Abstract
We tested two hypotheses, first that exercise training reverses age-related decrements in endothelium-dependent dilation in soleus muscle feed arteries and second that this improved endothelium-dependent dilation is the result of increased nitric oxide (NO) bioavailability due to increased content and phosphorylation of endothelial NO synthase (eNOS) and/or increased antioxidant enzyme content. Young (2 mo) and old (22 mo) male Fischer 344 rats were exercise trained (Ex) or remained sedentary (Sed) for 10–12 wk, yielding four groups of rats: 1) young Sed (4–5 mo), 2) young Ex (4–5 mo), 3) old Sed (24–25 mo), and 4) old Ex (24–25 mo). Soleus muscle feed arteries (SFA) were isolated and cannulated with two glass micropipettes for examination of endothelium-dependent (ACh) and endothelium-independent [sodium nitroprusside (SNP)] vasodilator function. To determine the mechanism(s) by which exercise affected dilator responses, ACh-induced dilation was assessed in the presence of Nω-nitro-l-arginine (l-NNA; to inhibit NO synthase), indomethacin (Indo; to inhibit cyclooxygenase), and l-NNA + Indo. Results indicated that ACh-induced dilation was blunted in old Sed SFA relative to young Sed SFA. Exercise training improved ACh-induced dilation in old SFA such that vasodilator responses in old Ex SFA were similar to young Sed and young Ex SFA. Addition of l-NNA, or l-NNA + Indo, abolished the exercise effect. Immunoblot analysis revealed that extracellular superoxide dismutase (SOD) protein content was increased by training in old SFA, whereas eNOS and SOD-1 protein content were not altered. Addition of exogenous SOD, or SOD + catalase, improved ACh-induced dilation in old Sed SFA such that vasodilator responses were similar to young Sed SFA. Addition of l-NNA abolished the effect of exogenous SOD in old Sed arteries. Collectively, these results indicate that exercise training reverses age-induced endothelial dysfunction in SFA by increasing NO bioavailability and that increases in vascular antioxidant capacity may play an integral role in the improvement in endothelial function.
Keywords: endothelial dysfunction, endothelial nitric oxide synthase, superoxide dismutase
aging is associated with a decline in endothelial function characterized, in part, by impaired endothelium-dependent vasodilator responses in central and peripheral arteries (1, 4, 8, 29, 45, 53, 54). The resulting endothelial dysfunction is believed to contribute to an increased risk of cardiovascular disease in older adults. In addition, the decline in endothelial function may contribute to impaired muscle blood flow and reduced exercise tolerance in the elderly (2, 18, 25, 31, 34, 37, 49). The mechanism(s) for the age-related decrement in endothelium-dependent dilation is not fully understood; however, previously published studies indicate that a decline in the bioavailability of nitric oxide (NO) plays an integral role (1, 5, 6, 29, 44, 54).
Endurance exercise training improves endothelium-dependent dilation in some conduit arteries in young healthy subjects (7, 22, 28, 30, 32, 42, 43) and in skeletal muscle arterioles/resistance arteries (24, 27, 39, 40). Importantly, examination of the effects of training in the arteriolar tree of skeletal muscle indicates that the improvement in endothelium-dependent dilation induced by exercise training is not uniformly distributed throughout the arteriolar tree (24, 27). The beneficial effect of exercise training has also been reported to be associated with increased expression of endothelial nitric oxide synthase (eNOS) (23, 38, 52), enhanced production of NO (31), and improved NO-mediated, endothelium-dependent dilation (30). In addition, exercise training has been reported to increase expression of cytosolic and extracellular superoxide dismutases (SOD-1 and ecSOD) in the aorta of mice and pigs, which may improve endothelial function by enhancing the capacity to scavenge superoxide and prolonging the biological half-life of NO (15, 36).
Previous studies indicate that endurance exercise training is also an effective intervention for attenuating or reversing age-induced endothelial dysfunction in first-order (1A) arterioles perfusing skeletal muscle (39, 40). Specifically, Spier et al. reported that endurance exercise training improves endothelium-dependent vasodilator responses in 1A arterioles from soleus and gastrocnemius muscles of aged rats, and that the improved endothelium-dependent dilation was mediated by enhanced NO bioavailability (39, 40). In addition, exercise training appears to enhance antioxidant status and improve endothelium-dependent dilation in conduit arteries of aged human subjects (8, 12, 14).
In skeletal muscle it has been established that a primary control point for regulating total muscle blood flow during exercise is the feed artery (50). Indeed, previous research indicates that feed arteries, which lie immediately external to skeletal muscle, provide the principal site of resistance to flow through individual skeletal muscles and play an integral role in mediating increases in skeletal muscle blood flow during physical activity (21, 50, 51). Thus an exercise training-induced improvement in vasodilator responses in skeletal muscle feed arteries could potentially work in concert with enhanced endothelial function previously reported in skeletal muscle arterioles (39, 40) by increasing the capacity to augment total muscle blood flow (feed arteries) and the ability to redistribute the augmented blood flow (arterioles) to active skeletal muscle fibers. Given that exercise in young animals has been shown to exert changes in endothelium-dependent dilation in the resistance artery network of skeletal muscles in a nonuniform manner and the importance of feed arteries to perfusion of skeletal muscle, it is important to know whether exercise training in aged animals has a beneficial impact on soleus muscle feed arteries. We know that exercise training has been shown to improve endothelium-dependent dilation in aged soleus muscle 1A arterioles (39, 40); however, the efficacy of endurance exercise training to improve endothelial function in senescent soleus muscle feed arteries is not known. Therefore, the purpose of this study was to test the hypothesis that exercise training reverses age-related decrements in endothelium-dependent dilation in soleus muscle feed arteries (SFA). We also hypothesized that endurance exercise training would improve endothelium-dependent dilation in senescent SFA by increasing NO bioavailability due to increased content and phosphorylation of eNOS and/or increased antioxidant enzyme content.
