Abstract
We have previously shown that microgravity and simulated microgravity induce an increase in human and rat aortic stiffness. We attempted to elucidate the mechanism(s) responsible for this increase in stiffness. We hypothesize that an alteration in vessel wall collagen or elastin content or in extracellular matrix (ECM) cross-linking either individually or in a combination is responsible for the increased vessel stiffness. Rats underwent hindlimb unweighting (HLU) for a period of 7 days to simulate microgravity. The contribution of ECM cross-linking to the vessel wall stiffness was evaluated by measuring aortic pulse wave velocity following inhibition of the cross-linking enzymes lysyl oxidase (LOX) and transglutaminase (tTG) and the nonenzymatic advanced glycation end product cross-linking pathway during HLU. Aortic collagen and elastin content was quantified using established colorimetric assays. Collagen subtype composition was determined via immunofluorescent staining. The increase in aortic pulse wave velocity after HLU was significantly attenuated in the LOX and tTG inhibition groups compared with saline (1.13 ± 0.11 vs. 3.00 ± 0.15 m/s, LOX vs. saline, P < 0.001; 1.16 ± 0.25 vs. 3.00 ± 0.15 m/s, tTG vs. saline, P < 0.001). Hydroxyproline content, a measure of collagen content, was increased in all groups after HLU (2.01 ± 0.62 vs. 3.69 ± 0.68% dry weight, non-HLU vs. HLU, P = 0.009). Collagen subtype composition and aortic elastin content were not altered by HLU. Together, these data indicate that HLU-induced increases in aortic stiffness are due to both increased aortic collagen content and enzyme cross-linking activity.
Keywords: arterial stiffness, hindlimb unweighting, vascular remodeling
we have recently demonstrated a dramatic microgravity-induced increase in aortic stiffness in 63% of short term spaceflight astronauts (25). An animal model simulating microgravity demonstrated similar increases in arterial stiffness, confirming this effect (25). Increased arterial stiffness has been associated with increased cardiovascular disease risk and can culminate with cardiac hypertrophy and heart failure (20, 32). Additionally, it is possible that increased vascular stiffness could exacerbate conditions such as atherosclerosis and hypertension in which arterial stiffening preexists (32). While the short duration of increased arterial stiffness endured by these astronauts most likely does not pose a significant health risk, prolonged increases in vascular stiffness during extended missions such as those seen on the International Space Station or those expected for missions to the moon or Mars may endanger the astronauts' cardiovascular health.
Changes in vascular stiffness can be mediated via a multitude of acute and chronic pathways. Acute dynamic changes are thought to be primarily due to neurohormonally mediated changes in vascular smooth muscle tone (mediated, at least in part, by the endothelium). Chronic alterations in vascular stiffness are thought to include changes in vessel wall smooth muscle content, vessel wall collagen and elastin content, the proportions of collagen subtypes in the vessel wall, and the extent of vessel wall extracellular matrix (ECM) cross-linking (32). Factors that increase vessel stiffness include increased vascular smooth muscle tone and content, increased vessel wall collagen content, decreased elastin content, increased ratio of type I to type III collagen, and an increase in ECM cross-linking. Each of these mechanisms has been found to individually modulate vessel stiffness (32). However, it is common for two or more of the pathways to interact, resulting in an aggregate change in vascular stiffness (32). By understanding the exact mechanism(s) responsible for increased arterial stiffness, it may be possible to develop proper countermeasures to protect astronaut cardiovascular health.
The results from our previously published in vitro vessel experiments suggest that the simulated microgravity-induced increase in vascular stiffness is due primarily to local structural alterations as opposed to changes in acute neurohormonal influences (25). Thus we hypothesized that (simulated) microgravity-induced increases in vascular stiffness are due to one or more of the following: an increase in vessel wall collagen content, a decrease in vessel wall elastin content, a shift in the vessel wall collagen type I to type III ratio, or an increase in vessel wall ECM cross-linking. The goal of this study was to elucidate the vascular modifications that occur during (simulated) microgravity that result in increased vascular stiffness. To address these questions, we used the same ground-based rat model to simulate microgravity that was originally used to investigate alterations in vascular stiffness.
