Abstract
It is now accepted that dopamine plays an important neuromodulatory role in the central nervous control of respiration. D1, D2, and D4 subtypes of the receptor seem to be important players, but the assignment of various respiratory tasks to specific subtypes of the dopamine receptor is a work in progress. In the present investigation, dihydrexidine (DHD), a full dopamine receptor agonist with affinity for both D1- and D2-subtypes of receptor, was tested for its effects on inspiratory neurons and motor output and on membrane potential properties of medullary bulbospinal expiratory augmenting expiratory neurons in the pentobarbital anesthetized adult cat. The effects of DHD were compared with those of the highly selective D1-dopamine receptor (D1R) agonists SKF-38393 and 6-chloro-APB. DHD increased the intensity and duration of inspiratory motor output. Phrenic nerve discharge intensity was increased and prolonged, contributing to elevated inspiratory effort and duration when spontaneous breathing was monitored with tracheal pressure measurements. Intracellular recording from rostral medullary inspiratory neurons revealed that DHD, like SKF-38393, increases and prolongs inspiratory phase membrane depolarization, resulting in a longer and more intense discharge of action potentials. Remarkably, DHD had opposite effects on Aug-E neurons. Membrane potential was hyperpolarized, and action potential discharges were suppressed or abolished. In association with reduction of discharge intensity, action potential half width was reduced and after-hyperpolarization increased. The stimulatory action of DHD on inspiratory motor output is attributed to D1R effects, while the depression of Aug-E neurons seems to be linked to D2R actions on the postsynaptic membrane.
Keywords: tracheal pressure, bulbar expiratory neurons
after over 30 yr of investigation using a variety of approaches, a strong case can now be made for an important role for dopamine and its receptors in modulating respiratory network activity. First indications for its importance came from fluorescence and immunohistochemical imaging, starting in the late 60s and extending into present times, which demonstrated the presence of dopamine and its converting enzyme, tyrosine hydroxylase, in respiratory-related regions of the brain stem (26, 36, 46). The physiological importance of dopamine is further emphasized in more recent studies by the inability of knockout mice lacking dopaminergic neurons or dopamine transport proteins to breathe properly (33, 48).
Pharmacological studies have been less discriminating and convincing in assessing the vital respiratory-related tasks of dopamine and its receptors. Perhaps this is due to manifold receptor-related respiratory functions of dopamine or to a lack of sufficient selectivity for different subtypes of the receptor by synthetic ligands. Experiments using dopamine receptor ligands with varying degrees of selectivity have provided a rather sketchy, empirical framework with which to pursue further studies. They do, however, suggest significant neuromodulatory roles for D1-, D2-, and D4-dopamine receptors (D1R; D2R; D4R) (8, 9, 14, 18, 19, 21, 24, 25, 31, 34, 44).
D1R agonists received considerable attention in this laboratory after it was previously discovered (3) that one, 6-chloro-APB (APB), reverses opiate depression of the respiratory network in the in vitro neonatal rat preparation. APB and two other D1R agonists, dihydrexidine (DHD) and SKF-38393 (SKF), were subsequently found to be effective antidotes against opiate respiratory depression in the cat in vivo, and to increase CO2-dependent respiratory drive (18, 19, 21).
The present report describes a serendipitous effect of DHD, discovered after its ability, when used as pretreatment to prevent opiate induction of tonic discharges in late-expiratory bulbospinal (Aug-E) neurons, was tested. DHD, a full receptor agonist with D1R/D2R affinity and selectivity of ∼10–12:1 for D1Rs (4, 29) was unexpectedly found to depress Aug-E neuron excitability while stimulating inspiratory motor output. The observation prompted further experiments and resulted in findings that are herein described.
METHODS
Animal preparation and surgical procedures.
Research reported here adheres to The American Physiological Society's Guiding Principles in the Care and Use of Animals, and was approved by the University of Wisconsin Medical School's Institutional Animal Care and Utilization Committee. Results were obtained in experiments performed on 17 pentobarbital-anesthetized adult male cats. The procedures have been previously described in detail (17–20). The animals were initially anesthetized with halothane and then given pentobarbital sodium (40 mg/kg iv) as halothane was gradually withdrawn. During surgery and recording, supplemental intravenous doses (4–8 mg/kg) were administered if symptoms indicating lightening of anesthesia occurred: sudden or gradual increases of arterial pressure, irregular breathing, or discharges of phrenic nerve activity (PNA) that decreased in duration and increased in frequency, shivering, nociceptive withdrawal reflexes. At least 3 h elapsed between the termination of halothane anesthesia and the beginning of recording. Atropine methyl nitrate (0.2 mg/kg ip) was given to minimize airway secretions and dexamethasone sulfate (0.3 mg/kg im) to prevent brain edema. A femoral artery and both femoral veins were cannulated to monitor arterial pressure and administer glucose-Ringer solution and drugs. The Ringer solution was continually infused to maintain tissue hydration. A cannula was inserted into the trachea below the larynx. Blood pressure, tracheal pressure, rate of ventilation, body temperature, end-tidal CO2, and inspired O2 were monitored continuously and recorded on chart paper. End-tidal CO2 was maintained within the range recorded during spontaneous breathing (4.6–5.1 vol, %) by adjusting ventilator rate and stroke volume. Temperature was measured rectally and maintained at 36–38°C by external heating. Animals were mounted in a stereotaxic head holder and suspended by thoracic and lumbar spinal clamps. Phrenic nerves (C4-C5) and cervical vagus nerves were exposed bilaterally through a dorsal approach and prepared for recording or stimulation with bipolar silver hook electrodes. The head of the animal was ventroflexed to allow wide exposure of the dorsal surface of the medulla by occipital craniotomy. Immobilization was produced with gallamine triethiodide (4–8 mg/kg initially followed by 4–8 mg/h), except in five experiments that required measurements of spontaneous breathing. Immobilized animals were ventilated with oxygen-enriched air (65–75 vol, % O2). Pneumothorax was performed bilaterally to increase stability of recording from medullary respiratory neurons and in experiments confined to phrenic nerve recording to achieve lung volumes that were equivalent to those during intracellular recording. Applying 1–2 cmH2O pressure to the expiratory outflow prevented atelectesis. The dura and arachnoid membranes were reflected, and patches of pia membrane were removed to allow insertion of glass microelectrodes. A pressure foot was placed gently on the surface of the medulla over the site of microelectrode insertion. A cervical laminectomy (C2-C4) was performed to insert spinal cord stimulating electrodes. All exposed tissue except the medulla was covered with a mixture of Vaseline and mineral oil.
