Abstract
Previous studies have demonstrated that electroacupuncture (EA) attenuates sympathoexcitatory reflex responses by activating a long-loop pathway involving the hypothalamic arcuate nucleus (ARC), midbrain ventrolateral periaqueductal gray (vlPAG), and rostral ventrolateral medulla (rVLM). Neurons in the ARC provide excitatory input to the vlPAG, whereas the vlPAG inhibits neuronal activity in the rVLM. γ-Aminobutyric acid (GABA) and glutamate (Glu) have been identified in the vlPAG. Endocannabinoids (ECs), acting as atypical neurotransmitters, inhibit the release of both neurotransmitters in the hypothalamus and midbrain through a presynaptic cannabinoid type 1 (CB1) receptor mechanism. The EC system has been observed in the dorsal but not in the vlPAG. Since it is uncertain whether ECs influence GABA and Glu in the vlPAG, the present study tested the hypothesis that EA modulates the release of these neurotransmitters in the vlPAG through a presynaptic CB1 receptor mechanism. We measured the release of GABA and Glu simultaneously by using HPLC to assess samples collected with microdialysis probes inserted unilaterally into the vlPAG of intact anesthetized rats. Twenty-eight min of EA (2 Hz, 2–4 mA, 0.5 ms) at the P5–6 acupoints reduced the release of GABA by 39% during EA and by 44% 15 min after EA. Thirty-five minutes after EA, GABA concentrations returned to pre-EA levels. In contrast, sham EA did not change the vlPAG GABA concentration. Blockade of CB1 receptors with AM251, a selective CB1 receptor antagonist, reversed the EA-modulated changes in GABA concentration, whereas microinjection of vehicle into the vlPAG did not alter EA-modulated GABA changes. In addition, we observed no changes in the vlPAG Glu concentrations during EA, although the baseline concentration of Glu was much higher than that of GABA (3,541 ± 373 vs. 33.8 ± 8.7 nM, Glu vs. GABA). These results suggest that EA modulates the sympathoexcitatory reflex responses by decreasing the release of GABA, but not Glu, in the vlPAG, most likely through a presynaptic CB1 receptor mechanism.
Keywords: somatic afferents, sympathoexcitatory reflex, cannabinoid type 1 receptor, microdialysis
electroacupuncture (EA), a potent alternative to manual acupuncture, has been suggested to be effective in treating certain cardiovascular diseases including hypertension, arrhythmias, and angina pectoris (5, 6, 41). Clinically, EA at the Neiguan-Jianshi (P5-6) acupoints has been used to treat cardiovascular diseases in Eastern and, more recently, Western countries (5, 21, 41). We and others have demonstrated that EA at P5-6 acupoints overlying the median nerve on the wrist modulate blood pressure elevation evoked by gastric distension (GD) in rats (27) or by gallbladder stimulation in cats (50) through a long-loop neural pathway, extending from the arcuate nucleus (ARC) in the hypothalamus to the ventrolateral periaqueductal gray (vlPAG) in the midbrain and, ultimately, to the rostral ventrolateral medulla (rVLM) (13, 26, 50, 58).
The midbrain periaqueductal gray (PAG) processes signals concerned with pain and analgesia, fear and anxiety, vocalization, lordosis, and cardiovascular control (9). Previous studies have demonstrated that stimulation of the vlPAG produces depressor responses in cats and rats (32, 33), whereas excitation of the dorsolateral PAG increases blood pressure in cats (16), and as noted above, it is the vlPAG that interacts with the ARC and rVLM in EA modulation of cardiovascular responses (19, 30, 50). Specifically, EA at P5-6 acupoints attenuates sympathoexcitatory reflex responses through excitation of the ARC and vlPAG neurons, which, in turn, inhibit the premotor sympathoexcitatory rVLM neuronal activity in rats and cats (11, 19, 50). Using c-Fos and opioid immunohistochemical labeling, we have documented that EA activates ARC and vlPAG neurons (19). Conversely, inactivation of neurons in the vlPAG with kainic acid largely abolishes the ARC-induced inhibition of rVLM activity (30). Thus the vlPAG is considered to be an important integrative region in the EA regulation of cardiovascular function. However, dynamic changes of neurotransmitters in the vlPAG that underlie the modulating action of EA have not been evaluated.
A number of neurotransmitters, including acetylcholine, β-endorphins, glutamate, and γ-aminobutyric acid (GABA), have been identified in various regions of the PAG (9). The latter two neurotransmitters may be involved in regulation of vlPAG neuron activity with respect to EA modulation of cardiovascular responses. In this regard, we have shown that microinjection of the glutamate agonist dl-homocysteic acid (DLH) into the vlPAG mimics the modulating effect of EA on sympathoexcitatory reflexes (50). Formalin injection into the hindpaw of conscious rats increases glutamate concentration generally in the PAG, although there has been no subdivision identification (47). Systemic injection of morphine modulates veratridine-induced release of GABA in the lateral PAG of rats (40, 48). Immunocytochemical studies have demonstrated GABA-immunoreactive terminals in the dorsolateral and ventrolateral PAG and the presence of GABAA receptors in the ventrolateral PAG of rats and cats (7, 56). GABAB receptors have been identified in the dorsomedial, lateral, and ventrolateral PAG but sparsely in the dorsolateral PAG of rats (8). There also appear to be high densities of glutamate receptors in the dorsolateral PAG and somewhat lower densities of these receptors in the vlPAG (1). Despite these observations, the roles of GABA and glutamate in the vlPAG, particularly with respect to the action of EA on cardiovascular function, are unclear. However, on the basis of the scant information available, we hypothesized that stimulation of somatic afferents during EA would modulate the release of glutamate and GABA in the vlPAG.
Previous studies have suggested that endocannabinoids (ECs) can modulate the function of central autonomic pathways by regulating presynaptic and postsynaptic neurotransmission. ECs are produced in various regions of the brain in response to noxious stimuli and bind to cannabinoid type 1 (CB1) receptors (14, 55). Administration of exogenous cannabinoids into the nucleus tractus solitarii (NTS) facilitates baroreflex inhibition of renal sympathetic nerve activity, suggesting that ECs modulate cardiovascular reflex responses (46). Blockade of CB1 receptors in the NTS of intact animals eliminates the facilitating effect that the EC anandamide has on baroreflex responses, indicating that ECs regulate cardiovascular reflexes through a GABAergic neurotransmission mechanism (46). In an activity recording study in brain slices from the PAG (without detailing the PAG subdivision), investigators have documented that the EC system inhibits glutamatergic and GABAergic synaptic transmission through a CB1 receptor mechanism (52, 53). ECs also are capable of inhibiting glutamatergic and GABAergic synaptic transmission in the hippocampus and basal ganglia (24, 49). Hentges et al. (20) reported that administration of an exogenous cannabinoid agonist inhibits the release of both GABA and glutamate from cells in hypothalamic arcuate nucleus proopiomelanocortin (POMC) neurons, whereas endogenous cannabinoids inhibit the release of GABA but not glutamate in the POMC. Although there is no direct evidence to implicate an action for the EC system in the vlPAG, we hypothesized that ECs in this region might participate in EA modulation of sympathoexcitatory reflexes by regulating the release of GABA and, possibly, glutamate.
