Abstract
Untrained rats selectively bred for either high (HCR) or low (LCR) treadmill running capacity previously demonstrated divergent physiological traits as early as the seventh generation (G7). We asked whether continued selective breeding to generation 15 (G15) would further increase the divergence in skeletal muscle capillarity, morphometry, and oxidative capacity seen previously at G7. At G15, mean body weight was significantly lower (P < 0.001) in the HCR rats (n = 11; 194 ± 3 g) than in LCR (n = 12; 259 ± 9 g) while relative medial gastrocnemius muscle mass was not different (0.23 ± 0.01 vs. 0.22 ± 0.01% total body weight). Normoxic (FiO2 = 0.21) V̇o2max was 50% greater (P < 0.001) in HCR despite the lower absolute muscle mass, and skeletal muscle O2 conductance (measured in hypoxia; FiO2 = 0.10) was 49% higher in HCR (P < 0.001). Muscle oxidative enzyme activities were significantly higher in HCR (citrate synthase: 16.4 ± 0.4 vs. 14.0 ± 0.6; β-hydroxyacyl-CoA dehydrogenase: 5.2 ± 0.2 vs. 4.2 ± 0.2 mmol·kg−1·min−1). HCR rats had ∼36% more total muscle fibers and also 36% more capillaries in the medial gastrocnemius. Because average muscle fiber area was 35% smaller, capillary density was 36% higher in HCR, but capillary-to-fiber ratio was the same. Compared with G7, G15 HCR animals showed 38% greater total fiber number with an additional 25% decrease in mean fiber area. These data suggest that many of the skeletal muscle structural and functional adaptations enabling greater O2 utilization in HCR at G7 continue to progress following additional selective breeding for endurance capacity. However, the largest changes at G15 relate to O2 delivery to skeletal muscle and not to the capacity of skeletal muscle to use O2.
Keywords: oxidative capacity, diffusive conductance, capillarity
endurance exercise capacity has been shown to correlate with the maximal rate of oxygen uptake (V̇o2max) of a given individual or organism. V̇o2max is determined by 1) a series of O2 transport steps that encompass ventilation, diffusion from the lung to blood, bulk delivery by the cardiovascular system, and the transfer of O2 from blood to skeletal muscle (1, 35) and 2) metabolic capacity to use the O2 delivered to generate ATP. For years, investigators sought to define the one limiting step to maximal oxygen uptake, but it is now recognized that there is no single step that alone limits V̇o2max (12, 36).
From the work of Krogh (19), the final step in oxygen transfer—from hemoglobin bound oxygen to skeletal muscle mitochondria—was thought to be affected primarily by diffusion distance. However, this process has been shown to be fairly complex, comprised of several diffusional steps (35, 36), and may present a functional limitation to the transfer of oxygen to tissue (10). Maximal extraction of oxygen by the muscle reflects the tissue diffusional conductance (DTO2) and cardiac output (essentially, at peak exercise, muscle blood flow; Q) by the relationship DTO2/(βQ), where β is the capacitance coefficient (slope of the blood O2 content − PO2 dissociation curve) (26). Oxygen conductance is determined by all of the resistances from erythrocyte to mitochondria—essentially dissociation of O2 from red blood cells and diffusion through blood plasma, capillary wall, interstitium, plasma membrane, and cytoplasm, the latter process possibly aided by myoglobin (35). Oxygen conductance from blood to skeletal muscle has been demonstrated to increase with endurance training (29), most likely stemming from several enabling adaptations, including increased capillarity and increased oxidative capacity. These same adaptations are seen in athletic species (12), such as horses, which have very high metabolic capacity and O2 extraction at V̇o2max despite high mass-specific cardiac outputs (37).
In general, V̇o2max can be increased by endurance training through increases in both oxygen delivery to, and use by, skeletal muscle (1). However, while exercise training can increase V̇o2max, a large portion (estimated to be as high as 70–90%) of oxygen uptake capacity is determined by genetic factors (3–5). A recently generated animal model in which untrained rats are selectively bred over several generations as high capacity runners (HCR) or low capacity runners (LCR) (18) provides a unique genetic substrate to study the mechanisms for increased running capacity and attendant changes in V̇o2max. We previously used this rat model to study central and peripheral differences in oxygen transport and use (8, 15). We previously showed at generation 7 (G7) that differences in V̇o2max and endurance capacity between LCR and HCR rats were supported primarily by peripheral muscle adaptations (15). In particular, we found essentially no differences in central cardiovascular or pulmonary function (8).
