Abstract
32-amino acid B-type natriuretic peptide (BNP 1-32) plays an important role in cardiovascular homeostasis. Recently, it was reported that BNP 1-32 is cleaved by the metalloprotease meprin A to BNP 8-32, the bioactivity of which is undefined. We hypothesized that BNP 8-32 has reduced vasodilating and natriuretic bioactivity compared with BNP 1-32 in vivo. Human BNP 8-32 and BNP 1-32 were compared in a crossover study in eight anesthetized normal canines. After a preinfusion clearance, BNP 1-32 was infused at 30 ng·kg−1·min−1 for 45 min followed by a 60-min washout and a second preinfusion clearance. Then, equimolar BNP 8-32 was infused. In half of the studies, the peptide sequence was reversed. Changes with peptides from the respective preinfusion clearance to infusion clearance were compared with paired tests. Mean arterial pressure was reduced by both BNP 8-32 and BNP 1-32 (−8 ± 3 vs. −6 ± 2 mmHg, P = 0.48). Changes in right atrial pressure, pulmonary capillary wedge pressure, heart rate, cardiac output, and glomerular filtration rate were similar. However, urinary sodium excretion increased less with BNP 8-32 than with BNP 1-32 (+171 ± 24 vs. +433 ± 43 μEq/min; P = 0.008), as did urinary potassium excretion, urine flow, and renal blood flow. While BNP 8-32 has similar vasodilating actions as BNP 1-32, its diuretic and natriuretic actions are reduced, suggesting a role for meprin A in the regulation of BNP 1-32 bioactivity in the kidney. Meprin A inhibition may be a potential strategy to increase the bioactivity of endogenous and exogenous BNP 1-32 in cardiovascular diseases.
Keywords: hormone, peptidase, cardiorenal regulation, natriuretic peptides
the cardiac hormone b-type natriuretic peptide (BNP), together with atrial NP and C-type NP is a member of the natriuretic peptide family, and plays an important role in cardiovascular and renal homeostasis (17, 24, 25). It is cleaved from an inactive precursor, 108-amino acid proBNP, to the biologically active 32-amino acid BNP 1-32 (30). Its pleiotropic actions, which include vasodilation, natriuresis, and inhibition of the renin-angiotensin-aldosterone system (RAAS), are mediated by the second messenger cGMP, which is produced when BNP 1-32 binds to the natriuretic peptide A receptor (NPR-A). BNP 1-32 is cleared from the circulation by binding to the natriuretic peptide clearance receptor, glomerular filtration, and enzymatic degradation.
About 2% of the human genome is estimated to code for peptidases; however, because of their high specificity, only a few are usually involved in the degradation of a specific peptide. Of note, enzymatic processing of a peptide hormone does not necessarily only have to be a matter of degradation, but it can also modify the profile of action of a hormone. Indeed, processing can even result in peptides with qualitatively quite different actions; for example, ANG III, which is cleaved from the vasoconstrictor ANG II by ACE II is a vasodilator, a cleavage product of the vasodilator adrenomedullin is a vasoconstrictor, and the dipeptidyl peptidase IV (DPP4) processed form of glucagon-like peptide 1 has cardiovascular actions that its precursor lacks (2, 9, 11, 20). With regard to BNP, its profile of action could be differentially affected by receptor density and peptidase activity in its target tissues.
Our knowledge regarding the processing of BNP 1-32 is limited, but neprilysin (EC 3.4.24.11, also called neutral endopeptidase 24.11, peptidase M13.001), dipeptidyl peptidase IV (DPP4, EC 3.4.14.5; S09.003), and most recently meprin A (EC 3.4.14.; M12.002) have been implicated (4, 23). Brandt et al. (4) reported that in EDTA blood BNP 1-32 was cleaved by DPP4 to BNP 3-32. They also reported that neither BNP 1-32 nor BNP 3-32 were substrates of neprilysin. Lam et al. (16) reported that BNP 3-32 is present in human plasma and is increased in patients with heart failure (HF). Indeed, it has been speculated that BNP 1-32 is actually low in human heart failure plasma and that may in part be due to reduced processing of proBNP 1-108 but also to accelerated degradation of BNP 1-32 to smaller molecular weight forms of BNP (12, 22).
