE cells. In addition, we have determined the effect of lovastatin on RCEC proliferation. Our results demonstrated that lovastatin at this concentration did not affect cell viability in RCEC under normoxia (normal glucose), hypoxia or high glucose conditions (data not shown). However, higher concentrations of lovastatin (10–100 μM) inhibited cell proliferation (data not shown). These results are in keeping with a recent study showing that high doses of simvastatin inhibited retinal microvascular endothelial cell growth (Medina et al., 2008).
Over-expression of pro-inflammatory factors in the retina is associated with the blood-retinal barrier breakdown in diabetic retinopathy (Funatsu et al., 2003; Joussen et al., 2002a; Joussen et al., 2002b; Zhang et al., 2006). In the present study, expression of ICAM-1 and TNF-α was significantly increased in parallel with vascular leakage and tight junction damage in the retina of db/db mice. Systemic administration of lovastatin significantly ameliorated pro-inflammatory factor expression and reversed retinal vascular leakage. Moreover, lovastatin attenuated hypoxia- and TNF-α-induced ICAM-1 expression in cultured RCEC and RPE cells. Lovastatin also decreased TNF-α expression-induced by hypoxia. In addition, we observed a potent inhibitory effect of lovastatin on TNF-α expression even under normal condition. This could provide an explanation why lovastatin decreased TNF-α and consequent ICAM-1 expression in the cells exposed to hypoxia to a level even lower than that in control cells cultured under normal conditions.
NF-κB is a transcription factor regulating expression of a wide range of inflammation-related genes, such as ICAM-1 and VEGF (Ledebur and Parks, 1995). NF-κB activation was observed in the retina in diabetic animal models and in diabetic patients (Kern, 2007; Romeo et al., 2002). In the present study, we demonstrated that TNF-α treatment induced a robust activation of NF-κB in ARPE-19 cells. Pre-treatment with lovastatin ameliorated NF-κB activation, but did not completely block NF-κB activation. Other mechanisms, such as inhibition of activator protein-1 (AP-1) activation (Haslinger et al., 2003) and Rho-mediated pathway (Gegg et al., 2005) may be involved and may contribute to the potent effects of lovastatin on the inflammation in diabetic retinopathy.
Taken together, our study demonstrated that systemic administration of lovastatin significantly reversed retinal vascular leakage and protected retinal tight junction in db/db mice. The beneficial effect of lovastatin on blood-retinal barrier is at least in part mediated by the inhibition of TNF-α activity via amelioration of NF-κB activation in retinal vascular endothelial cells and RPE cells.
Acknowledgments
This study was supported by NIH grant P20RR024215, JDRF grants 5-2007-793 and 18-2007-860, and a research award from OCAST.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Diabetes Association. Standards of medical care in diabetes--2007. Diabetes Care. 2007;30 (Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- Bamforth SD, Lightman S, Greenwood J. The effect of TNF-alpha and IL-6 on the permeability of the rat blood-retinal barrier in vivo. Acta Neuropathol. 1996;91(6):624–632. doi: 10.1007/s004010050476. [DOI] [PubMed] [Google Scholar]
- Clausen P, Jacobsen P, Rossing K, Jensen JS, Parving HH, Feldt-Rasmussen B. Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with Type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabetic Medicine. 2000;17(9):644–649. doi: 10.1046/j.1464-5491.2000.00347.x. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Fuller JH CARDS Investigators. Rapid emergence of effect of atorvastatin on cardiovascular outcomes in the Collaborative Atorvastatin Diabetes Study (CARDS) Diabetologia. 2005;48(12):2482–2485. doi: 10.1007/s00125-005-0029-y. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleight P, Peto R Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363(9411):757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J. Lowering the risk of visual impairment and blindness. Diabet Med. 1998;15 (Suppl 4):S47–50. doi: 10.1002/(sici)1096-9136(1998120)15:4+<s47::aid-dia739>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- Cusick M, Chew EY, Chan CC, Kruth HS, Murphy RP, Ferris FL., 3rd Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110(11):2126–2133. doi: 10.1016/j.ophtha.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Danesh FR, Kanwar YS. Modulatory effects of HMG-CoA reductase inhibitors in diabetic microangiopathy. FASEB J. 2004;18(7):805–815. doi: 10.1096/fj.03-0839rev. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for DME Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(5 Suppl):766–785. [PubMed] [Google Scholar]