From G7 to generation 15 (G15), there was a substantial further divergence for endurance capacity between LCR and HCR. That is, at G7 the female HCR and LCR rats tested differed in endurance running capacity by ∼7-fold (1,590 ± 77 vs. 222 ± 17 m) (8) and this difference increased to over 10-fold (2,349 ± 243 vs. 229 ± 27 m) at G15 (6). We hypothesized that this increase in capacity could not be solely supported by continued divergence in peripheral muscle structure and function alone but would require additional enhancements, especially in O2 delivery. However, this would not exclude the potential for continued divergence in peripheral tissues. The evaluation of changes in cardiovascular and pulmonary structure and function are reported elsewhere (6, 17) and do demonstrate that G15 animals show divergent cardiovascular and pulmonary adaptations. This is consistent with the idea that no part of the oxygen uptake system will adapt much beyond the scope of the other steps (38).
The present study was therefore undertaken to test how further generations of selective breeding for endurance running capacity would affect muscle structure and metabolic function compared with earlier generations. Our hypothesis was that, while adaptations in central factors would occur at G15, peripheral adaptations beyond those seen in the previous study at G7 would also be evident. Perhaps more importantly, one of the strengths of the current study is the opportunity to study groups of animals at different generations.
METHODS
Animal subjects.
Adult female rats of ∼8 mo of age (n = 23), selectively bred for high capacity (n = 11, HCR) or low capacity (n = 12, LCR) endurance treadmill running as previously outlined (18), were used for this study. These were the same animals reported in the earlier study (6). Rats were initially tested for maximal treadmill running capacity at 11 wk of age; the 12 LCR ran on average for 229 m and the 11 HCR for 2,349 m at exhaustion at that time. Prior to all experimental trials, all rats from both groups were familiarized to (but not trained on) the single-lane sealed treadmill used in the current study. All animal care and experimental procedures were approved by the University of California, San Diego Animal Care and Use Committee and conform to NIH and American Physiological Society standards.
Surgery.
Approximately 24 h prior to exercise testing, rats were anesthetized (pentobarbital sodium 30 mg/kg ip) and indwelling catheters were placed in the pulmonary artery and carotid artery as previously described (8). Rats were placed on a heating pad for recovery (2–4 h) and then returned to their cages overnight.
Testing.
On the experimental day, rats underwent two exercise bouts to maximal V̇o2, once in hypoxia (FiO2 = 0.10) and once in normoxia (FiO2 = 0.21), essentially as previously described (8). The order of inspired PO2 was balanced within both HCR and LCR groups, and rats were rested ∼3 h between each bout. Arterial lactate concentrations were measured using a YSI 2300 STAT Plus lactate analyzer (YSI, Yellow Springs, OH).
Tissue preparation.
Following the second exercise bout, each rat rested in a cage for 1–2 h. They were then anesthetized with pentobarbital sodium (60 mg/kg iv) and both left and right gastrocnemius muscles were removed intact. Each muscle was divided into lateral and medial portions, which were weighed individually. An entire transverse slice from the widest point of the middle belly portion of either the left or right medial gastrocnemius muscle (randomly selected) was excised and frozen in precooled isopentane (−140°C) and stored at −80°C until additional processing. Transverse 8-μm serial sections were cut on a cryotome (Cryostat) at −26°C and mounted on slides for histochemical analysis of capillary number and fiber morphology. Great care was taken to ensure that the widest part of the muscle was sectioned and that sectioning was perpendicular to the orientation of the fibers.
The contralateral medial gastrocnemius was prepared for enzyme/metabolite measurements. To ensure a representative outcome that could be compared with functional variables, the entire muscle was ground to a fine powder under liquid nitrogen. Subsequent aliquots were removed for enzyme measurements.
Capillary staining.
A combined protocol of alkaline phosphatase (AP) and dipeptidylpeptidase (DPP) reactions was used to stain the capillaries (7, 20). The sections were presoaked in a precooled (−20°C) 1:1 mixture of acetone and chloroform at room temperature for 5 min and then allowed to air dry. The slides were transferred to an incubation mixture containing 0.08% gly-pro 4-methoxy-β-naphtylamide and 0.034% fast blue in 0.1 M phosphate buffer at pH 7.2. Sections were incubated for 60 min at 37°C. Slides were briefly rinsed in PB before being transferred to a mixture containing 0.04% naphthol ASMX phosphate and 0.21% variance blue in 0.1 M Tris buffer, pH 9.2. Sections were incubated for 2 h at 37°C. The treatment of AP stains the arterial ends of the capillary segments blue while the DPP stains the venous ends of the capillary segments red (22). The sections were air-dried overnight before mounting with Permount.