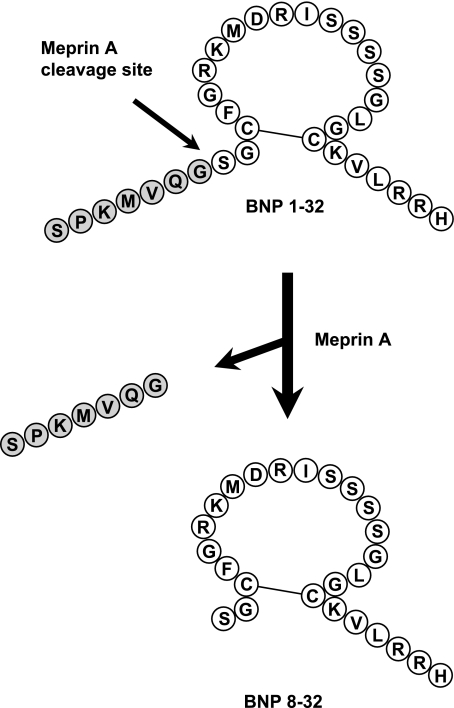
Importantly, we have recently reported that BNP 3-32 has reduced natriuretic and diuretic properties including a lack of vasodilating actions compared with BNP 1-32 in healthy canines (3). This is consistent with the concept that forms of BNP 1-32, which occur in response to further processing by proteases like DPP-IV, result in BNP forms with reduced biological activity. Most recently, Pankow et al. (23), using murine kidney brush border membranes, reported that murine BNP 1-32 is cleaved by the metalloprotease meprin A (EC 3.4.14.; M12.002) to BNP 7-32, while Walther (29) reported that human BNP 1-32 is cleaved by murine meprin A to BNP 8-32 (Fig. 1). Both murine BNP 7-32 and human BNP 8-32 were substrates of neprilysin. To date, the bioactivity of human BNP 8-32 in cardiorenal regulation compared with mature biologically active BNP 1-32 is unknown.
Fig. 1.
Schematic showing cleavage of BNP 1-32 to BNP 8-32 by the metalloprotease meprin A. BNP, B-type natriuretic peptide.
Underscoring the importance of the these previous reports and the current study is the increasing evidence that there is a heterogeneity of forms of BNP present in the blood of patients with HF, which may be detected by widely used BNP assays, but which may have reduced biological activity (14, 16, 22, 26). In the current study, we used synthetic BNP 8-32 to evaluate for the first time the integrative renal, cardiovascular, and endocrine properties of this lower molecular weight form of BNP 1-32. We hypothesized that BNP 8-32 has reduced vasodilating and natriuretic bioactivity compared with BNP 1-32 in vivo. We also assessed the direct action of BNP 1-32 and BNP 8-32 on cGMP-generation in human aortic endothelial cells (HAEC) in vitro.
MATERIALS AND METHODS
The current study was performed in eight male mongrel dogs (weight 20–28 kg) in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Animal Care and Use Committee.
We investigated the cardiorenal actions of intravenous synthetic human BNP 8-32 compared with equimolar human BNP 1-32 in eight anesthetized normal canines using the same crossover design recently used to characterize BNP 3-32 (3). Dogs were fed a sodium controlled diet (Hill's I/d diet, Hill's Pet Nutrition, Topeka, KS). On the evening before the acute experiment, they were fasted with ad libitum access to water. On the day of the acute experiment, animals were anesthetized with pentobarbital sodium and fentanyl, intubated, and mechanically ventilated with 3 l/min supplemental oxygen. A flow-directed balloon tipped thermodilution catheter was inserted via the right external jugular vein for hemodynamic measurements. Lines were placed in the femoral vein for continuous infusions. A catheter was advanced via the femoral artery into the aorta for mean arterial pressure measurements and blood sampling. Pressures were recorded and analyzed digitally (Sonometrics, London, Ontario, Canada). Via a left flank incision, the left ureter was cannulated for urine sampling and the left renal artery was equipped with a flow probe (Carolina Medical Electronics, King, NC). Cardiac output was measured by thermodilution (Cardiac output model 9510-A computer, American Edwards Laboratories, Irvine, CA).
At the beginning of the study protocol, a weight-adjusted inulin bolus was administered, and continuous inulin and saline infusions at a rate of 1 ml/min each were started. A preinfusion clearance was done after 60 min of equilibration. All clearances lasted 30 min and consisted of urine collection, blood sampling, and hemodynamic measurements. After the preinfusion clearance, the saline infusion was replaced with an infusion of synthetic human BNP 1-32 (Phoenix Peptide, Belmont, CA; diluted in saline) at a concentration of 30 ng·kg−1·min−1 (infusion rate 1 ml/min). The dose selected is the same as used in a previous study by our group (3). After a lead-in period of 15 min, a 30-min clearance was done. Subsequently, BNP 1-32 was replaced with a saline infusion (1 ml/min), and after a washout period of 60 min, a second preinfusion clearance was done. Thereafter, the saline infusion was replaced with an infusion of synthetic human BNP 8-32 (synthesized by Phoenix Peptide, Belmont, CA; diluted in saline) on an equimolar basis compared with the BNP 1-32 infusion (i.e., 23.7 ng·kg−1·min−1, infusion rate 1 ml/min). After a 15-min lead-in period, a 30-min clearance was done. To compensate for a possible carryover effect, the sequence of BNP 1-32 and BNP 8-32 infusions was reversed in half of the studies. Volume loss was replaced with saline at the end of each clearance.
