Abstract
Suppressor of cytokine signaling (SOCS) proteins and/or activation of the proinflammatory pathway have been postulated as possible mechanisms that may contribute to skeletal muscle insulin resistance. Thus, the aims of the present investigation were to determine in high-fat-fed skeletal muscle: 1) whether SOCS-3 protein concentration is increased, 2) whether coimmunoprecipitation of SOCS-3 with the insulin receptor-β subunit and/or IRS-1 is increased, and 3) whether select components of the proinflammatory pathway are altered. Thirty-two male Sprague-Dawley rats were assigned to either control (CON, n = 16) or high-fat-fed (HF, n = 16) dietary groups for 12 wk and then subjected to hind limb perfusions in the presence (n = 8/group) or absence (n = 8/group) of insulin. Insulin-stimulated skeletal muscle 3-MG transport rates and PI-3 kinase activity were greater (P < 0.05) in CON. IRS-1 tyrosine phosphorylation was decreased (P < 0.05), and IRS-1 serine 307 phosphorylation was increased (P < 0.05) in HF. Insulin receptor-β (IR-β) subunit coimmunoprecipitation with IRS-1 was reduced in HF. SOCS-3 protein concentration and SOCS-3 coimmunoprecipitation with both the IR-β subunit and IRS-1 was increased (P < 0.05) in HF. IKKα/β serine phosphorylation was increased (P < 0.05), IκBα protein concentration was decreased (P < 0.05) and IκBα serine phosphorylation was increased (P < 0.05) in HF. Increased colocalization of SOCS-3 with both the IR-β subunit and IRS-1 may provide steric hindrance that prevents IRS-1 from interacting with IR-β, while increased IKKβ serine phosphorylation may contribute to increasing IRS-1 serine phosphorylation, both of which independently can have deleterious effects on insulin-stimulated PI-3 kinase activation in high-fat-fed rodent skeletal muscle.
Keywords: insulin resistance, insulin signaling, proinflammatory signaling
in rodent skeletal muscle, high-fat feeding impairs insulin-stimulated glucose transport and uptake rates, in part, due to decreased insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol-3 (PI-3) kinase activity and GLUT4 translocation to the plasma membrane (14, 23, 31, 33, 38–40). However, it has not been clearly elucidated why high-fat feeding imparts these impairments on the insulin-signaling cascade. Several recent studies have implicated suppressor of cytokine signaling (SOCS) proteins as being capable of impairing insulin signaling (17, 27). Emanuelli et al. (9, 10) using 3T3-L1 adipocytes and COS7 cells have observed that insulin stimulation causes translocation of SOCS-3 to the plasma membrane, where it interacts with phosphotyrosine 960 in the juxtamembrane region of the insulin receptor (IR). Colocalization of SOCS-3 with the insulin receptor β-subunit (IR-β) prevents association of IRS-1 with IR-β, resulting in decreased activation of the downstream components of the insulin signaling cascade (9). It has been reported that overexpression of SOCS-3 in liver decreases insulin-stimulated PI-3 kinase activity (34). Additionally, SOCS-3 can bind to tyrosine-phosphorylated IRS-1 (28), which may also contribute to impaired insulin signaling. These observations when taken collectively with reports that SOCS-3 expression is elevated in adipocytes and liver of ob/ob and db/db mice (9, 35) and that in high-fat-fed rodent skeletal muscle (32), SOCS-3 mRNA is elevated, suggest that insulin resistance may result from a greater SOCS-3 protein concentration, which, in turn, increasingly binds to the IR-β and IRS-1, resulting in suppressed insulin signaling in skeletal muscle. However, it has not been reported whether the provision of a high-fat diet to rodents increases SOCS-3 protein concentration in skeletal muscle. Thus, the first aim of this investigation was to assess whether SOCS-3 protein concentration is increased in the skeletal muscle of high-fat-fed rodents and whether in response to insulin stimulation, coimmunoprecipitation of SOCS-3 with IR-β and/or IRS-1 is increased.
It has also been suggested that activation of the proinflammatory pathway may contribute to skeletal muscle insulin resistance (4, 30). Itani et al. (18) showed that lipid infusion-induced insulin resistance in humans decreased skeletal muscle IκBα protein concentration, which is indicative of increased IKKβ activation. In contrast, the IKKβ knockout mouse does not develop insulin resistance in response to lipid infusion (21, 42). Activated IKKβ has been shown to directly phosphorylate IRS-1 on serine 307 (11). This is of interest, as increased IRS-1 serine phosphorylation prevents the insulin-signaling cascade from becoming fully activated (16, 20, 36, 41). Despite the possibility that the IκB/NF-κB proinflammatory pathway may be involved in the pathogenesis of insulin resistance, it has not been extensively explored whether chronic consumption of a high-fat diet alters this pathway in rodent skeletal muscle. Therefore, the second aim of this investigation was to assess whether select components of the proinflammatory pathway are altered in high-fat-fed rodent skeletal muscle.
