Abstract
Autopsy/cadaver data indicate that many organs and tissues are smaller in the elderly compared with young adults; however, in vivo data are lacking. The aim of this study was to determine whether the mass of specific high-metabolic-rate organs is different with increasing age, using MRI. Seventy-five healthy women (41 African-Americans and 34 Caucasians, age range 19–88 yr) and 36 men (8 African-Americans and 28 Caucasians, age range 19–84 yr) were studied. MRI-derived in vivo measures of brain, heart, kidneys, liver, and spleen were acquired. Left ventricular mass (LVM) was measured by either echocardiography or cardiac gated MRI. Total body fat mass and fat-free mass (FFM) were measured with a whole body dual-energy X-ray absorptiometry (DXA) scanner. Multiple regression analysis was used to investigate the association between the organ mass and age after adjustment for weight and height (or DXA measures of FFM), race, sex, and interactions among these variable. No statistically significant interaction was found among age, sex, and race in any regression model. Significant negative relationships between organ mass and age were found for brain (P < 0.0001), kidneys (P = 0.01), liver (P = 0.001), and spleen (P < 0.0001). A positive relationship between LVM and age was found after adjustment for FFM (P = 0.037). These findings demonstrate that age has a significant effect on brain, kidneys, liver, spleen, and heart mass. The age effect was independent of race and sex.
Keywords: organs, magnetic resonance imaging, race, ethnicity
aging of organs and tissues is of clinical and research interest. Elucidating the extent of organ and tissue atrophy has important implications for understanding resting energy expenditure (REE) changes with age and REE-related diseases such as obesity (2, 18). Although the combined weight of the brain, heart, kidneys, and liver is less than 6% of total body weight, these four high-metabolic-rate organs account for 60–70% of REE in adults (3, 5, 10).
The in vivo weight of these organs and tissues may serve as a reference when evaluating the stage of a patient's disease. In normal (disease free) aging, the degree to which organ and tissue atrophy correlates with functional decline varies by organ and tissue. Most reference data for organ weights have been derived from autopsy studies (1, 9, 12, 20). Autopsy data have shown a linear decline in organ weight with increasing age for brain, liver, and kidneys, while the weight for heart increased with age (9). Limitations associated with the use of autopsy data as a reference for in vivo organ mass include a significant loss of organ weight that occurs during the first 15 min after being freed from the surrounding tissue (9). It is also unclear how the mass of these organs changes from a living to a cadaver state. An organ weight measured in vivo is likely more meaningful to guide clinical practice for treatment of a patient and evaluation of disease progression. The availability of imaging techniques, such as MRI, allows for the in vivo determination of organ weight (5, 6, 17).
The aim of this study was to investigate the association between age and the weight of specific high-metabolic-rate organs in vivo, including brain, heart, kidneys, liver, and spleen measured by MRI in healthy African-American and Caucasian men and women, and how the association will be influenced by sex and race.
METHODS
Subjects
Study volunteers were recruited through advertisements in local newspapers, on radio stations, and flyers posted in the local community. Based on self-report, only persons with all four grandparents of either African-American or European Caucasian ancestry were selected. Inclusion criteria required that volunteers be nonsmoking, ambulatory, not vigorously exercising, weight stable (weight change < 2.5 kg within past 6 mo), and not taking any medications or with any known medical condition that could potentially affect the variables under investigation. A body mass index (BMI) < 37 kg/m2 was set as a requirement for participation to accommodate MRI scanner limitations. A screening test that included physical examination and blood tests was conducted on each potential subject. Only healthy individuals without serious medical diagnoses or medical conditions that would affect body composition, organ size, and with normal thyroid hormone and cortisol values were finally enrolled. The age range for the study population was 19–88 yr of age. The study was approved by the Institutional Review Board of St. Luke's-Roosevelt Hospital, and each subject gave written consent to participate.
Body Composition Measurement
All body composition evaluations were carried out within a 2-wk period. On the test day, the subject reported to the laboratory in the morning after an overnight fast, and the tests were conducted with the subject clothed in a hospital gown and wearing foam slippers.