The aim of the present study, therefore, was to determine whether EA modulates the release of GABA and glutamate in the vlPAG through an EC-CB1 receptor mechanism. We employed microdialysis followed by HPLC analysis to test the overall hypothesis that EA modulates the release of GABA and glutamate in the vlPAG through a CB1 receptor mechanism. A preliminary report of this study has been published (17).
METHODS
Surgical Preparation
Adult Sprague-Dawley male rats (320–560 g) were anesthetized initially with ketamine (100 mg/kg im) followed by α-chloralose (5 mg/kg iv). Additional doses of α-chloralose were given as necessary to maintain an adequate level of anesthesia, assessed by observing the absence of a conjunctival reflex. A femoral vein was cannulated to administer drugs and fluids. Systemic arterial blood pressure was monitored by a pressure transducer attached to a femoral artery cannula. The trachea was intubated, and respiration was maintained artificially (model 661 Harvard ventilator; Ealing, South Natick, MA). Arterial blood gases and pH were measured with a blood gas analyzer (ABL 5; Radiometer America, West Lake, OH) and maintained within physiological limits (Po2 >100 mmHg, Pco2 30–40 mmHg, pH 7.35–7.45) by adjusting the respiratory rate or tidal volume or by administering NaHCO3 (1 M, iv). Body temperature was monitored with a rectal thermistor and maintained at 36–38°C with a circulating water heating pad and a heat lamp. All experimental preparations and protocols were reviewed and approved by the Animal Care and Use Committee at the University of California, Irvine. The investigation conformed to the American Physiological Society's “Guiding Principles in the Care and Use of Animals.”
In some rats, excitatory cardiovascular reflex responses were induced by GD as described previously (27). In brief, a 2-cm diameter (unstressed dimension) latex balloon (Traub) was attached to a polyurethane tube (3-mm diameter) that was inserted into the stomach through the mouth and esophagus. A syringe was attached to the cannula to inflate and deflate the balloon with air, while a manometer was used to monitor intragastric pressure through a T connection. The balloon was inflated inside the stomach, which evoked an increase in blood pressure within 30 s of inflation. After the maximum increase in blood pressure occurred and was maintained for at least 45 s, the balloon was deflated slowly over a 30-s period. At least 20-min intervals were maintained between two consecutive balloon inflations. We excluded one animal in the study after we found at the end of the experiment that the balloon was not positioned within the stomach.
Microdialysis and HPLC Measurement of GABA and Glutamate
The rat's head was fixed in a stereotaxic apparatus (Kopf Instruments) with the floor of the fourth ventricle positioned horizontally. A partial craniotomy was performed to expose the dorsal cortex to allow access to the vlPAG. A microdialysis probe (membrane diameter 240 μm, CMA/11; CMA, Stockholm, Sweden) was inserted perpendicularly (90°) to the dorsal surface of the cortex, 0.5 mm lateral to the midline, and 7.0–7.4 mm caudal to the bregma, to a depth of 6.5 mm from the dura, as depicted in the atlas of Paxinos and Watson (36). The probe was connected to the microdialysis syringe pump (CMA 402) and was perfused with artificial cerebral spinal fluid (aCSF; pH 7.4) at 1.5 μl/min. The aCSF contained 0.2% bovine serum albumin and the following compositions (in mM): 2.5 KCl, 125 NaCl, 1.26 CaCl2, and 1.18 MgCl2. Gel foam was used to minimize bleeding during this procedure.
Extracellular fluid samples were collected continuously for 7 min (1.5 μl/min, total 10.5 μl) with a refrigerated fraction collector and were stored at −80°C until assay. Initial confirmation of accurate placement of the microdialysis probes into a depressor region of the midbrain (i.e., the vlPAG) was verified by noting an immediate decrease in blood pressure following microperfusion of 5 mM l-glutamate (RBI, Natick, MA) into the area.
Glutamate and GABA in the microdialysate were measured simultaneously with a modified method using high-performance liquid chromatography (HPLC), which we have described previously (59). In brief, 10 μl of microdialysate was mixed with 5 μl of o-phthaldialdehyde (OPA) solution (20 mg of OPA, 0.5 ml of 1 M sodium sulfite, and 10 ml of 0.2 M sodium borate solution) for pre-column derivation for 30 min at 25°C (30). The mixed sample was analyzed immediately thereafter for glutamate and GABA. The mobile phase (pH 5.3) for isocratic elution of glutamate and GABA consisted of 100 mM NaH2PO4, 20% methanol, 3% acetonitrile, and 0.1 mM Na2EDTA and run through an HR-80, 3-μm HPLC analytical column (ESA, Chelmsford, MA). The mobile phase was pumped (510 Pump; Waters, Milford, MA) at a flow rate of 0.6 ml/min. Glutamate and GABA were detected coulometrically by a Coulochem II detector (ESA) with a 5020 guard cell and a 5011 analytic cell (ESA). The applied working potentials were 720 mV for the guard cell and 220 (E1) and 590 mV (E2) for the analytic cell. The detection limits for this assay were 800 fmol for glutamate and 10 fmol for GABA. Confirmation of peak identity was accomplished by concurrently running samples with known standards.
Glutamate in the microdialysate collected from another group of rats was measured using a modified method, as described previously (59), which was designed for high-resolution measurement of glutamate alone (detection limit 50 fmol). In brief, the sample pre-column derivation process was the same as the original method for measuring both GABA and glutamate. The mobile phase (pH 6.0) for isocratic elution of glutamate consisted of 100 mM NaH2PO4, 10% methanol, and 0.5 mM Na2EDTA and was run through an HR-80, 3-μm HPLC analytical column (ESA). The mobile phase was pumped (510 Pump; Waters) at a flow rate of 0.6 ml/min. Glutamate was detected coulometrically as described above. The applied working potentials were 780 mV for the guard cell and 260 (E1) and 640 mV (E2) for the analytic cell. Confirmation of peak identity was accomplished by concurrently running samples with known standard.