METHODS
The following animal protocol was approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine. In total, 43 male Wistar rats (12 wk old, Harlan) were utilized for these experiments. Hindlimb unweighting (HLU) was utilized as a ground-based microgravity exposure analog in animals. HLU in rats has been shown to closely mimic the cardiovascular changes seen in humans and rats exposed to microgravity, including alterations in vascular stiffness (13, 16, 25, 29). Twenty-four rats underwent HLU for 7 days. The other 19 animals served as non-HLU controls and spent 7 days in the same facility as the HLU animals. The HLU method is described in detail in Ref. 17. Briefly, the tail was placed in traction tape that was attached to a swivel-and-crossbar setup that allowed the animal full mobility in the cage with use of the forelimbs. The rats were maintained at a ∼30° angle, head-down tilt. All animals had ad libitum access to standard rat chow and water. Before HLU or non-HLU holding, all animals were subjected to blood pressure and pulse wave velocity measurements.
Blood pressure.
The blood pressure was measured using the XBP1000 noninvasive tail blood pressure system from Kent Scientific. Ten supine measures were taken and averaged while the rat was anesthetized and body temperature was held at 37°C. These supine blood pressure measurements were taken to enable the comparison of the pulse wave velocity (PWV) results pre- and post-HLU (or control). Blood pressure was taken both before the animal was placed in HLU or non-HLU holding and immediately after the 7-day experimental period.
PWV.
The PWV was measured using a Doppler Signal Processing Workstation by Indus Instruments as previously described (25). The PWV for the thoracic aorta was obtained by measuring the aortic blood velocity via recording ultrasonic Doppler signals at two different points (one proximal and one distal) on the thoracic aorta, with the distance between the two points representing the separation distance (SPD). For each aortic blood velocity profile, a time-synchronized electrocardiogram (ECG) was recorded. At each point, pulse wave arrival time (PAT) was measured and is defined as the time lapsed from the R-wave on the ECG to the foot of the blood velocity profile. From two measures, the pulse transit time (PTT) between the two recording sites can be calculated from the equation:
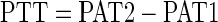 |
From the pulse transit time and the separation distance (SPD), the PWV for the thoracic aorta can be calculated from the equation:
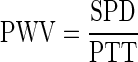 |
The PWV for the thoracic aorta was measured before HLU or the non-HLU period and measured again immediately after the animal was removed from HLU or non-HLU holding.
ECM cross-linking inhibition.
The role of ECM cross-linking in simulated microgravity-induced aortic stiffening was evaluated by inhibiting each of three potential cross-linking pathways during HLU. Two enzymatic pathways, lysyl oxidase (LOX) and transglutaminase (tTG), and one nonenzymatic pathway, advanced glycation end product (AGE), were inhibited during HLU. Beta-aminopropionitrile (BAPN), cystamine, and pyridoxamine were administered to inhibit LOX, tTG, and AGEs, respectively. Of the 24 rats that underwent HLU, 6 each were treated with saline (vehicle), BAPN, cystamine, and pyridoxamine daily, respectively, starting the first day of HLU and lasting for the duration of the HLU protocol. Of the 19 non-HLU animals, 3 were given saline, 6 were given BAPN, 4 were given cystamine, and 6 were given pyridoxamine daily starting on the first day of the non-HLU period and lasting for the duration of the non-HLU protocol. BAPN was administered subcutaneously at a dose of 650 mg·kg−1·day−1 (6), cystamine was administered intraperitoneally at a dose of 50 mg·kg−1·day−1 (10), pyridoxamine was administered orally through drinking water at a concentration of 1 g/l (1), and saline was given subcutaneously at the same volume as BAPN injections, as they were the largest. All injections were given without interrupting the HLU protocol. Rats receiving pyridoxamine drank, on average, 25 ± 2.5 ml of water per day, and this amount did not differ between HLU and non-HLU groups. Based on this, the average pyridoxamine intake was 63.6 ± 6.36 mg·kg−1·day−1, which was demonstrated as an effective dose to inhibit AGE-related pathologies in a prior study (1). Thus a constant dose of pyridoxamine was received by all animals in the group. At the end of the HLU or non-HLU period, the animals were removed from hindlimb suspension and anesthetized (1% isoflurane), and the blood pressures and thoracic aortic PWVs were measured. The thoracic aortic collagen and elastin quantification and collagen type protocols were performed on thoracic aortic tissue obtained from these animals after they were euthanized.
Collagen quantification.