Anthropometry.
Body weight was measured to the nearest 0.1 kg (Weight Tronix, New York) and height to the nearest 0.5 cm using a stadiometer (Holtain; Crosswell, United Kingdom).
MRI.
Subjects were positioned on the 1.5-T scanner (General Electric, 6X Horizon, Milwaukee, WI) platform with their arms extended above their heads. All MRI tests were carried out without prior sedation in a postabsorptive state. All organ volume values (in liters) were converted to mass (in kg) using the assumed density for each organ, 1.05 kg/l for liver, kidneys, and spleen and 1.03 kg/l for heart and brain (21). The MRI protocol has no known health risks to the subjects such as radiation, and it provides a measurement to quantify body composition at the organ level.
LIVER, KIDNEY, SPLEEN, AND BRAIN MEASUREMENT.
Liver, kidney, and spleen volumes were measured using an axial T1-weighted spin echo sequence with 5-mm slice thickness, no interslice gap, and a 40 × 40 cm2 field of view. For brain volumes, two protocols were used during the course of the study: an axial orientation for data collected before 2001 and a coronal orientation for data collected after 2001. Approximately 29 brain images acquired using the axial protocol were produced using a body coil with a fast-spin echo T2-weighted sequence with 5-mm contiguous axial images and a 40 × 40 cm2 field of view with a matrix of 256 × 256 and number of excitations of 1. For the coronal protocol, a transaxial T1-weighted sequence with 1.5-mm slice thickness was acquired in a coronal plane orthogonal to the anterior commissure-posterior commissure (AC-PC) plane over the whole brain with the acquisition time of 11 min. To combine the data, a study was conducted on five subjects using both protocols, and an equation was generated to convert the axially derived volume to the coronal volume as previously described (8). SliceOmatic 4.2 image analysis software (Tomovision, Montreal, Canada) was used to analyze all MRI images in the Image Reading Center (New York). The coefficient of variation for the same five scans read by two analysts for MRI-derived kidneys, liver, spleen, and brain volumes in adults is 1.6%, 4.3%, 15.7%, and 8%, respectively.
HEART MEASUREMENT.
For the heart mass, left ventricular mass (LVM) was measured, and total heart mass can be estimated from LVM by multiplying by a factor of 1.50 (13). Two protocols were used during the course of the study: echocardiography for data collected before 2001 and cardiac gated MRI for data collected thereafter as previously described (8). All measurements were analyzed by a single radiologist (L. Boxt). The mean intraobserver variability for estimating LVM was 5.13 ± 2.9% and the inter-observer variability was 9.01 ± 1.65% in an analysis of 10 healthy subjects (14).
Dual-energy X-ray absorptiometry measures of fat and fat-free mass.
Total body fat mass and fat free mass (FFM) were measured with a whole-body dual-energy X-ray absorptiometry (DXA) scanner, model DPX (software version 3.6Y) or model DPXL (software version 4.7E; both: GE Lunar, Madison, WI). The between-measurement technical error for FFM in the same subject is 1.2% (7). The daily quality control and calibration measures practiced in the DXA laboratory have been described previously (22).
Statistical Analysis
Descriptive subject data were expressed as means ± SD. Student's t-test was used to compare the means of select subject characteristics. Multiple regression analysis was used to determine the relationship between organ mass and age after adjustment for covariates. Log transformation was used to transform the dependent variable as needed to achieve normal distribution of the residuals. All statistics were computed using SAS software version 8 (19), and statistical significance was set at P < 0.05, two-tailed.