At the end of each experiment, the perfusion site was marked with injection of 50 nl of Chicago Sky blue dye (5% in 0.5 M sodium acetate) through a modified microdialysis probe; after removal of the probe membrane, the probe was replaced in the same position as the original microdialysis probe in the vlPAG using the same stereotaxic coordinates. Thereafter, rats were euthanized under deep anesthesia with 50 mg/kg of α-chloralose and saturated KCl. The stomach was exposed to confirm correct placement of the balloon. The midbrain was removed and submerged in 4% paraformaldehyde for at least 2 days. Frozen 40-μm coronal sections then were cut with a CM 1850 cryostat microtome (Leica) to confirm histologically the microinjection sites. The dye spots were identified with a binocular microscope. Microinjection sites in the vlPAG were plotted with Corel Presentation software on a single coronal section, extending from 1.20 to 1.68 mm rostral to the interaural line, and reconstructed according to the atlas of Paxinos and Watson (36). We excluded three animals from the study when the microdialysis probe was observed not to be in the vlPAG during histological examination.
Experimental Procedure
After insertion of the microdialysis probe into the vlPAG, animals were allowed to stabilize for 100 min to allow for equilibration of neurotransmitters released during positioning of the probe. During this period, aCSF was continuously dialyzed at 1.5 μl/min while 7-min collections of dialysates (10 μl) were sampled to establish a stable baseline (control) extracellular concentration of GABA and glutamate. We then initiated the experimental protocol, including blood pressure recordings and continuous collection of CSF samples.
Experimental Protocols
Effect of EA on release of GABA and glutamate in vlPAG.
We examined the influence of EA on extracellular vlPAG glutamate ([Glu]) and GABA concentrations ([GABA]) in the absence of GD. In the first group of animals (n = 8), we bilaterally placed 32-gauge stainless steel acupoint needles at the Neiguan-Jianshi (P5-6) acupoints, located over the median nerves above the paw (along the pericardial meridian). Acupuncture needles were inserted perpendicularly to a depth of 1–3 mm. Correct placement of the needles at the acupoints was confirmed by observing slight repetitive paw twitches (i.e., motor threshold) during EA stimulation. We have found previously that muscle twitches, evoked by stimulation of large-diameter α-motor neurons in the mixed median nerve, are a reliable way to confirm the appropriate amount of current that is necessary to also activate smaller diameter group III and IV sensory fibers in the median nerve. This degree of sensory nerve activation evokes a strong modulatory influence of EA on cardiovascular reflex responses (13, 26, 27). After the animal had been stabilized for 100 min after probe insertion, EA (1–2 mA, 0.5-ms duration, 2 Hz) was initiated and maintained for 28 min. Dialysate samples were collected continuously before, during, and after EA. Blood pressure also was recorded before and during EA.
In another six animals, an identical procedure was conducted, with the exception that EA needles at P5-6 acupoints were placed but not stimulated. This group of animals served as a sham EA control, since our previous study suggested that needle insertion without stimulation at active acupoints is an adequate control for EA (58).
As noted in results, we found that [Glu] was unchanged during EA. We therefore studied four additional rats to verify that the absence of any change in [Glu] was not due to an inability to resolve small changes below the limit of detection (800 fmol) when we simultaneously measured [Glu] and [GABA]. This was accomplished by increasing the sensitivity of measurement to allow detection of [Glu] down to 50 fmol by optimizing the HPLC technique in which [Glu] alone was measured (see Fig. 3, C and D). In this group, each rat was treated in an identical manner as the first group of animals, except that only [Glu] was measured using the optimized HPLC method.
Influence of EA on extracellular vlPAG [Glu] and [GABA] induced by visceral reflex stimulation.
To determine the effect of EA on visceral reflex stimulation-mediated vlPAG [Glu] and [GABA] during GD, we divided 12 rats into two groups. In the first group of rats (n = 6), acupuncture electrodes were inserted at P5-6 acupoints bilaterally but were not stimulated. This group served as sham controls for the EA group. GD was induced by inflating the balloon with a volume ranging from 5 to 8 ml of air after the animal had been stabilized for at least 60 min. Once the maximum blood pressure response was attained (generally within 30 s), distension was maintained for a total of at least 45 s, after which the injected air was slowly withdrawn from the balloon over a 20-s period. The peak blood pressure reflex response typically occurred within 20–30 s following inflation (27, 57). In this protocol, GD was repeated at least 20 min after the preceding GD during six separate inflation periods to prevent tachyphylaxis. CSF samples from the vlPAG were collected continuously during the entire period, and blood pressure was recorded before and during each GD.
In the second group of six rats, we recorded two mean arterial blood pressure (MAP) responses to repeated GD before EA and collected two CSF samples during a control period. Next, 28 min of EA (1–2 mA, 0.5-ms duration, 2 Hz) were performed using 32-gauge acupuncture needles placed bilaterally at the P5-6 acupoints. GD was repeated during EA as well as 10, 30, and 50 min after termination of EA. CSF samples were collected continuously, and blood pressure was recorded.
Influence of CB1 receptor blockade on EA-mediated change in [GABA].
In this protocol, two groups of animals were studied to determine the influence of CB1 receptor blockade with the selective receptor antagonist AM251 on [GABA] in the vlPAG during and after EA (22). In the first group, eight rats were subjected to EA, followed by blockade with AM251 in the vlPAG. Thus, after stabilization, we instituted EA (1–2 mA, 0.5-ms duration, 2 Hz) at P5-6 acupoints bilaterally for 28 min. Twenty-one minutes after initiation of EA, we administered AM251 (1 mg/ml) through the microdialysis probe in the vlPAG at the rate of 1.5 μl/min over a 7-min period. AM251 (Tocris, Ellisville, MS) was dissolved in ethanol to achieve a concentration of 10 mg/ml and was stored before use at −70°C. On the day of the experiment, we mixed 10 μl of the AM251 solution with Tocrisolve 100 to achieve a concentration of 5 mg/ml and further diluted with aCSF to yield a final concentration of 1 mg/ml. As in the previous protocol, CSF samples were collected continuously, and blood pressure was recorded.
In the second group of five control animals, we performed an identical procedure, except the administration of AM251 was replaced by the vehicle solution (10% Tocrisolve 100 and 10% ethanol).