METHODS
Experimental Design
Endothelium-dependent dilation in response to application of ACh was examined in SFA isolated from young and old sedentary and exercise-trained rats, using standard techniques. The relative contribution of NO synthesis by NOSs was evaluated by examining vasodilator responses in the presence of Nω-nitro-l-arginine (l-NNA; 300 μM) to inhibit nitric oxide synthase (NOS). The contribution of cyclooxygenase (COX) was evaluated by examining vasodilator responses in the presence of indomethacin (Indo; 5 μM) to inhibit COX, and the role of non-NOS and non-COX pathways to the responses was determined by examining vasodilator responses in the presence of l-NNA + Indo to inhibit NOS and COX. Feed arteries were also harvested for examination of expression of eNOS, Ser 1177 phospho-eNOS (p-eNOS), SOD-1, and ecSOD using immunoblot analysis. When these results revealed that ecSOD content was increased by exercise training, we did a series of experiments to determine whether the improvement in endothelium-dependent dilation could be produced simply by increasing antioxidant levels by adding exogenous antioxidants.
Animals
All of the protocols used in the present study were approved by the Animal Care and Use Committees at the University of Missouri and Texas A&M University. To determine the efficacy of exercise training to improve endothelium-dependent dilation in aged SFA, male Fischer 344 rats (2 and 22 mo of age; n = 20/age group) were purchased from a commercial dealer (Harlan Sprague-Dawley, Indianapolis, IN) and housed in the University of Missouri College of Veterinary Medicine's Animal Care Facility. One week after arrival, rats were exercise trained (Ex) or remained sedentary (Sed) for 10–12 wk. Thus at the end of the training program, the ages of the young and old rats were 4–5 mo or 24–25 mo, respectively. The resulting experimental design consisted of four groups of rats: 1) young Sed (n = 10), 2) young Ex (n = 10), 3) old Sed (n = 10), and 4) old Ex (n = 10). To determine whether exogenous antioxidants produce exercise-like effects on aged SFA, a separate group of male Fischer 344 rats (4 and 24 mo of age) was purchased and housed at the Texas A&M University Comparative Medicine Program Facility. Both animal facilities were maintained at 24°C with a 12:12-h light-dark cycle. Food and water were provided ad libitum, and the rats were examined daily by the investigators and by veterinarians affiliated with their respective institutions.
Training Program
The exercise training protocol used in the present study has been published previously in detail (39). In brief, rats were familiarized with running on a motorized treadmill and randomly assigned to an Ex or Sed group for 10–12 wk. Rats assigned to the Ex group ran 60 min/day, 5 days/wk, at 15 m/min (15° incline). Rats assigned to the Sed group were restricted to their cages and did not exercise. The efficacy of the exercise-training protocol was assessed from measurements of citrate synthase activity in the vastus lateralis muscle (41).
Isolation of Feed Arteries
Procedures used to isolate SFA have been published previously (53–56). In brief, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (50–60 mg/kg body wt ip). Soleus muscles from the left and right hindlimb were removed and placed in MOPS-buffered physiological saline solution (PSS) containing (in mM) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 25.0 MOPS, at pH 7.4. SFA from one hindlimb were dissected free of paired veins and connective tissue under a dissection microscope, cut on both ends, and transferred to a Lucite chamber containing MOPS-PSS (4°C) for cannulation. SFA from the contralateral hindlimb were dissected free, transferred to a microcentrifuge tube, snap-frozen, and stored at −80°C for subsequent immunoblot analysis.
Determination of Vasodilator Responses
Preparation of arteries.
SFA were prepared for functional analysis as described previously (19, 42). Specifically, arteries were cannulated with two resistance-matched glass micropipettes and secured with 11-0 surgical silk. The micropipettes were subsequently attached to separate pressure reservoirs filled with MOPS-PSS supplemented with albumin (1 g/100 ml). The height of each reservoir was initially adjusted to set intraluminal pressure in each SFA to 60 cmH2O (1 mmHg = 1.36 cm H20) for 20 min. After 20 min, intraluminal pressure was raised to 90 cmH2O, and the feed arteries were allowed to equilibrate for an additional 40 min at 37°C. At the end of the 60-min equilibration period, feed arteries that did not develop at least 25% spontaneous tone were constricted with phenylephrine. All experimental protocols were subsequently conducted at an intraluminal pressure of 90 cmH2O to approximate in vivo intraluminal pressure (50).
Endothelium-dependent dilation.
Endothelium-dependent dilation was assessed in feed arteries by adding increasing doses of ACh to the bath solution in cumulative doses over the range of 10−9–10−4 M in whole log increments as described previously (19, 53, 54). A total of 4 SFA were studied in parallel from each rat. In SFA 1, ACh-induced vasodilator responses were assessed in the absence of enzyme inhibitors. In SFA 2, vasodilator responses were assessed in the presence of l-NNA (300 μM) to inhibit NOS. In SFA 3, vasodilator responses were assessed in the presence of Indo (5 μM) to inhibit COX. In SFA 4, vasodilator responses were assessed in the presence of l-NNA + Indo to inhibit NOS and COX.
In a separate series of experiments, endothelium-dependent dilation was assessed in SFA from young Sed and old Sed rats in the absence and presence of exogenous antioxidants. In these studies, a total of 3 SFA were studied in parallel from each rat. In SFA 1, ACh-induced vasodilator responses were assessed in the absence of exogenous antioxidants. In SFA 2, vasodilator responses were assessed in the presence of superoxide dismutase (SOD; 120 U/ml) to scavenge superoxide. In SFA 3, vasodilator responses were assessed in the presence SOD and catalase (CAT; 100 U/ml) to scavenge superoxide and hydrogen peroxide. When results revealed that exogenous antioxidants improved endothelium-dependent dilation, a subsequent set of experiments was performed to determine whether NO mediated the improvement in endothelial function. In these studies 3 SFA were studied in parallel from each rat. In SFA 1, ACh-induced vasodilator responses were assessed in the absence of exogenous antioxidants. In SFA 2, vasodilator responses were assessed in the presence of SOD. In SFA 3, vasodilator responses were assessed in the presence SOD and l-NNA.
Endothelium-independent dilation.
Endothelium-independent dilation was assessed by adding increasing doses of sodium nitroprusside (SNP) to the bath solution in cumulative doses over the range of 10−9–10−4 M in whole log increments (19, 53, 54). SNP-induced dilation was also assessed in SFA from young and old Sed rats in the presence of SOD, SOD + CAT, and SOD + l-NNA.
Passive diameter.
At the end of each experiment, SFA were incubated for 30 min in Ca2+-free PSS to determine passive diameter at an intraluminal pressure of 90 cmH2O.
Solutions and Drugs
All reagents used in dose-response experiments were obtained from Sigma Chemical (St. Louis, MO). Reagents were prepared on the day of the experiment.