RESULTS
Subject Characteristics
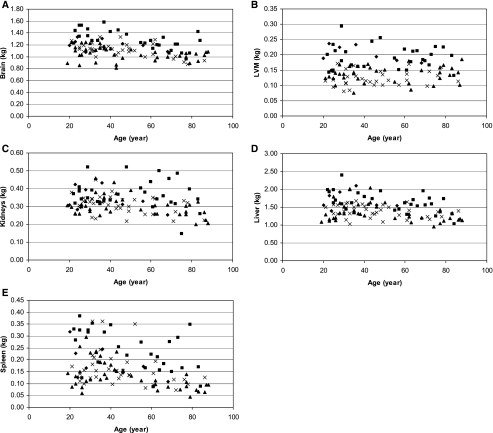
Seventy five healthy women (41 African-Americans and 34 Caucasians, age range 19–88 yr) and 36 men (8 African-Americans and 28 Caucasians, age range 19–84 yr) were studied. The mean and SD values for age, height, weight, BMI, organ weight from MRI, and fat mass and FFM from DXA, were computed by sex for African-Americans and Caucasians (Table 1). Since the African-American women were significantly heavier than the Caucasian women (P = 0.001) and the African-American men were slightly younger than the Caucasian men (P = 0.09), the group means for all other variables were not compared. The relationship between organ mass and age (unadjusted for covariates) is presented in Fig. 1, A–E. Except for the LVM, the mass of all other organs was smaller with increasing age.
Table 1.
Subject characteristics
| Women |
Men | |||
|---|---|---|---|---|
| African-American (n = 41) | Caucasian (n = 34) | African-American (n = 8) | Caucasian (n = 28) | |
| Age, yr | 48.1±22.3 | 45.2±17.2 | 37.6±17.5 | 51.6±20.6 |
| Weight, kg | 72.4±15.3 | 62.1±9.1 | 80.0±12.2 | 77.6±11.0 |
| Height, m | 1.62±0.06 | 1.63±0.05 | 1.78±0.06 | 1.77±0.08 |
| BMI, kg/m2 | 27.30±5.04 | 23.41±3.60 | 25.36±4.25 | 24.81±3.09 |
| Brain (MRI), kg | 1.05±0.11 | 1.11±0.11 | 1.21±0.09 | 1.29±0.15 |
| LVM (MRI), kg | 0.14±0.03 | 0.12±0.02 | 0.20±0.03 | 0.19±0.04 |
| Kidneys (MRI), kg | 0.31±0.06 | 0.31±0.05 | 0.34±0.07 | 0.37±0.08 |
| Liver (MRI), kg | 1.36±0.26 | 1.39±0.17 | 1.64±0.30 | 1.66±0.28 |
| Spleen (MRI), kg | 0.13±0.06 | 0.16±0.07 | 0.19±0.07 | 0.24±0.09 |
| Fat mass (DXA), kg | 26.48±11.27 | 19.51±7.92 | 16.53±8.74 | 14.89±8.11 |
| Fat-free mass (DXA), kg | 45.91±6.44 | 41.89±3.99 | 62.90±15.53 | 62.41±7.14 |
Values are means ± SD. BMI: body mass index; MRI: magnetic resonance imaging; LVM: left ventricular mass; DXA: dual-energy X-ray absorptiometry.
Fig. 1.
A: the relationship between the mass of brain and age (scatterplots of the raw data). B: the relationship between left ventricular mass (LVM) and age (scatterplots of the raw data). C: the relationship between the mass of kidneys and age (scatterplots of the raw data). D: the relationship between the mass of liver and age (scatterplots of the raw data). E: the relationship between the mass of spleen and age (scatterplots of the raw data). Symbols: ⧫, African-American men; ▪, Caucasian men; ▴, African-American women; ×, Caucasian women.