Recording procedures and measurements.
PNA recorded from cut and desheathed nerve trunks was amplified (×2,000–10,000), band-pass filtered (100–3,000 Hz) and recorded as raw discharges (neurograms) and as moving averages (τ =100 ms) of compound action potential frequency. Effects of D1-dopamine agonists on PNA were recorded in 12 animals; DHD, n = 7 (Table 1); SKF, n = 4; 6-chloro-APB, n = 1. Intracellular recordings from respiratory neurons in the ventral respiratory column (VRC) of the medulla were obtained with fine-tipped pipettes containing 2 M potassium methylsulfate (60–70 MΩ). Membrane potential (MP) was recorded in either bridge or discontinuous constant current mode (switching frequency, 30 KHz) with an intracellular amplifier (SEC-05 LX, npi, 10× gain; band width, DC −10 kHz). Electrophysiological data were displayed on an oscilloscope and stored on magnetic tape (Vettor, frequency response, DC to 5 kHz), chart recorder (DC to 10 kHz) and a data acquisition system (PowerLab S-8; AD Instruments, Apple G4 computer, Gould TA-2000 thermal oscillograph recorder and Wavemetric IGOR PRO software).
Table 1.
Effects of dihydrexidine (DHD) on phrenic nerve activity
| Parameters | Control | DHD | P, t-test* |
|---|---|---|---|
| Cycle frequency, cycles·min−1 | 16.2±1.4 | 14.4±1.4 | 0.007† |
| Peak AP frequency‡ | 983±49.6 | 1162±73.2 | 0.030† |
| Inspiratory phase, s§ | 1.55±0.20 | 1.68±0.19 | 0.050† |
| Postinspiratory phase§ | 0.90±0.15 | 1.20±0.27 | 0.290 |
| Expiratory phase§ | 1.48±0.20 | 1.51±0.20 | 0.560 |
Parameter values are presented as means ± SE; n =7 experiments. DHD doses, 0.8 or 1.0 mg/kg iv.
Significance of differences derived from two-tailed t-tests;
difference is significant;
peak compound action potential (AP) frequency (APs·s−1) at end of augmenting inspiratory phase discharge;
phase durations.
Identification of respiratory bulbospinal neurons.
To identify bulbospinal respiratory neurons, two concentric coaxial electrodes were positioned bilaterally in the cervical reticulospinal tracts at the C3 level. Stimulation with single shocks (5–15 V, 0.1-ms pulse duration) evoked a maximal, short-latency orthodromic volley in phrenic nerve recordings and antidromically conducted action potentials in bulbospinal respiratory neurons, as verified by constant latency and ability to faithfully follow 100 Hz shocks when not firing spontaneously, absence of evoked firing after collision with spontaneous action potentials, and all-or-nothing responses following just threshold stimulation.
Spontaneous breathing measurements.
To assess the effects of D1R agonists on spontaneous breathing, tracheal pressure and end-tidal CO2 were recorded in cats with intact phrenic nerves and not subjected to immobilization. Changes in tracheal pressure were measured by connecting the tracheal cannula to a T-tube, with one side connected to a pressure transducer and the other open to room air through a variable resistor. The resistor clamp was adjusted by first calibrating a pressure transducer (Spectramed; Statham, Costa Mesa, CA) and transducer amplifier (Gould, Cleveland, OH) with a manometer and passing a calibration pulse equivalent to 10 mmHg from the amplifier to the data acquisition system. A 50-cc glass syringe was then connected to the system, and the resistor was adjusted so that injection of 40 cc of air, the average tidal volume of adult anesthetized cats, produced a signal equivalent to 5 mmHg. Measurements were made thereafter in cats with the same side arm outlet resistance and transducer amplifier gain. Control values of ETCO2 were unchanged when the cats breathed spontaneously into the pressure recording system.
Intravenous administration of drugs.