METHODS
Experimental Design
Thirty-two male Sprague-Dawley rats, ∼6 wk of age weighing between 185 and 220 g, were obtained from Harlan (San Diego, CA). On arrival, animals were randomly assigned to either control (CON, n = 16) or high-fat-fed (HF, n = 16) dietary groups. The control diet consisted of 20% fat-derived calories (coconut oil) (#112386; Dyets, Bethlehem, PA), and the high-fat diet consisted of 59% fat-derived calories (coconut oil) (#112387 Dyets ) Throughout the 12-wk investigation, both groups received water and their respective diets ad libitum. This high-fat diet has previously been shown to induce skeletal muscle insulin resistance (23, 31, 40). Animals were housed two per cage in a temperature-controlled environment at 21°C with an artificial 12–12-h light-dark cycle.
All experimental procedures were approved by the Institutional Animal Care and Use Committee at California State University, Northridge, and conformed to the guidelines for the use of laboratory animals, published by the U.S. Department of Health and Human Services.
Surgical Preparation and Hind Limb Perfusions
Following the 12-wk dietary period, all animals were prepared for hind limb perfusions. Animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (6.5 mg/100 g body wt) and surgically prepared for hind limb perfusion as previously described by Ruderman et al. (27a) and modified by Ivy et al. (19). Following surgical preparation, cannulas were inserted into the abdominal aorta and vena cava, and the animals were killed via an intracardiac injection of pentobarbital sodium, as the hind limbs were washed out with 30 ml of Krebs-Henseleit buffer (KHB) (pH 7.55). The cannulas were placed in line with a nonrecirculating perfusion system, and the hind limbs were allowed to stabilize during a 5-min washout period. The perfusate was continuously gassed with a mixture of 95% O2-5% CO2 and warmed to 37°C. Perfusate flow rate was set at 7.5 ml/min during the stabilization and subsequent perfusion, during which rates of glucose transport were determined.
Perfusions were performed in the presence (n = 8/group) or absence (n = 8/group) of 500 μU/ml of insulin. The basic perfusate medium consisted of 30% washed time-expired human erythrocytes (Ogden Medical Center, Ogden, UT), KHB, 4% dialyzed BSA (Fisher Scientific, Fair Lawn, NJ) and 0.2 mM pyruvate. The hind limbs were washed out with perfusate containing 1 mM glucose for 5 min in preparation for the measurement of glucose transport. Glucose transport was measured over an 8-min period using an 8 mM concentration of nonmetabolized glucose analog 3-O-methylglucose (3-MG) (32 μCi 3-[3H] MG/mM; PerkinElmer Life Sciences, Boston, MA) and 2 mM mannitol (60 μCi-[1-14C] mannitol/mM; PerkinElmer Life Sciences). Rates of basal and insulin-stimulated skeletal muscle 3-MG transport were calculated as previously described (36). Immediately after the transport period, portions of the red (RQ) and white quadriceps (insulin-signaling analysis) and red (RG) and white gastrocnemius (SOCS-3 and proinflammatory signaling analysis) were excised from both hind limbs, blotted on gauze dampened with cold KHB, freeze-clamped in liquid N2, and stored at −80°C for later analysis. The white tissue was not further analyzed when rates of 3-MG transport were observed to be similar between groups (data not shown).
Muscle Lysate Preparation
Muscle samples were weighed and homogenized 1:10 in homogenization buffer that contained 50 mM HEPES, 150 mM NaCl, 200 mM sodium pyrophosphate, 20 mM α-glycerolphosphate, 20 mM NaF, 2 mM sodium vanadate, 20 mM EDTA, 1% IGEPAL, 10% glycerol, 2 mM PMSF, 1 mM MgCl2, 1 mM CaCl2, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Muscles were homogenized on ice using a glass Pyrex homogenizer and centrifuged at 18,300 g for 15 min at 4°C (Micromax RF; International Equipment, Needham Heights, MA). The supernatant was collected and quantified for protein content by the Bradford Method using a Benchmark microplate reader (Bio-Rad, Richmond, CA).
Insulin-Signaling Cascade
Western blot analysis.
Western blot analysis for insulin receptor-β subunit (IR-β) and IRS-1 was determined using lysate samples, as we have described previously (3, 39).
IR-β tyrosine phosphorylation, IRS-1 tyrosine phosphorylation, and IRS-1 serine phosphorylation.