Quantification of eNOS, Ser1177 p-eNOS, SOD-1, and ecSOD protein Content
Relative differences in eNOS, Ser1177 p-eNOS, SOD-1, and ecSOD protein contents were assessed in feed arteries using immunoblot analysis as described previously in detail (19). eNOS protein content was evaluated with a monoclonal antibody (1:1,250; catalog no. 610297, BD Transduction Laboratories). Ser1177 p-eNOS protein content was assessed with a monoclonal antibody (1:250; catalog no. 612393, BD Transduction Laboratories). SOD-1 protein content was assessed with a polyclonal antibody (1:3,300; catalog no. SOD-100, Stressgen). ecSOD protein content was assessed with a polyclonal antibody (1:1,000; catalog no. SOD-105, Stressgen). Immunoblots were evaluated by enhanced chemiluminescence (ECL, Amersham) and densitometry by using a LAS-3000 Luminescent Image Analyzer and Multi-Gauge Image Analysis Software (FUJIFILM Medical Systems). All protein data were expressed relative to GAPDH to control for small differences in protein loading. GAPDH protein content was assessed with a monoclonal antibody (1:10,000; catalog no. AB374, Millipore). To determine whether the ratio of Ser1177 p-eNOS to total eNOS protein content was altered with aging or exercise training, immunoblots were probed with the Ser1177 p-eNOS antibody, stripped with Restore Western Blot Stripping Buffer (Thermo catalog no. 21059), and reprobed with the eNOS antibody.
Statistical Analysis
All values are means ± SE. Between-group differences in body mass, citrate synthase activity, percent tone, relative protein content, and passive diameter were assessed using one-way ANOVA. Concentration-response curves were analyzed by two-way ANOVA with repeated measures on one factor (dose) to determine whether vasodilator responses to ACh and SNP differed by group. In addition, between-group differences in IC50 values and maximal responses were assessed with one-way ANOVA. Concentration-response data were expressed as a percentage of maximal possible dilation. Percent possible dilation was calculated as [(Ddose − DB)/(DP − DB)] × 100, where Ddose is measured diameter for a given dose, DB is baseline diameter before an intervention was started, and DP is maximal passive diameter. A total of 230 SFA were used in experiments to assess vasodilator function. Fifteen young (8 Sed; 7 Ex) and 21 old (11 Sed; 10 Ex) SFA required phenylephrine to achieve 25% tone. Deletion of these arteries from the statistical analyses did not alter interpretation of the results; therefore, all 230 SFA were included in the final analyses. When a significant F value was obtained, post hoc analyses were performed with Duncan's multiple-range test and Fisher's least significant difference (LSD) test. Statistical significance was set at the P ≤ 0.05 probability level.
RESULTS
Characteristics of rats and SFA
Skeletal muscle citrate synthase activity was increased by training in young and old rats, confirming the efficacy of the exercise training program (Table 1). Body weight of old Sed rats was significantly greater than young Sed rats (Table 1). Exercise training lowered body weight in the old rats such that the body weight of the old Ex rats was not significantly different from young Sed or young Ex rats. Maximal passive diameter was similar in all groups of arteries (Tables 2 and 3).
Table 1.
Body weight and citrate synthase activity in the vastus lateralis red muscle
| Young Sed | Young Ex | Old Sed | Old Ex | |
|---|---|---|---|---|
| Body weight, g | 391±9 | 353±8a,c,d | 425±12a | 402±12 |
| Citrate synthase activity; μmol·min−1·g wet wt−1 | 29.9±0.7 | 39.7±3.7a,c | 25.6±1.8 | 38.7±3.0a,c |
Values are means ± SE; n = 8–10 rats/group. Sed, sedentary; Ex, exercise trained. Significantly different from
young Sed,
byoung Ex,
old Sed,
old Ex, P ≤ 0.05.
Table 2.
Characteristics of soleus muscle feed arteries from young and old rats used in the exercise training study
| Parameter | Con | l-NNA | Indo | l-NNA + Indo |
|---|---|---|---|---|
| Maximal diameter, μm | ||||
| Young Sed | 199±5 | 193±7 | 195±11 | 180±13 |
| Young Ex | 196±12 | 178±16 | 191±12 | 184±15 |
| Old Sed | 170±8 | 173±10 | 174±17 | 187±12 |
| Old Ex | 200±10 | 194±8 | 171±11 | 171±10 |
| Initial tone, % | ||||
| Young Sed | 48.6±4.1 | 73.9±2.8a,c,d | 45.3±6.9b | 57.6±4.5b |
| Young Ex | 40.1±4.3 | 60.4±4.7a,c | 34.4±4.1b,d | 54.7±6.1a,c |
| Old Sed | 44.0±6.4 | 67.5±5.2a,c,d | 32.0±4.0b | 45.6±4.9b |
| Old Ex | 46.4±3.9 | 55.8±4.9c,d | 33.4±2.3a,b,d | 44.1±2.7b,c |
Values are means ± SE; n = 8–10 rats/group. Con, control; l-NNA, Nω-nitro-l-arginine; Indo, indomethacin. Significantly different from
Con,
l-NNA,
Indo,
l-NNA+ Indo, P ≤ 0.05.
Table 3.
Characteristics of soleus muscle feed arteries from young and old rats used in the antioxidant studies
| Parameter | Con | SOD | SOD + CAT | SOD + l-NNA |
|---|---|---|---|---|
| Maximal diameter, μm | ||||
| Young | 162.6±5.5 | 178.1±8.0 | 175.8±7.3 | 181.9±8.7 |
| Old | 158.3±7.6 | 183.2±9.4 | 175.3±13.9 | 169.0±14.2 |
| Initial tone, % | ||||
| Young | 41.1±2.3 | 38.3±2.9 | 38.2±3.9 | 47.4±5.2 |
| Old | 40.8±3.2 | 39.0±2.8 | 37.1±4.1 | 42.4±4.9 |
Values are means ± SE; n = 6–21 rats/group. SOD, superoxide dismutase; CAT, catalase.