Fiber typing by myosin-ATPase reaction.
A modified procedure of Ogilvie and Feeback (24) was used to delineate the muscle fiber types. Sections were preincubated for 8 min in a medium containing 0.49% potassium acetate and 0.26% calcium chloride at pH 4.4, and then briefly rinsed in 0.1 M Tris buffer at pH 7.8. Sections were then incubated at room temperature for 30 min in a medium containing 0.4% glycine, 0.42% calcium chloride, 0.38% sodium chloride, 0.19% sodium hydroxide, and 0.15% ATP at pH 9.4. Slides were rinsed in 1% calcium chloride and stained in 0.1% toluidine blue for 1 min, rinsed in distilled H2O, dehydrated in ethanol, cleared in HEMO-DE, and mounted with Permount.
Morphometry.
The stained sections were viewed under a light microscope at magnification ×25. The entire muscle cross section was digitally imaged (each rectangular image being 1.15 × 0.86 mm) and MATLAB 5.3 was used to perform morphometric measurements on the whole cross section, image by image. Total capillary number was determined from images of the AP-DPP slides prepared as above, and total number of fibers counted from the myosin-ATPase-stained sections. The myosin-ATPase slides were also used to determine the percentage of type I fibers. Finally, the total cross-sectional area of the muscle was measured from the entire set of digital images. From these data we calculated mean capillary-to-fiber ratio, mean capillary density, mean fiber area, and percentage of type I fibers over the entire cross section. In addition, type II only and mixed regions [which are easily demarcated (15)] were separately assessed for the same variables.
Skeletal muscle enzyme activities.
All enzymes measurements were performed at 20°C on a Beckman model 64 spectrophotometer. To assay citrate synthase (CS) and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities, whole muscle homogenates were prepared using 25–40 mg of pulverized wet tissue. Samples were homogenized in 100 volumes (wt/vol) of buffer (175 mM KCl, 2 mM EDTA; pH 7.4) with a Polytron mixer for 35–45 s and frozen in liquid N2 before undergoing three cycles of freezing/thawing in liquid N2. Before use, the homogenates were thawed a final time and centrifuged at 10,000 rpm for 1 min to spin down particulate matter. With the use of the supernatant, CS activity was assayed as per Srere (34). β-HAD activity was assayed as per Bergmeyer (2) by following the oxidation of NADH spectrophotometrically.
Statistics.
All data are presented as means ± SE because our focus is on group mean differences, and SE reflects confidence in the mean. Variation within each group may be assessed by SD. With 11 HCR rats, SD = 3.32 × SE and with 12 LCR rats, SD = 3.46 × SE. Our criterion for statistical significance was P < 0.05. To test for differences between the HCR and LCR groups at G15, independent unpaired t-tests were performed. In comparing the G7 (8, 15) data to G15 (6, 17), a two-way ANOVA (generation × running capacity) was used. When significance was reached, a Student Newman-Keuls post hoc test was used to determine differences between groups.
RESULTS
Oxygen transport and use.
These variables were previously reported in Gonzalez et al. (6) and are reiterated here only for background. Whole body V̇o2max in normoxia was 50% higher in HCR compared with LCR (69.2 ± 2.0 vs. 46.3 ± 1.1 ml O2·min−1·kg−1; P < 0.001). Concomitant with this difference was a higher tissue diffusing capacity (DTO2) during hypoxic testing in HCR rats compared with LCR (1.58 ± 0.07 vs. 1.06 ± 0.03 ml O2·min−1·Torr−1·kg−1; P < 0.001).
Muscle fiber morphometry.
Mean body weight, as reported in Gonzalez et al. (6), was significantly lower in HCR than LCR (194 ± 3 g vs. 259 ± 9 g; P < 0.001). The morphometric characteristics of the gastrocnemius from both groups are shown in Table 1. Mean medial gastrocnemius mass was also significantly lower in HCR than LCR (P < 0.001), while relative medial gastrocnemius mass (%body mass) was not significantly different (P > 0.05). Figure 1 demonstrates representative micrographs of part of a cross section of medial gastrocnemius in LCR and HCR fibers. Total fiber number for the medial gastrocnemius cross section was 36% higher in HCR compared with LCR (P < 0.01). Likewise, the mean cross-sectional area of each fiber was 35% lower in the HCR compared with LCR (P < 0.005), and, therefore, the total muscle area was similar between groups (P > 0.05). The percentage of total fibers that were type I was not different between the two groups (P > 0.05).