Drugs and chemicals were dissolved in glucose-Ringer's solution and injected slowly over durations of 30–60 s to minimize changes of blood pressure. As in previous tests of D1R ligands (18), tests for volume effects were made with bolus injections of the vehicle without test drug. The control tests did not reveal changes in PNA or MP of VRC neurons. Drug administration was always preceded by at least 5 min of stable recording just before intravenous injection that served as the control period. Drugs tested were D1R agonists 6-chloro-APB, SKF, and DHD purchased from Sigma-Aldrich, St. Louis, MO. Solution concentrations were 3–6 mg/ml. Doses were based on dose-response relationships established in a previous study from this laboratory (18). D1R selectivity was confirmed (18) by showing that the drug effects were blocked by the selective D1R antagonist SCH-23390.
Euthanasia.
Experiments were terminated by intravenous injection of pentobarbital sodium in sufficient quantity to produce irreversible cardiac arrest.
Statistical analysis.
Pooled measurements of control and D1R agonist responses were tested for significance of difference by paired Student's t-tests using SigmaPlot version 4.11 software (Jandel Scientific). Differences were accepted as significant if P < 0.05. SigmaPlot was also used to derive means and standard errors.
RESULTS
DHD increases inspiratory neuron motor output to the diaphragm.
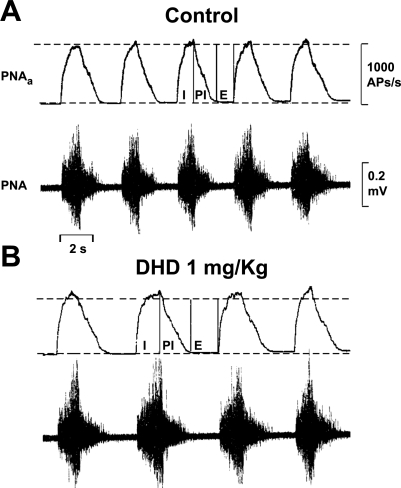
Estimates of the effects of DHD on inspiratory motor output to the diaphragm were revealed in phrenic nerve electroneurograms (raw PNA), as well in moving averages of PNA (PNAa) that more clearly show time-related changes in patterns of activity. An example of DHD effects on the intensity and time course of PNA in one animal is illustrated in Fig. 1. In this experiment, peak action potential frequency (estimated from consecutive PNAa measurements over 1-min intervals) was increased by about 10%, and cycle frequency was slowed by 20%. Slowing was due to lengthening of inspiratory and postinspiratory discharges, and to prolongation of the late-expiratory silent periods.
Fig. 1.
Effects of dihydrexidine (DHD) given intravenously on phrenic nerve activity (PNA). A and B: electroneurograms of PNA (lower traces) and the moving average of compound action potential (AP) frequency (PNAa, top trace). I, PI, and E are inspiratory, postinspiratory, and expiratory, respectively, phases of the PNA cycle.
Measurements made in seven animals, including those illustrated in Fig. 1, are summarized in Table 1. They reveal a significant increase in peak inspiratory phase discharge intensity, on average by 11.9%. Cycle frequency was slowed significantly, by 12.2%, due to lengthening of the inspiratory phase discharge. The postinspiratory discharge and late expiratory silent period were not greatly affected in most experiments. The DHD effects on PNA were similar (18) to those of the highly selective D1R agonists 6-chloro-APB and SKF (32).
As reported in a previous study from this laboratory (18), DHD also decreased mean arterial pressure. The fall in blood pressure (12 ± 3.8 mmHg) occurred without a change in heart rate and lasted for 18 ± 6.3 min.
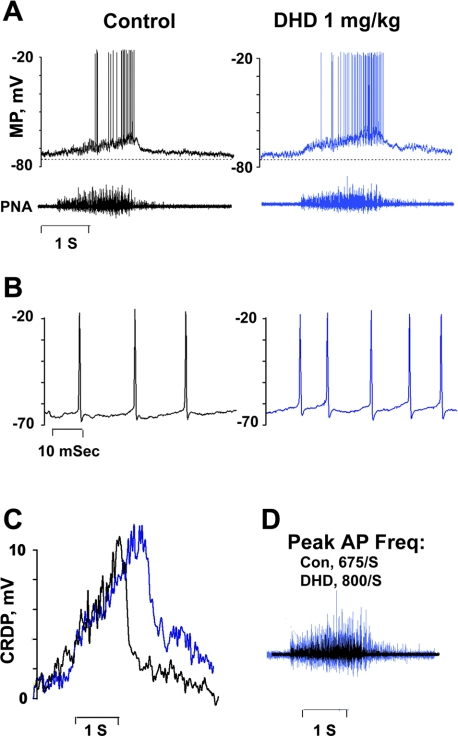
DHD produced changes in discharge intensity and duration in a nonantidromically activated inspiratory neuron of the VRC that are consistent with its effects on PNA, as seen in Fig. 2. Inspiratory phase discharge in the neuron was prolonged, and peak intensity exceeded control values (Fig. 2, A–D). The effects are evidently linked to elevated excitatory synaptic drive because the amplitude and duration of the central respiratory drive potentials (CRDP) (Fig. 2C), a summed wave of excitatory postsynaptic potentials (EPSPs) (41) that shapes respiratory neuron discharge properties via glutamatergic transmission (37), increased. DHD did not, however, alter action potential amplitude, duration, or after-hyperpolarization (Fig. 2B). Overall, the changes in MP, discharge properties, and PNA produced by DHD in Fig. 3 are similar to those evoked by intravenous SKF in bulbospinal inspiratory neurons tested in this study (n = 2 cells; not illustrated) and in a previous investigation (n = 3 cells) (18). Similar stimulatory effects were evoked in this investigation in a bulbospinal inspiratory neuron and in PNA by topical application of SKF into a well positioned on the dorsal surface of the medulla rostral to the obex (not illustrated).
