Abstract
Solute delivery to avascular cartilaginous plates is critical to bone elongation, and impaired transport of nutrients and growth factors in cartilage matrix could underlie many skeletal abnormalities. Advances in imaging technology have revolutionized our ability to visualize growth plates in vivo, but quantitative methods are still needed. We developed analytical standards for measuring solute delivery, defined by amount and rate of intravenous tracer entry, in murine growth plates using multiphoton microscopy. We employed an acute temperature model because of its well-established impact on bone circulation and tested the hypothesis that solute delivery changes positively with limb temperature when body core and respiration are held constant (36°C, 120 breaths/min). Tibial growth plates were surgically exposed in anesthetized 5-wk-old mice, and their hindlimbs were immersed in warm (36°C) or cool (23°C) saline (n = 6/group). After 30 min of thermal equilibration, we administered an intracardiac injection of fluorescein (50 μl, 0.5%) and captured sequentially timed growth plate images spanning 10 min at standardized depth. Absolute growth plate fluorescence was normalized to vascular concentrations for interanimal comparisons. As predicted, more fluorescein infiltrated growth plates at 36°C, with standardized values nearly double those at 23°C. Changing initial limb temperature did not alter baseline values, suggesting a sustained response period. These data validate the sensitivity of our system and have relevance to strategies for enhancing localized delivery of therapeutic agents to growth plates of children. Applications of this technique include assessment of solute transport in models of growth plate dysfunction, particularly chondrodysplasias with matrix irregularities.
Keywords: bone elongation, chondrocyte, extracellular matrix, nutrient supply, blood flow
bone elongation occurs through the process of endochondral ossification in cartilaginous growth plates located at the ends of immature long bones (Fig. 1) (7, 21). Like articular cartilage, growth plates lack a direct blood supply and thereby receive essential nutrients and growth factors via transport of solutes from the surrounding vasculature (9, 11). The structure and composition of collagen and other extracellular matrix proteins are thought to be important for allowing molecules such as hormones, oxygen, nutrients, wastes, and paracrine signaling factors, to move through cartilage and regulate its metabolism (5, 42, 54, 55, 58, 59, 67). Components of extracellular matrix can be altered by disuse or pathology (15, 35), as well as by environmental influences such as high or low temperature (33, 52, 58). The delivery and movement of systemic and paracrine regulators to and within growth plates are necessary for bone elongation (60), so changes in the matrix that affect its transport capabilities may also impact skeletal growth.
Fig. 1.
Long bone schematic illustrating the growth plate and its principal blood supplies: epiphyseal vessels, metaphyseal vessels, and a subperichondrial ring vessel plexus around the periphery. Vasculature is shown at left. The growth plate comprises a heterogeneous collection of chondrocytes located between the ossified epiphysis and metaphysis of immature long bones. Growth plates are conventionally subdivided into 4 morphologically and functionally distinct zones: reserve (R), proliferative (P), proliferative-hypertrophic transition (T), and hypertrophic (H) (right). Bone elongation occurs through a series of well-orchestrated events in which chondrocytes in columns divide, mature, and are replaced with mineral at the chondro-osseous junction, where bone-forming osteoblasts invade from the metaphyseal vasculature (V).
Significant changes in total vascular supply to limb bones have been demonstrated in a variety of natural and experimental conditions (10, 19, 20, 24, 29, 39, 45, 51, 52, 56, 57), but it is unclear how these changes translate to growth plates. Solute transport in articular cartilage has been well studied because of its key role in normal joint function and the debilitating and costly impact of its damage, particularly in the aged population (5, 35, 42, 54, 55, 58, 59, 67). However, despite the widespread prevalence of chondrodysplasias and other skeletal disorders involving the growth plate in children (1, 3), comparably few studies have focused on dynamic molecular transport in growth plate cartilage, and even fewer have examined these processes in a living organism (22, 62). Due primarily to the inherent challenges of imaging connective tissue in real time (17, 63), there is a major gap in our understanding of the role of solute transport in normal and abnormal growth plate physiology.
Most current approaches to bone and cartilage imaging are limited to macroscopic studies that lack cellular resolution in vivo (12, 20, 65) or postmortem analyses that lack the dynamic physiology of an intact system (16, 27, 36), particularly an intact circulatory system. Only recently have techniques been developed that enable visualization of growth plate dynamics at detailed cellular resolution in vivo using imaging capabilities of two-photon microscopy (22, 62); however, quantitative standards are still needed. The mouse is the most widely studied laboratory animal (34) for which dozens of existing research models are well characterized to mimic bone and cartilage disease states in humans (26), including those with disrupted blood flow (19) and extracellular matrix integrity (27, 43, 49, 66). For progress to be made in understanding the nature of the disorders in these valuable model systems and in developing effective strategies for their treatment, there is a paramount need for methodologies to quantitatively assess solute transport in growth cartilage.
Building on prior work in our laboratory (22, 62), we developed analytical standards for measuring solute delivery, defined by amount and entry rate of intravenous tracers, in murine growth plates using multiphoton microscopy. We employed an acute temperature model because of the well-established impact of temperature on bone circulation and tested the hypothesis that solute delivery changes positively with limb temperature when body core is held constant. We present biological data that validate the sensitivity of our system and demonstrate practical relevance to understanding and enhancing delivery of therapeutic agents to growth plates of children. This technique provides a means for elucidating the role of solute transport in normal and aberrant bone growth processes. The methodology can be applied broadly to other intact connective tissues such as articular cartilage or intervertebral disk.
There are three vascular routes by which blood reaches the growth plate (Fig. 1): epiphyseal vessels, metaphyseal vessels, and a ring vessel and subperichondrial plexus around the periphery (11). The epiphyseal and metaphyseal vessels have a deep origin within the bone, whereas the ring plexus is located just slightly deep in the surrounding perichondrium. Blood flow through these vessels can be significantly altered by local heat or cold treatment by means of sympathetically activated vasodilation or vasoconstriction (11). Such a model of acute temperature exposure is well established for its effects on local bone circulation. Feucht et al. (24) were among the earliest to quantitatively demonstrate effects of warm temperature on increasing blood flow to the hindlimbs of dogs. Schoutens et al. (51) later found that warming (∼40°C) or cooling (∼23°C) the hindlimbs of rats resulted in bone blood flow rates that were 188 and 34%, respectively, of those measured under normal physiological conditions. With a similar model, Genant et al. (29) showed a positive correlation between temperature and the uptake of radiolabeled bone tracers, with significantly more tracer localizing at the growth plate of the warmed limb.