The amount of collagen contained in the rat aortic vessel wall was assessed by using hydroxyproline as a marker (28). Hydroxyproline was quantified using a colorimetric assay as described by Stegemann and Stalder (23). Briefly, vessel sections were lyophilized overnight and subsequently incubated with papain for 18 h at 60°C. The digested solution was then hydrolyzed in 6 N HCl at 115°C for 18 h. The samples were titrated to neutral pH. Chloramine-T solution was added to each sample and allowed to sit at room temperature for 20 min. Next, a p-dimethylaminebenzaldehyde solution was added to each sample and incubated at 60°C for 30 min. The absorbance of each sample was measured on a spectrophotometer set at 550 nm. Each sample was completed in triplicate. The absorbance value of each sample was compared with a set of standard hydroxyproline samples prepared in parallel to determine the amount of hydroxyproline in each sample. The hydroxyproline content of each rat aorta sample is an average of the triplicates and is expressed as a percentage of the dry weight of the vessel.
Collagen type I to type III ratio.
Immunofluorescence staining was used to evaluate the ratios of type I to type III collagen in the rat aorta. Sections of rat aorta were harvested, fixed in formalin, and set in paraffin wax blocks. Sections of aorta were cut at 5 μm. For each sample, three slides were prepared with multiple aortic sections per slide. One slide was treated with a mouse collagen type I antibody (Santa Cruz Biotechnology, sc-59772) and a secondary cy3-conjugated anti-mouse IgG antibody (Jackson Laboratories). The second slide was treated with a mouse collagen type III antibody (Santa Cruz Biotechnology, sc-80564) and the same secondary antibody. Neither antibody, according the manufacturer, is cross-reactive with the opposite collagen type. The last slide was treated with only the secondary antibody. Average intensity values were calculated for each slide (average of 3 aortic rings). The intensity value of the control slide was subtracted from both the type I and type III slide values to account for any autofluorescence and nonspecific staining. In all cases, the intensity of the control never exceeded the other groups. The data are expressed as a percentage of total collagen intensity (type I plus type III).
Elastin quantification.
Rat aortic elastin content was quantified by using the Fastin elastin assay kit (Biocolor). Briefly, rat aorta vessel samples taken in triplicate were homogenized and centrifuged, and the supernatant was collected. The sample supernatants were then treated with an elastin precipitating reagent and centrifuged, and the supernatant was discarded. The elastin pellet was treated with a dye reagent that binds elastin for 90 min. All unbound dye was then washed out. Finally, the bound dye-elastin complex was resuspended and the dye was disassociated from the elastin. The samples were then transferred to a 96-well plate, and the absorbance of each sample was measured at 513 nm. The absorbance value of each sample was compared with a set of standard elastin samples prepared in parallel to determine the amount of elastin in each sample. The elastin content of each rat aorta sample is an average of the triplicates and is expressed as a percentage of the dry weight of the vessel.
Statistics.
The PWV data were analyzed by performing a repeated-measures ANOVA test, with Tukey's post test comparing each group. The collagen quantification, collagen type quantification, and elastin quantification data were analyzed using Student's t-test. All groups of data are reported as means ± SE.
RESULTS
Rat aortic ECM cross-linking.
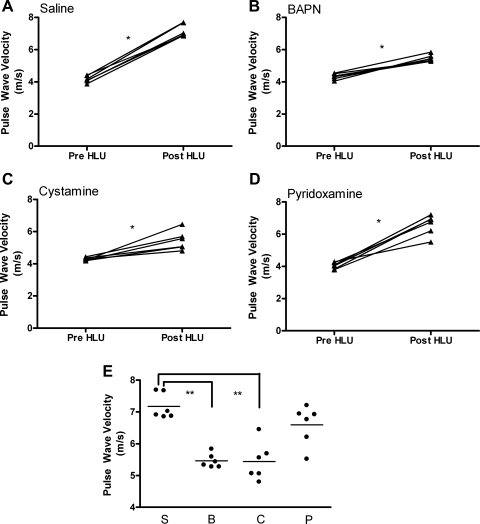
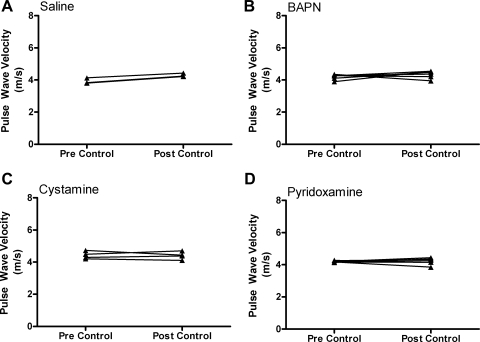