ACh-Induced Dilation
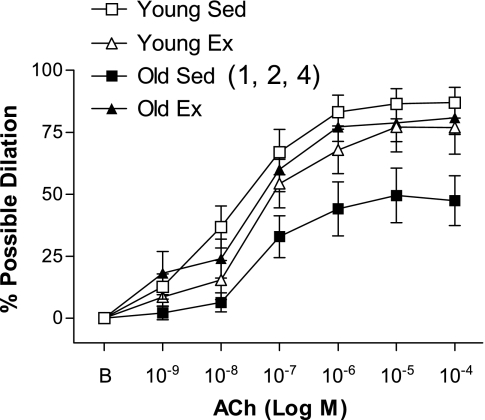
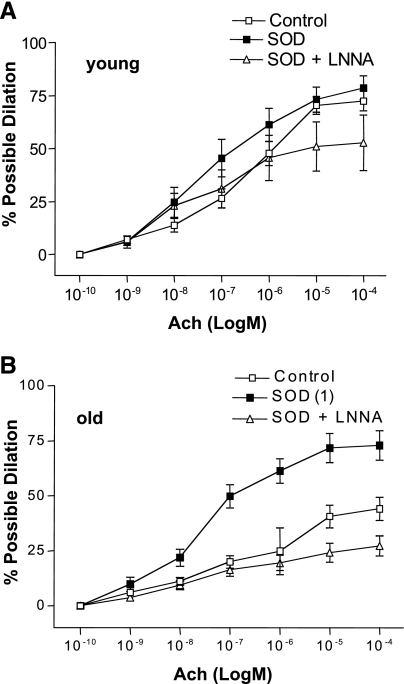
ACh-induced dilation was significantly blunted in the old Sed arteries relative to the young Sed arteries (Fig. 1). Ex improved ACh-induced dilation in old (not young, P = 0.21) SFA, such that ACh-induced dilation was significantly greater in old Ex SFA than in old Sed SFA (Fig. 1). In addition, ACh-induced dilation of old Ex SFA was not different from that of young Sed and young Ex SFA (Fig. 1).
Fig. 1.
Values are means ± SE; n = 8–10 rats/group. ACh-induced dilation in soleus muscle feed arteries (SFA). Sed, sedentary; Ex, exercise trained; B, baseline diameter before the 1st dose of ACh. Dose-response curve significantly different from 1Young Sed, 2Young Ex, and 4Old Ex, P ≤ 0.05.
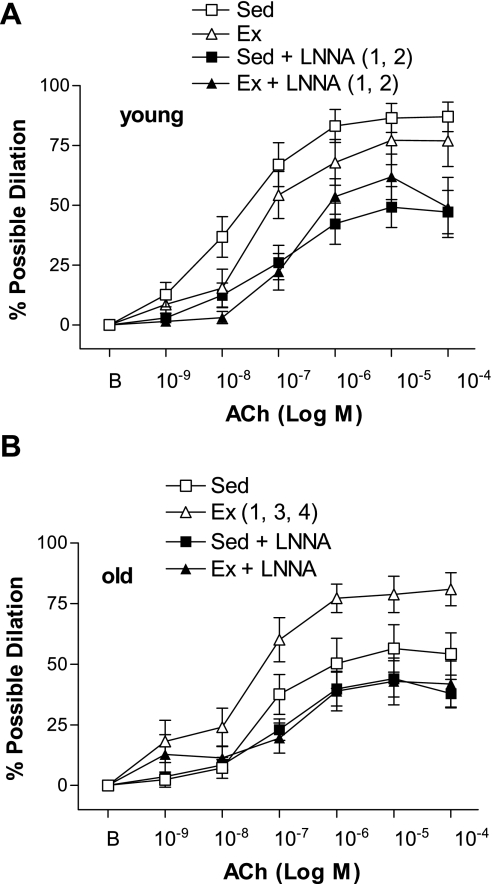
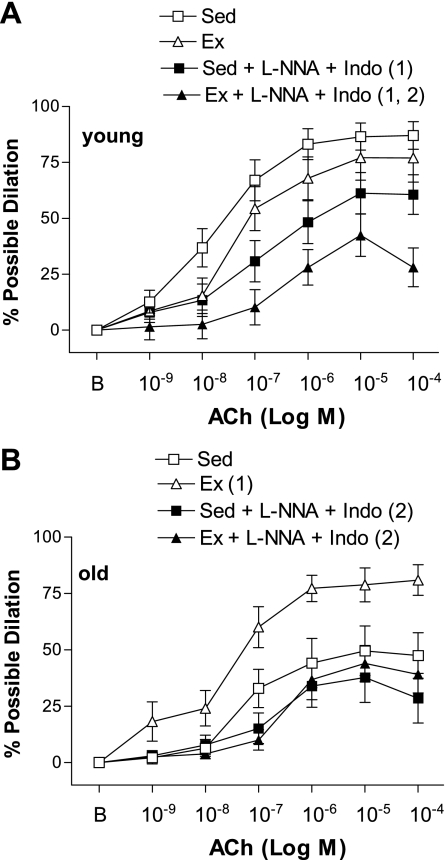
ACh-induced dilation was inhibited by l-NNA (Fig. 2) and l-NNA + Indo (Fig. 3) in young Sed, young Ex, and old Ex arteries. In contrast, ACh-induced dilation was not significantly inhibited by l-NNA (Fig. 2) or l-NNA + Indo (Fig. 3) in old Sed arteries. In the presence of l-NNA, or l-NNA + Indo, ACh-induced dilation of old Ex SFA was no longer greater than that of old Sed SFA (Figs. 2 and 3). ACh-induced dilation was not significantly inhibited by Indo alone in any group of arteries (data not shown). Alterations in the response to the maximal dose of ACh (10−4 M) followed a similar pattern to the alterations observed in the dose-response curves. Sensitivity (IC50) to ACh was not altered by age, training status, or treatment (Table 4).
Fig. 2.
Values are means ± SE; n = 8–10 rats/group. ACh-induced dilation in young (A) and old (B) SFA in the absence or presence of Nω-nitro-l-arginine (l-NNA; 300 μM) to inhibit nitric oxide synthase. B, baseline diameter before the 1st dose of ACh. Dose-response curve significantly different from 1Sed, 2Ex, 3Sed + l-NNA, and 4Ex + l-NNA, P ≤ 0.05.
Fig. 3.
Values are means ± SE; n = 8–10 rats/group. ACh-induced dilation in young (A) and old (B) SFA in the absence or presence of l-NNA (300 μM) and indomethacin (Indo; 5 μM) to inhibit nitric oxide synthase and cyclooxygenase. B, baseline diameter before the 1st dose of ACh. Dose-response curve significantly different from 1Sed and 2Ex, P ≤ 0.05.