Following these methods, we employed a model of hindlimb immersion in mice to study the effects of local heating (36°C) and cooling (23°C) on solute transport in growth plate cartilage using in vivo multiphoton microscopy. To determine whether a differential response to temperature occurred in the same mouse over time, we administered single and repeated bolus injections of fluorescein at different temperatures and examined tracer accumulation in the growth plate. We chose fluorescein because small size (332.3 Da), low toxicity, and neutral charge (2) render it a good general model for passively transported agents in the bloodstream. We have shown previously that fluorescein uniformly enters the growth plate with relative ease (22, 62). We focused on the deeper vessels of the epiphysis and metaphysis, which we thought might be less reactive to acute temperature stimuli compared with the less deep plexus (48). Our goal was to develop a system with a wide detection range that could be applied to natural and experimental models of chronic growth plate dysfunction where changes in solute delivery might be subtle, although biologically meaningful. We observed qualitative changes in size of the subperichondrial plexus vessels in response to acute temperature change (Fig. 2), so we chose to focus imaging in a deeper plane where vessels might not exhibit the same degree of vasoactivity.
Fig. 2.
Multiphoton images of vessels in the subperichondrial plexus from the same mouse with its limb bathed in cool (23°C) and then changed to warm (37°C) lactated Ringer saline. The growth plate is oriented as in Fig. 1. Images were captured 30 min apart at the same growth plate depth and location, verified in a series of optical sections imaged superficial to deep. Vessels were visualized at 780-nm illumination after an intravenous injection of fluorescein (FL) so that the plasma is fluorescent (white) and blood cells appear as dark shadows within the vessels. Vessels were enlarged after the switch to the warmer temperature (arrows).
MATERIALS AND METHODS
Animals and preparation.
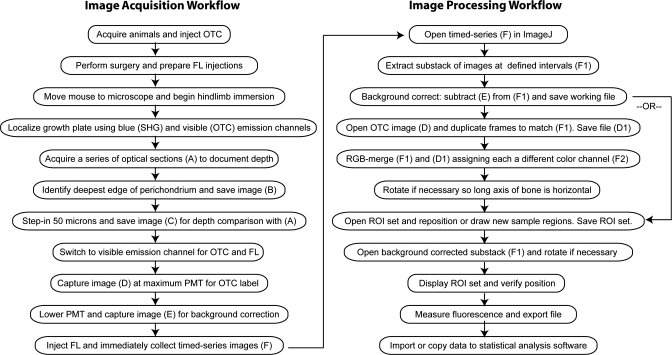
Steps for image acquisition and analysis are illustrated in a workflow diagram (Fig. 3). All procedures were approved by the Institutional Animal Care and Use Committee at Cornell University (protocol 2007-0179). Mice (n = 12) were bred from a line carrying the green fluorescent protein (GFP) reporter controlled by the type II collagen (Col2) promoter/enhancer that serves as a fluorescent marker for chondrocytes (30). We used a mixed sex sample of positive and negative mice, identified by ear punch fluorescence as previously described (22). Although Col2-GFP expression is useful for visualizing detailed morphology of growth plate chondrocytes, this feature was not relevant to the experiments here, and sex was not considered a critical variable in our model system. However, both sex and genotype were documented to assess their potential variation. All mice were between 4 and 5 wk old, with a modal age of 30 days and a mass of 15 g.
Fig. 3.
Workflow diagram illustrating key steps in image acquisition and processing procedures. See text for details.
The morning of imaging, mice were weighed and injected with oxytetracycline (OTC; Pfizer LA-200; 0.05 mg/g ip) to facilitate growth plate localization. OTC binds calcium at the mineralizing chondro-osseous junction (32) and enables growth plate identification under multiphoton excitation. OTC injections were administered a minimum of 30 min and a maximum of 5 h before surgery and imaging.
Mice were anesthetized in an induction chamber of 3.5% isoflurane with 1 l/min oxygen flow. After 1–2 min, when fully induced, the animal was moved to a two-chamber stage (22) and maintained under 1.5% isoflurane anesthesia delivered through a nose cone for surgery and imaging. The mouse was positioned in dorsal recumbency on one side of the stage atop a heating pad with a rectal feedback probe set to maintain core temperature at 36.5°C (FHC model 40-90-8C A720D DC temperature control module set to 6, model 40-90-2 heating pad, and model 40-90-5B rectal thermistor probe), while its left limb was extended into a separate chamber for perfusion with lactated Ringer (LR) solution (Baxter, product code 2B2324X) for acute temperature exposure and water-immersion imaging. The limb was stabilized by placing a pin through the toe webbing, and skin was moistened with 75% ethanol to clean the area and moisten the fur. A longitudinal skin incision was made proximally from the crus to the inguinal canal using fine dissection shears, and skin was loosely tacked back with pins.
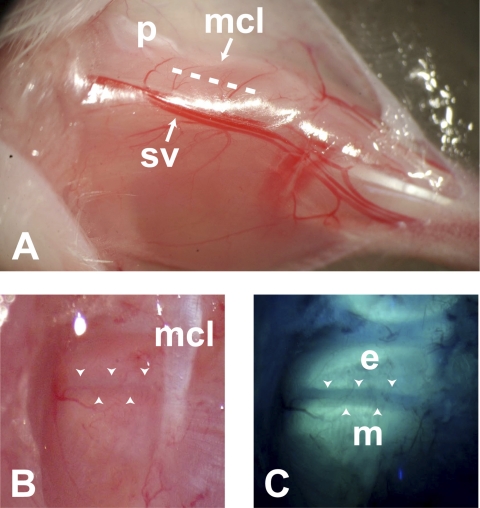
Surgical exposure of the growth plate was performed under an Olympus fluorescent dissection microscope (SZX12; ×0.5 objective) as previously described (22) by incising the superficial fascia of the biceps femoris and gastrocnemius muscles between the medial collateral ligament and saphenous vessels with a no. 11 scalpel (Fig. 4A). This minor incision exposed the medial aspect of the proximal tibial growth plate just caudal to the medial collateral ligament, leaving the perichondrium, its vascular network, and joint capsule intact (Fig. 4B). When this procedure was performed carefully, minimal bleeding occurred. The limb was kept moist with saline throughout the procedure. OTC labeling was verified under UV excitation (Fig. 4C). After the hindlimb was prepared, a mediolateral skin incision was made between the forelimbs to expose the rib cage for intracardiac injection of fluorescein, which was later performed at the level of the left fourth intercostal space. All surgical procedures were completed within 20 min, and the stage was then moved to the multiphoton microscope where the growth plate was first oriented using an Olympus ×4/0.28 NA objective under bright-field illumination.
Fig. 4.