Phosphorylation of the IR-β subunit and IRS-1 was determined using immunoprecipitation followed by Western blot analysis, as we have previously described (3, 39).
Coimmunoprecipitation of IRS-1 with IR-β.
Sixty microliters of PRO-A bead slurry were incubated with 5 μg of anti-phospho-IRS-1 (cat. no. 07–848; Millipore, Billerica, MA) overnight at 4°C with rotation. Following the overnight incubation, the conjugated IRS-1/PRO-A beads were washed 3 times with PBS. Five hundred micrograms of muscle lysate protein were then incubated overnight with the conjugated IRS-1/PRO-A beads followed by single washes of PBS, 2% Triton X-PBS, a final PBS wash followed by the addition of 30 μl of Laemmli sample buffer and 5 min incubation at 100°C. Ten microliters of eluted sample were loaded in duplicate and subjected to SDS-PAGE run under reducing conditions on a 7.5% resolving gel. Resolved proteins were transferred to polyvinylidene difluoride (PVDF) membranes, blocked with 5% nonfat dry milk-Tris-Tween buffered saline, incubated overnight at 4°C with anti-IR-β (cat. no. 07–724; Millipore) followed by incubation with a species-specific secondary antibody conjugated to horseradish peroxidase (HRP). Antibody binding was visualized and quantified, as we have described previously (3, 39).
IRS-1-associated PI-3 kinase activity.
IRS-1-associated PI-3 kinase activity was determined, as we have described previously (31).
SOCS and Proinflammatory Signaling
Skeletal muscle TNF-α.
TNF-α concentration was determined in lysate samples. Protein-A conjugated beads (50 μl slurry) were incubated with anti-TNF-α antibody (cat. no. ARC3012; Biosource, Camarillo, CA) overnight at 4°C, while rotating. Following the overnight incubation, samples were washed 3 times with PBS. Samples were then incubated overnight with lysate (250 μg of protein) followed by washing once with PBS, once with 2% Triton X-PBS and a final PBS wash before the addition of Laemmli solution. Ten microliters of supernatant were loaded in duplicate and subjected to SDS-PAGE under reducing conditions on a 15% resolving gel. Resolved proteins were transferred to PVDF membranes and blocked with 5% NFDM-TTBS. The membranes were then incubated overnight at 4°C with affinity-purified rabbit polyclonal anti-TNF-α (cat. no. ARC3012, Biosource) primary antibody followed by incubation with species-specific secondary antibody conjugated to HRP. Antibody binding was visualized and quantified as we have described previously (3, 39).
Analysis of select components of the IκB/NF-κB proinflammatory pathway.
Skeletal muscle lysate samples were used for Western blot analysis of IKKα, IKKβ, IKKα/β pS, IκBα, and IκBα pS. Protein samples were combined with Laemmli sample buffer (1:1) and heated at 100°C for 5 min. One hundred micrograms of muscle lysate protein were subjected to SDS-PAGE run under reducing conditions on a 10% resolving gel and transferred to PVDF membranes. The PVDF membranes were blocked using a 5% NFDM-TTBS solution followed by incubation with either anti-IKKα (cat. no. 2682; Cell Signaling, Beverly, MA), anti-IKKβ (cat. 05–535; Millipore), anti-IKKα/β pS (cat. no. 2694S; Cell Signaling), anti-IκBα (cat. no. sc-371; Santa Cruz Biotechnology, Santa Cruz, CA), or anti-IκBα pS (cat. no. 9246L; Cell Signaling), primary antibody solutions followed by incubation with species-specific secondary antibody conjugated to HRP. Images were captured and quantified, as we have described previously (3, 39).
SOCS-3 protein concentration.
SOCS-3 protein content was determined in lysate samples. Fifty micrograms of skeletal muscle lysate were subjected to SDS-PAGE run under reducing conditions on a 15% resolving gel. Resolved proteins were transferred to PVDF membranes and blocked overnight at 4°C with 5% BSA-TTBS. Membranes were then incubated with affinity-purified rabbit polyclonal anti-SOCS-3 (cat. no. sc-9023; Santa Cruz Biotechnology) primary antibody solutions for 2 h at room temperature followed by incubation with goat anti-rabbit IgG-HRP secondary antibody solution. Antibody binding was visualized and quantified as we have previously described (3, 39).
Coimmunoprecipitation of SOCS-3 with IR-β and IRS-1.