Table 4.
IC50 −log M values and response to 10−4 M ACh of soleus muscle feed arteries from young and old rats used in the exercise training study
| Parameter | Con | l-NNA | Indo | l-NNA + Indo |
|---|---|---|---|---|
| IC50, −log M ACh | ||||
| Young Sed | −7.75±0.27 | −6.97±0.26 | −7.37±0.26 | −7.06±0.38 |
| Young Ex | −7.53±0.30 | −6.91±0.18 | −7.25±0.29 | −6.47±0.45 |
| Old Sed | −7.52±0.35 | −7.13±0.16 | −6.55±0.24 | −6.5±0.73 |
| Old Ex | −7.83±0.55 | −7.15±0.26 | −7.20±0.60 | −6.55±0.60 |
| Response to 10−4 M ACh, % possible dilation | ||||
| Young Sed | 87.0±6.2 | 52.4±8.2a | 64.6±9.3 | 60.6±8.8a |
| Young Ex | 76.9±10.7 | 49.1±12.6a | 73.6±9.4d | 28.1±8.6a,c |
| Old Sed | 54.2±8.7f | 37.9±6.0 | 67.4±9.0b,d | 28.5±11.1c |
| Old Ex | 80.9±6.8b,e | 41.8±9.5a | 61.8±11.3 | 39.1±8.3a |
Values are means ± SE; n = 8–10 rats/group. Significantly different from
Con,
L-NNA,
Indo,
l-NNA+ Indo,
Sed,
Young, P ≤ 0.05.
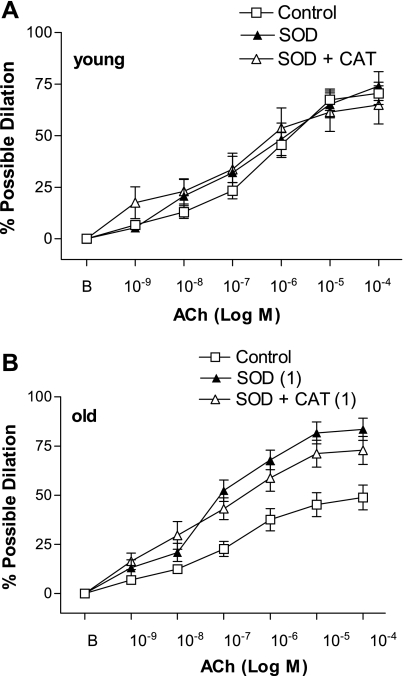
To determine if exogenous antioxidants restore ACh-induced dilation in a manner similar to exercise, experiments were carried out in the absence and presence of SOD or SOD + CAT. In the absence of antioxidants, the response to ACh was significantly attenuated in the old Sed SFA compared with the young Sed SFA (P = 0.003) (Fig. 4). SOD, and SOD + CAT, significantly improved ACh-induced dilation in old Sed SFA (Fig. 4). SOD improved ACh-induced dilation in old Sed SFA such that the dilator response was greater than that seen in young Sed SFA, while addition of SOD + CAT improved dilation in old Sed SFA to the extent that dilation was comparable to young Sed SFA (Fig. 4). In young Sed SFA, addition of SOD, or SOD + CAT, did not alter the ACh dose-response curve (Fig. 4). In old Sed SFA, the SOD-induced improvement in ACh-induced dilation was abolished in the presence of SOD + l-NNA (Fig. 5). In young Sed, SOD + l-NNA tended (P = 0.12) to inhibit ACh-induced dilation (Fig. 5) and significantly attenuated sensitivity (IC50) to ACh (Table 5).
Fig. 4.
Values are means ± SE; n = 6–14 rats/group. ACh-induced dilation in young (A) and old (B) SFA in the absence or presence of exogenous antioxidants superoxide dismutase (SOD, 120 U/ml) or SOD + catalase (CAT, 100 U/ml). B, baseline diameter before the 1st dose of ACh. Dose-response curve significantly different from 1control, P ≤ 0.05.
Fig. 5.
Values are means ± SE; n = 8–21 rats/group. ACh-induced dilation in young (A) and old (B) SFA in the absence or presence of SOD or SOD + l-NNA. B, baseline diameter before the 1st dose of ACh. Dose-response curve significantly different from 1control, P ≤ 0.05.
Table 5.
IC50 −log M values and response to 10−4 M ACh of soleus muscle feed arteries from young and old rats used in the antioxidant studies
| Parameter | Con | SOD | SOD + CAT | SOD + l-NNA |
|---|---|---|---|---|
| IC50, −log M ACh | ||||
| Young | −6.61±0.20 | −7.28±0.26 | −6.72±0.20 | −8.18±0.62a,c |
| Old | −7.01±0.18 | −7.39±0.14 | −7.44±0.35 | −7.33±0.46 |
| Response to 10−4 M ACh, % possible dilation | ||||
| Young | 72.5±4.7 | 78.7±5.4d | 64.9±9.3 | 52.7±13.2b |
| Old | 44.1±5.3b,c,e | 72.9±6.6a,d | 72.8±7.1a,d | 27.2±4.5b,c |
Values are means ± SE; n = 6–21 rats/group. Significantly different from
Con,
SOD,
SOD + CAT,
SOD + l-NNA,
Young, P ≤ 0.05.
SNP-Induced Dilation
SNP elicited a concentration-dependent dilation of all arteries. Statistical analysis revealed no significant between-group differences (data not shown).