Table 1.
Skeletal muscle morphometry and capillarity for the medial gastrocnemius cross section from both HCR and LCR groups of rats
| HCR | LCR | P Value | |
|---|---|---|---|
| Total muscle area, mm2 | 21.9±1.3 | 22.3±1.2 | NS |
| Total fiber number | 6621±405 | 4837±385 | 0.01 |
| Mean fiber area, μm2 | 3400±166 | 4594±245 | 0.005 |
| % type I fibers | 6.6±0.8 | 9.6±1.9 | NS |
| Total capillary number | 9588±384 | 7068±691 | 0.01 |
| Mean capillary density, mm−2 | 435±26 | 321±17 | 0.005 |
| Capillary/fiber ratio | 1.46±0.06 | 1.46±0.06 | NS |
| Medial gastrocnemius mass, g | 0.46±0.02 | 0.57±0.03 | 0.0056 |
| Medial gastrocnemius mass, % body wt | 0.23±0.01 | 0.22±0.01 | NS |
Values are means ± SE; n = 6 in each group. HCR, LCR, high and low, respectively, treadmill running capacity.
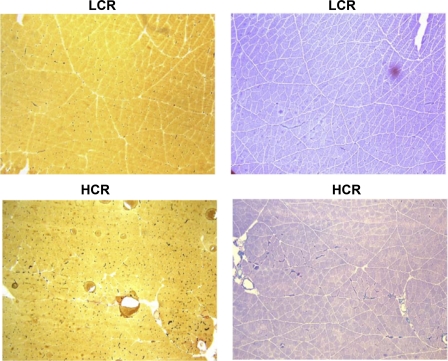
Fig. 1.
Representative photomicrographs of the rat medial gastrocnemius sections for a single low treadmill running capacity (LCR; top) and high treadmill running capacity (HCR; bottom) subject showing the staining for capillarity (left) and fiber type (right). Both the increase in number of capillaries and decrease in average fiber size are visible in these micrographs.
Capillarity.
Figure 1 demonstrates staining of capillaries in a micrograph from part of a single cross section of medial gastrocnemius from representative HCR and LCR samples. Table 1 also lists the capillarity measurements for the medial gastrocnemius cross section for both groups. Total capillary number, determined by counting all the capillaries in the medial gastrocnemius cross section, was 36% higher in HCR than in LCR (P < 0.01). Therefore, due to the higher fiber number in HCR, the mean capillary-to-fiber ratio was not different between LCR and HCR. However, because the HCR group had significantly smaller muscle fiber areas, the mean capillary density was 35% higher in HCR compared with LCR (P < 0.05).
Muscle oxidative enzyme activities.
The whole mixed gastrocnemius muscle enzyme activities for two representative oxidative enzymes are found in Table 2. The whole muscle activities of oxidative enzymes CS and β-HAD were both significantly higher (by 18 and 23%, respectively; P < 0.005) in HCR compared with LCR. These differences in muscle oxidative capacity are considerably less than the differences in maximal V̇o2, which was higher by 50% in HCR (6).
Table 2.
Whole skeletal muscle enzyme activities from the mixed gastrocnemius for both HCR and LCR groups of rats
| HCR | LCR | P Value | |
|---|---|---|---|
| Citrate synthase | 16.4±0.4 | 14.0±0.6 | 0.002 |
| β-HAD | 5.2±0.2 | 4.2±0.2 | 0.002 |
Values are means ± SE. β-HAD, β-hydroxyacyl-CoA dehydrogenase.
Blood lactate.
At rest, arterial lactate concentrations were not different between LCR and HCR during either normoxic or hypoxic exposure prior to exercise (Table 3). At maximal exercise, both HCR and LCR groups showed the expected significant increase in arterial lactate compared with rest. However, compared with LCR, HCR rats showed significantly higher arterial lactate concentrations at maximal exercise in both normoxia and hypoxia (both P < 0.05).
Table 3.
Arterial lactate values for HCR and LCR subjects at rest and maximal exercise for both hypoxic and normoxic conditions
| Condition | HCR | LCR | P Value | |
|---|---|---|---|---|
| Rest | Normoxia | 1.7±0.4 | 2.3±0.5 | NS |
| Hypoxia | 2.1±0.2 | 2.4±0.8 | NS | |
| V̇o2max | Normoxia | 10.1±0.9 | 6.6±0.9 | 0.02 |
| Hypoxia | 13.2±1.0 | 9.4±1.1 | 0.03 |
Values are means ± SE in mM.