Association Between Age and Organ Mass
Multiple regression analysis was used to examine the associations between the mass of each organ (kg) and age after adjustment for other variables: weight and height (Table 2). No statistically significant two- or three-way interactions were found among age, sex, and race in any regression analysis. Significant negative relationships between organ mass and age were seen in brain (P < 0.0001), kidneys (P = 0.01), liver (P = 0.001), and spleen (P < 0.0001), indicating per year decreases of 0.002 kg for brain, 0.001 kg for kidneys, 0.003 kg for liver, and 0.001 kg for spleen. For LVM, a nonstatistically significant positive relationship was found with age (P = 0.15). This finding was the same when the analysis was run using the method (echocardiography vs. cardiac gated MRI) as a covariate (results not shown here). The SEs of estimate for the regression equations were 0.107 kg for brain, 0.026 kg for LVM, 0.143 kg for kidneys, 0.188 kg for liver, and 0.319 kg for spleen. Similar results were found using FFM and fat mass as covariates instead of weight and height for body size (Table 3). Organ weights were positively related to fat mass, except for the brain; and age did not modify this relationship as the interaction between fat mass and age was not significant in all models. The SEs of estimate for the regression equations were 0.106 kg, 0.026 kg, 0.145 kg, 0.185 kg, and 0.322 kg for brain, LVM, kidneys, liver, and spleen, respectively. In summary, age explained 10% of the variance for brain, 1% for LVM, 4% for kidneys, 5% for liver, and 11% for spleen in the weight and height model (Table 2). Similar values were found (1–11%) in the FFM and fat mass model (Table 3).
Table 2.
Age and organ weight regression model with weight and height as covariates
| Model | Variable | Regression Coefficient ± SE | P Value | R2 Explained Variance |
|---|---|---|---|---|
| Brain | Intercept | 0.701±0.276 | 0.013 | |
| Age | −0.002±0.001 | <0.0001 | 0.10 | |
| Weight | −0.001±0.001 | 0.460 | 0.00 | |
| Height | 0.353±0.173 | 0.043 | 0.02 | |
| Race | −0.064±0.023 | 0.006 | 0.04 | |
| Sex | 0.132±0.033 | 0.0001 | 0.07 | |
| Total: 0.54 | ||||
| LVM | Intercept | 0.015±0.071 | 0.830 | |
| Age | 0.0002±0.0001 | 0.153 | 0.01 | |
| Weight | 0.001±0.0002 | <0.0001 | 0.09 | |
| Height | 0.016±0.044 | 0.717 | 0.00 | |
| Race | 0.002±0.006 | 0.755 | 0.00 | |
| Sex | 0.050±0.008 | <0.0001 | 0.13 | |
| Total: 0.64 | ||||
| Kidney* | Intercept | −1.017±0.378 | 0.008 | |
| Age | −0.002±0.001 | 0.003 | 0.04 | |
| Weight | 0.008±0.001 | <0.0001 | 0.22 | |
| Height | −0.348±0.237 | 0.145 | 0.01 | |
| Race | −0.111±0.030 | 0.0004 | 0.06 | |
| Sex | 0.119±0.045 | 0.009 | 0.03 | |
| Total: 0.50 | ||||
| Liver | Intercept | 0.051±0.486 | 0.916 | |
| Age | −0.003±0.001 | 0.001 | 0.05 | |
| Weight | 0.009±0.002 | <0.0001 | 0.15 | |
| Height | 0.552±0.304 | 0.073 | 0.01 | |
| Race | −0.110±0.040 | 0.007 | 0.03 | |
| Sex | 0.073±0.058 | 0.210 | 0.01 | |
| Total: 0.56 | ||||
| Spleen* | Intercept | −2.850±0.826 | 0.0008 | |
| Age | −0.008±0.002 | <0.0001 | 0.11 | |
| Weight | 0.014±0.003 | <0.0001 | 0.12 | |
| Height | 0.273±0.517 | 0.60 | 0.00 | |
| Race | −0.340±0.068 | <0.0001 | 0.10 | |
| Sex | 0.169±0.099 | 0.09 | 0.01 | |
| Total: 0.57 |
Race: 0 = Caucasian; 1 = African-American. Sex: 0 = Women, 1= Men.
Log-transformed values.
Table 3.