eNOS, Ser1177 p-eNOS, SOD-1, and ecSOD Protein Content
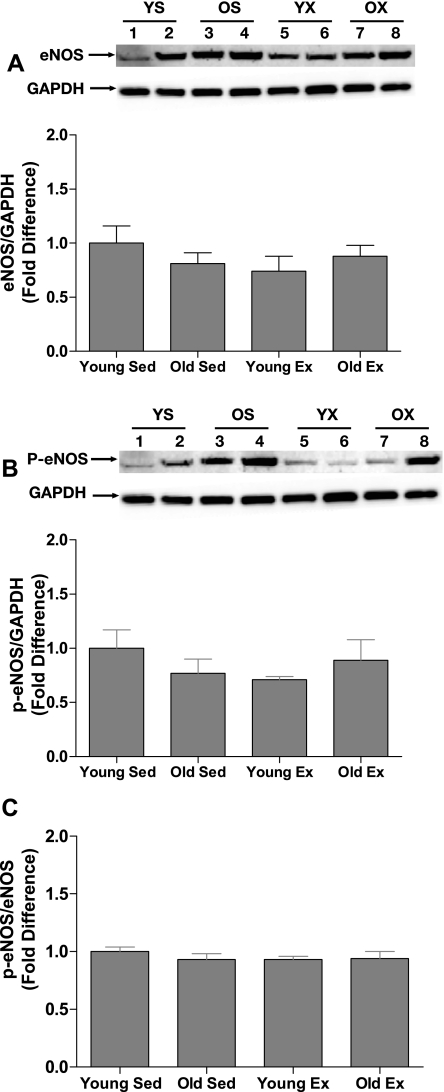
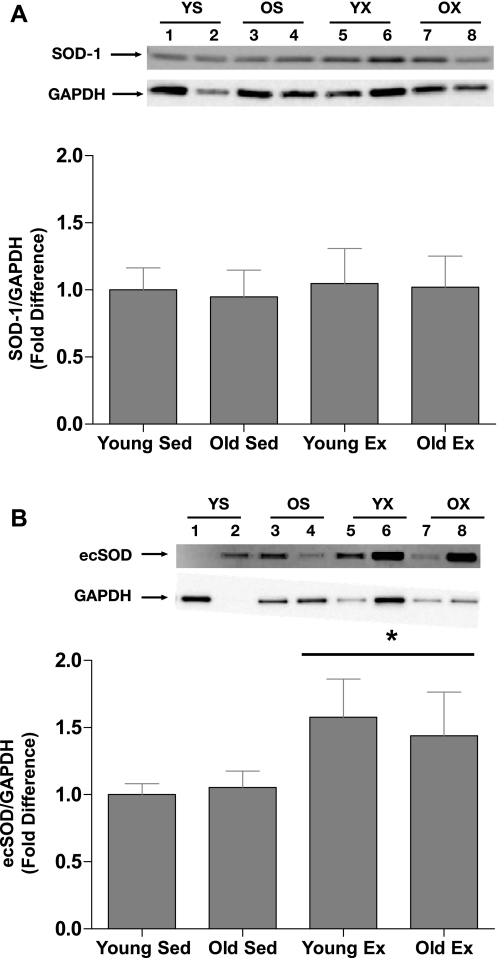
Immunoblot analysis revealed that eNOS (Fig. 6A), Ser1177 p-eNOS (Fig. 6B), and SOD-1 (Fig. 7A) protein contents were not significantly altered by age or exercise training. The Ser1177 p-eNOS-to-total eNOS protein content ratio was also not altered by age or training status (Fig. 6C). In contrast, ecSOD protein content was significantly increased by exercise training in young and old SFA such that ecSOD content was greater in young Ex and old Ex arteries than in young Sed and old Sed arteries (Fig. 7B).
Fig. 6.
Values are means ± SE; n = 9–10 rats/group. Comparison of endothelial nitric oxide synthase (eNOS) (A), Ser1177 phospho-eNOS (p-eNOS; B) protein content, and Ser1177 p-eNOS-to-eNOS ratio (C) in SFA from young and old rats. A and B, insets: representative immunoblots for the target protein (top) and the same blot reprobed for GAPDH (bottom). YS, young sedentary; OS, old sedentary, YX, young exercise trained; OX, old exercise trained. Statistical analysis revealed no significant differences.
Fig. 7.
Values are means ± SE; n = 8 rats/group. Comparison of Cu,Zn-dependent superoxide dismutase (SOD-1; A) and extracellular superoxide dismutase (ecSOD) (B) protein content in SFA from young and old rats. A and B, insets: representative blots for the target protein (top) and the same blot reprobed for GAPDH (bottom). *Significantly different from Sed rats, P ≤ 0.05.
DISCUSSION
The purpose of this study was to test the hypothesis that exercise training reverses age-related decrements in endothelium-dependent dilation in SFA by increasing NO bioavailability due to increased content and phosphorylation of eNOS and/or increased antioxidant enzyme content. The primary new findings of this study are that: 1) exercise training improved ACh-induced dilation in old SFA such that vasodilator responses in old Ex SFA were similar to young Sed and young Ex SFA; 2) ecSOD protein content was increased by training in old SFA, whereas the SFA protein content of eNOS, Ser1177 p-eNOS, and SOD-1 were not altered by training; and 3) exogenous SOD, and SOD + CAT, restored ACh-induced dilation in old SFA in an NO-dependent manner similar to the effects of exercise training. Results also demonstrate that treatment with l-NNA, or l-NNA + Indo, inhibited ACh-induced dilation in old Ex SFA such that the response was not greater than the response in the old Sed SFA. Also, ACh-induced dilation was not significantly inhibited by Indo alone in young or old rats. Collectively, these results indicate that exercise training reverses the detrimental effects of aging on endothelium-dependent dilation in SFA and that increased vascular ecSOD protein content may contribute to this improvement. To our knowledge, this is the first study to demonstrate that exercise training reverses age-induced endothelial dysfunction in SFA or any skeletal muscle feed artery. Given that skeletal muscle feed arteries contribute importantly to regulating total skeletal muscle blood flow during exercise (47), this exercise-induced adaptation may have substantial functional significance.
Reduced exercise capacity with age is well documented in humans and animals (2, 3, 26). The mechanism(s) for the age-related decline in exercise tolerance is not fully understood; however, an impaired ability to increase skeletal muscle blood flow during exercise may contribute to the reduction in exercise capacity (2, 18, 31, 34, 37). There are numerous potential mechanisms for the impairment in the blood flow response to exercise, including age-related increases in vasoconstrictor responsiveness (9–11, 37, 47) and blunted NO-mediated endothelium-dependent dilation (37). Although NO does not appear to be obligatory for increasing muscle blood flow during exercise in young subjects (13, 35), Schrage et al. (37) have shown that the contribution of NO to exercise hyperemia is reduced in older subjects.