Lysate samples (250 μg of protein) were subjected to immunoprecipitation with 4 μg of either anti-IR-β (cat. no. 06–492; Millipore) antibody or anti-IRS-1 (cat. no. 06–248; Millipore). Immunocomplexes were allowed to form overnight at 4°C. Following overnight incubation, samples were loaded with 80 μl of Protein-A bead slurry and rotated for 3 h at 4°C. Samples were then washed, 25 μl of Laemmli buffer were added, and samples were boiled for 5 min at 100°C. Ten microliters of eluted sample were loaded in duplicate onto a 15% SDS-PAGE resolving gel. Following electrophoresis, resolved proteins were transferred onto PVDF membranes, blocked with 5% NFDM-TTBS and then probed with anti-SOCS-3 (cat. no. sc-9023, Santa Cruz Biotechnology) primary antibody for 2 h followed by incubation with species-specific secondary antibody conjugated to HRP. Antibody binding was visualized and quantified, as we have previously described (3, 39).
Statistical analysis.
An ANOVA was used on all variables to determine whether significant differences existed between groups. When a significant F ratio was obtained, a Tukey honestly significant difference post hoc test was used to identify statistically significant differences (P < 0.05) among the means. Statistical analyses were performed using JMP software (SAS Institute, Cary, NC), and all values are expressed as means ± SE.
RESULTS
Skeletal Muscle 3-MG Transport
Basal rates of 3-MG transport (μmol·g−1·h−1) of the CON and HF animals were not different in the RG (CON: 3.92 ± 0.1 vs. HF: 3.40 ± 0.2) and RQ (CON: 4.82 ± 0.1 vs. HF 4.14 ± 0.3). In the presence of insulin, 3-MG transport rates of the CON animals were greater (P < 0.05) than that of the HF animals in both the RG (CON: 7.04 ± 0.4 vs. HF: 5.06 ± 0.3) and RQ (CON: 6.92 ± 0.2 vs. HF: 5.07 ± 0.3).
Insulin-Signaling Cascade
IR-β subunit protein concentration and tyrosine phosphorylation.
IR-β protein content was similar between groups under both basal [CON: 91.58 ± 7.2 arbitrary units (AU) vs. HF: 91.23 ± 9.9 AU] and insulin-stimulated (CON: 102.90 ± 8.2 AU vs. HF: 102.5 ± 11.2 AU) conditions. Tyrosine phosphorylation of the IR-β subunit was similar between both groups in the absence (CON: 45.56 ± 4.1 AU vs. HF: 44.65 ± 5.6 AU) and the presence of insulin (CON: 89.69 ± 3.8 AU vs. HF: 89.96 ± 5.6 AU) but was significantly elevated (P < 0.05) above basal levels in both groups.
IRS-1 protein content, tyrosine phosphorylation, and serine 307 phosphorylation.
IRS-1 protein concentration was similar in the CON and HF animals in the presence (CON: 52.64 ± 4.1 AU vs. HF: 53.20 ± 5.8 AU) and absence of insulin (CON: 47.30 ± 3.2 AU vs. HF: 50.80 ± 5.5 AU). IRS-1 tyrosine phosphorylation was similar between the CON (72.03 ± 6.9 AU) and HF (65.04 ± 3.8 AU) animals under basal conditions. Although insulin increased IRS-1 tyrosine phosphorylation in both the CON and HF animals, insulin-stimulated IRS-1 tyrosine phosphorylation was greater (P < 0.05) in the CON animals (178.89 ± 7.4 AU) compared with HF animals (82.18 ± 6.3 AU). IRS-1 serine 307 phosphorylation was significantly elevated (P < 0.05) in the HF animals compared with CON animals under both basal (CON: 64.47 ± 4.3 AU vs. HF: 85.57 ± 5.7 AU) and insulin-stimulated (CON: 102.29 ± 1.2 AU vs. HF: 120.78 ± 3.0 AU) conditions.
Coimmuniprecipitation of IRS-1 with IR-β.
We assessed IRS-1 coimmunoprecipitation with IR-β only in insulin-stimulated muscle. We observed that IRS-1 coimmunoprecipitation with IR-β in the CON animals was significantly (P < 0.05) greater (108.8 ± 6.0 AU) compared with the HF animals (67.2 ± 6.7 AU). Our rationale for limiting this measurement to insulin-stimulated tissue was that as both IR-β and IRS-1 protein concentrations were not different between groups either in the absence or presence of insulin, potential differences in protein-protein interaction would, therefore, not likely be due to alterations in protein expression, and additionally, this interaction predominantly occurs in the presence of insulin.
PI-3 kinase activity.
PI-3 kinase activity was similar between the CON (69.55 ± 17.8 AU) and HF (53.94 ± 18.3 AU) animals in the absence of insulin. Insulin increased PI-3 kinase activity in both the CON and HF animals, but the insulin-stimulated activity was significantly lower (P < 0.05) in the HF (134.07 ± 25.4 AU) animals compared with CON (255.99 ± 31.7 AU) animals.
SOCS and Proinflammatory Signaling Cascade
Skeletal muscle TNF-α.