Statistical Analysis
Data are means ± SE. We used the Kolmogorov-Smirnoff test to determine whether the data were normally distributed. Changes in blood pressure and GABA and glutamate concentrations over time were analyzed using a one-way repeated-measures ANOVA, followed by multiple comparisons for individual differences using the Tukey test, if the ANOVA showed statistically significant changes. Values were considered significantly different when P < 0.05. All statistical calculations were performed with a statistical software package (SigmaStat for Windows version 3.0; Jandel Scientific Software, San Rafael, CA).
RESULTS
Effect of EA on vlPAG Glu and GABA Release
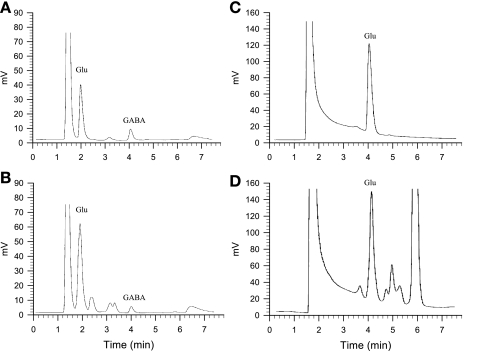
Figure 1 shows the chromatographic spectra of glutamate and GABA in both standards and in a dialysate sample collected from one animal as detected coulometrically following HPLC. We observed glutamate and GABA peaks in dialysate that corresponded closely to the standard peaks using the method optimized for simultaneous assay of glutamate and GABA (Fig. 1, A and B).
Fig. 1.
Representative chromotographs of glutamate (Glu) and γ-aminobutyric acid (GABA) from 10-μl standards and of microdialysis sample collected from the midbrain ventrolateral periaqueductal gray (vlPAG) of rat. A: 2 μM Glu and 100 nM GABA standard peaks in artificial cerebrospinal fluid (aCSF) that were measured simultaneously. B: 3.2 μM Glu and 38 nM GABA peaks in microdialysate sample. Concentrations were calculated from the peak integrations. Detection was accomplished coulometrically. Limits of detection with this method were 800 fmol for glutamate and 10 fmol for GABA. C: 2 μM Glu standard alone in CSF measured by the high-resolution method of glutamate assay. D: 2.96 μM Glu in microdialysis sample. The limit of detection for glutamate with the latter method was 50 fmol.
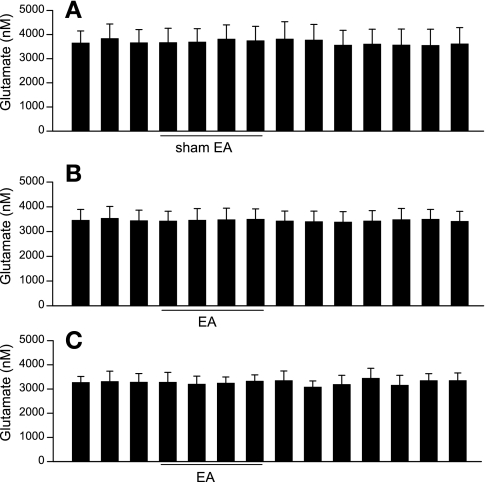
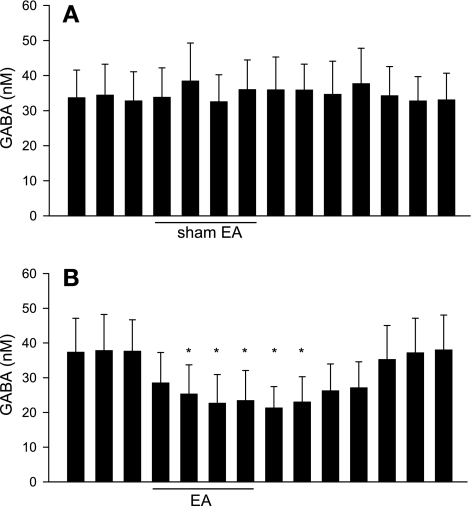
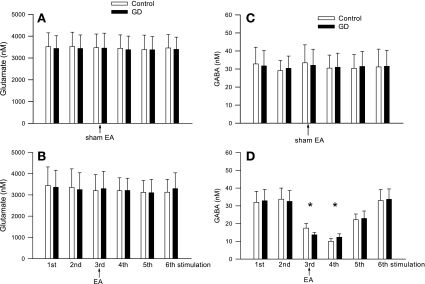
The modified HPLC method allowed us to simultaneously measure both [Glu] and [GABA] in microdialysate collected from the vlPAG. In a preliminary study with three rats, we observed that the dialysate [Glu] and [GABA] initially were very high, but they stabilized 98 min after insertion (3,248 ± 246 vs. 3,319 ± 326 and 37 ± 8 vs. 38 ± 9 nM at 91 vs. 98 min after probe insertion, respectively, n = 3). Therefore, in the remainder of the study we chose to assess [Glu] and [GABA] in dialysates 100 min after probe insertion. We observed that baseline levels of glutamate were much higher than those of GABA in the vlPAG of rats (Figs. 2 and 3). The vlPAG [Glu] was unchanged by EA compared with either the concentration in the pre-EA period or at the same relative time in the sham EA group (Fig. 2, A and B). Similarly, we observed that the vlPAG [Glu] measured using the method optimized for higher resolution in the assay of glutamate alone also was unchanged by EA (Fig. 2C). However, vlPAG [GABA] was significantly decreased by EA and remained low for at least 21 min after termination of EA in eight rats (Fig. 3B). Conversely, we observed no changes in the vlPAG [GABA] during sham EA (n = 6, Fig. 3A).
Fig. 2.
Dynamic changes in vlPAG glutamate concentrations ([Glu]) every 7 min before, during, and after sham electroacupuncture (EA; A; n = 6) and before, during, and after 28 min of low-frequency EA at P5-6 acupoints (B; n = 8). C: vlPAG [Glu] using the method optimized for glutamate measurement in 4 other rats treated with EA. Data are means ± SE. There were no significant changes in the vlPAG [Glu] during EA compared with pre-EA.
Fig. 3.
Dynamic changes in the vlPAG GABA concentrations every 7 min before, during, and after sham EA (A; n = 6). and before, during, and after 28 min of EA at low-frequency P5-6 acupoints (B; n = 8). Low-frequency EA decreased GABA release in the vlPAG. Data are means ± SE. *P < 0.05 vs. pre-EA.