DISCUSSION
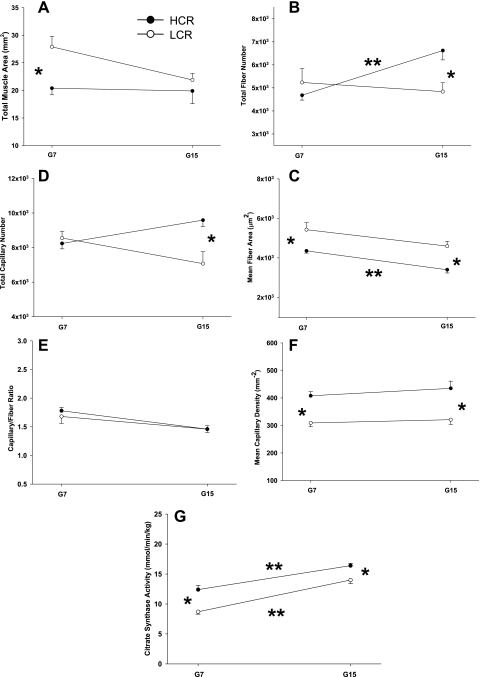
Data from the present study demonstrate that some of the adaptations in skeletal muscle structure and function that enabled greater oxygen uptake and use in high capacity runners studied previously at G7 (15) continue to progress during selective breeding through G15. Figure 2 compares the major skeletal muscle structural differences at G7 and G15, which include smaller fibers, more capillaries, and greater oxidative capacity in HCR rats. As reported elsewhere, these changes at G15 occur simultaneously with adaptations in cardiovascular (6) and pulmonary (17) function in the present cohort of rats that were not previously seen at G7 (8). At G7, endurance capacity was ∼7-fold greater in HCR rats and V̇o2max was 12% higher (8). The central and peripheral adaptations in the present group of animals result in a further divergence in endurance capacity (now a 10-fold difference) and whole body oxygen uptake (now 50%) between HCR and LCR groups (6).
Fig. 2.
Comparison of skeletal muscle structural and functional variables between HCR and LCR animals at both generations 7 and 15 (G7 and G15, respectively). *Significant difference between HCR and LCR at a given generation. **Significant difference between G7 and G15 within HCR or LCR.
Comparison between G7 and G15.
The data from the experiments in the present study and those from the prior evaluation of G7 animals (8, 15) represent a unique and powerful opportunity to compare differences in whole body and skeletal muscle adaptations to artificial selection for endurance performance. That is they allow comparisons not only of 1) LCR and HCR at G15 to each other; 2) HCR and LCR at G7 to each other (8, 15); but also of 3) G15 and G7 groups within the HCR and LCR conditions (see Fig. 2).
Oxygen delivery and conductance.
While V̇o2max has long been thought to be limited by O2 delivery to the muscle, there is strong evidence of a diffusional limitation in transport of O2 into the muscle that imposes additional limits to O2 availability (10, 30). The experiments at G7 demonstrated that the difference in V̇o2max between LCR and HCR rats could be nearly fully attributed to peripheral oxygen extraction (15) as no differences in oxygen delivery by the cardiovascular and/or pulmonary systems were seen between HCR and LCR at G7 (8). The increased muscle extraction, coupled with similar oxygen delivery, was reflected in the much greater oxygen skeletal muscle conductance (33% higher than LCR) in the HCR group at G7 (8). Increases in conductance have been previously shown with endurance training (9, 29).
By G15, compared with G7, the magnitude of the difference in V̇o2max between groups had further increased (6), but since peripheral O2 conductance was already much higher in HCR at G7, it seemed unlikely that additional changes only in peripheral oxygen transport could account for greater oxygen uptake. We hypothesized that central factors could become limiting if not improved and cardiovascular and/or pulmonary changes would need to occur to compensate for some of the potential delivery limitations. As reported separately, this has indeed occurred (2, 6). However, despite the manifestation of central changes at G15 relative to G7, there was still further divergence between HCR and LCR groups in terms of peripheral extraction at G15. In the present study, HCR animals show much higher (>49% more than LCR) tissue oxygen conductance to working skeletal muscle than their LCR counterparts (6).
Skeletal muscle morphometry.