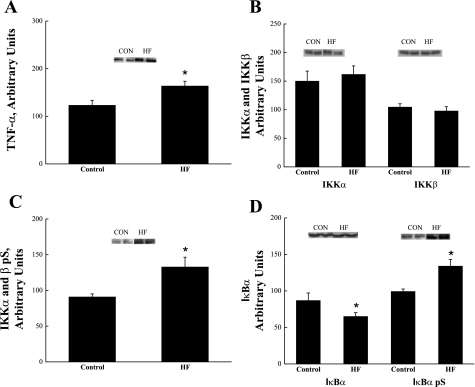
Skeletal muscle TNF-α concentration was significantly elevated (P < 0.05) in the HF animals compared with CON animals (Fig. 1A).
Fig. 1.
TNF-α protein concentration (A), IKKα/β protein concentration (B), IKKα/β serine phosphorylation (pS) (C), and IκBα protein concentration and IκBα serine phosphorylation (pS) (D) in skeletal muscle obtained from control (CON) and high-fat fed (HF) animals. *Significantly different from CON (P < 0.05). Values are expressed as means ± SE.
IKKα and IKKβ protein concentration and IKKα/β serine phosphorylation.
IKKα and IKKβ protein concentration was similar between the CON and HF animals (Fig. 1B). Serine phosphorylation of IKKα/β was significantly (P < 0.05) increased in the HF animals (Fig. 1C).
IκBα protein concentration and IκBα serine 32/36 phosphorylation.
High-fat feeding significantly (P < 0.05) decreased skeletal muscle IκBα protein concentration (Fig. 1D). To further investigate the decrease in IκBα protein content, IκBα serine phosphorylation was evaluated and was observed to be significantly (P < 0.05) increased in HF animals (Fig. 1D).
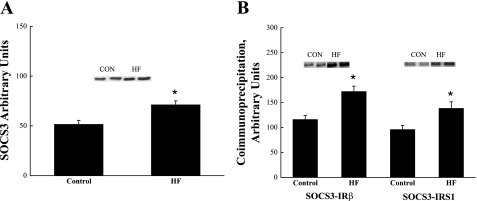
SOCS-3 protein concentration and coimmunoprecipitation of SOCS-3 with IRS-1 and IR-β.
High-fat feeding significantly increased (P < 0.05) SOCS-3 protein concentration (Fig. 2A). In the presence of insulin SOCS-3 colocalization with IR-β (Fig. 2B) and IRS-1 (Fig. 2B) was significantly increased (P < 0.05) in the HF animals compared with CON animals. To further explore the relationship between SOCS-3 and components of the insulin-signaling cascade, we ran a correlational analysis and found the following: IR-β coimmunoprecipitated with SOCS3 correlated to total SOCS3 protein concentration (r = 0.79, P = 0.01), IRS-1 coimmunoprecipitated with SOCS3 correlated to total SOCS3 protein concentration (r = 0.68, P = 0.04), IRS-1 coimmunoprecipitated with IR-β correlated to total SOCS3 protein concentration (r = 0.77, P = 0.01), and PI-3 kinase activity correlated to total SOCS3 protein concentration (r = −0.68, P = 0.03).
Fig. 2.
SOCS-3 protein concentration (A), and SOCS-3 coimmunoprecipitation (B) with insulin receptor β-subunit and SOCS-3 coimmunoprecipitation with IRS-1 in skeletal muscle obtained from CON and HF animals. *Significantly different from CON (P < 0.05). Values are expressed as means ± SE.
DISCUSSION
The high-fat-fed animals exhibited significantly impaired insulin-stimulated skeletal muscle carbohydrate metabolism as evidenced by decreased rates of 3-MG transport and PI-3 kinase activity compared with animals on the normal diet. These observations are in agreement with a number of previous reports that have also shown a high-fat diet to decrease insulin-stimulated glucose transport (1, 7, 13, 14, 22, 23, 31, 37–39) and PI-3 kinase activity (23, 24, 31, 33, 40). We have previously reported that insulin-stimulated plasma membrane-associated aPKCζ and aPKCλ protein concentration and activity, and cytosolic Akt2 and aPKCζλ activities are reduced in the skeletal muscle of the rodents that were used in the present investigation (15). While activation of these downstream components of the insulin-signaling cascade were reduced by high-fat feeding, it appeared that the impairments were largely a result of an insufficient activation of PI-3 kinase. On the basis of this observation, it was of interest to ascertain whether a mechanism could be identified that could account for why the provision of a high-fat diet decreases insulin-stimulated PI-3 kinase activity.