Fig. 2.
Effects of DHD given intravenously on a nonantidromically activated inspiratory neuron in the ventral medullary respiratory column (VRC). A: records of membrane potential (MP) and PNA electroneurograms before (left) and 2 min after injection of DHD (right). B: AP discharges presented on an expanded time scale, showing responses before (black, left) and after (blue, right) DHD. Discharges occurred at peak MP depolarization. C: central respiratory drive potentials (CRDP) recorded before (black) and after (blue) administration of DHD. CRDPs were extracted from the records in A by binomial smoothing: 30,000 iterations in each trace. D: superimposed PNA traces before (black) and after (blue) DHD. The blue trace is enlarged to reveal differences in temporal patterns of discharge. Con, control.
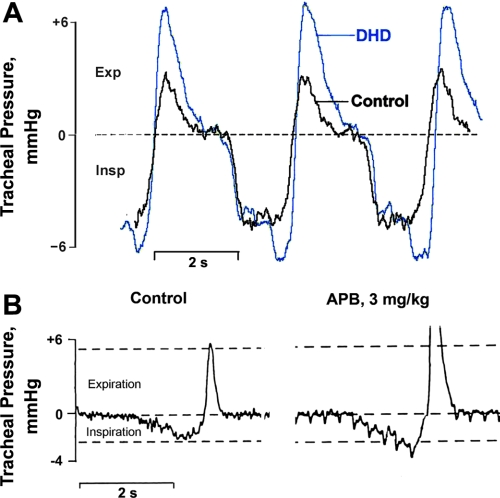
Fig. 3.
Effects of DHD (A) and 6-chloro-APB (B) on tracheal pressure during spontaneous breathing in 2 cats. Negative pressure during inspiration (Insp) is downward, positive pressure during expiration (Exp) is upward. In B, right, the relatively rapid, uniformly spaced downward deflections are associated with increased arterial pulse pressure pulsations produced by 6-chloro-APB.
Augmented inspiratory phase discharge activity was also reflected in changes of spontaneous breathing when tracheal pressure measurements were made in five animals, including two whose responses are shown in Fig. 3. Inspiration was prolonged and greater at its peak in all animals given DHD (Fig. 3A), and in one experiment by 6-chloro-APB that was administered 1 h after recovery from DHD (Fig. 3B). Expiratory pressure was also elevated and prolonged by either agonist, which was most likely the consequence of increased inspiratory effort and lung recoil, since there was no visual evidence of active expiratory effort. In addition, intracellular measurements, described next, point to quite the opposite effect of DHD on expiratory motor output.
DHD depresses medullary expiratory neurons.
Bulbospinal Aug-E neurons of the caudal VRC provide excitatory synaptic drive to expiratory neurons that regulate the tone of the chest wall and abdominal expiratory muscles (37). In tests made on four neurons, DHD hyperpolarized MP and suppressed discharge activity. In each neuron, DHD evoked a hyperpolarizing shift of MP that affected all phases of the cycle and depressed action potential discharge either partially or completely. Figure 4 is representative of the changes that occurred in three neurons when action potential frequency was reduced by DHD. The reduced firing frequency is related to a combination of MP hyperpolarization and increased fast and slow action potential after-hyperpolarization (Fig. 4, C and G). Additional effects produced by DHD were reduced action potential duration (half width), increased fast and slow after-hyperpolarization, and increased spike amplitude (4, B, E, and G). CRDP amplitude and duration were essentially unchanged (Fig. 4F), while the duration of the inspiratory wave of MP hyperpolarization was lengthened in parallel with inspiratory motor output, as seen in PNA.
Fig. 4.
Effects of DHD given intravenously on a bulbospinal Aug-E neuron in the caudal region of the VRC. Labeling of traces, same as in Fig. 2. Records in B are superimposed APs recorded before (black) and after (blue) DHD. Note shortened spike duration and increased after-hyperpolarization produced by DHD (also evident in E and G). F: CRDPS before (black) and after (blue) DHD. Record of PNA taken after DHD in B is enlarged.
Figure 5 demonstrates effects that accompanied abolition of discharges in one of the four Aug-E neurons. DHD produced a steady hyperpolarizing shift of MP in the neuron that kept the CRDP from reaching discharge threshold. These depressant effects were brought about without changes in PNA activity and without effects on inspiratory phase waves of synaptic inhibition in the Aug-E neuron, indicating that depression of expiratory neuron excitability was affected without effects on network inspiratory properties.
Fig. 5.
DHD given intravenously evokes membrane hyperpolarization and arrest of discharge in a bulbospinal Aug-E neuron of the caudal VRC without changing network inspiratory activity. Labeling of traces, same as in Fig. 2. Blue shading highlights the magnitude and duration of inspiratory phase waves of membrane hyperpolarization, which are unchanged after giving DHD. PNA is also unchanged.