Surgical approach to the proximal tibial growth plate. The limb is extended with the hindpaw to the right and the patella (p) at the top of the image (A). An incision (dashed line) is made through the superficial fascia of the biceps femoris and gastrocnemius muscles between the medial collateral ligament (mcl) and saphenous vessels (sv). Images at bottom are oriented as in Fig. 1. Under bright-field dissection (B), the growth plate appears as a dark band (between the arrowheads) caudal to the medial collateral ligament. The perichondrium, vascular network, and joint capsule remain intact. UV illumination (C) reveals oxytetracycline (OTC) fluorescence in epiphyseal (e) and metaphyseal (m) bone, which denotes boundaries of the growth plate (arrowheads).
Once positioned under the microscope, the extended hindlimb was immersed in warm or cool LR using a perfusion pump (Master-Flex; Cole-Parmer model 7553-60 with 7021-24 pump head at inflow/outflow setting 3) with a flow rate of ∼5.8 ml/min. The limb was warmed to 36°C using an in-line heater coupled to the perfusion system (Warner Instruments TC344A dual heater controller) with ∼15-cm tubing between the heater and stage chamber. To account for heat loss in the perfusion line, we set the heater to a maximum of 49.1°C, which kept the chamber bath at a steady 36°C at this flow rate. The limb was cooled to 23°C by chilling a flask of LR perfusate in an ice bucket and placing a bag of ice over the inflow line to account for warming. LR bath temperature was monitored and recorded using a thermocouple thermometer (Physitemp BAT-12). Temperatures were stabilized for at least 30 min to allow thermal equilibration before imaging. Core temperature and respiration were recorded to gauge depth of anesthesia, and respiration was maintained at 120 breaths/min by adjusting the isoflurane level as appropriate.
In vivo multiphoton microscopy.
The multiphoton system has been described previously (22, 25, 62) and consists of a Ti:Sapphire laser (Milenia/Tsunami combination; Spectra Physics, Mountain View, CA) and Bio-Rad 600 MRC laser scanner interfaced to a modified Olympus AX-70 upright microscope. The beam intensity was modulated with a 350-80 BKLA Pockel's Cell (Conoptics, Danbury, CT) with custom-made electronics. Excitation light was focused on the growth plate using an Olympus ×20/0.95 NA water-immersion objective that provided a large field of view and several millimeters of necessary working distance. The Pockel's Cell was set to blank the beam on scanner flyback so that the specimen was only illuminated while imaging data were collected. The growth plate was oriented using second-harmonic generation (SHG) from collagen in the perichondrium and OTC labeling as previously detailed (22, 61, 68, 69). The illumination wavelength was tuned to 880 nm (68). Nonlinear emissions were collected in epifluorescence mode and separated from the excitation beam using a 670DCXXRU long-pass dichroic filter (Chroma Technology, Rockingham, VT). Emission filters provided a blue (400–490 nm for collagen SHG) and visible (510–650 nm for fluorescein and OTC) separation using BGG22 and 580/150 filters with a separating 500DCXR dichroic filter (Chroma Technology). The emission filters provided a 107 rejection ratio of exciting to emitting wavelengths, after which filtered emissions were detected using HC125-02 bialkali photomultiplier tube assemblies (Hamamatsu, Bridgewater, NJ).
Image acquisition.
Simultaneous imaging of collagen SHG in the perichondrium and OTC fluorescence at the two chondro-osseous junctions delineated growth plate architecture and provided landmarks for consistent orientation and depth standardization (22). To reference imaging depth, we acquired sequential optical sections at 5-μm intervals from superficial to deep using blue and visible emission channels. The arrival pattern of fluorescein differs with imaging depth (22), and so we standardized imaging at a focal plane 50 μm deep to the deep surface of the perichondrium. At this depth, the epiphyseal and metaphyseal chondro-osseous junctions are approximately parallel (securing visual confirmation of location), and fluorescein arrives primarily from the epiphyseal and metaphyseal vasculature, rather than the subperichondrial plexus vessels encircling the growth plate (22). In the optical sections taken superficial to deep, the first appearance of OTC fluorescence in epiphyseal or metaphyseal bone indicated the location of the deepest edge of the perichondrium. To standardize depth, the objective was then focused in 50 μm deeper, where all subsequent imaging occurred using only the visible channel for fluorescein detection.
Fluorescein was prepared from a standard 25% ophthalmic stock (Ak-Fluor; Akorn, Buffalo Grove, IL) and diluted to 0.5% in phosphate-buffered saline (PBS). Just before the injection, two sequential images were captured for use in later processing. The first image was taken at maximum photomultiplier tube (PMT) voltage (∼1,200 V) to record the OTC label that defined boundaries of the growth plate (image D in Fig. 3 workflow). The PMT was then lowered in the second image to ∼1,050 V (virtually eliminating any OTC signal) in preparation for fluorescein arrival (image E in Fig. 3). The second image (image E) was critical for background correction during image processing. Without delay, 50 μl of 0.5% fluorescein was then injected ventrally into the heart through the intact rib cage at the level of the left fourth intercostal space using a 28-gauge insulin syringe (BD; catalog no. 329461). This technique was established and verified in a pilot study aimed at directing the needle into the left ventricle. In such a way, consistent intracardiac injections could be performed obviating the need for thoracotomy and ventilation. Sequential images of fluorescein entering the growth plate were collected in a series spanning 10 min with ∼6 s between frames. This produced a timed-series stack of 100 images (image F in Fig. 3).
To determine whether a differential response to acute temperature could be observed in the same mouse over time, we also administered repeated bolus injections of fluorescein (50 μl of 1 or 2% dilutions) after changing the initial temperature of the LR perfusate bath. Images were collected at the same depth by making a slight temperature-mandated focus adjustment as determined in a pilot study. All other procedures described above were the same.
Image processing and data collection.
Images were processed using ImageJ software (1.37v; National Institutes of Health) on a Macintosh computer running Mac OS X. To facilitate data analysis, we extracted a substack of frames (image F1 in Fig. 3) at 1-min intervals from the time series using the “Substack Maker” plug-in (http://rsbweb.nih.gov/ij/plugins/substack-maker.html). Background correction was accomplished by using the “Image Calculator” function to subtract the image captured immediately before the timed series (image E in Fig. 3) from the substack. This process eliminated background from the image and ensured that any signal detected was incoming fluorescein.