Three separate ECM cross-linking pathways were inhibited during 7 days of HLU to determine their relative contributions to the simulated microgravity-induced increase in aortic stiffness. Stiffness was evaluated by measuring PWV pre- and post-HLU. Rat aortic PWV significantly increased in the saline HLU group, from 4.17 ± 0.21 to 7.17 ± 0.40 m/s (pre- vs. post-HLU, P < 0.001), which will be considered a normal PWV response to HLU (Fig. 1A). Although PWV was also significantly increased following HLU in the BAPN-treated group (4.33 ± 0.18 to 5.46 ± 0.22 m/s, pre- vs. post-HLU, P < 0.001) (Fig. 1B), the magnitude of this increase was profoundly attenuated compared with saline-treated HLU animals (1.13 ± 0.11 vs. 3.00 ± 0.15 m/s, BAPN HLU vs. saline HLU, P < 0.001). Similar results were observed in the cystamine-treated animals. There was a significant increase in PWV following HLU in cystamine-treated animals (4.28 ± 0.10 vs. 5.43 ± 0.58 m/s, pre- vs. post-HLU, P < 0.001) (Fig. 1C), but the magnitude of this increase was profoundly attenuated compared with saline HLU (1.16 ± 0.25 vs. 3.00 ± 0.15 m/s, cystamine HLU vs. saline HLU, P < 0.001). In contrast to BAPN and cystamine, pyridoxamine administration was associated with an increase in PWV of similar magnitude to that observed in saline HLU animals (4.05 ± 0.20 vs. 6.59 ± 0.62 m/s, pre vs. post, P < 0.001) (Fig. 1D). The PWV did not change in any of the non-HLU groups after 7 days of treatment (saline, BAPN, cystamine, or pyridoxamine) with both pre- and post-non-HLU measures being similar to those reported for the pre-HLU animals (Fig. 2). The blood pressures of the rats did not differ significantly between the groups or over time: e.g., in the HLU group treated with saline, 119/88 ± 9/8 mmHg vs. 116/88 ± 6/5 mmHg, pre- vs. post-HLU; and in the non-HLU group treated with saline, 115/78 ± 7/6 mmHg 113/82 ± 9/4 mmHg, pre- vs. post-non-HLU; P > 0.40 for all comparisons. Based on blood pressure measurements at the time of PWV measurement, we can conclude that the changes in PWV most likely reflect actual changes in aortic stiffness (rather than being influenced by changes in blood pressure).
Fig. 1.
Rat thoracic aorta pulse wave velocity (PWV) reported in m/s measured from saline (A)-, beta-aminopropionitrile (BAPN; B)-, cystamine (C)-, and pyridoxamine (D)-treated groups. Post-hindlimb unweighting (post-HLU) measures are compared in E, where S = saline, B = BAPN, C = cystamine, and P = pyridoxamine. Paired measures are illustrated with a connecting line. Pre- and post-HLU measures were made before and after the 7 day HLU period. *P < 0.001, **P < 0.001; n = 6 for each group.
Fig. 2.
Rat thoracic aorta PWV reported in m/s measured from saline-treated (n = 3) (A), BAPN-treated (n = 6) (B), cystamine-treated (n = 4) (C), and pyridoxamine-treated (n = 6) (D) groups. Paired measures are illustrated with a connecting line. Pre- and post-control measures were made before and after the 7-day control period in the animal facility.
Rat aortic hydroxyproline content.
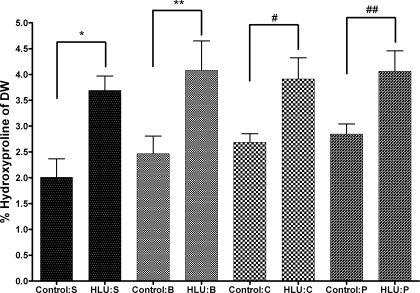
The rat thoracic aorta hydroxyproline content was significantly higher after 7 days of HLU compared with the non-HLU animals in all treatment groups. The saline-treated non-HLU group had a thoracic aorta hydroxyproline content of 2.01 ± 0.62% of the dry weight of the vessel, while the saline HLU group had hydroxyproline content of 3.69 ± 0.68%. The difference between the saline non-HLU and saline HLU groups was statistically significant (P = 0.009). Each of the treatment groups demonstrated similar changes across non-HLU and HLU groups, respectively, and were all significantly different (Fig. 3).
Fig. 3.
Rat thoracic aorta hydroxyproline content expressed as a percentage of the dry weight (DW) of the vessel. Control and HLU measures were compared for saline (S)-, BAPN (B)-, cystamine (C)-, and pyridoxamine (P)-treated groups. The data were analyzed using a standard t-test for all comparisons; n = 6 for all HLU groups, and n =4 for control S group, n = 6 for control B group and for control P group, and n = 4 for control C group. All data are reported as means ± SE. *P = 0.009, **P = 0.03, # P = 0.049, ## P = 0.02.