DISCUSSION
The present study has revealed a rather startling and unexpected dichotomy in the effects of DHD on inspiratory and expiratory components of the respiratory motor output. Such partitioning of receptor subtypes and their associated effects could not be anticipated because no receptor labeling studies have been reported nor investigations that otherwise identify relative expression of the receptor subtypes in the brain stem respiratory network. The conclusions expressed here therefore hinge on what is known about the receptor and effector properties of DHD in other neurons and other types of excitable cells.
DHD is a full dopamine receptor agonist that binds to both D1R and D2R with greater selectivity for the D1-subtype. These properties provide an explanation for the different effects on the respiratory neural network that is based on relative D1R and D2R affinities and their associated intracellular transduction mechanisms.
Stimulation of the inspiratory motor output and slowing of cycle frequency are linked to D1R activation.
D1R agonists depolarize MP and increase discharge intensity in central nervous system (CNS) neurons by stimulating the adenylyl cyclase-cAMP transduction pathway, or by acting on protein kinases that regulate ion channel conductances independent of AC-cAMP (15). Ultimately, K-channel conductance is decreased and conductances of Ca-channels, Na-channels, and nonspecific cation-channels are increased (2, 11, 32, 50).
In the earliest reports of binding affinity and in vitro pharmacology, DHD was described as a full dopamine receptor agonist with a D1:D2 receptor selectivity of ∼70:1 and very low, if any, affinity for other dopamine receptor subtypes or other types of receptor (23). This meant a high probability of relating effects to D1R activation and to functional selectivity mediated by adenylyl cyclase and cyclic AMP upregulation (29). Since then, the D1:D2 selectivity has been downgraded to ∼10–12:1 (4, 15, 29, 30, 40), and weak D3R (49) as well as α2 (28) receptor agonist affinity have been reported.
The present study and an earlier one from this laboratory indicate on several counts that the stimulatory effects of DHD on the inspiratory motor output are associated with D1R-agonist effects: 1) DHD produces effects on inspiratory neurons and PNA (Figs. 1 and 2) that are similar to those of 6-chloro-APB and SKF (18), both highly selective D1R agonists (32); 2) inspiration during spontaneous breathing is stimulated and prolonged in identical fashion by DHD and 6-chloro-APB (Fig. 3); and 3) DHD, SKF, and 6-chloro-APB consistently reverse opiate depression of the respiratory network, but SCH-23390, a highly selective D1R blocker (30), prevents reversal (18).
The sites and mechanisms of D1R action that result in stimulation of inspiratory motor output are not yet known. Dopaminergic axonal projections, recognized by the presence of tyrosine hydroxylase, have been found in the vicinities of medullary respiratory neurons (46), but the subtypes of dopamine receptor they engage have not yet been identified. Previous results from this laboratory implicate a brain stem site of action (18). The increase in inspiratory bulbospinal neuron and PNA excitability evoked in this study by topical application of SKF to the medulla in the approximate region of underlying VRC inspiratory neurons further supports the likelihood. However, additional spinal cord actions cannot be excluded. It seems unlikely that carotid body chemoreceptor stimulation plays a significant role because, as in an earlier investigation (18), the cats were ventilated with oxygen-enriched air (∼60%) during phrenic nerve recording, and CO2 was maintained at levels that, for the cat, are eucapnic, so constitiutive activation of carotid body chemoreceptors should have been minimal. In addition, while blockade of D1Rs with SCH-23390 prevents the stimulatory effects of DHD, SKF, and 6-chloro-APB on PNA, it fails to block stimulation of phrenic nerve discharges by the carotid body chemoreceptor stimulant doxapram (18).
The DHD and SKF effects on MP and discharge properties of VRC inspiratory neurons, although not yet linked to specific cellular sites, do appear to be initiated by afferent neurons that increase drive on bulbospinal inspiratory neurons. Excitatory synaptic drive is elevated (Fig. 2), as illustrated by increases in amplitude and duration of the CRDP (Fig. 2C), a summed wave of EPSPs (41) that shapes respiratory neuron discharge properties via glutamatergic transmission (37). On the other hand, there is no evidence for postsynaptic mechanisms of action. DHD did not alter action potential amplitude, duration, or after-hyperpolarization (Fig. 2B). Changes in these action potential properties would be expected if there were significant postsynaptic actions, because DHD and other D1R agonists decrease K-channel conductance and increase several depolarizing ion channel conductances that affect spike properties and after-hyperpolarization (10, 11, 50). Further evidence against D1R-dependent postsynaptic effects is that microiontophoresis of SKF does not alter discharge properties of bulbospinal inspiratory neurons (18).
Although the DHD-related effects on inspiratory activity in this study reflect a preeminent, D1R-based influence, it cannot be concluded that D2R activation is altogether without effect on inspiratory drive. In fact, during acute episodes of hypercapnea or hypoxia, D2R (−/−) knockout mice show a greater increase in tidal volume, reflecting an increase of inspiratory motor output, than their wild-type counterparts (14). Dopamine-D2Rs in the central respiratory network may thus modulate acute hypoxic and hypercapneic responses. In the respiratory network, as in other CNS regions involved in motor control, functional dominance by one or another dopamine receptor subtype may depend on many factors, including locations of dopamine release sites and receptors, and the efficacy of coupling between the receptor, the intracellular transduction machinery, and membrane ion channels (28). Whether MP is depolarized or hyperpolarized when D1R agonists are applied can also affect the response (10).