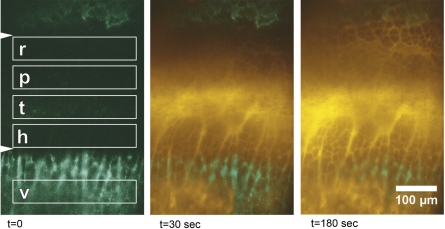
We collected all fluorescence data from the background-corrected substack (image F1 in Fig. 3); however, we found it useful to first define the growth plate sampling regions in a separate pseudocolored image in which both fluorescein and OTC label could be viewed together for the most accurate positioning (Fig. 5). To accomplish this, the OTC image captured before the fluorescein injection (image D in Fig. 3) was duplicated to match the number of frames in the fluorescein substack (ImageJ can only merge stacks that contain an equal number of frames) using the “Stack Sorter” plug-in (http://www.optinav.com/Stack-Sorter.htm). The duplicated OTC stack (image D1) was then merged with the fluorescein substack (image F1) using the “RGB Merge” function, where each was assigned to a different color channel. The merged image was rotated if necessary so that the long axis of the bone was horizontal, defined by parallel OTC labeling in the epiphysis and metaphysis. Five rectangular regions of interest (ROI; 18,963 μm2) were then displayed from a predefined ROI set that encompassed subdivisions of the growth plate and the metaphyseal vasculature (Fig. 5). The epiphyseal vasculature was not sampled because it could not be imaged reproducibly, since the structure of the bone in this region was significantly more scattering. The four growth plate boxes were positioned between the bounding OTC labels, and the vasculature box was positioned distal to the growth plate within the metaphyseal bone (Fig. 5). The newly positioned ROI set was saved, and the native uncolored background-corrected substack (image F1) was reopened and rotated as determined above. The ROI set was displayed and verified for position, and average fluorescence (mean gray value) was quantified using the ROI “Measure” command. This produced 55 measurements for each of 5 regions in an 11-frame substack.
Fig. 5.
Time-lapse multiphoton images illustrating FL (yellow pseudocolor) entering the growth plate within seconds (t) after intracardiac injection. Orientation matches that in Fig. 1. OTC label (green pseudocolor) of epiphyseal (top) and metaphyseal (bottom) bone facilitates localization of the growth plate, which appears between the arrowheads in the far left frame. Boxes depict sample regions for measuring fluorescence in reserve (r), proliferative (p), transitional (t), and hypertrophic (h) subdivisions of the growth plate, as well as in the vasculature (v) of the metaphyseal bone. Although the conventional terminology used in Fig. 1 is upheld for simplicity, these sample regions were not strictly confined to the standard growth plate zones, and boxes may have included cells from adjacent morphological territories.
Data analysis and statistical testing.
Data were exported from ImageJ to an Excel spreadsheet and manually copied into SPSS 11.0 statistical analysis software on a personal computer running Windows XP. To facilitate interanimal comparisons, we standardized mean fluorescein levels in the growth plate to the maximum local vascular saturation in metaphyseal bone by calculating a ratio of average growth plate fluorescence to the average vascular fluorescence measured 8 min after tracer injection (i.e., Ref. 22). This time point was chosen because fluorescein had uniformly saturated the vascular compartment by 8 min in both groups, as evident by the plateau in the accumulation curves (Fig. 6B), and was therefore considered as the peak local vascular concentration to which the total amount entering the adjacent growth plate could be compared over time. Although analyses of absolute values are often preferred over ratios, this standardization factor was necessary to account for potential variation in imaging conditions (mostly due to differential tissue scattering), injectant quantity and/or tracer dye load, and any fat or fascia obscuring the growth plate due to surgical approach, animal size, or slight tilt in orientation. These conditions could produce absolute fluorescence values that appear unusually high or low in the growth plate (e.g., Fig. 6A), inflating the level of interanimal variability. However, when vascular levels were used as an internal reference point (Fig. 6B), the range of variation is considerably lower (Fig. 6C), since fluorescence values in the growth plate and vasculature were highly correlated (Pearson's r = 0.94, P < 0.001) (Fig. 6D). We therefore found it more appropriate to analyze values normalized to an internal standard. Potential caveats on selecting an appropriate reference point are noted in the discussion. All statistical analyses were performed using SPSS. Groups were compared at set time points using the Student's t-test, and sex and genotype effects were assessed using factorial ANOVA. Relationships among variables were assessed using Pearson's product-moment correlation. The level of accepted significance was set at α = 0.05 for all procedures.
Fig. 6.
Scatter plots illustrating absolute fluorescence in combined sample regions of the growth plate (A) and vasculature (B) at warm and cool limb temperatures over time. The warm outlier with unusually high values in the growth plate and vasculature inflates interanimal variation. Standardizing growth plate values to 8-min vascular concentrations (C) considerably reduces the range of variation, since fluorescence values in the growth plate and vasculature are highly correlated (D) (Pearson's r = 0.94, P < 0.001). See text for discussion of reference point selection.
RESULTS
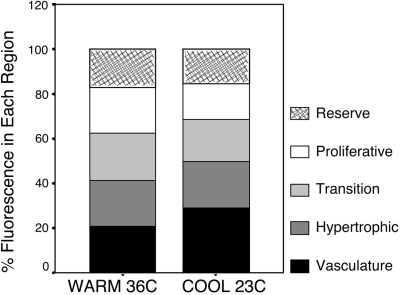
There were no significant differences due to sex or genotype; however, the size of our sample precluded a robust statistical assessment of interactions (see discussion). When the vascular compartment reached saturation at 8 min, fluorescein was distributed approximately equally among all sample regions of the growth plate and vasculature at warm temperature (∼20% per region); in particular, concentrations in the growth plate matched those in the vasculature (Fig. 7). In the cold, fluorescein was distributed nearly equally among growth plate compartments, with slightly higher proportions in the transition (18.6%) and hypertrophic (21%) regions compared with reserve (15.7%) and proliferative (15.8%) regions (Fig. 7). However, there was nearly one-half more tracer in the vascular compartment (28.9% of total) compared with all growth plate regions; less tracer had infiltrated the growth plate in the cold (Fig. 7). Timed accumulation curves illustrate the significant effect of temperature on fluorescein entry into the growth plate (Fig. 8). There was over twice as much tracer in the growth plate at warm temperature within 1 min after injection (Student's t = 2.81; P < 0.01). The difference persisted over time; there was still 1.5-fold more tracer in the warm growth plates at the 10-min end point (Student's t = 4.10; P < 0.001). On average, standardized values at warm temperature were approximately double those measured in the cold (Fig. 8).
Fig. 7.
Stacked bar graph illustrating the spatial distribution of FL in subdivisions of the growth plate and vasculature 8 min after intracardiac injection. Regions correspond to boxes shown in Fig. 5. Each box represents a percentage of the total fluorescence measured among all 5 regions so that the total shown is 100%. At warm temperature, FL was distributed evenly among all sample areas (∼20% per region). In the cold, FL was distributed nearly equally among growth plate compartments, with slightly more in the transition (18.6%) and hypertrophic (21%) regions compared with reserve (15.7%) and proliferative (15.8%), but a greater percentage of the tracer (28.9%) remained in the cold vasculature compared with the warm group.