Analysis of electrolytes and neurohormones.
Electrolytes were measured by flame photometry (IL943, Instrumentation Laboratory, Lexington, MA). Inulin was measured with the anthrone method (10). Glomerular filtration rate was assessed by inulin clearance. Plasma renin activity, ANG II, and aldosterone were determined by commercially available radioimmunoassays, as described previously (18). Immunoreactivity for human BNP was measured with the Triage BNP assay (Biosite, San Diego, CA). Importantly, this assay system uses a monoclonal mouse antibody (Scios Mab 106.3) directed against amino acids 5 to 13 on the N terminus and ring structure of BNP 1-32, an epitope that is partially missing in BNP 8-32 (22). Cyclic GMP was measured using a competitive RIA cGMP kit (Perkin-Elmer, Boston, MA).
Cell culture and measurement of cGMP.
For the in vitro studies, HAECs (Clontech, Mountain View, CA) were cultured in manufacturer endothelial cell medium (Clontech) supplemented with FBS and penicillin/streptomycin supplied as supplements with the media. Cells were treated at 80% to 90% confluency. Only cell passages 1 through 4 were used for experiments (15, 28). Cells were treated as described previously (28). Briefly, cells were incubated in Hank's balanced salt solution (Invitrogen, Carlsbad, CA) containing 20 mmol/l HEPES, 0.1% BSA, and 0.5 mmol/l 3 isobutyl-1-methylxanthine (Sigma, St. Louis, MO). In one experiment, cells were treated for 10 min with 10−10, 10−9, 10−8, 10−7, and 10−6 M human BNP 1-32 or BNP 8-32. In a separate experiment, cells were treated for 10 min with 10−6 M human BNP 1-32 or BNP 8-32 without and with 10−6 M HS-142–1, an NPR-A antagonist. In both experiments, cells were then lysed in 6% TCA and sonicated for 10 min. The samples were ether extracted four times in 4 volumes of ether, dried, and reconstituted in 300 μl cGMP assay buffer. The samples were assayed using a competitive RIA cGMP kit (Perkin-Elmer, Boston, MA). Briefly, samples and standards are incubated with 100 μl anti-human cGMP polyclonal antibody and I125-antigen for 18 h. Cyclic GMP assay buffer was added to the samples, and they were centrifuged for 20 min at 2,500 rpm. The free fraction was aspirated off, and the bound fraction was counted and concentrations determined. Samples are corrected for dilution factors and protein concentration, and values are expressed as femtomoles per milliliter. There is no cross-reactivity with ANP, BNP, CNP, ET, and <0.001% cross-reactivity with cAMP, GMP, GDP, ATP, and GTP.
Statistical analysis.
Values are expressed as means ± SE of the mean. For each peptide, changes from preinfusion levels were analyzed with paired t-test for normally distributed data. Peptides were compared with each other by analyzing the changes from the respective preinfusion clearance to the respective infusion clearance with paired t-test for normally distributed data. BNP values were log-transformed before analysis. Wilcoxon signed rank test was used for not normally distributed data, specifically urinary sodium excretion and urinary cGMP excretion. In the cell culture experiment, treatments were compared with one-way ANOVA with post hoc Bonferroni test (treatments vs. control, BNP-32 vs. BNP 8-32, BNP 8-32 vs. BNP 8-32+HS142). Statistical significance was accepted at P < 0.05. Analyses were performed with GraphPad Prism 4.03 (GraphPad Software, San Diego, CA).
RESULTS
Cardiorenal and humoral function are reported in Table 1 and Figs. 2 and 3. Preinfusion parameters for BNP 8-32 and BNP 1-32 were similar with the exception of plasma renin activity (PRA) and ANG II, which were higher before BNP 8-32 infusion [PRA: 6.4 ± 1.5 vs. 10.3 ± 1.5 ng·ml−1·h−1 (P = 0.05), BNP 8-32 vs. BNP 1-32; ANG II: 13.3 ± 2.7 vs. 7.2 ± 1.8 pg/ml (P = 0.02)].