Our first line of inquiry was directed toward determining whether SOCS-3 could account for the reduced insulin-stimulated PI-3 kinase activity. Our interest in this molecule as a potential modulator of PI-3 kinase activity was based on reports that SOCS-3 is elevated in adipocytes and liver obtained from ob/ob and db/db mice (9, 35), and overexpression of SOCS-3 in liver decreases insulin-stimulated PI-3 kinase activity (34). We are unaware of any investigations that have evaluated whether the chronic consumption of a high-fat diet affects skeletal muscle SOCS-3 protein concentration in rodents. Thus, our observation that SOCS-3 protein concentration was elevated in the skeletal muscle of the high-fat-fed animals appears novel. Of note, although it has been reported in human skeletal muscle myotubes that an increase in SOCS-3 mRNA may not be related to insulin resistance (26).
However, simply increasing the expression of this protein may not be directly accountable for impairments in PI-3 kinase activation. Rather, it appears that for SOCS-3 to impair the insulin-signaling cascade, it must colocalize with phosphotyrosine 960 in the juxtamembrane region of the insulin receptor β-subunit (IR-β) (9, 10). This, in turn, has been observed in 3T3-L1 adipocytes and COS7 cells to prevent association of IRS-1 with IR-β, which results in decreased activation of the downstream components of the insulin-signaling cascade (9, 10). Consistent with this report, we noted that when we immunoprecipitated the insulin receptor β-subunit from lysates prepared from insulin-stimulated skeletal muscle and probed for SOCS-3, there was a significantly greater coimmunoprecipitation of SOCS-3 with the IR-β subunit in the high-fat-fed skeletal muscle. Similarly, we observed that there was a greater coimmunoprecipitation of SOCS-3 with IRS-1 in the high-fat-fed skeletal muscle, which is in agreement with a previous report, indicating that SOCS-3 can also bind to tyrosine phosphorylated IRS-1 (28). Thus, our data suggest that in skeletal muscle, the chronic consumption of a high-fat diet increases the expression of SOCS-3 and colocalization of SOCS-3 with both the IR-β subunit and IRS-1 under insulin stimulation. In turn, this colocalization possibly results in a physical barrier that impairs IRS-1 from interacting with the IR-β subunit. Although we cannot definitively confirm this hypothesis, we did note that IR-β subunit and IRS-1 coimmunoprecipitation was reduced in insulin-stimulated skeletal muscle collected from the HF animals. Furthermore, we observed that PI-3 kinase activity was decreased in the HF animals. As IRS-1 must associate with the IR-β subunit to activate PI-3 kinase, it is not implausible to suggest that reduced colocalization between the IR-β subunit and IRS-1 in the HF animals was a key factor that contributed to the decreased PI-3 kinase activity and reduced rates of 3-MG transport. Alternatively, increased SOCS-3 mRNA has been reported to inhibit leptin signaling and fat oxidation in skeletal muscle (32), which, in turn, may impair insulin signaling by other related mechanisms.
We have previously observed that a high-fat diet increases IRS-1 serine phosphorylation in skeletal muscle (39) and again show this effect in the present investigation. The observation that IRS-1 serine phosphorylation was increased in the HF animals is of interest, as it has been reported that increased IRS-1 serine phosphorylation prevents the insulin signaling cascade from becoming fully activated (16, 20, 36, 41) and is related to increased diacylglycerol (DAG) accumulation in the skeletal muscle (20, 29). Additionally, we have found that high-fat feeding increases DAG accumulation because of increased rates of palmitate uptake without a concomitant increase in fatty acid oxidation (24). Although it has not been investigated in skeletal muscle, it has been reported that accumulation of DAG in hepatocytes activates components of the proinflammatory cascade and contributes to the development of insulin resistance (5). Of interest, a component of the proinflammatory cascade, IκB kinase β (IKKβ), when activated, has been reported to directly phosphorylate IRS-1 on serine 307 (11). Taken collectively, these data suggest that high-fat feeding might also impair the insulin-signaling cascade in skeletal muscle as a result of components of the proinflammatory cascade being activated and contributing to increased IRS-1 serine phosphorylation.
Thus, our second line of inquiry was to assess whether select components of the proinflammatory pathway are activated in high-fat-fed rodent skeletal muscle. We initially evaluated whether components of the IκB kinase complex were altered by high-fat feeding due to IKKβ being implicated as an IRS-1 serine kinase. The IκB kinase complex exists as a trimolecular complex that contains a regulatory subunit, IκB kinase gamma (IKKγ), and two catalytic subunits IκB kinase α (IKKα) and IKKβ (12). Although IKKα and IKKβ protein concentration was not altered by the high-fat diet, we did note that IKKα/β serine phosphorylation was significantly increased in the HF animals, which indicated that the IKKs were activated.