Prolongation of discharge duration, a second common effect of D1R agonists, occurred in all VRC inspiratory neurons so far tested, and probably accounts for the same effects observed in PNA, because phrenic nerve motoneurons receive excitatory synaptic drive from them (37). The neurons responsible and the mechanisms affected have not been identified. According to the three-phase network hypothesis, tentative target neurons include inspiratory off-switching neurons (37). The slowing of respiratory cycle frequency activity seen in this study and reported previously by this laboratory (18) is primarily the consequence of inspiratory phase discharge prolongation. Along with increased discharge intensity, a longer inspiratory phase would tend to increase the pressure-generating capacity of the respiratory muscles (51).
In summary, it appears that DHD stimulates inspiratory motor output by activating D1Rs that affect MP depolarization, increased excitatory synaptic drive, and prolongation of discharge in bulbospinal inspiratory neurons. Various types of network neurons, other than bulbospinal inspiratory neurons, as well as brain stem chemoreceptor neurons are likely cellular targets.
DHD depression of excitability in medullary bulbospinal Aug-E neurons: indirect or direct?
In three of four bulbospinal Aug-E neurons tested, depression of excitability evoked by DHD was characterized by a steady hyperpolarizing shift of MP that reduced action potential discharge frequency. Action potential duration (half-width) was decreased and after-hyperpolarization increased in magnitude and duration, indicating that DHD depressed excitability postsynaptically. On the other hand, upstream network drive on Aug-E neurons was not suppressed; at least at it was expressed in the CRDPS (Figs. 4F, and 5). Based on what is known concerning postsynaptic inhibition linked to activation of D2Rs (5, 28), it seems to be linked to suppressed adenylyl cyclase and reduced cAMP production (40). Similar transduction mechanisms affect excitability in medullary Aug-E neurons of the cat, because intracellular injection of cAMP-PKA inhibitors evokes MP hyperpolarization and effects on action potential properties (38) that are comparable to those of DHD in this study. D2R agonists have many postsynaptic conductance effects that decrease neuron excitability. They depress α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid current (11), high voltage-activated Ca current (12, 43), and Na current (27), and they increase K current (7, 35).
Effects of DHD summarized in Fig. 5 also favor a primary postsynaptic D2R effect. MP hyperpolarization and discharge arrest occurred without change of PNA and without effects on inspiratory phase waves of MP hyperpolarization. Inspiratory phase-dependent mechanisms, such as reciprocal inhibition of Aug-E neurons by inspiratory neurons (37, 42), can therefore be excluded, even if dopamine receptor activation recruits inhibitory neurons elsewhere in the CNS (47).
Physiological and clinical implications.
Several laboratories have attempted to define the physiological role of dopamine in the control of breathing. They suggest that in the absence of oxygen deprivation, dopaminergic modulation within the brain stem respiratory network reinforces CO2-dependent respiratory drive and increases minute ventilation, mainly by increasing tidal volume, and it seems likely that the D1-subtype of receptor is a major player (18, 19, 21, 24, 25, 31, 34).
The effects of DHD in the present study point to a two-component dopaminergic control mechanism, in which expiratory neurons are depressed via D2Rs, even as inspiratory neurons are stimulated via D1Rs. The implications are exciting, namely, that predominance of dopamine receptor subtype, and therefore function, are strikingly different between inspiratory and expiratory motor outflows.
Physiological circumstances that necessitate this type of partitioning are unknown. One possibility is that homeostatic adjustments in functional residual capacity (FRC) and ventilation-to-perfusion ratio (VPR) are made by dopamine released at D1Rs and D2Rs in response to increased inspiratory effort. Lung inflation activates vagal pulmonary afferents (1) that promote dopamine release in the brain stem (13). An increase in lung volume also results in vagally mediated inhibition of expiratory muscle activity, which can increase FRC (39) and VPR (45). Thus when metabolic demand and CO2 production increase inspiratory effort, suppression of expiratory motor output might improve chest wall compliance with favorable consequences on FRC and VPR.
Clinically, analogs of DHD (30) currently under investigation for human use (40) might be useful against opiate disturbances of ventilation. During surgery and postoperatively, opiates like fentanyl can, even in small doses, produce chest wall and abdominal muscle rigidity that results in reduced FRC and respiratory distress (6, 16). These side effects are evidently caused by tonic discharge of bulbospinal Aug-E neurons (18, 22), and DHD prevents opiate-induced tonic discharges (19).
Study limitations and suggestions for future research.
Depression of excitability in Aug-E neurons by D2Rs seems likely based on results presented here. The importance of D2Rs can only be suggested, however, because the only D2R ligand tested was DHD. In addition, the effects of DHD on motor output to expiratory muscles were not measured by nerve recording or electromyography. Additional studies are needed to compare the effects of DHD with more selective D2R ligands, such as the agonist quinpirole and the antagonist sulpiride. They need to be tested for effects on Aug-E neuron MP and discharge properties, on the expiratory motor output and on spontaneous breathing.