Table 1.
Cardiorenal and humoral function
| Baseline | Δ with BNP 8-32 | Δ with BNP 1-32 | P value† | |||||
|---|---|---|---|---|---|---|---|---|
| Hemodynamic function | ||||||||
| Heart rate, beats/min | 123±12 | +7±3* | +17±5* | 0.15 | ||||
| Right atrial pressure, mmHg | 1.3±0.4 | −0.5±0.2* | −0.8±0.3* | 0.39 | ||||
| Pulmonary artery pressure, mmHg | 12.6±0.7 | −1.2±0.7 | −0.5±0.4 | 0.33 | ||||
| Pulmonary capillary wedge pressure, mmHg | 4.2±0.6 | −0.9±0.3* | −1.5±0.4* | 0.31 | ||||
| Cardiac output, l/min | 4.0±0.2 | −0.4±0.3 | −0.1±0.1 | 0.44 | ||||
| Systemic vascular resistance, mmHg·l−1·min | 33±2 | +1.5±2.0 | +0.5±0.8 | 0.67 | ||||
| Renal vascular resistance, mmHg·l−1·min | 432±37 | −55±23* | −81±20* | 0.18 | ||||
| Renal function | ||||||||
| Glomerular filtration rate, ml/min | 47±6 | +9±3* | +10±5 | 0.67 | ||||
| Urinary potassium excretion, μEq/min | 23±3 | +28±4* | +48±4* | 0.02 | ||||
| Humoral function | ||||||||
| Hematocrit, % | 40±1 | +0.9±0.9 | +3.2±0.4* | 0.09 | ||||
| Atrial natriuretic peptide, pg/ml | 11±1 | +5±1* | +5±1* | 0.74 | ||||
| Plasma renin activity, ng·ml−1·h−1 | 9.9±1.6 | −4.5±0.9* | −2.2±0.7* | 0.10 | ||||
| Angiotensin II, pg/ml | 12.5±2.9 | −4.3±2.5 | −0.4±0.9 | 0.13 | ||||
| Aldosterone, ng/dl | 10.5±2.4 | +4.9±6.2 | −2.6±1.2 | 0.22 | ||||
| Plasma sodium, mmol/l | 145±1 | +0.7±1.0 | +2.1±0.9 | 0.34 | ||||
| Plasma potassium, mmol/l | 4.1±0.1 | −0.0±0.1 | −0.0±0.0 | 0.91 | ||||
Values are expressed as means ± SE. Baseline denotes levels during the first clearance before any peptide infusion
P < 0.05 vs. respective preinfusion level.
P value for comparison of changes from respective preinfusion levels induced by BNP 8-32 vs. BNP 1-32. BNP, B-type natriuretic peptide; Δ, change from preinfusion level.
Fig. 2.
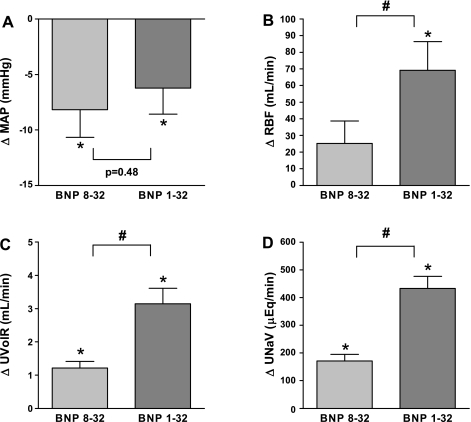
BNP 8-32 and BNP 1-32-induced changes from preinfusion levels of mean arterial pressure (A), renal blood flow (B), urine flow (C), and urinary sodium excretion (D). *P < 0.05 vs. respective preinfusion level. #P < 0.05 BNP 8-32 vs. BNP 1-32. Levels during the first preinfusion clearance were (means ± SE): Mean arterial pressure: 131 ± 5 mmHg; renal blood flow: 312 ± 20 ml/min; urine flow: 0.28 ± 0.04 ml/min; urinary sodium excretion: 21 ± 5 μEq/min. BNP, B-type natriuretic peptide; MAP, mean arterial pressure; RBF, renal blood flow; UNaV, urinary sodium excretion; UVolR, urine flow.
Fig. 3.