Fig. 8.
Timed accumulation curves of fluorescein in the growth plate (all subdivisions combined) standardized to 8-min vascular concentrations. Values are means ± SE for each group at 1-min intervals spanning 10 min after intracardiac injection. Mean values in the warm temperature were nearly double those measured at cool temperature.
Effects of temperature change.
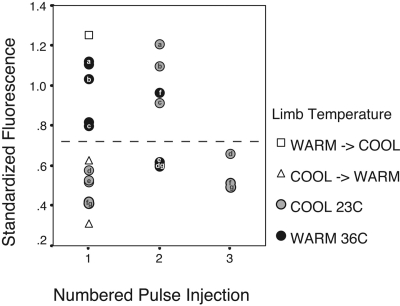
To determine whether a differential acute temperature response could be observed in the same mouse over time, we also administered repeated bolus injections of fluorescein (50 μl of 1 or 2% dilutions) after changing the initial temperature of the LR perfusate bath and allowing the animal to equilibrate for at least 30 min. Surprisingly, we found that switching the temperature of the perfusion bath had little impact on fluorescein accumulation in the growth plate in the same animal (Fig. 9). Fluorescein levels remained similar to those measured at the baseline temperature, regardless of whether the secondary or tertiary injection was done at warm or cold temperature. A cold baseline temperature resulted in low fluorescence even after another injection was given at warm temperature, and a warm baseline temperature resulted in high fluorescence even after a repeated injection in the cold (Fig. 9).
Fig. 9.
Repeated FL injections after a change in limb immersion temperature. Plots show fluorescence in the growth plate standardized to 8-min vascular concentrations. Up to 3 FL injections were administered to each animal at different limb temperatures, allowing at least 30 min of thermal equilibration between injections. Letters a–g correspond to individual mice. The dashed horizontal line separates the ranges found at warm and cool baseline (injection 1). For each repeated injection after a temperature change (injections 2 and 3), FL levels were primarily found within the range of those measured at baseline, regardless of the secondary or tertiary injection temperature. When limb temperature was changed before the first injection was administered, FL values were found within the baseline ranges, rather than those of the actual injection temperature (□, warm baseline switched to cool temperature for the first injection; ▵, cool baseline switched to warm temperature for the injection). These results suggest a sustained physiological response to the initial temperature exposure. See text for further discussion.
To determine whether these results represented a sustained biological response to initial temperature exposure (i.e., a “recovery period”) and to eliminate the possibility that it could be an artifact of image acquisition or processing, we conducted additional experiments in which the limb temperature was changed before the first tracer injection was administered. The null hypothesis was that fluorescein levels would reflect the temperature at which the injection was given and would suggest that prior results were likely an experimental artifact. The alternative hypothesis was that fluorescein levels would reflect those expected at baseline temperature, suggesting that there was a sustained biological effect of the initial temperature exposure. Our results unequivocally support the latter hypothesis. In a sample of three mice in which the limb was either warmed (Fig. 9, □) or cooled (Fig. 9, ▵) initially and then subjected to a temperature change before any tracer was injected, fluorescein levels were all found within the range of those expected at the initial baseline temperature at which no injection occurred.
DISCUSSION
We used a model of acute temperature exposure to develop in vivo multiphoton imaging methods for quantitatively assessing solute transport in growth plate cartilage using fluorescein, a small-molecular-weight tracer, as a proxy for biological substances in the bloodstream. We found a significant and sizeable impact of limb temperature on fluorescein accumulation in the growth plate, complementing prior studies that have shown similar effects on total bone blood flow (24, 29, 51). Solute delivery was twofold higher in warm (36°C) vs. cool (23°C) limbs (Fig. 8), and tracer concentrations were distributed evenly in the growth plate and vasculature at warm temperature (Fig. 7). In contrast, substantially less tracer infiltrated the cold growth plates, with nearly one-half more tracer remaining in the vascular compartment compared with all growth plate regions (Fig. 7). These results validate the sensitivity of our method as a viable tool for detecting differences in the movement of solutes within growth plate cartilage and have broad applications for studying natural and experimental models of skeletal growth variation. Furthermore, by demonstrating that warm temperature significantly increases solute delivery to the growth plate, these results have clinical utility for developing strategies to enhance targeted delivery of therapeutic drugs to injured growth plates of children through localized application of heat at injury sites.
Limitations.
Our model holds promise for elucidating the role of solute transport in normal and abnormal growth plate functioning, but it is important to acknowledge several caveats. Our studies employed fluorescein because of the ease in which this small tracer has been shown to enter the growth plate (22, 62). However, transport of biological substances involves complex interactions in which molecular weight, charge, carrier proteins, and receptor affinities all play a critical role (40). Prior work in our laboratory using dextrans of differing molecular mass demonstrates that small molecules (up to 10 kDa) enter the growth plate without hindrance, but larger molecules (40 kDa and larger) do not enter to an appreciable degree (22). We did not examine the potential of a temperature-molecular weight interaction in our present experiments, but we expect there could be differential tracer access (larger molecules entering at warm temperature), especially since warmer temperature increases vascular endothelial permeability (4) and alters properties of the extracellular matrix (discussed below). An ideal future study might employ fluorescence-labeled hormones or growth factors to determine how closely these inert tracers mimic the actual transport of bioactive molecules in the growth plate, although the sensitivity range required to detect low levels of circulating hormones, particularly those with short half-lives, makes this a challenging task.
Age and size of the study animal are additional points for consideration. At present, size constraints have limited the application of our technique to mice, because depth penetration would be inadequate in a larger animal. However, this limitation is matched by the benefit that mice are the most common research models in the life sciences. Perhaps a more challenging concern is the age at which imaging experiments must be conducted for analyses of active growth plates, which are only present in young animals (70). We have found that 4–5 wk is an ideal age range for visualizing the growth plate in vivo (22, 62). Since imaging a single mouse is time intensive (between 2 and 4 mice can usually be imaged in a single day) and microscope access can be limited in shared-user facilities, it may be necessary to maintain a large breeding colony of animals if specialized strains are needed to assure continuous availability of appropriate-age mice for imaging. This could likewise render sampling particularly difficult if sex and/or genotype are important research considerations, since so few mice can be imaged on any given day and, as with any sensitive in vivo experimentation, a variety of issues could impair data collection (i.e., poor surgical approach, suboptimal growth plate visualization, equipment malfunction). Therefore, it could take multiple repeated imaging sessions to secure a large enough sample for robust statistical testing. However, it should be clarified that this age restriction is only applicable to growth plate studies, since growth cartilage becomes reduced in size and/or degenerates at maturity (46, 53, 70). These methods could readily be applied to the study of other tissues such as articular cartilage or intervertebral disk in adult animals of any age.