Release of Glutamate and GABA in vlPAG during GD and Response to EA
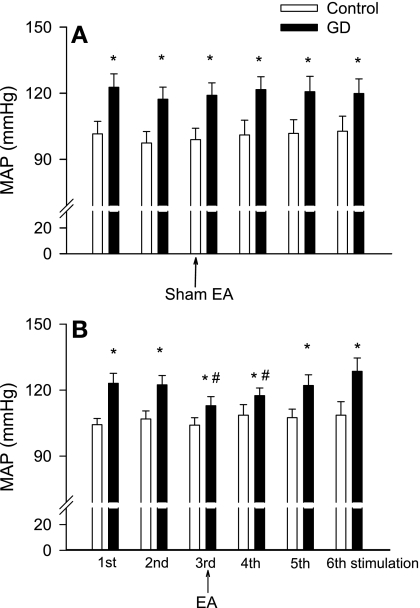
Similar to our previous studies (27, 57), GD significantly elevated MAP compared with prestimulation values (Fig. 4A). However, GD did not alter the vlPAG [Glu] or [GABA] in the dialysate of six rats (Fig. 5, A and C).
Fig. 4.
Peak changes in mean arterial blood pressure (MAP) during repeated gastric distension (GD) with sham EA (A; n = 6) and EA (B; n = 6). Repeated GD was conducted at intervals of least 20 min. Data are means ± SE. *P < 0.05 vs. control. #P < 0.05 vs. pre-EA.
Fig. 5.
Changes in the vlPAG [Glu] (A and B) and [GABA] (C and D) in response to GD before, during, and after sham EA (A and C; n = 6) and before, during, and after 28 min of low-frequency EA at P5-6 acupoints (B and D; n = 6). Repeated GD was conducted at intervals of least 20 min. Data are means ± SE. *P < 0.05 vs. pre-EA.
In six other rats, we evaluated the influence of EA on GD-mediated glutamate and GABA release in the vlPAG as well as the MAP responses. Like our previous observations (27), 28 min of EA at P5-6 attenuated the MAP responses evoked by GD by ∼50% (Fig. 4B). We found that EA did not alter the vlPAG [Glu] (Fig. 5 B). In contrast, we observed that EA decreased the vlPAG [GABA] and that this depression was present for more than 10 min after termination of EA (Fig. 5D).
Blockade of CB1 Receptor on EA-Induced Changes in [Glu] and [GABA]
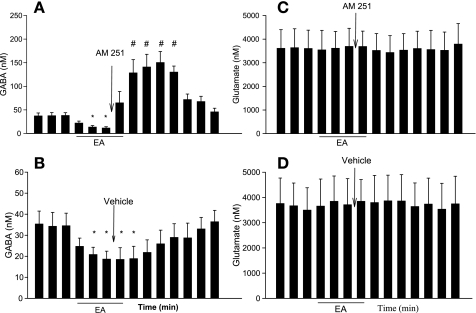
In eight rats, microperfusion of the EC CB1 receptor antagonist AM251 into the vlPAG immediately reversed the decrease in vlPAG [GABA] that was associated with EA (Fig. 6A). In contrast, microperfusion with vehicle for AM251 into the vlPAG did not alter the EA-associated decrease in the vlPAG [GABA] in five separate animals (Fig. 6B). [Glu] in the vlPAG were unchanged during EA or microperfusion of AM251 or vehicle (Fig. 6, C and D). We also observed no change in MAP during and after microperfusion of AM251 compared with preperfusion (105 ± 5 vs. 103 ± 6 mmHg, before vs. after AM251, P > 0.05).
Fig. 6.
Blockade of cannabinoid type 1 (CB1) receptors in the vlPAG reversed EA-related inhibition of GABA release. A: microdialysis of AM251 into the vlPAG reversed the dynamic changes in [GABA] every 7 min induced by EA (n = 8). B: microdialysis of vehicle into the vlPAG did not affect EA-induced inhibition of GABA release (n = 5). There were no changes in the vlPAG [Glu] following microperfusion of AM251 (C) or vehicle (D). Data are means ± SE. *P < 0.05 vs. control. #P < 0.05 vs. EA inhibition.
[GABA] in regions outside of the vlPAG
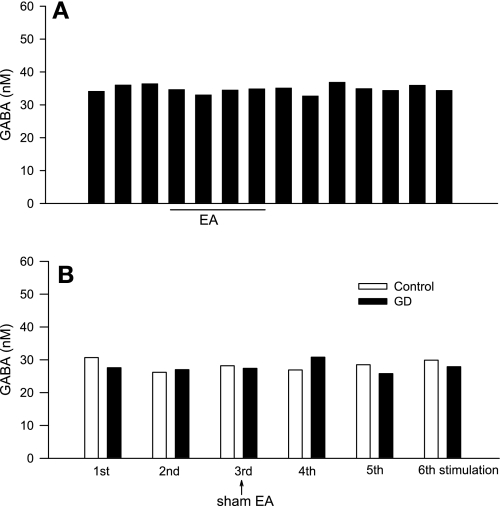
In the present study we observed that, occasionally (n = 3), microdialysis probes were placed inadvertently outside the vlPAG, including one following EA, one in the EA group treated with AM251, and one following GD and sham EA. GABA concentrations in the first two rats during and after EA were unchanged (Fig. 7A). Likewise, GABA concentrations in the GD and sham EA rat were unchanged (Fig. 7B), similar to the results noted in Fig. 5C.
Fig. 7.
Dynamic changes in [GABA] in a region outside the vlPAG from samples collected every 7 min before, during, and after EA at P5-6 acupoints (A; n = 2, 1 from AM251 perfusion group) as well as in response to GD before, during, and after sham EA (B; n = 1). Repeated GD was conducted at intervals of least 20 min.
Histology
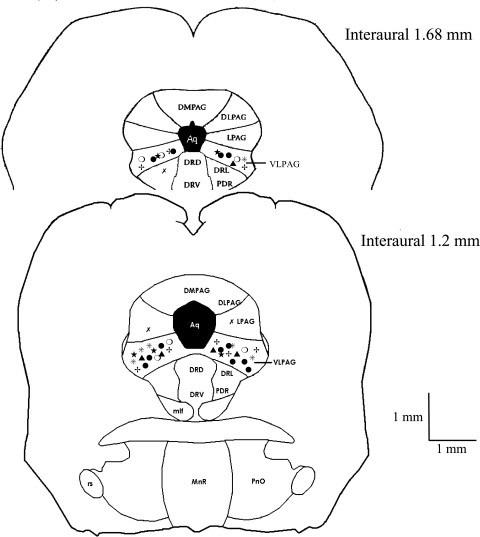
Figure 8 provides histological data showing locations of placement of the microdialysis probe in the various protocols. Among the 43 sites of placement of the probe in the vlPAG, 6 were related to GD and sham EA, 6 others to GD and EA, 8 to EA, 6 to sham EA, 4 to EA associated with the optimized HPLC assay, and 13 to CB receptor blockade or vehicle control. There were three other sites outside the vlPAG.