Rat aortic collagen type I-to-type III ratio.
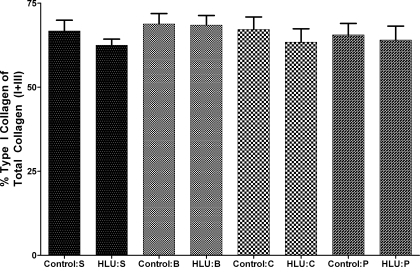
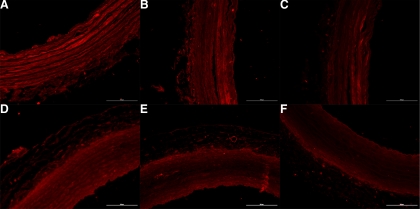
There were no significant changes in the relative amount of aortic type I collagen to type III collagen due to HLU or cross-linking treatments. Type I collagen represented 66.71 ± 5.55% of total collagen (defined as combined type I + type III) in the saline non-HLU group and 62.47 ± 4.50% in the saline HLU group. This was not statistically different. The values reported for the saline groups are representative for the other treatment groups (Fig. 4). Figure 5 shows the aortic collagen type distributions in a non-HLU and HLU saline-treated animal, which was representative of all groups. Figure 5, A–C, represents aortic cross-sectional staining from one non-HLU saline animal, and Fig. 5, D–F, represents aortic cross-sectional staining from one HLU saline animal. It is clear from Fig. 5 that the relative intensities of type I collagen (A and D) compared with type III collagen (B and E) are the same for both the HLU and non-HLU animals.
Fig. 4.
Rat thoracic aorta collagen type quantity distribution expressed as percentage of type I collage out of total collagen (which is type I + type III). Control and HLU measures were compared for saline (S)-, BAPN (B)-, cystamine (C)-, and pyridoxamine (P)-treated groups; n = 6 for all HLU groups, and n = 4 for control S, n = 6 for control B and for control P, and n = 4 for control C group. The data were analyzed using a standard t-test for all comparisons. All data are reported as means ± SE.
Fig. 5.
Immunofluorescence staining for rat aortic collagen type I (A, D), collagen type III (B, E), and secondary antibody control (C, F) for one non-HLU (A–C) and one HLU (D–F) saline-treated animal. The measure bar represents 100 μm. Contrast levels were adjusted by an identical factor for all images for presentation.
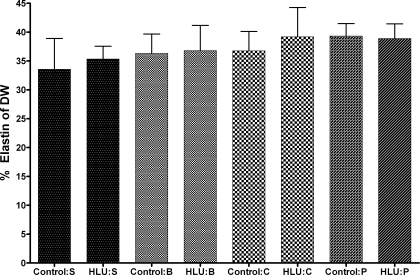
Rat aortic elastin content.
The rat thoracic aorta elastin content was not different between non-HLU and HLU groups. The aortic elastin content was 33.55 ± 9.25% of the dry weight of the vessel in the saline non-HLU group, while the elastin content of the saline HLU group was 35.33 ± 5.46%. The differences between the HLU and non-HLU groups (in any treatment group) were not statistically different. The values reported for the saline groups are representative for the other treatment groups (Fig. 6).
Fig. 6.
Rat thoracic aorta elastin content expressed as a percentage of the dry weight of the vessel. Control and HLU measures were compared for saline (S)-, BAPN (B)-, cystamine (C)-, and pyridoxamine (P)-treated groups. The data were analyzed using a standard t-test for all comparisons; n = 6 for all HLU groups, and n = 4 for control S group, n = 6 for control B group and for control P group, and n = 4 for control C group. All data are reported as means ± SE.
DISCUSSION
The present results are consistent with our previously published work on HLU and aortic stiffness (25). Specifically, all animals subjected to 7 days of HLU demonstrated some degree of aortic vascular stiffening as evaluated by PWV (which we demonstrated to directly correlate with in vitro measures of aortic stiffness) (25). We have confirmed our hypothesis that during HLU, structural and compositional changes take place within the aortic vessel wall that contribute to the simulated microgravity-induced increase in aortic stiffness.