The next question of interest was why IKKα and IKKβ serine phosphorylation was increased. DiDonato et al. (8) have reported that TNF-α increases IKK activity. Thus, our observation that skeletal muscle TNF-α concentration was increased in the high-fat-fed rodents provides a plausible mechanism that accounts for the increased IKK serine phosphorylation. Additionally, our finding that skeletal muscle TNF-α content was increased is consistent with that of Borst and Conover (6), who have reported that Wistar rats that consumed a high-fat diet exhibited elevated TNF-α levels in the RG. However, NF-κB can autoregulate the inflammatory cascade, and thus, the possibility exists that TNF-α was increased as a consequence rather than a cause of NF-κB activation (2). On a side note, TNF-α also activates JNK, a member of the MAPK family, and when activated, JNK can also induce IRS-1 serine 307 phosphorylation (16). It has been reported that a high-fat diet increases JNK activation in rodent skeletal muscle (25). Thus, it is possible that a portion of the IRS-1 serine 307 phosphorylation observed in the present investigation may have also resulted from TNF-α-induced JNK activation.
To this point, our measurements did not directly address whether, in fact, the proinflammatory pathway was fully activated in the high-fat-fed skeletal muscle. It is well known that in the proinflammatory pathway, IKKβ is the catalytic subunit that activates NF-κB. NF-κB and IκBα exist as a complex under steady-state conditions until acted on by IKKβ, causing phosphorylation of IκBα on serine residues 32 and 36. This, in turn, causes the IκBα/NF-κB complex to disassociate, allowing NF-κB to translocate to the nucleus and targets IκBα to the proteosomal degradation pathway. Our finding that high-fat feeding decreased skeletal muscle IκBα protein concentration and increased IκBα serine 32/36 phosphorylation confirms that the proinflammatory pathway was indeed activated, and consistent with the model, whereby activation of the proinflammatory pathway contributes to the development of skeletal muscle insulin resistance (30).
Perspectives and Significance
In summary, we identified two independent mechanisms that were altered in the skeletal muscle of high-fat-fed rodents that could have deleterious effects on insulin-stimulated activation of PI-3 kinase. We found that high-fat feeding increased the expression of SOCS-3 and coimmunoprecipitation of SOCS-3 with both the IR-β subunit and IRS-1. Colocalization of SOCS-3 with both the IR-β subunit and IRS-1 may result in a steric hindrance that possibly prevents IRS-1 from interacting with the IR-β subunit, thereby reducing PI-3 kinase activity. Additionally, we observed that components of the proinflammatory pathway were activated in the skeletal muscle collected from high-fat-fed animals. Of particular note, IKK serine phosphorylation was significantly increased, which may have contributed to increasing IRS-1 serine phosphorylation, and, in turn, may also prevent PI-3 kinase from being fully activated in response to insulin stimulation.
GRANTS
This study was supported by grants from the National Institutes of Health (GM-48680, GM-08395, and DK-57625).
Acknowledgments
We thank Jose J. Limon and Donald W. Reeder for excellent technical assistance.
REFERENCES
- 1.Barnard RJ, Roberts CK, Varon SM, Berger JJ. Diet-induced insulin resistance precedes other aspects of the metabolic syndrome. J Appl Physiol 84: 1311–1315, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Karin M. Nuclear factor-κB - A pivitol transcription factor in inflammatory diseases. N Engl J Med 336: 1066–1071, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Bernard JR, Reeder DW, Herr HJ, Rivas DA, Yaspelkis BB, III. High fat feeding effects on components of the novel insulin signaling cascade in Sprague-Dawley rat skeletal muscle. Metabol 55: 203–212, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt BA, Dube JJ, Dedousis N, Reider JA, O'Doherty RM. Diet-induced obesity and acute hyperlipidemia reduce IκBα levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol 290: R233–R240, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-κB pathway in rat liver. Diabetes 54: 3458–3465, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Borst SE, Conover CF. High-fat diet induces increased tissue expression of TNF-α. Life Sci 77: 2156–2165, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Buettner R, Newgard CB, Rhodes CJ, O'Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am J Physiol Endocrinol Metab 278: E563–E569, 2000. [DOI] [PubMed] [Google Scholar]
- 8.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388: 548–554, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Emanuelli B, Peraldi P, Filloux C, Chavey C, Friedinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of mice. J Biol Chem 276: 47944–47949, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275: 15985–15991, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J Biol Chem 277: 48115–48121, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE 357: re13, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Halseth AE, Bracy DP, Wasserman DH. Limitation to basal and insulin-stimulated skeletal muscle glucose uptake in the high-fat-fed rat. Am J Physiol Endocrinol Metab 279: E1064–E1071, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Hansen PA, Han DH, Marshall BA, Nolte LA, Chen MM, Mueckler M, Holloszy JO. A high fat diet impairs stimulation of glucose