The decreased ACh-induced dilation observed in SFA from old Sed rats in the present study (Figs. 1, 4, and 5) is in accord with previous studies indicating that endothelium-dependent dilation is impaired in feed arteries (53, 54) as well as 1A arterioles (29) of the soleus muscle of aged rats. Also, previous studies indicate that exercise training attenuates the detrimental effects of aging on endothelium-dependent dilation of skeletal muscle 1A arterioles (39, 40). Present results indicate that Ex improved ACh-induced dilation in old SFA such that vasodilator responses in old Ex SFA were similar to young Sed and young Ex SFA (Fig. 1). The exercise-induced improvement in endothelial function in SFA may be functionally significant given that feed arteries serve as the primary control point for regulating total muscle blood flow to soleus muscle at rest and during exercise (50). In addition to enhancing NO-mediated dilation in aged skeletal muscle arterioles, exercise training has also been shown to attenuate vasoconstrictor responses in skeletal muscle arterioles (10, 11, 39, 40). Thus it is likely that the exercise-induced improvement in endothelium-dependent vasodilator responses in SFA observed in the present study works in concert with enhanced vasodilator, and attenuated vasoconstrictor, responses in skeletal muscle arterioles to enhance the capacity to increase skeletal muscle blood flow and to distribute blood flow to the actively contracting muscle fibers during exercise.
Mechanisms Responsible for increased Vasodilator Responses
Results of the present study indicate that the beneficial effect of exercise training on ACh-induced vasodilator function is primarily due to increases in NO bioavailability, since the exercise effect was eliminated in the presence of l-NNA (Fig. 2B). Importantly, the improvement in NO-mediated dilation could not be attributed to an exercise-induced improvement in the ability of vascular smooth muscle cells to respond to NO, since vasodilator responses to SNP (an NO donor) were similar in all groups of arteries. To determine whether the beneficial effect of exercise on endothelium-dependent dilation also involved COX products, ACh-induced dilation was assessed in the presence of Indo to block COX. ACh-induced dilation was not significantly inhibited by Indo alone in young or old rats regardless of training status. Thus the beneficial effect of exercise training on vasodilator responses in aged SFA does not to appear to be mediated by altered COX signaling.
To determine whether enhancement of a NOS- and COX-independent vasodilator mechanism contributed to the beneficial effect of exercise training, ACh-induced dilation was assessed in the presence of l-NNA + Indo (double blockade). In the presence of l-NNA + Indo, residual dilation to ACh can be attributed to vasodilators other than NO and prostacyclin (PGI2), primarily endothelium-derived hyperpolarizing factors (EDHF). Double blockade had no statistically significant impact on old Sed arteries (Fig. 3B), suggesting that endothelium-dependent dilation in old Sed SFA is mediated entirely by non-NOS, non-COX mechanisms. Double blockade inhibited, but did not eliminate, ACh-induced dilation in old Ex SFA (Fig. 3B). Equally important was the finding that ACh-induced dilation in old Ex SFA in the presence of l-NNA + Indo was not different from the old Sed arteries in the presence of double blockade (Fig. 3B). These data indicate that a vasodilator pathway independent of NOS and COX contributed to ACh-induced dilation in young and old arteries; however, the beneficial effect of exercise training in aged arteries cannot be attributed to enhancement of this pathway. In conclusion, our pharmacological experiments indicate that exercise training reverses the detrimental effects of aging on endothelial function of SFA primarily by enhancing NO bioavailability. This conclusion is similar to previous reports for 1A arterioles of soleus muscle of aged rats (39, 40).
Results of the present study also indicate that exercise training did not induce increases in eNOS protein content in young or old SFA (Fig. 6A), nor did exercise training increase basal levels of Ser 1177 p-eNOS (Fig. 6B) or alter the Ser 1177 p-eNOS-to-total eNOS ratio (Fig. 6C). These data suggest that enhanced NO-mediated, endothelium-dependent dilation in old Ex SFA is not due to increased eNOS protein content or eNOS phosphorylation on Ser 1177. In addition, these results indicate that while exercise training increases NO bioavailability in SFA (Fig. 6) and 1As (39, 40) of aged soleus muscle, the vascular adaptation induced by exercise in SFA is not the same as that reported in soleus 1A arterioles by Spier et al. where exercise training increased eNOS protein content (39). Thus, while exercise training improves endothelial function in SFA and 1A arterioles perfusing soleus muscle, the mechanism by which exercise improves NO-mediated endothelium-dependent dilation is different in SFA and 1A arterioles of this muscle (39, 40). Exercise training has also been shown to increase eNOS mRNA and protein content in aged rat aorta (46). The differences in aging- and exercise training-induced alterations in different vessels suggest that findings in one vessel cannot necessarily be applied to other vessels.
Another mechanism whereby exercise training could increase NO bioavailability in aged SFA is through an exercise-induced reduction in the rate of NO degradation. The primary mechanism for NO degradation in blood vessels is rapid interaction with superoxide anion, leading to the formation of peroxynitrite (16). This mechanism has been implicated in aging-induced endothelial dysfunction (16, 48). Vascular superoxide dismutases play a role in preserving the bioactivity of NO by scavenging superoxide. Consequently, an exercise-induced increase in the expression of superoxide dismutases could lead to the exercise-induced improvement in NO-mediated, endothelium-dependent dilation in aged SFA. Therefore we tested the hypothesis that exercise training increases the expression of SOD-1 and ecSOD in old SFA. The rationale for this hypothesis was based on experimental evidence indicating that exercise training increases expression of SOD-1 and ecSOD in the aorta of young mice and pigs (15, 36). Our results reveal that ecSOD (not SOD-1) content was increased by training in aged SFA (Fig. 7B). These results support the hypothesis that the beneficial effect of exercise on endothelium-dependent dilation observed in the old SFA is, in part, the result of an enhanced capacity of ecSOD to scavenge superoxide, resulting in an increase in the bioavailability of NO. Because ecSOD activity is regulated by a number of factors including protein folding, reactions with NO, and hydrogen peroxide and copper availability (15, 17, 20, 33), further study is needed to fully elucidate the role of ecSOD in the beneficial effects of exercise training in aged SFA.