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R, American Diabetes A. Diabetic retinopathy. Diabetes Care. 2003;26(1):226–229. doi: 10.2337/diacare.26.1.226. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110(9):1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Harry R, Hankey D, Zambarakji H, Pryce G, Baker D, Adamson P, Calder V, Greenwood J. Suppression of autoimmune retinal disease by lovastatin does not require Th2 cytokine induction. J Immunol. 2005;174(4):2327–2335. doi: 10.4049/jimmunol.174.4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girach A, Lund-Andersen H. Diabetic macular oedema: a clinical overview. Int J Clin Pract. 2007;61(1):88–97. doi: 10.1111/j.1742-1241.2006.01211.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137(4):675–682. doi: 10.1016/j.ajo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Kleemann R, Toet KH, Kooistra T. Simvastatin suppresses tissue factor expression and increases fibrinolytic activity in tumor necrosis factor-alpha-activated human peritoneal mesothelial cells. Kidney Int. 2003;63(6):2065–2074. doi: 10.1046/j.1523-1755.2003.t01-2-00004.x. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB Journal. 2002a;16(3):438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. American Journal of Pathology. 2002b;160(2):501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Moon SO, Park SK, Kwang S, Chae SW, Koh GY. Angiopoietin-1 Reduces VEGF-Stimulated Leukocyte Adhesion to Endothelial Cells by Reducing ICAM-1, VCAM-1, and E-Selectin Expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, Kociok N, Kirchhof B, Joussen AM. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44(5):2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995;270(2):933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77(2):170–175. doi: 10.1034/j.1600-0420.1999.770211.x. [DOI] [PubMed] [Google Scholar]
- Ludwig S, Shen GX. Statins for diabetic cardiovascular complications. Curr Vasc Pharmacol. 2006;4(3):245–251. doi: 10.2174/157016106777698388. [DOI] [PubMed] [Google Scholar]
- Medina RJ, O'Neill CL, Devine AB, Gardiner TA, Stitt AW. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS ONE. 2008;3(7):e2584. doi: 10.1371/journal.pone.0002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura Y, Takeuchi S, Matsuda A, Tagawa Y, Mizue Y, Nishihira J. Monocyte chemotactic protein-1 in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmologica. 2001;215(6):415–418. doi: 10.1159/000050900. [DOI] [PubMed] [Google Scholar]
- Pelzek C, Lim JI. Diabetic macular edema: review and update. Ophthalmololgy Clinics of North America. 2002;15:1–11. doi: 10.1016/s0896-1549(02)00043-3. [DOI] [PubMed] [Google Scholar]
- Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42(10):2408–2413. [PubMed] [Google Scholar]
- Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51(7):2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56(1):1–11. doi: 10.1016/s0168-8227(01)00341-2. [DOI] [PubMed] [Google Scholar]
- Shestakova MV, Kochemasova TV, Gorelysheva VA, Osipova TV, Polosukhina EP, Baryshnikov A, Dedov II. [The role of adhesion molecules (ICAM-1 and E-selectin) in development of diabetic microangiopathies] Terapevticheskii Arkhiv. 2002;74(6):24–27. [PubMed] [Google Scholar]
- Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, Nishihira J. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med. 2004;21(12):1292–1297. doi: 10.1111/j.1464-5491.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- The Eye Diseases Prevalence Research Group. The Prevalence of Diabetic Retinopathy Among Adults in the United States. Archives of Ophthalmology. 2004;122(4):552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol. 1999;97(3–4):217–228. doi: 10.1023/a:1002136712070. [DOI] [PubMed] [Google Scholar]
- Wu M, Chen Y, Wilson K, Chirindel A, Ihnat M, Yu Y, Boulton ME, Szweda LI, Ma JX, Lyons TJ. Intra-retinal Leakage and Oxidation of LDL in Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2008 Mar 24; doi: 10.1167/iovs.07-1440. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma J-x. Pigment epithelium-derived factor (PEDF) is an endogenous anti-inflammatory factor. FASEB J. 2006;20(2):323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Mott R, Chen Y, Knapp RR, Cao W, Ma JX. Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294(5):F1166–1173. doi: 10.1152/ajprenal.00375.2007. [DOI] [PubMed] [Google Scholar]