Age and organ weight regression model with FFM and FM as covariates
| Model | Variable | Regression Coefficient ± SE | P Value | R2 Explained Variance |
|---|---|---|---|---|
| Brain | Intercept | 1.194±0.084 | <0.0001 | |
| Age | −0.003±0.001 | <0.0001 | 0.10 | |
| FFM | 0.002±0.002 | 0.293 | ||
| FM | −0.001±0.022 | 0.257 | ||
| Race | −0.063±0.023 | 0.006 | ||
| Sex | 0.130±0.041 | 0.002 | ||
| Total: 0.54 | ||||
| LVM | Intercept | 0.022±0.021 | 0.292 | |
| Age | 0.0003±0.0001 | 0.070 | 0.01 | |
| FFM | 0.002±0.0004 | <0.0001 | ||
| FM | 0.001±0.0003 | 0.006 | ||
| Race | 0.003±0.006 | 0.637 | ||
| Sex | 0.038±0.010 | 0.0003 | ||
| Total: 0.65 | ||||
| Kidney* | Intercept | −1.629±0.115 | <0.0001 | |
| Age | −0.002±0.001 | 0.040 | 0.02 | |
| FFM | 0.009±0.002 | <0.0001 | ||
| FM | 0.006±0.002 | <0.0001 | ||
| Race | −0.106±0.031 | 0.001 | ||
| Sex | 0.030±0.057 | 0.600 | ||
| Total: 0.50 | ||||
| Liver | Intercept | 0.688±0.147 | <0.0001 | |
| Age | −0.003±0.001 | 0.005 | 0.03 | |
| FFM | 0.017±0.003 | <0.0001 | ||
| FM | 0.007±0.002 | 0.001 | ||
| Race | −0.116±0.040 | 0.004 | ||
| Sex | −0.007±0.072 | 0.918 | ||
| Total: 0.58 | ||||
| Spleen* | Intercept | −2.327±0.256 | <0.0001 | |
| Age | −0.009±0.002 | <0.0001 | 0.11 | |
| FFM | 0.012±0.005 | 0.016 | ||
| FM | 0.017±0.003 | <0.0001 | ||
| Race | −0.353±0.069 | <0.0001 | ||
| Sex | 0.263±0.124 | 0.037 | ||
| Total: 0.58 |
Race: 0 = Caucasian; 1 = African-American. Sex: 0 = women; 1 = men. FFM, fat-free mass; FM, fat mass.
Log-transformed values.
DISCUSSION
To our knowledge, this is the first study to present in vivo values for brain, liver, kidneys, spleen, and LVM in healthy African-Americans and Caucasians across the adult age range (19–88 yr). These findings confirm and expand our knowledge of organ weights from previous autopsy studies, i.e., older people have a smaller mass of brain, kidneys, liver, and spleen, but not the heart, compared with younger subjects.
The decrease in organ mass with aging (except for the heart) is an important finding. The finding of no significant interaction between age, sex, and race in any of the analyses that we report indicates that, within the power of this sample size to detect differences (sex or race) in rate of decline, none are seen. It is well known that muscle mass decreases with aging (4, 11, 15, 16); here we also see a disproportionate decrease in organ mass, even after adjusting for FFM. The reasons for organ mass decrease and associated functional implications are worthy of future studies. One possible reason for the failure of the heart to decrease with age is that blood pressure increases with age, which means that the heart has to work harder to maintain its cardiac output. This would predispose to cardiac hypertrophy, an increase in heart mass.
It is observed based on the R2 values from the multiple regression equations that age, race, sex, and weight and height (or FFM and fat mass) explain only about one-half of the variability in organ size. Most of the variation not explained by regression analysis is due to interindividual variation, e.g., differences in muscularity either through training or constitution; one is more muscular than the other (i.e., ratio of muscle to FFM will be affected). The same could apply to organs. The effect of alcohol on liver size is complex and depends on the presence of fat at least in some individuals. It is also important to note that MRI measures organ size including fat (and this varies from individual to individual). Other factors such as familial traits, diet, physical activity, and disease in childhood may also be contributing significantly to the variation in organ size.