Fig. 8.
Composite map showing locations of the microdialysis sites in the vlPAG (total of 43 sites) for EA and Glu (•, n = 12), sham EA (★, n = 6), GD + sham EA (○, n = 6), GD + EA (*, n = 6), AM251 (+, n = 8), and vehicle (▴, n = 5) and of microdialysis sites outside the vlPAG (χ, n = 3). Sections represent 1.2 and 1.68 mm rostral to interaural line.
DISCUSSION
Electrophysiological and immunohistochemical studies in intact animals have demonstrated that the vlPAG serves as part of a long-loop pathway involved in EA modulation of sympathoexcitatory cardiovascular reflex responses evoked by visceral afferent stimulation (19, 30, 50). Since glutamate and GABA have been identified in the vlPAG (9, 39), we speculated that EA stimulation would regulate vlPAG activity through both neurotransmitters in the EA-associated modulation. The present study, using a combined microdialysis and intact animal neural reflex approach, has demonstrated that EA at the Neiguan-Jianshi (P5-6) acupoints over the median nerve decreases the concentration of vlPAG GABA, but not glutamate. A reduction of GABA in this region leads to disinhibition of neuronal activity that ultimately could increase inhibitory input from the vlPAG to the rVLM, which we have documented previously (50).
Numerous studies have demonstrated that activation of the vlPAG neurons during EA regulates sympathoexcitatory reflex responses by modulating the sympathetic outflow (3, 30, 50). In this respect, using immunohistochemical and electrophysiological approaches, we previously showed that stimulation of somatic afferents during EA activates neurons in the vlPAG (18, 19) mainly through activation of neurons in the ARC in the hypothalamus that provide excitatory projections to the vlPAG. In fact, chemical stimulation of the ARC by microinjection of DLH enhances the response of vlPAG neurons to EA at P5-6 acupoints (23, 30), whereas depolarization blockade of the ARC by microinjection of kainic acid into this region essentially eliminates the excitatory influence of EA on the vlPAG (30). However, there is little information on the vlPAG neurotransmitter mechanism(s) that underlie this EA-associated modulation.
More than 10 neurotransmitters or modulators, including both glutamate and GABA, have been identified in the different subdivisions of PAG (9, 38, 39), although there is no information regarding glutamate and GABA release in the vlPAG during somatic afferent stimulation and/or during EA. The PAG is organized into four longitudinal columns or subdivisions: the dorsomedial, dorsolateral, lateral, and ventrolateral. As noted earlier, injection of formalin into the hindpaw of the rat increases the concentration of glutamate in the caudal ventrolateral PAG (38, 47). Peripheral inflammation in conscious rats alters the extracellular concentrations of glutamate and GABA in the PAG, although the specific subdivision(s) participating in this release has not been identified (34). In addition, investigators have reported that glutamate concentration in the dorsal lateral PAG increases during stimulation of muscle afferents (31). Increases in glutamate and GABA concentrations have been discovered in the PAG following chemical stimulation of the PAG neurons, although, again, the particular subdivisions were not identified (39). In addition, immunocytochemical studies have documented that ∼40% of terminals distributed in the four subdivisions of the PAG, including the vlPAG, are GABAergic (7). The present study thus provides the first evidence demonstrating that EA decreases the vlPAG release of GABA but not glutamate.
Cannabinoid CB1 receptors are located mainly on axon terminals (including central and peripheral neurons), whereas the CB2 receptors are present on immune cells (37). A number of studies have documented that ECs mediate stress-induced analgesia in the dorsolateral PAG and other regions of the brain by inhibiting glutamatergic and/or GABAergic synaptic transmission through a CB1 receptor mechanism (22, 45, 53, 54). The present study provides the first evidence that ECs also can modulate the release GABA in the vlPAG through a CB1 receptor mechanism. In this regard, investigators have observed that ECs, including anandamide and 2-arachidonoylglycerol (2-AG), increase in the other PAG regions following foot shock in rats (22); Seagard et al. (46) also have observed that anandamide increases in hypertensive animals in the nucleus of the solitary tract. Moreover, as noted above, microinjection of the selective CB1 receptor antagonist rimonabant in the dorsolateral PAG decreases stress antinociception. Interestingly, this antagonist does not alter nociception in the lateral or vlPAG (22). In vitro recordings in brain slices have shown that activation of CB1 presynaptic receptors can inhibit GABAergic neurotransmission in the PAG, although the specific location of this effect has not been documented in previous investigations (53, 54). Inhibition of GABAergic and/or glutamatergic synaptic transmission by the cannabinoid agonists, however, has been documented in a number of other brain regions, including the hippocampus, cerebellum, and basal ganglia (12, 24, 25, 49). Also, recordings in brain slices suggest that cannabinoid agonists can regulate the release of neurotransmitters such as glutamate and GABA through presynaptic receptor mechanisms in many regions of brain (45, 46, 53). Our data generally are consistent with these observations, although GABA rather than glutamate in the vlPAG appears to be preferentially regulated during EA. Furthermore, our observation that blockade of CB1 receptors in the vlPAG does not alter the glutamate release in the vlPAG implies that the lack of influence of EA on glutamate release is not due to EC-mediated inhibition.
The extracellular GABA concentration is a function of release vs. metabolism or reuptake by GABA transporters located on the membrane of presynaptic terminals and on glial cells (10, 15). Although postsynaptic CB1 receptors have been suggested to exist in the brain (2, 35, 42–44), there is no evidence that CB1 receptors located postsynaptically influence GABA transporter function. Furthermore, Vaughan et al. (53) did not find postsynaptic cannabinoid inhibition in the PAG, which is consistent with the observation of a high prevalence of CB1 receptors on PAG axons and fibers rather than cell bodies (52). The present study shows that the EA-associated reduction in GABA concentrations can be reversed by CB1 receptor blockade in the vlPAG. Taking these findings together with prior investigations, we believe that our demonstration of EA modulation of vlPAG release of GABA most likely is due to its action on presynaptic CB1 receptors.