transport in muscle. Functional evaluation of potential mechanisms. J Biol Chem 273: 26157–26163, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Herr HJ, Bernard JR, Reeder DW, Rivas DA, Limon JJ, Yaspelkis BB, III. Insulin-stimulated plasma membrane association and activation of Akt2 and, aPKC ζ and λ in high-fat fed rodent skeletal muscle. J Physiol 565: 627–636, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirosumi J, Tuncman G, Chang LF, Gorgun CZ, Uysal KT, Maedda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17: 365–371, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Itani S, Ruderman N, Schmeider F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51: 2005–2011, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Ivy JL, Brozinick JT Jr, Torgan CE, Kastello GM. Skeletal muscle glucose transport in obese Zucker rats after exercise training. J Appl Physiol 66: 2635–2641, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI. PKC-τ knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114: 823–827, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108: 437–446, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraegen EW, James DE, Storlein LH, Burleigh KM, Chisholm DJ. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia 29: 192–198, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Krisan AD, Collins DE, Crain AM, Kwong CC, Singh MK, Bernard JR, Yaspelkis BB, III. Resistance training enhances components of the insulin signaling cascade in normal and high-fat-fed skeletal muscle. J Appl Physiol 96: 1691–1700, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MF, Reeder DW, Kemp BE, Yaspelkis III BB, Hawley JA. Tissue-specific effects of Rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56: 1856–1864, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Prada PO, Zecchin HG, Gasparetti AL, Torsoni MA, Ueno M, Hirata AE, Corezola do Amaral ME, Hoer NF, Boschero AC, Saad MJ. Western diet modulates insulin signaling, c-Jun-N-terminal kinase activity, and insulin receptor substrate-1Ser307 phosphorylation in a tissue-specific fashion. Endocrinology 146: 1576–1587, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, Laville M, Vidal H. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes 53: 2232–2241, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Ronn SG, Billestrup N, Mandrup-Poulsen T. Diabetes and suppressors of cytokine signaling proteins. Diabetes 56: 541–548, 2007. [DOI] [PubMed] [Google Scholar]
- 27a.Ruderman NB, Houghton CRS, Helms R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J 124: 639–651, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rui L, Yuan M, Frantz D, Shoelson S, White M. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277: 42394–42398, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz-Peiffer C Protein kinase C and lipid-induced insulin resistance in skeletal muscle. Ann NY Acad Sci 967: 146–157, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int J Obesity 27: S49–S52, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Singh MK, Krisan AD, Crain AM, Collins DE, Yaspelkis BB, III. High-fat diet and leptin treatment alter skeletal muscle insulin-stimulated phosphatidylinositol 3-kinase activity and glucose transport. Metabolism 52: 1196–1205, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg GR, Smith AC, Wormald S, Malenfant P, Collier C, Dyck DJ. Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle. Am J Physiol Endocrinol Metab 286: E57–E63, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Tremblay F, Lavigne C, Jacques H, Marette A. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (ζλ) activities. Diabetes 50: 1901–1910, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of the insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 24: 5434–5446, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci 101: 10422–10427, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Um SH, Frigerio F, Watanaabe M, Picard F, Joaqui M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200–2005, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Wilkes JJ, Bonen A, Bell RC. A modified high-fat diet induces insulin resistance in rat skeletal muscle but not adipocytes. Am J Physiol Endocrinol Metab 275: E679–E686, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Yaspelkis BB, III, Davis JR, Saberi M, Smith TL, Jazayeri R, Singh M, Fernandez V, Trevino B, Chinookoswong N, Wang J, Shi ZQ, Levin N. Leptin administration improves skeletal muscle insulin responsiveness in diet-induced insulin-resistant rats. Am J Physiol Endocrinol Metab 280: E130–E142, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Yaspelkis BB, III, Lessard SJ, Reeder DW, Limon JJ, Saito M, Rivas DA, Kvasha I, Hawley JA. Exercise reverses high-fat diet-induced impairments on compartmentalization and activation of components of the insulin signaling cascade in skeletal muscle. Am J Physiol Endocrinol Metab 293: E941–E949, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Yaspelkis BB, III, Singh MK, Krisan AD, Collins DE, Kwong CC, Bernard JR, Crain AM. Chronic leptin treatment enhances insulin-stimulated glucose disposal in skeletal muscle of high-fat fed rodents. Life Sci 74: 1801–1816, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Yu CL, Chen Y, Cline GW, Zhang DY, Zong HH, Wang YL, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li Z, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293: 1673–1677, 2001. [DOI] [PubMed] [Google Scholar]