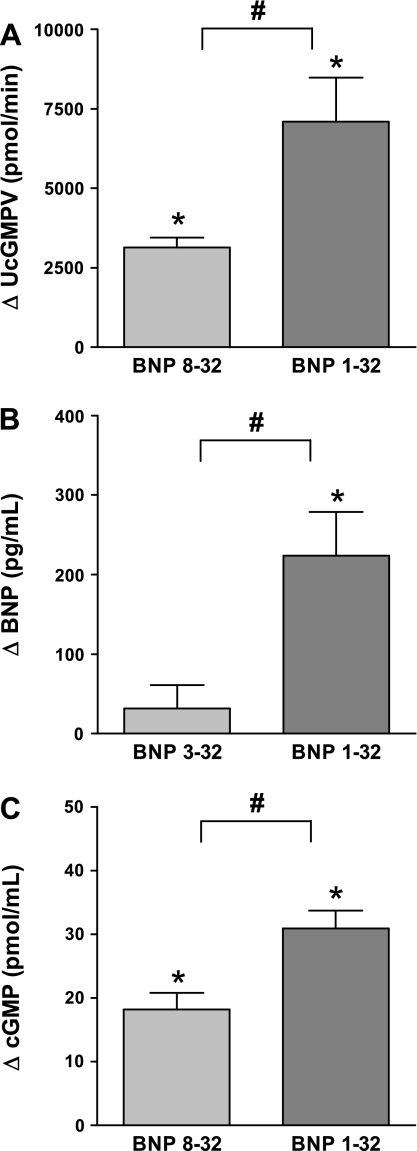
BNP 8-32 and BNP 1-32 induced changes from preinfusion levels of urinary cGMP excretion (A), human B-type natriuretic peptide plasma immunoreactivity (B), and plasma cyclic guanosine monophosphate (C). *P < 0.05 vs. respective preinfusion level. #P < 0.05 BNP 8-32 vs. BNP 1-32. Levels during the first preinfusion clearance were urinary cGMP excretion: 988 ± 132 pmol/min; cGMP: 9.7 ± 1.0 pg/ml. BNP, B-type natriuretic peptide; cGMP, cyclic guanosine monophosphate; UcGMPV, urinary cGMP.
Hemodynamic function.
Mean arterial pressure was reduced by both BNP 8-32 and BNP 1-32 to similar degrees (Fig. 2A), as were right atrial pressure and pulmonary capillary wedge pressure. Heart rate increased with both peptides, while pulmonary artery pressure and systemic vascular resistance remained unchanged. Cardiac output was unchanged with both peptides (P = 0.19 and P = 0.13 for BNP 8-32 and BNP 1-32, respectively).
Renal function.
Renal blood flow did not significantly change with BNP 8-32 (P = 0.10), while it increased significantly with BNP 1-32, and this was significant between peptides (Fig. 2B). Both peptides reduced renal vascular resistance. Glomerular filtration rate increased with BNP 8-32 but not with BNP 1-32 (P = 0.09) with no difference between peptides. Both peptides increased urine flow (Fig. 2C), urinary sodium excretion (Fig. 2D), and urinary potassium excretion, but these effects were significantly reduced with BNP 8-32. The same was true for urinary cGMP excretion (Fig. 3A).
Humoral function.
Plasma BNP immunoreactivity increased significantly less with BNP 8-32 compared with BNP 1-32 (Fig. 3B), using however, as mentioned, an assay that should not detect BNP 8-32. Indeed, when BNP 8-32 was infused as the first peptide, human BNP immunoreactivity was completely absent in 3 of 4 studies. Plasma cGMP, the second messenger of BNP, increased with both peptides, but again to a significantly lesser degree with BNP 8-32 (Fig. 3C). Atrial natriuretic peptide levels also increased, with no difference between peptides. Both peptides decreased PRA, with no difference between peptides (P = 0.10). ANG II remained unchanged with both BNP 8-32 (P = 0.13) and BNP 1-32. Again, the difference between peptides was not significant (P = 0.13). Aldosterone was unchanged with BNP 8-32 (P = 0.46) and BNP 1-32 (P = 0.06), with no difference between peptides. Hematocrit increased with BNP 1-32 but remained unchanged with BNP 8-32, with no significant difference between peptides (P = 0.09). There were no significant changes in plasma sodium or potassium with either peptide.