Chemical and electrical stimulation of visceral spinal afferents activates vlPAG neurons and hence has the potential to change glutamate and/or GABA concentrations in the vlPAG (19, 30). The degree of activation of these neurons during visceral stimulation is less than that observed during somatic simulation associated with acupuncture (30). In the present study, we observed that stimulation of visceral afferents by GD changed neither the microdialysate GABA nor the glutamate concentrations collected from the vlPAG, although GD indeed evoked a pressor reflex response, as we have observed previously (27). Activation of premotor sympathetic neurons in the rVLM that do not involve the long-loop ARC and midbrain vlPAG nuclei likely accounts for much of the reflex sympathoexcitation (28, 58). In this regard, depolarization blockade (or deactivation) of vlPAG neurons with kainic acid does not alter sympathoexcitatory reflex responses to visceral afferent stimulation by bradykinin (BK), whereas it does eliminate EA modulation of reflex activation following application of BK to the gallbladder (50). Furthermore, consideration of the experimental technique may explain our current observations, since the microdialysate GABA and glutamate measurements reflect changes in neurotransmitters pools from many neurons, whereas the electrophysiological and immunohistochemical studies reveal a specific group of neurons that are activated by stimulation of visceral afferents. Altogether, it appears that stimulation of visceral afferents by GD is not a sufficient stimulus to change GABA or glutamate concentrations in the vlPAG, likely because this region is not required for sympathoexcitatory reflex responses consequent to visceral afferent stimulation.
Previous neuronal electrophysiological and pharmacological studies from our group (29, 30, 50) suggest that EA increases vlPAG glutamate, since EA at P5-6 increases splanchnic nerve stimulation of vlPAG neuronal activity and because microinjection of DLH into the vlPAG decreases blood pressure and the rVLM neuronal activity. However, in the present study we did not observe an increase in glutamate in the vlPAG during EA. There are several possible explanations for this discrepancy. First, as discussed above, electrophysiological studies involving glutamate suggest that EA activates small populations of neurons in the vlPAG, whereas microdialysis samples reflect changes in glutamate pools from much bigger populations of neurons and glial cells in the vlPAG (4). Second, there also are methodological concerns that may explain this discrepancy. In this regard, we initially thought that the EA might cause small alterations in glutamate (likely an increase) that were undetected by the lower HPLC resolution of glutamate when we simultaneously assayed both glutamate and GABA. However, we were still unable to detect consistent responses even after increasing the sensitivity of the HPLC method to allow detection of smaller changes (threshold of 50 vs. 800 fmol) when the technique was optimized for assessment of glutamate alone. Using the same technique, we have documented significant changes in glutamate concentrations in the rVLM (58, 59), indicating that this method is capable of detecting changes in extracellular glutamate. Also, we observed that variability of glutamate concentration in individual samples was fairly high (see Figs. 2 and 4). However, despite this variability, we found no consistent trend in the change of glutamate concentration using either the low- or high-resolution method. Third, we found that the vlPAG baseline glutamate concentration was ∼100 times higher than the baseline GABA concentration in rats (Figs. 2 and 3). This observation is consistent with previous findings from other laboratories that baseline concentration of glutamate is much higher than that of GABA in the vlPAG and other brain stem region of rats (39). We recognize that it may be more difficult to detect small changes in the very large concentrations of glutamate that are normally present in the vlPAG. Finally, during insertion of the microdialysis probe, there is cellular disruption, which leads to artificially high extracellular concentrations of glutamate (the preliminary data). The levels, however, equilibrated after 90 min, and we did not see a consistent downward trend in glutamate concentrations during control and acupuncture periods, although clearly EA was associated with consistent decreases in GABA. We therefore doubt that mechanically induced elevations in glutamate explain the high initial levels or the inability to resolve significant differences during EA. However, at this time we cannot rule out the possibility that the high baseline concentration and the associated variability of glutamate concentration in the vlPAG dialysate may have masked very small changes in the vlPAG glutamate concentration during EA in rats.
In summary, the present study provides the first evidence to show that EA stimulation at P5-6 acupoints modulates visceral sympathoexcitatory reflexes by decreasing the concentration of GABA in the vlPAG. The EA-mediated decrease in the vlPAG GABA release is reversed by blockade of EC CB1 receptors with AM251. Recently, we observed that blockade of CB1 receptors with AM251 reversed the EA-modulated sympathoexcitatory reflex responses evoked by GD (51). These two studies reinforce the conclusion that EA decreases GABA release, most likely through a presynaptic CB1 receptor mechanism in the vlPAG.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-72125 and the Larry K. Dodge and Susan Samueli Endowed Chairs (J. Longhurst).
Acknowledgments
We gratefully acknowledge the technical assistance of Wei Zhou, Alvin Nguyen, and Rainier Cabatbat. We also thank undergraduate students Willis Yuen, Marielle Reataza, and Sherwin Barvarz for help with experimental procedures.
REFERENCES
- 1.Albin RL, Makowiec RL, Hollingsworth Z, Dure LS, Penney JB, Young AB. Excitatory amino acid binding sites in the periaqueductal gray of the rat. Neurosci Lett 118: 112–115, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431: 312–316, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bago M, Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT1A receptors in the RVLM. Am J Physiol Regul Integr Comp Physiol 280: R976–R984, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Baker D, Xi Z, Shen H, Swanson C, Kalivas P. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22: 9134–9141, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballegaard S, Jensen G, Pedersen F, Nissen VH. Acupuncture in severe, stable angina pectoris: a randomized trial. Acta Med Scand 220: 307–313, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Bao YX, Yu GR, Lu HH, Zhen DS, Cheng BH, Pan CQ. Acupuncture in acute myocardial infarction. Chin Med J (Engl) 95: 824–828, 1982. [PubMed] [Google Scholar]
- 7.Barbaresi P GABA-immunoreactive neurons and terminals in the cat periaqueductal gray matter: a light and electron microscopic study. J Neurocytol 34: 471–487, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Barbaresi P Cellular and subcellular localization of the GABAB receptor 1a/b subunit in the rat periaqueductal gray matter. J Comp Neurol 505: 478–492, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Behbehani MM Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol 46: 575–605, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Bradford HF Glutamate, GABA and epilepsy. Prog Neurobiol 47: 477–511, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal: a correlative functional and anatomical study. Brain Res 541: 206–216, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Chan PK, Chan SC, Yung WH. Presynaptic inhibition of GABAergic inputs to rat substantia nigra pars reticulata neurones by a cannabinoid agonist. Neuroreport 9: 671–675, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Chao DM, Shen LL, Tjen-A-Looi S, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am J Physiol Heart Circ Physiol 276: H2127–H2134, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Devane WA, Hanus L, Breuer A, Pertwee R, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946–1949, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Dodd PR, Watson WE, Morrison MM, Johnston GA, Bird ED, Cowburn RF, Hardy JA. Uptake of gamma-aminobutyric acid and l-glutamic acid by synaptosomes from postmortem human cerebral cortex: multiple sites, sodium dependence and effect of tissue preparation. Brain Res 490: 320–331, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Duggan AW, Morton CR. Periaqueductal grey stimulation: an association between selective inhibition of dorsal horn neurones and changes in peripheral circulation. Pain 15: 237–248, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Fu LW, Zhou W, Guo ZL, Longhurst JC. EA modulates release of GABA but not glutamate in the vlPAG through a presynaptic cannabinoid CB1 receptor mechanism (Abstract). Neuroscience 33: 357.4, 2007. [Google Scholar]