Our earlier study on selectively bred rats at G7 showed differences in muscle morphometry between the HCR and LCR groups that strongly correlated with the differences in diffusional conductance (15). At G7, HCR and LCR had similar total fiber numbers, but HCR displayed significantly smaller individual muscle fiber cross-sectional areas because the total muscle area was much smaller in HCR. Because total capillary number differences were also smaller between groups at G7, the mean capillary density was much higher in HCR compared with LCR. In the present study (G15), HCR rats show an additional similar (36%) increase in both total fiber and total capillary numbers. The result is that mean capillarity density is still significantly greater in HCR. Increased capillary density (capillaries/mm2) has been shown to strongly correlate with increased skeletal muscle O2 conductance in this model previously (15). At G15, total muscle area was not different between HCR and LCR groups, but total fiber number rose significantly (25% compared with G7) in HCR rats. Mean fiber area was therefore significantly smaller in HCR. In the present study at G15, as in our previous work at G7 (15), we found an identical capillary-to-fiber ratio between groups. The lack of difference in total muscle area between groups may be somewhat surprising given the difference in medial gastrocnemius mass (Table 1) and body mass. However, a recent publication (23) demonstrates that HCR rats have shorter femur lengths and thus a smaller stature, suggesting that the difference in weight is attributable to muscle length.
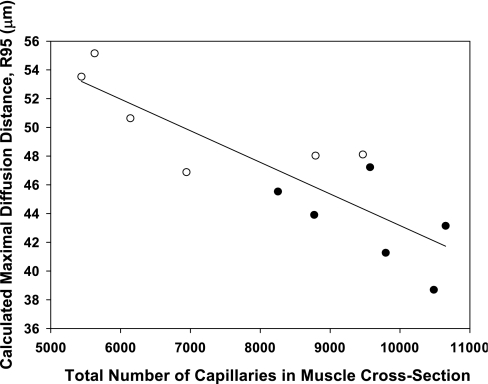
Comparing the total number of capillaries in the medial gastrocnemius to the maximal estimated diffusion distance (R95; see Ref. 33) for both HCR and LCR rats in the present study results in a strong negative correlation (Fig. 3). Therefore, the animals with the most capillaries also showed the smallest diffusion distance. While this correlation cannot be used to imply a causal relationship, it does suggest that the traits of both smaller muscle fiber size and greater capillary number are independently selected for in rats bred for endurance performance. Thus, because two factors, each of which could be argued to increase DTO2, are changing simultaneously on a per animal basis (Fig. 2) it is not possible to determine the individual roles of distance vs. capillarity to explain the divergence in diffusional conductance in this model.
Fig. 3.
Relationship between medial gastrocnemius total capillary number and maximal diffusion distance [R95; see Ref. (33) for both HCR (○) and LCR (•) groups]. A strong negative correlation (r2 = −0.724) exists between total number of capillaries in the muscle cross section and the estimated maximal diffusion distance for the gastrocnemius muscle across experimental groups.
Selecting for increased capillary number is an obvious advantage for oxygen transport to skeletal muscle. However, while smaller fibers may intuitively seem to reduce force-generating capacity, and thus performance, others have speculated that the increased benefits of enhanced aerobic metabolism and potentially reduced locomotory costs may offset this difference (13). A decrease in muscle size has been reported in some mice selectively bred for voluntary wheel running, regarded as the “mini muscle” phenotype (28). Smaller muscles and muscle fibers have also been reported in humans following exposure to chronic hypoxia (16) and in the aerobic flight muscles of hummingbirds (21).
Oxidative capacity.
During endurance exercise, the majority of energy is produced via oxidative phosphorylation in skeletal muscle mitochondria. Therefore, increased oxidative capacity or mitochondrial content is a common feature of both endurance training (11, 14, 25) and athletic species (12). These increases in oxidative capacity have also been seen in mice selectively bred and trained for voluntary wheel running capacity (13).
At G7, skeletal muscle oxidative capacity, assessed by maximal catalytic activities of two mitochondrial enzymes, CS and β-HAD, was significantly greater in HCR compared with LCR (15). However, the relative differences between HCR and LCR in oxidative enzyme activities remained similar between G7 and G15. These data demonstrate that, while increased mitochondrial content is advantageous for oxygen extraction, further divergence in oxidative enzyme activity at G15 was not necessary to support the increase in exercise capacity seen from G7 to G15. This suggests that in peripheral tissues, further increases in capillarity and/or decreases in fiber area between G7 and G15 are sufficient to increase V̇o2max in HCR animals in the face of increased bulk oxygen delivery to the muscle. It should be pointed out that in LCR animals, V̇o2max decreased from G7 to G15 while oxidative capacity increased, suggesting that increases in oxygen utilization ability cannot offset decreases in oxygen delivery and/or peripheral oxygen conductance.