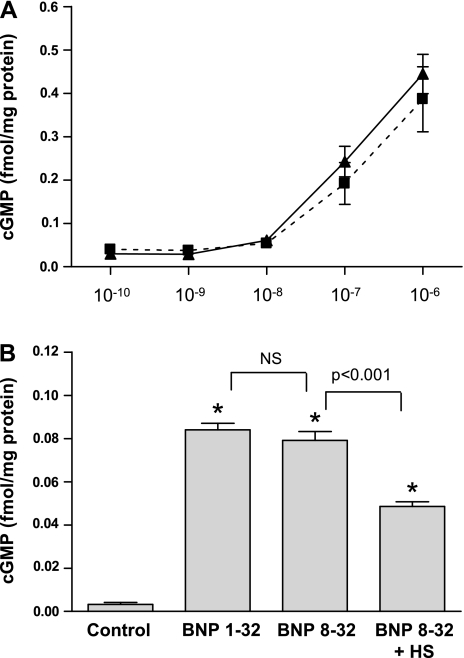
BNP 8-32 vs. BNP 1-32 in vitro: compared with control, BNP 8-32 and BNP 1-32 significantly increased cGMP generation in HAECs with no difference between peptides (Fig. 4A). Cyclic GMP generation was significantly reduced when the NPR blocker HS-142–1 was added to BNP 8-32 (Fig. 4B).
Fig. 4.
BNP 1-32 (dashed line) and BNP 8-32 (solid line) significantly increased cGMP generation in human aortic endothelial cells (HAEC) with no difference between peptides (A). Cyclic GMP generation by BNP 8-32 was significantly reduced when the natriuretic peptide receptor blocker HS-142-1 was present (B). *P < 0.05 vs. control. cGMP, cyclic guanosine monophosphate, HS, HS-142-1, NS, nonsignificant.
DISCUSSION
This study reports for the first time that BNP 8-32, which at least in vitro is produced from mature BNP 1-32 by meprin A, has similar hemodynamic but reduced renal actions compared with BNP 1-32 in vivo. These findings suggest that meprin A may play a role in the regulation of BNP bioactivity and that meprin A inhibition may be a strategy to augment the renal actions of endogenous and exogenous BNP 1-32.
Pankow et al. (23) and Walther (29) recently sought to better characterize the processing of BNP 1-32. They used membrane preparation of murine kidney brush borders and investigated the breakdown of BNP 1-32 in the presence and absence of peptidase inhibitors. With this approach, they were able to demonstrate that human BNP 1-32 is cleaved by murine meprin A to BNP 8-32, which unlike BNP 1-32, is also a substrate for neprilysin. Meprin A is a metallopeptidase, which is highly expressed in the kidney brush border, at least in rodents, and its substrates include matrix proteins. Studies suggest that it is involved in renal damage and meprin A inhibition has shown beneficial results in experimental models of kidney damage (5, 13, 27). Of note, as meprin A is also inhibited by EDTA, Brandt et al. (4), who investigated BNP degradation in EDTA plasma, were by study design not able to recognize a potential contribution of this peptidase (4).
To increase our knowledge regarding the potential physiological role of meprin A in BNP metabolism, we assessed the cardiorenal actions of synthetic BNP 8-32 compared with BNP 1-32. Both BNP 1-32 and BNP 8-32 increased plasma cGMP, the second messenger of BNP, which can be used as an indicator of NPR-A activation. BNP immunoreactivity increased significantly more with BNP 1-32, which can be explained by the fact that the employed assay system uses an antibody directed against an epitope on the N terminus of BNP 1-32, which is not present in BNP 8-32. The larger increase in plasma cGMP with BNP 1-32 infusion could be explained with a relatively lower receptor affinity of BNP 8-32; however, the similar cGMP activation in HAECs in vitro does not favor this explanation. An alternative explanation is that BNP 8-32 is more rapidly degraded to inactive fragments than BNP 1-32, which would be in keeping with the demonstration of Pankow et al. (23) that BNP 1-32 only becomes a substrate of neprilysin after cleavage by meprin A. In this case, reduced bioactivity of BNP 8-32 would be consistent with the presence of neprilysin in this target tissue. This would also be in keeping with the findings by Chen et al. (7) that neprilysin inhibition with the vasopeptidase inhibitor omapatrilat augmented urinary sodium and BNP excretion after subcutaneous administration of BNP 1-32.
The systemic hemodynamic actions observed with BNP 8-32 and BNP 1-32 were similar. Mean arterial pressure was reduced with both peptides, while systemic vascular resistance was unchanged. The decrease in blood pressure may be the combined result of the reduction in cardiac filling pressures due to venodilation and volume loss and the inhibitory actions of BNP on the RAAS and the sympathetic nervous system. Of note, these studies were done in healthy canines with physiologically hemodynamic and neurohumoral function at baseline; in pathophysiological conditions, such as heart failure, the cardiac unloading actions of BNP need not be associated with a decrease in cardiac output and can actually increase it (1, 6, 8, 19, 31).