- 18.Guo ZL, Li P, Longhurst J. Central pathways in the pons and midbrain involved in cardiac sympathoexcitatory reflexes in cats. Neuroscience 113: 433–444, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Guo ZL, Moazzami A, Longhurst J. Electroacupuncture induces c-Fos expression in the rostral ventrolateral medulla and periaqueductal gray in cats: relation to opioid containing neurons. Brain Res 1030: 103–115, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hentges S, Low M, Williams J. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci 25: 9746–9751, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho FM, Huang PJ, Lo HM, Lee FK, Chern TH, Chiu TW, Liau CS. Effect of acupuncture at Nei-Kuan on left ventricular function in patients with coronary artery disease. Am J Chin Med 27: 149–156, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature 435: 1108–1112, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Huangfu DH, Li P. The role of nucleus arcuatus in the inhibitory effect of deep peroneal nerve inputs on defence reaction. Chin J Physiol Sci 3: 37–46, 1987. [Google Scholar]
- 24.Isokawa M, Alger BE. Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell. J Physiol 567: 1001–1010, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levenes C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol 510: 867–879, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Pitsillides KF, Rendig SV, Pan HL, Longhurst JC. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 97: 1186–1194, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Rowshan K, Crisostomo M, Tjen-A-Looi SC, Longhurst JC. Effect of electroacupuncture on pressor reflex during gastric distention. Am J Physiol Regul Integr Comp Physiol 283: R1335–R1345, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Tjen-A-Looi S, Longhurst JC. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton Neurosci 89: 38–47, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Tjen-A-Looi SC, Guo ZL, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol 106: 620–630, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Tjen-A-Looi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535–H2542, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Mitchell JH. Glutamate release in midbrain periaqueductal gray by activation of skeletal muscle receptors and arterial baroreceptors. Am J Physiol Heart Circ Physiol 285: H137–H144, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Lovick TA Inhibitory modulation of the cardiovascular defence response by the ventrolateral periaqueductal grey matter in rats. Exp Brain Res 89: 133–139, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Lovick TA, Hilton SM. Vasodilator and vasoconstrictor neurones of the ventrolateral medulla in the cat. Brain Res 331: 353–357, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Maione S, Marabese I, Oliva P, de Novellis V, Stella L, Rossi F, Filippelli A. Periaqueductal gray matter glutamate and GAGA decrease following subcutaneous formalin injection in rat. Neuroreport 10: 1403–1407, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience 92: 1177–1191, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2005.
- 37.Piomelli D The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873–884, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Renno WM Microdialysis of excitatory amino acids in the periaqueductal gray of the rat after unilateral peripheral inflammation. Amino Acids 14: 319–331, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Renno WM, Alkhalaf M, Mousa A, Kanaan RA. A comparative study of excitatory and inhibitory amino acids in three different brainstem nuclei. Neurochem Res 33: 150–159, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Renno WM, Mullett MA, Beitz AJ. Systemic morphine reduces GABA release in the lateral but not the medial portion of the midbrain periaqueductal gray of the rat. Brain Res 594: 221–232, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Richter A, Herlitz J, Hjalmarson A. Effect of acupuncture in patients with angina pectoris. Eur Heart J 12: 175–178, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat caudate putamen nucleus. J Neurosci 21: 823–833, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salio C, Fischer J, Franzoni MF, Conrath M. Pre- and postsynaptic localizations of the CB1 cannabinoid receptor in the dorsal horn of the rat spinal cord. Neuroscience 110: 755–764, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M. CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport 12: 3689–3692, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci 22: 565–572, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Seagard JL, Dean C, Patel S, Rademacher DJ, Hopp FA, Schmeling WT, Hillard CJ. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am J Physiol Heart Circ Physiol 286: H992–H1000, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Silva E, Hernandez L, Contreras Q, Guerrero F, Alba G. Noxious stimulation increases glutamate and arginine in the periaqueductal gray matter in rats: a microdialysis study. Pain 87: 131–135, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Stiller CO, Bergquist J, Beck O, Ekman R, Brodin E. Local administration of morphine decreases the extracellular level of GABA in the periaqueductal gray matter of freely moving rats. Neurosci Lett 209: 165–168, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience 85: 395–403, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Tjen-A-Looi SC, Li P, Longhurst JC. Midbrain vlPAG inhibits rVLM cardiovascular sympathoexcitatory responses during acupuncture. Am J Physiol Heart Circ Physiol 290: H2543–H2553, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Tjen-A-Looi SC, Li P, Longhurst JC. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: roles of endocannabinoids and GABA. J Appl Physiol; doi: 10.1152/japplphysiol.00142.2009. [DOI] [PMC free article] [PubMed]
- 52.Tsou K, Brown S, Sanudo-Pena M, Mackie K, Walker J. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol 57: 288–295, 2000. [PubMed] [Google Scholar]
- 54.Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol 127: 935–940, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker J, Huang S, Tsou K, Strangman N, Sanudo-Pena M. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA 96: 12198–12203, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams FG, Beitz AJ. Ultrastructural morphometric analysis of GABA-immunoreactive terminals in the ventrocaudal periaqueductal grey: analysis of the relationship of GABA terminals and the GABAA receptor to periaqueductal grey-raphe magnus projection neurons. J Neurocytol 19: 686–696, 1990. [DOI] [PubMed] [Google Scholar]
- 57.Zhou W, Fu LW, Tjen-A-Looi SC, Li P, Longhurst JC. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol 98: 872–880, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Zhou W, Fu LW, Guo Z, Longhurst J. Role of glutamate in rostral ventrolateral medulla in acupuncture-related modulation of visceral reflex sympathoexcitation. Am J Physiol Heart Circ Physiol 292: H1868–H1875, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Zhou W, Fu L, Tjen-A-Looi S, Guo Z, Longhurst J. Role of glutamate in visceral sympathoexcitatory reflex in rostral ventrolateral medulla of cats. Am J Physiol Heart Circ Physiol 291: H1309–H1318, 2006. [DOI] [PubMed] [Google Scholar]