All previously published data on organ weight have come from autopsy studies. These data are useful for pathologists as a reference for understanding whether specific organs under investigation in autopsy show pathological changes based on the size. A comparison of organ weights across autopsy-derived databases is difficult given that these studies had quite different inclusion criteria for healthy subjects. For example, one study used the inclusion of “healthy” and “apparently healthy,” which were judged based on the statement from the general practitioner or relatives of the deceased (9), whereas the other study used “free of pathological changes” as an inclusion criterion to derive organ weight for healthy subjects (1).
It is not so meaningful to compare our MRI-derived organ weight with those autopsy data. First, organs after death are not the same in nature as the organs during life. To evaluate the clinical significance of a patient's organ weights, it is important to know the normal range of these organs in a living condition. Second, the methodology on the inclusion of associated tissues in an organ mass from the in vivo MRI analysis may be slightly different from the autopsy dissection. Table 4 shows the organ weights derived from our MRI measure (Caucasian subjects only) and the autopsy data cited from Garby et al. (Ref. 9; the majority of their subjects are white). Generally, the MRI organ weights were less than the autopsy organ weight. One of the explanations for this difference is that associated tissues were included in the autopsy measurement. For example, liver mass from MRI analysis did not include portal and inferior vena cava, gall bladder, and biliary ducts; however, all or a portion of these tissues were included as liver mass when measurements were made during autopsy. Another example is that the volumes of intraventricular fluid and blood within the chambers of heart were not included in the MRI measurements; however, autopsy calculation included these volumes in the total weight of the heart. The causes of death were not provided in the study by Garby et al. (9), which may have included unexpected deaths, accident, homicides, suspected crimes, etc., as these were the main reasons for requiring an autopsy in accordance with Danish law (9). Accordingly, the following reasons could result in the autopsy organ weights being greater than the in vivo MRI organ weights: death having occurred after fluid administration (before and after admission to hospital); heart failure that results in venous congestion and hepatomegaly; head injury that results in cerebral edema; and injury to other organs, which can cause them to swell.
Table 4.
Organ weight derived from the MRI measure and the autopsy data for healthy Caucasian subjects
| Women |
Men | |||
|---|---|---|---|---|
| MRI | Autopsy | MRI | Autopsy | |
| Age, yr | 45±17 | 48±16 | 52±21 | 45±17 |
| Weight, kg | 62±9 | 61±12 | 78±11 | 76±13 |
| Height, cm | 163±6 | 164±7 | 177±8 | 177±7 |
| BMI, kg/m2 | 23.4±4 | 22.3±4 | 24.8±3 | 24.0±4 |
| Brain, g | 1,114±111 | 1,332±112 | 1,293±147 | 1,498±128 |
| Heart, g | 184±36 | 320±67 | 291±57 | 423±87 |
| Spleen, g | 163±73 | 120 (median) | 243±87 | 170 (median) |
| Liver, g | 1,390±172 | 1,591±436 | 1,658±281 | 1,851±442 |
Study Limitations
This study is limited by its cross-sectional design. Ideally, elucidation of the effect of aging on features of interest should be based on serial measures of the same individuals as they progress through adulthood.
An assumed organ density was used in calculating mass for each organ and it is unclear whether the density of individual organs changes with increasing age or whether densities are similar across race/ethnic groups. We acknowledge that there may be individual differences in density, e.g., due to lipid in liver. We convert MRI measurement of organ volume to mass using an assumed density because this makes the units more comparable with other data (body weight, the DXA variable of FFM or autopsy organ weights). However, since the MRI measurements are of organ volume, and the conversions to mass are based on multiplying volume by a constant, at a minimum, the observed age-related changes can be applied to organ volumes. We acknowledge that this problem is not totally avoided by using volume because fatty infiltration of the liver is likely to affect liver volume also.
Summary
Our findings demonstrate that age has a significant effect on brain, kidneys, liver, spleen, and heart mass. The effect of age is consistent across sex and across the race groups studied.
GRANTS
This study was supported in part by National Institutes of Health Grants PO1-DK-42618 (Project 4 and Core C), RR-00645, DK-40414, P30-DK-26687, RO1-DK-072507, and RR-24156, and an educational grant from Knoll Pharmaceuticals.