A final major consideration involves selecting an appropriate reference point for standardizing fluorescence values in the growth plate. We demonstrated the importance of using an internal standard to normalize data for interanimal comparisons (Fig. 6), but selection of the reference point merits some discussion. Normalized values, or ratios, can be difficult to interpret in the case of a “shifting denominator,” that is, when the reference point itself significantly differs between groups. Such was the case in our experiments, where we found elevated fluorescein levels in both growth plate and vasculature at warm limb temperature (Fig. 6B). Although less pronounced in the vasculature compared with the growth plate, there was still a marked difference between the cold and warm groups. A potential explanation for the decreased values in the cold is that peripheral vasoconstriction shunts blood toward the core as a heat-conserving mechanism (23, 50), and less total blood (and therefore less fluorescein) reaches the extremities. However, in this particular situation, the higher vasculature values in the warm group tended to bias results in opposition to our hypothesis, since a larger denominator would by nature create a smaller ratio unless the numerator were concomitantly increased. Since growth plate and vascular values were always highly correlated (Fig. 6D), we found this method of standardization appropriate for our purposes. However, we must still acknowledge this important confounding issue and recommend selection of a standardization factor that is most appropriate to the research question.
Response to acute temperature change.
We identified an unexpected response to acute temperature change. This type of response would be difficult to observe using other methods. After the initial warm or cool limb immersion, we found that changing the temperature of the perfusion bath had little impact on fluorescein accumulation in the growth plate (Fig. 9). In all but one instance of temperature change (Fig. 9, mouse f), fluorescein levels were found within the range of those expected at the original baseline temperature even when the repeated injection was done at a warmer or colder temperature. By changing limb temperature before administering the first injection, we were able to eliminate the possibility of an experimental artifact, suggesting that there may be a refractory period of sustained biological response to the initial temperature exposure. These results could reflect changes in sensitivity of vessels supplying the growth plate and/or temperature-induced alterations in the structure and composition of the cartilage itself.
It is possible that the initial acute temperature “shock” elicits changes in blood viscosity, vessel permeability, elasticity, and/or extracellular matrix properties that are not immediately reversed, even after exposure to warmer or cooler temperatures. For example, Kandušer and et al. (38) recently showed that cold temperature (4°C) significantly decreases membrane fluidity of cultured mammalian cells without altering cell size or morphology. Acute cold exposure also increases blood viscosity by several orders or magnitude in humans (14, 18), birds (31), amphibians, and reptiles (41, 47), with accompanying increases in hematocrit (18). Such changes are consistent with the decreased fluorescein concentrations we observed in the vasculature exposed to a cold baseline temperature. On the other hand, warm temperature increases vascular endothelial permeability (4, 28, 44), potentially facilitating fluorescein entry in the growth plate from the vasculature, which we found to be more restricted at cooler temperature (Fig. 7).
With respect to the extracellular matrix, experiments have shown that the viscoelastic properties of collagen-rich ligaments in the spine (8) and nasal septal cartilage (13) are temperature dependent, and local application of heat and cold similarly has sustained effects on joint stiffness (64), which could potentially enhance or impair solute movement through the tissues. The rate of endogenous collagen lysis is nearly four times higher in rheumatoid knee joints that exhibit characteristically elevated temperature (36°C) compared with normal human intra-articular knee temperatures (33°C) (33). This temperature-dependent increase in intrinsic collagenase activity can be replicated under acute conditions in vitro (33), suggesting that changes in enzymatic activity in growth plate cartilage could potentially account for at least some of our results, since solute transport in cartilage is increased when enzymatic activity is high and/or cartilage structure is otherwise compromised (54, 55, 58). In fact, Torzilli (58) has shown that high temperature alone can increase solute transport in articular cartilage substantially more than the minimal diffusion increase expected using the Stokes-Einstein approximation (6). If such effects are due to transient changes in cartilage structure induced by temperature, there is likely to be some delay before the original configuration can be restored, especially if any change in tissue temperature persists beyond the initial exposure period. Johnson et al. (37) reported changes in intramuscular temperatures of the human lower limb following 30 min of single-limb immersion in cold water (10°C) that persisted for over 4 h after the treatment. Our experiments were only conducted over 3–4 h total time per mouse, with exposure to each temperature being 1 h or less, and so it is plausible that there may be several hours or more of recovery time required before normal transport conditions are reestablished.
Summary.
We have shown that in vivo multiphoton imaging is a reliable tool for measuring solute transport in tibial growth plates of mice. We demonstrated significant effects of acute temperature on fluorescein accumulation in the growth plate, and our results suggest that temperature could be a novel strategy for enhancing the localized delivery of therapeutic agents to injured growth plates of children. By administering repeated tracer injections at different limb temperatures, we also identified a sustained biological response to the initial temperature exposure that could reflect acute changes in vessel permeability and/or extracellular matrix composition. These analytical methods are a substantial advancement over traditional approaches to bone and cartilage imaging and will be important for elucidating the role of nutrient transport in natural and experimental models of growth plate dysfunction, particularly chondrodysplasias with known defects in components and structure of the extracellular matrix.
GRANTS
This work was made possible by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01 AR052003-05. M. Serrat is an American Association of Anatomists Scholar, and this research was funded in part by a Postdoctoral Fellowship from the American Association of Anatomists.
Acknowledgments
We thank Jesse McMullen and Warren Zipfel for assistance with the multiphoton microscope; Sylvia Allen for animal care; Barbara Linnehan for research aid; and Eve Donnelly, Serena Chang, and Wenhua Liu for technical advice and suggestions. We are grateful to Dr. William A. Horton at the Shriners Research Center, Portland OR, for generously supplying the Col2-GFP mice.
REFERENCES
- 1.Aigner T, Rau T, Niederhagen M, Zaucke F, Schmitz M, Pöhls U, Stöss H, Rauch A, Thiel CT. Achondrogenesis type IA (Houston-Harris): a still unresolved molecular phenotype. Pediatr Dev Pathol 10: 328–334, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Akorn. AK-FLUOR Fluorescein Injection, USP (Online). http://www.akorn.com/documents/catalog/sell_sheets/17478-253-10.pdf?PHPSESSID=30993e49aca5d5d47deef463268b2046 [2009].