Interestingly, renal blood flow was significantly more increased with BNP 1-32 compared with BNP 8-32. A possible explanation could be a relatively higher neprilysin activity in the renal vasculature, to which BNP 1-32 would be more resistant. With regard to renal excretory function, BNP 8-32 significantly increased diuresis, natriuresis, and kaliuresis; however, compared with BNP 1-32, these actions were reduced to similar degrees as renal blood flow, plasma cGMP, and urinary cGMP excretion. This again is in keeping with neprilysin activity in the renal vessels and tubules.
Of note, activation of RAAS was reduced or unchanged, despite the diuretic and hypotensive actions, in keeping with a direct effect of BNP on renin- and aldosterone-secreting cells. There was no difference between BNP 8-32 and BNP 1-32, which could mean they are equally efficacious at suppressing RAAS; alternatively, because of the smaller diuresis and natriuresis induced by BNP 8-32, there may have been less stimulation of RAAS secretion, offsetting a potential lesser RAAS-suppressing activity of BNP 8-32.
When the actions of BNP 8-32 are compared with those we previously reported for BNP 3-32 (3), which is produced from BNP 1-32 by cleavage with DPP4 (4), the effects of both peptides on renal excretory function were reduced to a similar extent compared with BNP 1-32. Surprisingly, however, BNP 8-32 reduced MAP to the same degree as BNP 1-32, whereas BNP 3-32 did not change MAP. These findings suggest that differential processing of BNP 1-32 can result in fragments with different profiles of action. Confirmation of these findings in other species and in other disease conditions is required.
These findings could be of clinical relevance. Plasma levels of BNP have been found to provide diagnostic and prognostic information in a variety of cardiovascular diseases, and various assay systems are currently employed to assess plasma BNP immunoreactivity. Of note, these BNP assays frequently cannot differentiate between the different processed forms of BNP, or, as with BNP 8-32 in this study, do not detect some forms that still retain some biological activity (14). Given the different activities of these forms, it may be worthwhile to develop more specific assay systems (e.g., including mass spectrometry) and assess their diagnostic and prognostic potential (22). The current findings suggest that meprin A may be involved in reducing the renal actions of BNP 1-32. Given that in disease conditions, there frequently is a resistance, particularly to the renal actions of natriuretic peptides, it may be worthwhile to investigate whether meprin A inhibition alone or in combination with exogenous BNP may be beneficial.
There are several limitations to our study. First, we infused human peptides into canines. We cannot exclude the possibility that for both BNP 1-32 and BNP 8-32, receptor binding and peptide metabolism may differ between humans and canines, and therefore, we cannot be sure that our findings can be extrapolated to humans. Second, this study was performed in healthy canines, and it is unknown whether results would be different in disease conditions that are usually associated with increased endogenous BNP levels, such as heart failure. Third, it is currently unknown whether BNP 8-32 circulates and whether meprin A is involved in BNP metabolism in vivo in humans or canines. Conventional BNP assays are not specific and detect BNP fragments and also proBNP (14). Niederkofler et al. (22) recently developed a quantitative mass spectrometry immunoassay and were able to demonstrate the presence of BNP 1-32, BNP 3-32, BNP 4-32, and BNP 5-32 in plasma samples from patients with heart failure. However, as one antibody of this assay system was the above-mentioned Mab 106.3, which requires the presence of amino acids 5–13 for epitope binding, no assessment of BNP forms with a shorter N-terminus, such as BNP 8-32, was possible. Finally, the anesthesia used in the current study may have affected the response to the peptides (21).
Perspectives and Significance
We demonstrated that BNP 8-32, which has been shown in vitro to be produced by meprin A, has similar systemic hemodynamic actions as equimolar BNP 1-32 but is less efficacious in increasing renal blood flow and renal excretory function. These findings suggest that meprin A may be involved in modulating the actions of BNP 1-32. It remains to be established to what degree BNP 1-32 is affected by meprin A in humans. If significantly so, meprin A inhibition may increase the renal actions of endogenous and exogenous BNP 1-32. Furthermore, specific characterization and quantification of cleavage products of BNP 1-32 may better reflect BNP bioactivity and improve the diagnostic and prognostic characteristics of BNP as a biomarker.
GRANTS
This research was supported by Grants HL-36634 (to J. C. Burnett, Jr.) and HL-07111 (to G. Boerrigter) from the National Institutes of Health, and by the Mayo Foundation.
Acknowledgments
We are very grateful to Denise M. Heublein, Sharon M. Sandberg, and Lynn K. Harstad for their technical assistance.