While all HLU groups demonstrated an increase in aortic PWV post-HLU, two of the groups had a significantly attenuated PWV response (Fig. 1). Both the BAPN- and cystamine-treated groups had significantly lower aortic PWVs post-HLU compared with the saline group. These results provide support for the idea that enzymatic ECM cross-linking by LOX and tTG is increased during simulated microgravity and that it contributes to the increase in aortic stiffness. In our previous publication, we suggested a local pressure-dependent mechanism was responsible for inducing the increase in thoracic aortic stiffness due to microgravity exposure (25). It has been previously demonstrated that a differential pressure gradient exists along the aorta during HLU. As a result of HLU, a greater than normal pressure is present in the proximal aorta (7, 30) and a less than normal pressure is present in the caudal artery (7). This establishes a pressure gradient along the whole of the aorta during HLU. This differential pressure gradient may induce the increase in stiffness of the proximal aorta, which experiences a greater than normal blood pressure during HLU. The finding that LOX and tTG appear to significantly contribute to the HLU-induced increase in aortic stiffness fits into this paradigm. Sheridan et al. (21) showed that LOX is instrumental in hypertension-induced vascular remodeling. By inhibiting LOX with BAPN, they reduced the hypertension-dependent increase in LOX activity and subsequently reduced collage incorporation into the aorta. Additionally, Hornstra et al. (11) demonstrated the importance of LOX in developing and maintaining the mechanical properties of blood vessels. tTG has also been implicated in constriction-dependent remodeling of arterial blood vessels that results in an increase in vessel stiffness (3). Finally, Eftekhari et al. (10) found that chronic inhibition of tTG prevents the inward remodeling associated with hypertension. Together, this research demonstrates the importance of LOX and tTG in maintaining vessel structure and in remodeling in response to changes in vascular pressure and supports our finding that LOX and tTG are active contributors to HLU-induced vascular remodeling, resulting in increased aortic stiffness.
Although inhibition of LOX and tTG during HLU resulted in an attenuated PWV response, inhibition of AGEs by pryidoxamine had no effect on the PWV response. This could be due to the fact that the animals were subjected to 7 days of HLU and that enzymatically mediated cross-links can be formed more quickly than the AGE-mediated cross-links (12, 24, 31, 32). AGEs have been shown to be major contributors to the increase in vascular stiffness associated with aging and diabetes, suggesting that the formation of these cross-links may be a long-term process of AGE formation and cross-link accumulation (27, 31, 32). It is possible that AGEs or other nonenzymatic cross-link pathways contribute to increased vascular stiffness during prolonged periods of actual or simulated microgravity. It is apparent from our data that the enzymatic-mediated cross-linking pathways predominate in the short-term response to simulated microgravity, while the AGE-mediated cross-linking pathway does not contribute to the HLU-induced increase in vascular stiffness under these conditions.
Collagen is the primary component in the vessel wall responsible for vascular stiffness (4). We found that simulated microgravity induced an increase in thoracic aortic wall hydroxyproline content in all groups (Fig. 3), which is an established marker for collagen (23). Increases in vessel wall collagen content have been associated with increased arterial stiffness in several different settings. Aging-related increases in vascular stiffness have been associated with increases in vascular wall collagen content (9). Additionally, hypertension has been shown to increase collagen deposition in arterial vessels, resulting in increased stiffness (32). Given that hypertension has been known to stimulate collagen accumulation in vessels, it is not surprising that we found an increase in aortic wall collagen content due to simulated microgravity. These results further support our hypothesis of a pressure-dependent mechanism for microgravity-induced aortic remodeling and stiffening.
There are subtypes of collagen that confer on the vessel wall different mechanical properties. The vascular wall has been shown to consist mainly of type I (70–75%) and type III (20–25%) collagen (15). Type I collagen is thought to provide tensile strength and stiffness to the vessel while type III collagen allows for vessel extensibility (2, 28). A significant change in the ratio of the types of collagen in the vessel wall would certainly influence vascular stiffness. However, in this study, we found no change in the proportion of type I to type III collagen in the aortic wall (Figs. 4 and 5). Additionally, the other major structural component of the vessel wall, elastin, which is responsible for the elasticity of the vessel, was found to be unaffected by HLU or by treatments in terms of actual content (Fig. 6).
It is clear from the data that the simulated microgravity-induced increase in aortic stiffness is due to a combination of increased aortic wall collagen deposition and enzymatic cross-linking activity. The blunted stiffness responses observed with BAPN and cystamine administration can be attributed to the action of the inhibitors since the change or lack of change in the collagen and elastin contents and types observed in the saline HLU group was also observed in these groups. Inhibition of LOX or tTG resulted in a blunted PWV response by nearly 55% separately. Based on this, we would hypothesize that dual inhibition of tTG and LOX would severely attenuate, but not completely abolish, the increase in aortic stiffness. Other work has shown that the cross-linking activity provided by LOX and tTG is necessary to stabilize and integrate the newly synthesize collagen fibers into the vessel wall (11, 12, 21). In fact, we would have expected the overall aortic collagen deposition in the inhibitor groups to have decreased based on those previous studies.