- 3.Alman BA Skeletal dysplasias and the growth plate. Clin Genet 73: 24–30, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Ang C, Dawes J. The effects of hyperthermia on human endothelial monolayers: modulation of thrombotic potential and permeability. Blood Coagul Fibrinolysis 5: 193–199, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Arkill KP, Winlove CP. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis Cartilage 16: 708–714, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Atkins P, de Paula J. Physical Chemistry. New York: Freeman, 2002.
- 7.Ballock RT, O'Keefe RJ. The biology of the growth plate. J Bone Joint Surg 85A: 715–726, 2003. [PubMed] [Google Scholar]
- 8.Bass CR, Planchak CJ, Salzar RS, Lucas SR, Rafaels KA, Shender BS, Paskoff G. The temperature-dependent viscoelasticity of porcine lumbar spine ligaments. Spine 32: E436–E442, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Brodin H Longitudinal bone growth and nutrition of the epiphyseal cartilages and the local blood supply: an experimental study in the rabbit. Acta Orthop Scand Suppl 20: 9–92, 1955. [PubMed] [Google Scholar]
- 10.Brookes M Blood supply of developing bone and its possible bearing on malformation of the limbs and face in congenital haemangiomatous disorders. Proc R Soc Med 65: 597–599, 1972. [PMC free article] [PubMed] [Google Scholar]
- 11.Brookes M, Revell WJ. Blood Supply of Bone: Scientific Aspects. New York: Springer, 1998.
- 12.Cairns R Magnetic resonance imaging of the growth plate: pictorial essay. Can Assoc Radiol J 54: 234–242, 2003. [PubMed] [Google Scholar]
- 13.Chae Y, Aguilar G, Lavernia EJ, Wong BJ. Characterization of temperature dependent mechanical behavior of cartilage. Lasers Surg Med 32: 271–278, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Çinar Y, Senyol M, Duman K. Blood viscosity and blood pressure: role of temperature and hyperglycemia. Am J Hypertens 14: 433–438, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Comper WD Extracellular Matrix. Amsterdam: Harwood Academic, 1996.
- 16.Draenert K, Draenert Y. The role of the vessels in the growth plate: morphological examination. Scan Electron Microsc 1: 339–344, 1985. [PubMed] [Google Scholar]
- 17.Dunn KW, Sutton TA. Functional studies in living animals using multiphoton microscopy. ILAR J 49: 66–77, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann DM, Bowers S, Stecker M, Cheung AT. Hematocrit, volume expander, temperature, and shear rate effects on blood viscosity. Anesth Analg 91: 539–545, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Embury SH, Mohandas N, Paszty C, Cooper P, Cheung AT. In vivo blood flow abnormalities in the transgenic knockout sickle cell mouse. J Clin Invest 103: 915–920, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etchebehere E, Caron M, Pereira JA, Lima M, Santos AO, Ramos CD, Barros FB, Sanches A, Santos-Jesus R, Belangero W, Camargo EE. Activation of the growth plates on three-phase bone scintigrapy: the explanation for the overgrowth of fractured femurs. Eur J Nucl Med 28: 72–80, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Farnum CE Postnatal growth of fins and limbs through endochondral ossification. In: Fins into Limbs: Evolution, Development, and Transformation, edited by Hall BK. Chicago, IL: University of Chicago Press, 2007, p. 118–151.
- 22.Farnum CE, Lenox M, Zipfel W, Horton W, Williams R. In vivo delivery of fluoresceinated dextrans to the murine growth plate: imaging of three vascular routes by multiphoton microscopy. Anat Rec A Discov Mol Cell Evol Biol 288: 91–103, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldhamer GA, Drickamer LC, Vessey SH, Merritt JF. Mammalogy: Adaptation, Diversity, Ecology. New York: McGraw-Hill, 2004.
- 24.Feucht BL, Richardson AW, Hines HM. Effect of hot foments on volume of blood flow in extremities of dogs. Arch Phys Med Rehabil 30: 687–690, 1949. [PubMed] [Google Scholar]
- 25.Flesken-Nikitin A, Williams RM, Zipfel W, Webb WW, Nikitin AY. Use of multiphoton imaging for studying cell migration in the mouse. Methods Mol Biol 294: 335–345. 2005. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs H, Gailus-Durner V, Adler T, Pimental J, Becker L, Bolle I, Brielmeier M, Calzada-Wack J, Dalke C, Ehrhardt N, Fasnacht N, Ferwagner B, Frischmann U, Hans W, Hölter SM, Hölzlwimmer G, Horsch M, Javaheri A, Kallnik M, Kling E, Lengger C, Maier H, Mossbrugger I, Mörth C, Naton B, Nöth U, Pasche B, Prehn C, Przemeck G, Puk O, Racz I, Rathkolb B, Rozman J, Schäble K, Schreiner R, Schrewe A, Sina C, Steinkamp R, Thiele F, Willershäuser M, Zeh R, Adamski J, Busch DH, Beckers J, Behrendt H, Daniel H, Esposito I, Favor J, Graw J, Heldmaier G, Höfler H, Ivandic B, Katus H, Klingenspor M, Klopstock T, Lengeling A, Mempel M, Müller W, Neschen S, Ollert M, Quintanilla-Martinez L, Rosenstiel P, Schmidt J, Schreiber S, Schughart K, Schulz H, Wolf E, Wurst W, Zimmer A, Hrabé de Angelis M. The German Mouse Clinic: a platform for systemic phenotypic analysis of mouse models. Curr Pharm Biotechnol 10: 236–243, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Garofalo S, Vuorio E, Metsaranta M, Rosati R, Toman D, Vaughan J, Guillermina L, Mayne R, Ellard J, Horton W, De Crombrugghe B. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen α1-chain gene. Proc Natl Acad Sci USA 88: 9648–9652, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrick RA, Ryan US, Chinard FP. Water permeability of isolated endothelial cells at different temperatures. Am J Physiol Cell Physiol 255: C311–C314, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Genant HK, Bautovich GJ, Singh M, Lathrop KA, Harper PV. Bone-seeking radionucleotides: an in vivo study of factors affecting skeletal uptake. Radiology 113: 373–382, 1974. [DOI] [PubMed] [Google Scholar]
- 30.Grant TD, Cho J, Ariail KS, Weksler NB, Smith RW, Horton WA. Col2-GFP reporter marks chondrocyte lineage and chondrogenesis during mouse skeletal development. Dev Dyn 218: 394–400, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Guard C, Murrish DE. Effects of temperature on the viscous behavior of blood from Antarctic birds and mammals. Comp Physiol Biochem A Comp Physiol 52: 287–290, 1975. [DOI] [PubMed] [Google Scholar]
- 32.Hansson LI Daily growth in length of diaphysis measured by oxytetracycline in rabbit normally and after medullary plugging. Acta Orthop Scand Suppl 101: 1–199, 1967. [DOI] [PubMed] [Google Scholar]
- 33.Harris ED, McCroskery PA. The influence of temperature and fibril stability on degradation of cartilage collagen by rheumatoid synovial collagenase. N Engl J Med 290: 1–6, 1974. [DOI] [PubMed] [Google Scholar]
- 34.Hedrich HJ, Bullock G. The Laboratory Mouse. Boston, MA: Elsevier Academic, 2004.