REFERENCES
- 1.Abraham WT, Lowes BD, Ferguson DA, Odom J, Kim JK, Robertson AD, Bristow MR, Schrier RW. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail 4: 37–44, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117: 2340–2350, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC Jr. Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol 292: R897–R901, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem 52: 82–87, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Carmago S, Shah SV, Walker PD. Meprin, a brush-border enzyme, plays an important role in hypoxic/ischemic acute renal tubular injury in rats. Kidney Int 61: 959–966, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Chen HH, Cataliotti A, Schirger JA, Martin FL, Burnett JC Jr. Equimolar doses of atrial and brain natriuretic peptides and urodilatin have differential renal actions in overt experimental heart failure. Am J Physiol Regul Integr Comp Physiol 288: R1093–R1097, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chen HH, Lainchbury JG, Harty GJ, Burnett JC Jr. Maximizing the natriuretic peptide system in experimental heart failure: subcutaneous brain natriuretic peptide and acute vasopeptidase inhibition. Circulation 105: 999–1003, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med 343: 246–253, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol 52: 750–754, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhr J, Kaczmarczyk K, Kruttgen C. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr 33: 729–733, 1955. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg B An ACE in the hole alternative pathways of the renin angiotensin system and their potential role in cardiac remodeling. J Am Coll Cardiol 52: 755–757, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC Jr, and Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA 102: 17442–17447, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog C, Seth R, Shah SV, Kaushal GP. Role of meprin A in renal tubular epithelial cell injury. Kidney Int 71: 1009–1018, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Heublein DM, Huntley BK, Boerrigter G, Cataliotti A, Sandberg SM, Redfield MM, Burnett JC Jr. Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension 49: 1114–1119, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, Burnett JC Jr. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol 209: 943–949, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Lam CS, Burnett JC Jr, Costello-Boerrigter L, Rodeheffer RJ, Redfield MM. Alternate circulating pro-B-type natriuretic peptide and B-type natriuretic peptide forms in the general population. J Am Coll Cardiol 49: 1193–1202, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 339: 321–328, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Lisy O, Lainchbury JG, Leskinen H, Burnett JC Jr. Therapeutic actions of a new synthetic vasoactive and natriuretic peptide, dendroaspis natriuretic peptide, in experimental severe congestive heart failure. Hypertension 37: 1089–1094, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Marcus LS, Hart D, Packer M, Yushak M, Medina N, Danziger RS, Heitjan DF, Katz SD. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized cross-over trial. Circulation 94: 3184–3189, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Martinez A, Oh HR, Unsworth EJ, Bregonzio C, Saavedra JM, Stetler-Stevenson WG, Cuttitta F. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J 383: 413–418, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsukawa K, Ninomiya I, Nishiura N. Effects of anesthesia on cardiac and renal sympathetic nerve activities and plasma catecholamines. Am J Physiol Regul Integr Comp Physiol 265: R792–R797, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Niederkofler E, Kiernan U, O'Rear J, Menon S, Saghir S, Protter A, Nelson R, Schellenberger U. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail 1: 258–264, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Pankow K, Wang Y, Gembardt F, Krause E, Sun X, Krause G, Schultheiss HP, Siems WE, Walther T. Successive action of meprin A and neprilysin catabolizes B-type natriuretic peptide. Circ Res 101: 875–882, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Rubattu S, Sciarretta S, Valenti V, Stanzione R, Volpe M. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens 21: 733–741, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Nakao K, Itoh H, Yamada T, Mukoyama M, Arai H, Hosoda K, Shirakami G, Suga S, Minamino N, Kanagawa K, Matsuo H, Imura H. Brain natriuretic peptide is a novel cardiac hormone. Biochem Biophys Res Commun 158: 360–368, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, Sugita K, Yamada K. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta 316: 129–135, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Takayama J, Takaoka M, Yamamoto S, Nohara A, Ohkita M, Matsumura Y. Actinonin, a meprin inhibitor, protects ischemic acute kidney injury in male but not in female rats. Eur J Pharmacol 581: 157–163, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC Jr. Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res 91: 1127–1134, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Walther T (WO/2007/003594) Screening methods for inhibitors of the metalloprotease meprin, 2007, http://www.wipo.int/pctdb/en/wo.jsp?IA=EP2006063734&DISPLAY=DESC.
- 30.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA 97: 8525–8529, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura M, Yasue H, Morita E, Sakaino N, Jougasaki M, Kurose M, Mukoyama M, Saito Y, Nakao K, Imura H. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation 84: 1581–1588, 1991. [DOI] [PubMed] [Google Scholar]