Our findings are consistent with other research in terms of the time course of vascular remodeling. Nuthakki et al. (19) showed that 3 days after a remodeling stimulus, LOX mRNA expression was significantly increased in carotid arteries and that it preceded measurable vascular remodeling. A similar study by Sluijter et al. (22) demonstrated a significant increase in femoral wall collagen deposition 7 days after a remodeling stimulus. Similarly, tTG has been shown to produce significant arterial inward remodeling and an increase in vessel stiffness after only 24 h (3).
Although the present study only represents vascular stiffness changes due to short-duration microgravity exposure, it is likely that thoracic aortic stiffness remains increased during extended exposures to microgravity such as those seen on the International Space Station or those expected for missions to the moon or Mars based on observations made during long-term human bed-rest studies (26). This increase in vascular stiffness could potentially adversely affect the astronauts' health. In addition to being a marker and consequence of cardiovascular diseases, increased large-artery stiffness will result in increased systolic and decreased diastolic pressure, as well as increased pulse pressure, which can have deleterious effects on human health (13, 18). The increased pulse pressure results in an increased afterload on the left ventricle, which increases the workload on the heart and can eventually lead to hypertrophy and heart failure. Additionally, the decreased diastolic pressure can decrease coronary perfusion and increase the risk of myocardial infarction (5). In our study, we have identified two enzymes that, when inhibited, significantly blunt the increase in aortic stiffness associated with microgravity. Thus they are potential targets should an increase in vascular stiffness become problematic for future long-term spaceflight missions. However, it is possible the specific treatment protocols presented in the present study may interfere with normal physiological function, even though no obvious symptoms manifested in any of the control animals. For example, high doses of cystamine can cause hepatocyte toxicity (18), and BAPN can cause aortic wall weakness and Purkinje cell toxicity, albeit at much higher doses than those given in this study (8, 14). It is possible that these specific inhibitors may pose too much risk to be utilized as countermeasures, but the most important point is that the pathways leading to increased vascular stiffness have been identified and work can continue to find a more specific, less toxic intervention for use as a countermeasure if needed.
There are some limitations in our study. First, we did not measure matrix metalloproteinase (MMP) activity. It is possible that HLU could influence the activity of this family of enzymes, which has the potential to influence vascular stiffness. Many studies have noted the role of MMPs in vascular remodeling; as their chief role is to degrade the ECM it is possible that they contribute to the HLU-induced increase in vascular stiffness through a decrease in degradation activity. This would enhance the remodeling activity that we have observed in this study. Second, we did not measure overall hydration status or extracellular vs. intracellular fluid distribution. It is possible that an accumulation of fluid in the extracellular compartments would lead to a stiffening of the vessels. We believe that this is not likely to be the case since our saline HLU group demonstrated similar increases in PWV as we observed in our previous study (25). Last, depositions of chondroitin sulfate, heparin sulfate, proteoglycans, and fibronectin have all been found to result in increased vascular stiffness (32). It may be the case that some or all of these are contributing to the simulated microgravity-induced increase in vascular stiffness.
In conclusion, we found that 7 days of HLU induced an increase in aortic wall collagen content and ECM-cross-linking enzyme activity and that this is chiefly responsible for the increase in aortic stiffness that accompanies exposure to simulated microgravity. On the basis of our data, we believe that the cross-linking activity mediated by LOX and tTG is the main contributor to the HLU-induced increase in aortic stiffness, followed by the increase in aortic collagen content. Our findings support our hypothesis that the aortic remodeling is due to a pressure-dependent mechanism as we suggested in our previous study. Additionally, we have identified two therapeutic targets in the event that prolonged exposure to microgravity, such as that expected for human missions to the moon or Mars, results in chronic, unhealthy increases in vascular stiffness. Further research should be directed toward long-term vascular stiffness studies in both actual and simulated microgravity as well as performing investigations into combination treatments.
GRANTS
This research is supported in part by National Space Biomedical Research Institute Grant CA-00405 and by National Institutes of Health Grant ROI-AG-021523.
Acknowledgments
We thank Janice Lee and Hanwei Li of the laboratory of Jennifer Elisseeff for technical help.