The increases in oxidative capacity in another group of HCR rats (at G10) similar to those used in the present study have been previously correlated with the upregulation of several genes involved in mitochondrial biogenesis such as PGC-1α (peroxisome proliferator-activated receptor-γ coactivator-1α) (39), although the upstream regulation of these factors through the selective breeding process is not currently understood. Similarly, endurance training has been shown to increase expression of many of these same genes (32). With training, relative increases in oxidative capacity often far outstrip parallel increases in V̇o2max, leading some to suggest that this increase in mitochondrial enzymes is related more to increased endurance than to increased V̇o2max (1, 11).
Capillarity vs. oxidative capacity.
The interaction between oxidative capacity and capillary density has been noted before and a tight coupling between oxidative capacity and capillarity has been demonstrated across species of differing sizes and between athletic and sedentary species of matched size (12). Increases in both capillarity and oxidative capacity are a common set of adaptations seen in several models (27, 31) to maintain the relationship between oxygen delivery and use. Interestingly, in the present study the divergence in actual running performance between groups is much larger (on the order of a 10-fold difference) than the differences in any of the variables such as V̇o2max (50%), oxidative capacity, as determined by CS (∼19%), or capillarity (36%). In the present study, we showed that increases in cardiovascular and/or pulmonary function alone are not the sole determinant of the divergence in V̇o2 between HCR and LCR animals but that increases in oxidative capacity are also present. Indeed, the divergence in oxidative capacity actually preceded the increase in central adaptations when studied in previous generations of HCR and LCR at G7 (15).
In conclusion, the data from the present study suggest that continued breeding for endurance running capacity in HCR and LCR rats has led to further divergence in terms of peripheral oxygen transfer and a maintained difference in utilization capacity. At the earlier generation (G7), peripheral adaptations for using delivered O2 accounted for all of the divergence in pulmonary V̇o2 between groups. At G15, while the hypothesized central adaptations allowing for increased O2 delivery have occurred (6), this study has shown further significant divergence at the skeletal muscle level.
GRANTS
This study was supported by Grants AR-40155, HL-17731, HL-64270, HL-39443 from National Institutes of Health (NIH) and RR17718 from the National Center for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. R. Howlett was a Parker B. Francis Fellow.
Acknowledgments
We thank Julie Allen, Jill Koehler, and Patrick Giuliano for invaluable technical expertise in completing this study.
REFERENCES
- 1.Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32: 70–84, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bergmeyer HU Methods of Enzymatic Analysis. New York: Academic, 1974.
- 3.Bouchard C, Daw EW, Rice T, Perusse L, Gagnon L, Province MA, Leon AS, Rao DC. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc 30: 252–258, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, Lesage R, Lortie G, Simoneau JA, Hamel P, Boulay MR, Perusse L, Theriault G, Leblanc C. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc 18: 639–646, 1986. [PubMed] [Google Scholar]
- 5.Bouchard C, Rankinen T, Chagnon YC, Rice T, Perusse L, Gagnon J, Borecki I, An P, Leon AS, Skinner JS, Wilmore JH, Province M, Rao DC. Genomic scan for maximal oxygen uptake and its response to training in the HERITAGE Family Study. J Appl Physiol 88: 551–559, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez NC, Kirkton SD, Howlett RA, Britton SL, Koch LG, Wagner H, Wagner PD. Continued divergence in VO2max of rats artificially selected for running endurance is mediated by greater convective O2 delivery. J Appl Physiol 101: 1288–1296, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Grim M, Carlson BM. Alkaline phosphatase and dipeptidylpeptidase IV staining of tissue components of skeletal muscle: a comparative study. J Histochem Cytochem 38: 1907–1912, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Henderson KK, Wagner H, Favret F, Britton SL, Koch LG, Wagner PD, Gonzalez NC. Determinants of maximal O2 uptake in rats selectively bred for endurance running capacity. J Appl Physiol 93: 1265–1274, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Hepple RT, Hogan MC, Stary CM, Bebout DE, Mathieu-Costello O, Wagner PD. Structural basis of muscle O2 diffusing capacity: evidence from muscle function in situ. J Appl Physiol 88: 560–566, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Hogan MC, Roca J, West JB, Wagner PD. Dissociation of maximal O2 uptake from O2 delivery in canine gastrocnemius in situ. J Appl Physiol 66: 1219–1226, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Hoppeler H, Weibel E. Limits for oxygen and substrate transport in mammals. J Exp Biol 201: 1051–1064, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Houle-Leroy P, Garland TJ, Swallow JG, Guderley H. Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol 89: 1608–1616, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Howald H, Hoppeler H, Claassen H, Mathieu-Costello O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pfluegers Arch 403: 369–376, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Howlett RA, Gonzalez NC, Wagner HE, Fu Z, Britton SL, Koch LG, Wagner PD. Skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J Appl Physiol 94: 1682–1688, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kayser B, Hoppeler H, Claasen H, Cerretelli P. Muscle structure and performance capacity of Himalayan Sherpas. J Appl Physiol 70: 1938–1942, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Kirkton SD, Howlett RA, Gonzalez NC, Giuliano PG, Britton SL, Koch LG, Wagner HE, Wagner PD. Continued selection for running endurance in rats is associated with improved lung function. J Appl Physiol; doi: 10.1152/japplphysiol.90419.2008. [DOI] [PMC free article] [PubMed]
- 18.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Krogh A The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol 52: 419–432, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lojda Z Studies on dipeptidyl(amino)peptidase IV (glycyl-proline napthylamidase). Histochemistry 59: 153–166, 1979. [DOI] [PubMed] [Google Scholar]
- 21.Mathieu-Costello O, Suarez RK, Hochachka PW. Capillary-to-fiber geometry and mitochondrial density in hummingbird flight muscle. Respir Physiol 89: 113–132, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Mrazkova O, Grim M, Carlson BM. Enzymatic heterogeneity of the capillary bed of rat skeletal muscles. Am J Anat 177: 141–148, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab 293: E1–E41, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Ogilvie RW, Feeback DL. A metachromatic dye-ATPase method for the simultaneous identification of skeletal muscle fiber types I, IIA, IIB and IIC. Stain Technology 65: 231–241, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on VO2 kinetics at the onset of submaximal exercise. J Appl Physiol 79: 1914–1920, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Piiper J, Scheid P. Modeling oxygen availability to exercising muscle. Respir Physiol 118: 95–101, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Poole DC, Mathieu-Costello O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirculation 3: 175–186, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Rezende EL, Garland T, Chappell MA, Malisch JL, Gomes FR. Maximum aerobic performance in lines of Mus selected for high wheel-running activity: effects of selection, oxygen availability and the mini-muscle phenotype. J Exp Biol 209: 115–127, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Roca J, Agustí AGN, Alonso A, Poole DC, Viegas C, Barberá JA, Rodríguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at V̇o2max. J Appl Physiol 73: 1067–1076, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, Wagner PD. Evidence for tissue diffusion limitation of V̇o2max in normal humans. J Appl Physiol 67: 291–299, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Rossiter HB, Howlett RA, Holcombe HH, Entin PL, Wagner H, Wagner PD. Age is no barrier to muscle structural, biochemical and angiogenic adaptations to training up to 24 months in female rats. J Physiol 565: 993–1005, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52: 2874–2881, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Snyder GK Capillarity and diffusion distances in skeletal muscles in birds. J Comp Physiol [B] 160: 583–591, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Srere PA Citrate synthase. In: Methods in Enzymology, vol. 13, edited by Lowenstein JM. New York: Academic, 1969, p. 3–5. [Google Scholar]
- 35.Wagner PD Determinants of maximal oxygen transport and utilization. In: Annual Reviews of Physiology, edited by Massaro D. Palo Alto, CA: Annual Reviews, 1996, p. 21–50. [DOI] [PubMed]
- 36.Wagner PD New ideas on limitations to V̇o2max. Exerc Sport Sci Rev 28: 10–18, 2000. [PubMed] [Google Scholar]
- 37.Wagner PD, Gillespie JR, Landgren GL, Fedde MR, Jones BW, DeBowes RM, Pieschl RL, Erickson HH. Mechanism of exercise-induced hypoxemia in horses. J Appl Physiol 66: 1227–1233, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Weibel ER, Taylor CR, Weber JM, Vock R, Roberts TJ, Hoppeler H. Design of the oxygen and substrate pathways VII. Different structural limits for oxygen and substrate supply to muscle mitochondria. J Exp Biol 199: 1699–1709, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005. [DOI] [PubMed] [Google Scholar]