- 35.Horton WE, Bennion P, Yang L. Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskelet Neuronal Interact 6: 379–381, 2006. [PubMed] [Google Scholar]
- 36.Hunter WL, Arsenault AL. Vascular invasion of the epiphyseal growth plate: analysis of metaphyseal capillary ultrastructure and growth dynamics. Anat Rec 227: 223–231, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Johnson DJ, Moore S, Moore J, Oliver RA. Effect of cold submersion on intramuscular temperature of the gastrocnemius muscle. Phys Ther 59: 1238–1242, 1979. [DOI] [PubMed] [Google Scholar]
- 38.Kandušer M, Šentjurc M, Miklavcic D. The temperature effect during pulse application on cell membrane fluidity and permeabilization. Bioelectrochemistry 74: 52–57, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Kiiskinen A, Suominen H. Blood circulation of long bones in trained growing rats and mice. Eur J Appl Physiol 34: 303–309, 1975. [DOI] [PubMed] [Google Scholar]
- 40.Krauss G Biochemistry of Signal Transduction and Regulation. Weinheim, Germany: Wiley-VCH, 2003.
- 41.Langille BL, Crisp B. Temperature dependence of blood viscosity in frogs and turtles: effect on heat exchange with environment. Am J Physiol Regul Integr Comp Physiol 239: R248–R253, 1980. [DOI] [PubMed] [Google Scholar]
- 42.Leddy HA, Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann Biomed Eng 31: 753–760, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Lian G, Lenkinski R, De Grand A, Vaid RR, Bryce T, Stasenko M, Boskey A, Walsh C, Sheen V. Filamin B mutations cause chondrocyte defects in skeletal development. Hum Mol Genet 16: 1661–1675, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Marcus BC, Gewertz BL. Measurement of endothelial permeability. Ann Vasc Surg 12: 384–390, 1998. [DOI] [PubMed] [Google Scholar]
- 45.McDonald F, Pitt Ford TR. Blood flow changes in the tibia during external loading. J Orthop Res 11: 36–48, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson O, Baron J. Fundamental limits on longitudinal bone growth: growth plate senescence and epiphyseal fusion. Trends Endocrinol Metab 15: 370–374, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Palenske NM, Saunders DK. Comparisons of blood viscosity between amphibians and mammals at 3°C and 38°C. J Therm Biol 27: 479–484, 2002. [Google Scholar]
- 48.Roberts M, Rivers T, Oliveria S, Texeira P, Raman E. Adrenoreceptor and local modulator control of cutaneous blood flow in thermal stress. Comp Physiol Biochem A Mol Integr Physiol 313: 485–496, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Rodgers K.D, Sasaki T, Aszodi A, Jacenko O. Reduced perlecan in mice results in chondrodysplasia resembling Schwartz-Jampel syndrome. Hum Mol Genet 16: 515–528, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Scholander PF, Hammel HT, Hart JS, LeMessurier DH, Steen J. Cold adaptation in Australian aborigines. J Appl Physiol 13: 211–218, 1958. [DOI] [PubMed] [Google Scholar]
- 51.Schoutens A, Bergmann P, Verhas M. Bone flood flow measured by 85Sr microspheres and bone seeker clearances in the rat. Am J Physiol Heart Circ Physiol 236: H1–H6, 1979. [DOI] [PubMed] [Google Scholar]
- 52.Serrat MA, King D, Lovejoy CO. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc Natl Acad Sci USA 105: 19347–19352, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serrat MA, Lovejoy CO, King D. Age- and site-specific decline in insulin-like growth factor-I receptor expression is correlated with differential growth plate activity in the mouse hindlimb. Anat Rec (Hoboken) 290: 375–381, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Silvast TS, Jurvelin JS, Aula AS, Lammi MJ, Töyräs J. Contrast agent-enhanced computed tomography of articular cartilage: associations with tissue composition and properties. Acta Radiol 50: 78–85, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Silvast TS, Jurvelin JS, Lammi MJ, Töyräs J. pQCT study on diffusion and equilibrium distribution of iodinated anionic contrast agent in human articular cartilage—associations to matrix composition and integrity. Osteoarthritis Cartilage 17: 26–32, 2009. [DOI] [PubMed] [Google Scholar]
- 56.Tøndevold E Haemodynamics of long bones: an experimental study on dogs. Acta Orthop Scand Suppl 54: 9–48, 1983. [PubMed] [Google Scholar]
- 57.Tøndevold E, Bülow J. Bone blood flow in conscious dogs at rest and during exercise. Acta Orthop Scand 54: 53–57, 1983. [DOI] [PubMed] [Google Scholar]
- 58.Torzilli P Effects of temperature, concentration and articular surface removal on transient solute diffusion in articular cartilage. Med Biol Eng Comput 31: S93–S98, 1993. [DOI] [PubMed] [Google Scholar]
- 59.Torzilli P, Grande DA, Arduino JM. Diffusive properties of immature cartilage. J Biomed Mater Res 40: 132–138, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev 24: 782–801, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J 88: 1377–1386, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams RM, Zipfel WR, Tinsley ML, Farnum CE. Solute transport in growth plate cartilage: in vitro and in vivo. Biophys J 93: 1039–1050, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams RM, Zipfel WR, Webb WW. Multiphoton microscopy in biological research. Curr Opin Chem Biol 5: 603–608, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Wright V, Dowson D, Unsworth A. The lubrication and stiffness of joints. Mod Trends Rheumatol 2: 30–45, 1971. [PubMed] [Google Scholar]
- 65.Yang KT, Yang AD. Evaluation of activity of epiphyseal plates in growing males and females. Calcif Tissue Int 78: 348–356, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Sivakumar P, Kunida Te, Tsutsui TW, Boskey A, Bonewald LF, Feng JQ. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem 280: 6197–6203, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Szeri AZ. Transport of neutral solute in articular cartilage: effect of microstructure anisotropy. J Biomech 41: 430–437, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci USA 100: 7075–7080, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21: 1369–1377, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Zoetis T, Tassinari MS, Bagi C, Walthall K, Hurtt ME. Species comparison of postnatal bone growth and development. Birth Defects Res B Dev Reprod Toxicol 68: 86–110, 2003. [DOI] [PubMed] [Google Scholar]