REFERENCES
- 1.Alderson NL, Chachich ME, Youssef NN, Beattie RJ, Nachtigal M, Thorpe SR, Baynes JW. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int 63: 2123–2133, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Arteaga-Solis E, Gayraud B, Ramirez F. Elastic and collagenous networks in vascular diseases. Cell Struct Funct 25: 69–72, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 96: 119–126, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol 34: 513–525, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 33: 1111–1117, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bruel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis: 135–145, 1998. [DOI] [PubMed]
- 7.Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol 89: 1046–1054, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Conklin DJ, Trent MB, Boor PJ. The role of plasma semicarbazide-sensitive amine oxidase in allylamine and beta-aminopropionitrile cardiovascular toxicity: mechanisms of myocardial protection and aortic medial injury in rats. Toxicology 138: 137–154, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Cox RH Age-related changes in arterial wall mechanics and composition of NIA Fischer rats. Mech Ageing Dev 23: 21–36, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Eftekhari A, Rahman A, Schaebel LH, Chen H, Rasmussen CV, Aalkjaer C, Buus CL, Mulvany MJ. Chronic cystamine treatment inhibits small artery remodelling in rats. J Vasc Res 44: 471–482, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 278: 14387–14393, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Maki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol 167: 927–936, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martel E, Champeroux P, Lacolley P, Richard S, Safar M, Cuche JL. Central hypervolemia in the conscious rat: a model of cardiovascular deconditioning. J Appl Physiol 80: 1390–1396, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Martin JE, Sosa-Melgarejo JA, Swash M, Mather K, Leigh PN, Berry CL. Purkinje cell toxicity of beta-aminopropionitrile in the rat. Virchows Arch A Pathol Anat Histopathol 419: 403–408, 1991. [DOI] [PubMed] [Google Scholar]
- 15.McNulty M, Mahmud A, Spiers P, Feely J. Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. J Hum Hypertens 20: 867–873, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med 10: 7–40, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol 92: 1367–1377, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Nicotera P, Hartzell P, Baldi C, Svensson SA, Bellomo G, Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem 261: 14628–14635, 1986. [PubMed] [Google Scholar]
- 19.Nuthakki VK, Fleser PS, Malinzak LE, Seymour ML, Callahan RE, Bendick PJ, Zelenock GB, Shanley CJ. Lysyl oxidase expression in a rat model of arterial balloon injury. J Vasc Surg 40: 123–129, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107: 2864–2869, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Sheridan PJ, Kozar LG, Benson SC. Increased lysyl oxidase activity in aortas of hypertensive rats and effect of beta-aminopropionitrile. Exp Mol Pathol 30: 315–324, 1979. [DOI] [PubMed] [Google Scholar]
- 22.Sluijter JP, Smeets MB, Velema E, Pasterkamp G, de Kleijn DP. Increased collagen turnover is only partly associated with collagen fiber deposition in the arterial response to injury. Cardiovasc Res 61: 186–195, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta 18: 267–273, 1967. [DOI] [PubMed] [Google Scholar]
- 24.Tang SS, Trackman PC, Kagan HM. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem 258: 4331–4338, 1983. [PubMed] [Google Scholar]
- 25.Tuday EC, Meck JV, Nyhan D, Shoukas AA, Berkowitz DE. Microgravity-induced changes in aortic stiffness and their role in orthostatic intolerance. J Appl Physiol 102: 853–858, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Tuday EC, Platts SH, Nyhan D, Shoukas AA, Berkowitz DE. A retrospective analysis on gender differences in the arterial stiffness response to microgravity exposure. J Grav Physiol. In press.
- 27.Ulrich P, Cerami A. Protein glycation, diabetes, aging. Recent Prog Horm Res 56: 1–21, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Vouyouka AG, Pfeiffer BJ, Liem TK, Taylor TA, Mudaliar J, Phillips CL. The role of type I collagen in aortic wall strength with a homotrimeric. J Vasc Surg 33: 1263–1270, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Wilkerson MK, Lesniewski LA, Golding EM, Bryan RM Jr, Amin A, Wilson E, Delp MD. Simulated microgravity enhances cerebral artery vasoconstriction and vascular resistance through endothelial nitric oxide mechanism. Am J Physiol Heart Circ Physiol 288: H1652–H1661, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Wilkerson MK, Muller-Delp J, Colleran PN, Delp MD. Effects of hindlimb unloading on rat cerebral, splenic, and mesenteric resistance artery morphology. J Appl Physiol 87: 2115–2121, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Williams ME Clinical studies of advanced glycation end product inhibitors and diabetic kidney disease. Curr Diab Rep 4: 441–446, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005. [DOI] [PubMed] [Google Scholar]