Perspectives and Significance
With the continuing development and refinement of receptor-labeling and imaging methods as well as improvements in receptor ligand selectivity, we are now in a far better position than in earlier years to effectively investigate the role of dopamine and its receptors in respiratory network modulation. The details at present are rather murky and in great need of further clarification, but it appears that several types of dopamine receptors play significant, distinctly different roles in dopaminergic modulation of respiration under physiological conditions. The present study suggests that dopaminergic modulation through D1Rs and D2Rs in the VRC achieves ventilatory coherence and effectiveness by contrasting effects on inspiratory and expiratory motor output neurons. Other pharmacological studies with dopamine receptor agonists and antagonists and those with knockout mice further persuade us that dopaminergic modulation is important for efficient ventilation. But the analysis of dopaminergic modulation in the CNS respiratory network is still in its embryonic stage. Experiments are needed to determine whether all of the five major subtypes of dopamine receptor are contributory, where in the network they are functional, the network synaptic mechanisms through which they operate, and what their relative importance and specific functional contributions to effective ventilation are.
GRANTS
The research for this study was supported by National Heart, Lung, and Blood Institute Grant HL-65526.
Acknowledgments
The author thanks Anne M. Bischoff, Centre for Physiology, University of Göttingen, Germany for valuable comments and criticisms concerning the manuscript figures.
REFERENCES
- 1.Adrian ED Afferent impulses in the vagus and their effect on respiration. J Physiol 79: 332–358, 1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aosaki T, Kiuchi K, Kawaguchi Y. Dopamine D1-Like receptor activation excites rat striatal large aspiny neurons in vitro. J Neurosci 18: 5180–5190, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballanyi K, Lalley PM, Hoch B, Richter DW. cAMP-dependent reversal of opioid- and prostaglandin-mediated depression of the isolated respiratory network in newborn rats. J Physiol 504: 127–134, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewster WK, Nichols DE, Riggs RM, Mottola DM, Lovenberg TW, Lewis MH, Mailman RB. Trans-10,11-dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo [a] phenanthridine: a highly potent selective dopamine D1 full agonist. J Med Chem 33: 1756–1764, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Castro SW, Strange PG. Differences in the ligand binding properties of the short and long versions of the D2 dopamine receptor. J Neurochem 60: 372–375, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Chawla G, Drummond GB. Fentanyl decreases end-expiratory lung volume in patients anaesthetized with sevoflurane. Br J Anaesth 100: 411–414, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Freedman JE, Weight FF. Single K+ channels activated by D2 dopamine receptors in acutely dissociated neurons from rat corpus striatum. Proc Natl Acad Sci USA 85: 3618–3622, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii M, Umezawa K, Arata A. Dopaminergic modulation on respiratory rhythm in rat brainstem-spinal cord preparation. Neurosci Res 50: 355–359, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Fujii M, Umezawa K, Arata A. Dopamine desynchronizes the pace-making neuronal activity of rat respiratory rhythm generation. Eur J Neurosci 23: 1013–1027, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an l-type Ca2+ conductance. J Neurosci 17: 3334–3342, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Echeagaray E, Starling AJ, Cepeda C, Levine MS. Modulation of AMPA currents by D2 dopamine receptors in striatal medium-sized spiny neurons: are dendrites necessary? Eur J Neurosci 19: 2455–2463, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce l-type Ca21 currents and excitability via a novel PLCb1-IP3-calcineurin-signaling cascade. J Neurosci 20: 8987–8995, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtzheimer PE, III, Nemeroff CB. Advances in the treatment of depression. NeuroRX, J Am Soc Exper NeuroTher 3: 42–56, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huey KA, Szewczak JM, Powell FL. Dopaminergic mechanisms of neural plasticity in respiratory control: transgenic approaches. Respir Physiol Neurobiol 135: 133–44, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Kilts JD, Connery HSHSH, Arrington EG, Lewis MM, Lawler CP, Oxford GS, O'Malley KL, Todd RD, Blake L, Nicholls DE, Mailman RB. Functional selectivity of dopamine receptor agonists. II. actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J Pharmacol Exper Ther 301: 1179–1189, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Knill R, Cosgrove JF, Olley PM, Levison H. Components of respiratory depression after narcotic premedication in adolescents. Can Anaesth Soc J 23: 449–458, 1976. [DOI] [PubMed] [Google Scholar]
- 17.Lalley PM μ-Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 285: R1287–R1304, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Lalley PM D1-dopamine receptor agonists reverse respiratory network depression by opioids and increase reactivity to CO2. Resp Physiol Neurobiol 139: 247–262, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Lalley PM D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat. Am J Physiol Regul Integr Comp Physiol 289: R45–R51, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lalley PM D1-Dopamine receptor blockade slows respiratory rhythm and enhances opioid-mediated depression. Resp Physiol Neurobiol 145: 13–22, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lalley PM Opioidergic and dopaminergic modulation of respiration. Resp Physiol Neurobiol 164: 160–167, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laubie MJ, Drouillet M, Schmitt H. Discharge patterns of bulbar respiratory neurons in response to the morphinometric agent, fentanyl, in chloralosed dogs. Eur J Pharmacol 122: 301–309, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Lovenberg TW, Brewster WK, Mottola DM, Lee RC, Riggs RM, Nichols DE, Lewis MH, Mailman RB. Dihydrexidine, a novel selective high potency full dopamine d-1 receptor agonist. Eur J Pharmacol 166: 111–113, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg D, Breese GR, Mueller RA. Dopaminergic interaction with the respiratory control system in the rat. Eur J Pharmacol 54: 153–159, 1979. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg D, Mueller RA, Breese GR. Effects of vagotomy and glossopharyngectomy on respiratory response to dopamine-agonists. Acta Physiol Scand 114: 81–89, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Maley BE Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem Senses 21: 367–376, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Maurice N, Mercer J, Savio Chan C, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. Cellular/molecular D2 dopamine receptor-mediated modulation of voltage-dependent Na channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci 24: 10289–10301, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, Lawler CP, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. I. selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exper Ther 301: 1166–1178, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Mottola DM, Brewster WK, Cook LL, Nichols DE, Mailman RB. Dihydrexidine, a novel full efficacy D1 dopamine receptor agonist. J Phramacol Exper Ther 262: 383–393, 1992. [PubMed] [Google Scholar]
- 30.Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol 474: 137–140, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen AM, Bisgard GE. Dopaminergic modulation of respiratory timing mechanisms in carotid body-denervated dogs. Respir Physiol 53: 71–86, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Nisenbaum ES, Mermelstein PG, Wilson CJ, Surmeier DJ. Selective blockade of a slowly inactivating potassium current in striatal neurons by (+/−) 6-chloro-APB hydrobromide (SKF82958). Synapse 29: 213–224, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Nsegbe E, Wallén-Mackenzie A, Dauger S, Roux JC, Shvarev Y, Lagercrantz H, Perlmann T, Herlenius E. Congenital hypoventilation and impaired hypoxic response in Nurr1 mutant mice. J Physiol 556: 43–59, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson LG, Saunders NA. The effect of central and peripheral dopamine-agonists on ventilation in the mouse. Respir Physiol 61: 335–345, 1985. [DOI] [PubMed] [Google Scholar]
- 35.Perez MF, White FJ, Hu XT. Dopamine D2 receptor modulation of K channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J Neurophysiol 96: 2217–2228, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Rea MA, Aprison MH, Felten DL. Catecholamines and serotonin in the caudal medulla of the rat: combined neurochemical-histofluorescence study. Brain Res Bull 9: 227–236 1982. [DOI] [PubMed] [Google Scholar]
- 37.Richter DW Neural regulation of respiration: rhythmogenesis, and afferent control. In: Comprehensive Human Physiology, edited by Gregor R and Windhorst U. Heidelberg, Germany: Springer-Verlag, 1996, vol. 2, chapt. 105, p. 2079–2095. [Google Scholar]
- 38.Richter DW, Lalley PM, Pierrefiche O, Haji A, Bischoff AM, Wilken B, Hanefeld F. intracellular signal pathways controlling respiratory neurons. Respir Physiol 110: 113–23, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Romaniuk JR, Dick TE, Kowalski KE, DiMarco AF. Effects of pulse lung inflation on chest wall expiratory motor activity. J Appl Physiol 102: 485–491, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Salmi P, Isacson R, Kull, B. Dihydrexidine–the first full dopamine D1 receptor agonist. CNS Drug Rev 10: 230–242, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saywell SA, Anissimova NP, Ford TW, Meehan CF, Kirkwood PA. The respiratory drive to thoracic motoneurones in the cat and its relation to the connections from expiratory bulbospinal neurons. J Physiol 579: 765–782, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid K, Foutz AS, Denavit-Saubié M. Inhibitions mediated by glycine and GABA-A receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res 710: 150–160, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Seabrook GR, Knowles M, Brown N, Myers J, Sinclair H, Patel S, Freedman SB, McAllister G. Pharmacology of high-threshold calcium currents in GH4C1 pituitary cells and their regulation by activation of human D2 and D4 dopamine receptors. Br J Pharmacol 112: 728–34, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan M, Yamamoto Y, Brodin E, Persson H. Treatment with SCH-23390, a selective dopamine D1 receptor blocker decreases preprotachykinin-A mRNA levels in nucleus tractus solitarii of the rabbit: role in respiratory control. Brain Res Mol Brain Res 3: 233–238, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Stokke DB Artificial ventilation with positive end-expiratory pressure (PEEP). Historical background, terminology and patho-physiology. Eur J Intens Care Med 2: 77–85, 1976. [DOI] [PubMed] [Google Scholar]
- 46.Sun QJ, Pilowsky P, Minson J, Arnolda L, Chalmers J, Llewelyn-Smith IJ. Close appositions between tyrosine hydroxylase immunoreactive boutons and respiratory neurons in the rat ventrolateral medulla. J Comp Neurol 340: 1–10, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Tseng KY, O'Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse 61: 843–850, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vincent SG, Waddell AE, Caron MG, Walker JKL, Fisher JT. A murine model of hyperdopaminergic state displays altered respiratory control. FASEB J 21: 1463–1471, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Watts VJ, Wiens BL, Cumbay MG, Vu MN, Neve RL, Neve KA. Selective activation of Gao by D2L dopamine receptors in NS20Y neuroblastoma cells. J Neurosci 18: 8692–8699, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witkowski G, Szulczyk B, Rola R, Szulczyk P. D1 dopaminergic control of G protein-dependent inward rectifier K+ (GIRK)-like channel current in pyramidal neurons of the medial prefrontal cortex. Neuroscience 155: 53–63, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Younes M, Riddell W. A model for the relation between respiratory neural and mechanical outputs. I. Theory. J Appl Physiol 51: 963–978, 1981. [DOI] [PubMed] [Google Scholar]