REFERENCES
- 1.Bean BR Composite study of weight of vital organs in man. Am J Phys Anthropol 9: 293–317, 1926. [Google Scholar]
- 2.Bosy-Westphal A, Eichhorn C, Kutzner D, Ner K 3rd, Heller M, Muller MJ. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J Nutr 133: 2356–2362, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Elia M Organ and tissue contribution to metabolic rate. In: Energy Metabolism. Tissue Determinants and Cellular Corollaries, edited by Kinney JM, Tucker HN. New York, Raven, p. 61–77, 1992.
- 4.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol 83: 229–239, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol Endocrinol Metab 275: E249–E258, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher D, Allen A, Wang Z, Heymsfield SB, Krasnow N. Smaller organ tissue mass in the elderly fails to explain lower resting metabolic rate. Ann NY Acad Sci 904: 449–455, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher D, Kovera AJ, Clay-Williams G, Agin D, Leone P, Albu J, Matthews DE, Heymsfield SB. Weight loss in postmenopausal obesity: no adverse alterations in body composition and protein metabolism. Am J Physiol Endocrinol Metab 279: E124–E131, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N, Elia M. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr 83:1062–1067, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garby L, Lammert O, Kock K, Thobo-Carlsen B. Weights of brain, heart, liver, kidneys, and spleen in healthy and apparently healthy adult Danish subjects. Am J Hum Biol 5: 291–296, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Grande F Energy expenditure of organs and tissues. In: Assessment of Energy Metabolism in Health and Disease. Report of the first Ross Conference on Medical Research. Columbus, OH: Ross Laboratories, p. 88–92, 1980.
- 11.He Q, Heo M, Heshka S, Wang J, Pierson-JRR, Albu J, Wang ZM, Heymsfield SB, Gallagher D. Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr 78: 72–77, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Inoue T, Otsu S. Statistical analysis of the organ weight in 1,000 autopsy cases of Japanese aged over 60 years. Acta Pathol Jpn 37: 343–359, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Jones RS Weight of the heart and its chambers in hypertensive cardiovascular disease with and without failure. Circulation 7: 357–369, 1953. [DOI] [PubMed] [Google Scholar]
- 14.Katz J, Whang J, Boxt LM, Barst RJ. Estimation of right ventricular mass in normal subjects and in patients with primary pulmonary hypertension by nuclear magnetic resonance imaging. J Am Coll Cardiol 21:1475–1481, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Kehayias JJ, Fiatarone MA, Zhuang H, Roubenoff R. Total body potassium and body fat: relevance to aging. Am J Clin Nutr 66: 904–910, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr 55: 663–672, 2001. [DOI] [PubMed] [Google Scholar]
- 17.McNeill G, Foster MA, Love J, Antfang V. Liver and kidney volume and their relationship to metabolic rate at rest. Proc Nutr Soc 54: 151A, 1995.7568249 [Google Scholar]
- 18.Puggaard L, Bjornsbo K, Kock K, Luders K, Thobo-Carlsen B, Lammert O. Age-related decrease in energy expenditure at rest parallels reductions in mass of internal organs. Am J Hum Biol 14: 486–493, 2002. [DOI] [PubMed] [Google Scholar]
- 19.SAS Institute. SAS User's Guide: Statistics: Version 8. Cary, NC: SAS Institute, 1999.
- 20.Seo JS, Lee SY, Won KJ, Kim DJ, Sohn DS, Yang KM, Cho SH, Park JD, Lee KH, Kim HD. Relationship between normal heart size and body indices in Koreans. J Korean Med Sci 15: 641–646, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder WS, Cook MJ, Nasset ES, Karhaussen LR, Howells GP, Tipton IH. Report of the task group on reference men. In: International Commission on Radiological Protection, No. 23. Oxford, UK: Pergamon, 1975.
- 22.Song M, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased muscle adipose tissue infiltration in elderly African American women. Am J Clin Nutr 79: 874–880, 2004. [DOI] [PubMed] [Google Scholar]