In light of the observation that ecSOD protein content was increased in old SFA with exercise training, we reasoned that the beneficial effects of exercise on endothelium-dependent dilation of SFA may be mimicked by treatment with exogenous antioxidant enzymes. To test this hypothesis we chose SOD (non-cell permeable) rather than PEG-SOD (cell permeable) because the primary exercise-induced alteration in antioxidant protein content occurred with the extracellular isoform of SOD (ecSOD). Results indicated that addition of exogenous SOD, or SOD + CAT, did not alter the ACh dose response in feed arteries from young rats but resulted in greater ACh-induced dilation in old Sed SFA compared with old Sed SFA without antioxidants (Fig. 4). In addition, ACh-induced dilation was either equal to (in the presence of SOD + CAT) or greater than (in the presence of SOD alone) ACh-induced dilation in young SFA (Fig. 4). Also, l-NNA abolished the improvement in ACh-induced dilation in old Sed SFA observed in the presence of SOD (Fig. 5). These results demonstrate that exogenous antioxidant enzymes mimic the effects of exercise in old SFA, consistent with our hypothesis. Based on the observation that exercise training increased ecSOD protein content and that exogenous SOD mimicked the effects of exercise on ACh-induced dilation in senescent SFA, we conclude that exercise training-induced improvements in NO-mediated, endothelium-dependent dilation in senescent SFA may be in part the result of enhanced scavenging of superoxide by ecSOD, resulting in increased stability of NO. These results are consistent with those obtained in human subjects where older athletes exhibited enhanced endothelium-dependent dilation and antioxidant capacity compared with their sedentary counterparts (14); also, addition of exogenous antioxidants enhanced endothelium-dependent dilation in old sedentary subjects (12). Complementing these observations, our study is the first to demonstrate enhanced ecSOD protein content after exercise training in the aged vasculature.
Conclusion
The results of this study indicate that exercise induces improvement in endothelium-dependent dilation in soleus muscle feed arteries of aged rats. The beneficial effect of exercise training in aged feed arteries was mediated by enhanced NO bioavailability that appears to be the result of increased ecSOD protein content in the aged SFA. The improved NO bioavailability was not associated with increased content or phosphorylation of eNOS or increased SOD-1 protein content. Exogenous SOD treatment of SFA from old Sed rats mimicked the effects of exercise training. Collectively, these results suggest that exercise training reverses the detrimental effects of aging on endothelial function in skeletal muscle feed arteries by enhancing the capacity to scavenge superoxide, increasing the bioavailability of NO. The exercise training-induced improvement in endothelial function in SFA may work in concert with enhanced endothelial function in skeletal muscle arterioles to improve skeletal muscle blood flow and increase exercise tolerance in the elderly.
GRANTS
This work was supported by American Heart Association, Texas Affiliate Grant 0765043Y (C. R. Woodman), National Institute on Aging Grant AG-00988 (C. R. Woodman), National Heart, Lung, and Blood Institute Grant HL-36088 (M. H. Laughlin), and an American College of Sports Medicine Foundation Research Grant (D. W. Trott).
Acknowledgments
We gratefully acknowledge the expert technical assistance of Pam Thorne.
REFERENCES
- 1.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension 30: 817–824, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Cartee GD, Farrar RP. Muscle respiratory capacity and VO2 max in identically trained young and old rats. J Appl Physiol 63: 257–261, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan A, More RS, Mullins PA, Taylor G, Petch MC, Schofield PM. Aging associated endothelial dysfunction in humans is reversed by l-arginine. J Am Coll Cardiol 28: 1796–1804, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Delp MD, McAllister RM, Laughlin MH. Exercise training alters aortic vascular reactivity in hypothyroid rats. Am J Physiol Heart Circ Physiol 268: H1428–H1435, 1995. [DOI] [PubMed] [Google Scholar]
- 8.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol 579: 115–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res 66: 393–401, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frandsenn U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol 531: 257–264, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105: 1631–1639, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived relaxing factor. Nature 320: 454–456, 1986. [DOI] [PubMed] [Google Scholar]
- 17.Hink HU, Santanam N, Dikalov S, McCann L, Nguyen AD, Parthasarathy S, Harrison DG, Fukai T. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol 22: 1402–1408, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol 42: 660–665, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise-trained rats. J Appl Physiol 86: 441–449, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Jeney V, Itoh S, Wendt M, Gradek Q, Ushio-Fukai M, Harrison DG, Fukai T. Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ Res 96: 723–729, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol 72: 2052–2062, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Laughlin MH Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc 27: 1135–1144, 1995. [PubMed] [Google Scholar]
- 23.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol 96: 233–244, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: the effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mazzeo RS, Brooks GA, Horvath SM. Effects of age on metabolic responses to endurance training in rats. J Appl Physiol 57: 1369–1374, 1984. [DOI] [PubMed] [Google Scholar]
- 27.McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol 98: 753–761, 2005. [DOI] [PubMed] [Google Scholar]
- 28.McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol 82: 1438–1444, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Muller JM, Meyers PR, Laughlin MH. Vasodilator response of coronary resistance arteries of exercise-trained pigs. Circulation 89: 2308–2314, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Parker JL, Oltman CL, Muller JM, Meyers PR, Adams HR, Laughlin MH. Effects of exercise training on regulation of tone in coronary arteries and arterioles. Med Sci Sports Exerc 26: 1252–1261, 1994. [PubMed] [Google Scholar]
- 33.Petersen SV, Valnickova Z, Oury TD, Crapo JD, Chr Nielsen N, Enghild JJ. The subunit composition of human extracellular superoxide dismutase (EC-SOD) regulates enzymatic activity. BMC Biochem 8: 19, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Rush JWE, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial nitric oxide synthase gene expression. Circ Res 74: 349–353, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spier SA, Delp MD, Stallone JN, Dominguez JM 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Srere PA Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 42.Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res 56: 54–61, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Sun D, Huang A, Koller A, Kaley G. Short term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol 76: 2241–2247, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Tanabe T, Maeda S, Miyauchi T, Iemitsu M, Takanashi M, Irukayama-Tomobe Y, Yokota T, Ohmori H, Matsuda M. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol Scand 178: 3–10, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol 103: 852–857, 2007. [DOI] [PubMed] [Google Scholar]
- 48.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33: 79–86, 1974. [DOI] [PubMed] [Google Scholar]
- 50.Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol 463: 631–646, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams DA, Segal SS. Microvascular architecture in rat soleus and extensor digitorum longus muscles. Microvasc Res 43: 192–204, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol Heart Circ Physiol 273: H2575–H2579, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Woodman CR, Price EM, Laughlin MH. Selected Contribution: Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol 95: 2164–2170, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol 98: 940–946, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Woodman CR, Trott DW, Laughlin MH. Short-term increases in intraluminal pressure reverse age-related decrements in endothelium-dependent dilation in soleus muscle feed arteries. J Appl Physiol 103: 1172–1179, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]