Abstract
The medullary 5-HT system has potent effects on heart rate and breathing in adults. We asked whether this system mitigates the respiratory instability and bradycardias frequently occurring during the neonatal period. 5,7-Dihydroxytryptamine (5,7-DHT) or vehicle was administered to rat pups at postnatal day 2 (P2), and we then compared the magnitude of bradycardias occurring with disruptions to eupnea in treated and vehicle control littermates at P5–6 and P10–12. We then used a novel method that would allow accurate assessment of the ventilatory and heart rate responses to near square-wave challenges of hypoxia (10% O2), hypercapnia (5 and 8% CO2 in normoxia and hyperoxia), and asphyxia (8% CO2-10% O2), and to the induction of the Hering-Breuer inflation reflex (HBR), a potent, apnea-inducing reflex in newborns. The number of 5-HT-positive neurons was reduced ∼80% by drug treatment. At both ages, lesioned animals had considerably larger bradycardias during brief apnea; at P5–6, average and severe events were ∼50% and 70% greater, respectively, in lesioned animals (P = 0.002), whereas at P10–12, events were ∼ 23% and 50% greater (P = 0.018). However, lesioning had no effect on the HR responses to sudden gas challenge or the HBR. At P5–6, lesioned animals had reduced breathing frequency and ventilation (V̇e), but normal V̇e relative to metabolic rate (V̇e/V̇o2). At P10–12, lesioned animals had a more unstable breathing pattern (P = 0.04) and an enhanced V̇e response to moderate hypercapnia (P = 0.007). Within the first two postnatal weeks, the medullary 5-HT system plays an important role in cardiorespiratory control, mitigating spontaneous bradycardia, stabilizing the breathing pattern, and dampening the hypercapnic V̇e response.
Keywords: neonate, CO2, breathing, SIDS, Hering-Breuer, chemoreflex
sporadic apnea and bradycardia are hallmarks of early postnatal life (20). Although progress has been made toward characterizing inhibitory neurotransmitters responsible for this cardiorespiratory instability [e.g., GABA, adenosine (1)], there is little information regarding the role of serotonin (5-HT). This is of interest because 5-HT not only projects to multiple brain stem loci participating in cardiorespiratory control, but also interacts extensively with GABA and adenosine signaling within these loci (1, 10, 11). Apnea, bradycardia, and the resulting desaturation are highly associated with an increase in morbidity and mortality (12, 22). Infants also display overt apnea and bradycardia shortly before succumbing to the sudden infant death syndrome (SIDS) (26, 35), an affliction that is strongly associated with disorders within the medullary 5-HT system, located predominantly in the raphé nuclei (32).
Accumulating data obtained from a variety of unanesthetized, intact animal models suggests that the medullary 5-HT system makes a significant contribution to eupnea as well as the ventilatory response to an increase in Pco2/[H+] within central chemosensitive regions (16, 17, 19, 25, 28, 30, 39). There is a lack of information, however, pertaining to the role of the 5-HT system within the neonatal respiratory control system. In piglets, focally inhibiting the medullary raphé with 8-hydroxy-2-(di-N-propylamino) tetralin, a 5-HT1A agonist (i.e., promoting mainly inhibition), has been shown to have an age-dependent effect on the ventilatory CO2 response: in animals older than approximately postnatal day 8 (∼P8) the response is inhibited, while in animals younger than ∼P8 the response is enhanced (27). Pet-1−/− mice (lacking ∼70–80% of their hindbrain 5-HT) breathe irregularly during the first week of life and at P4.5 have normal hypercapnic ventilatory responses (13). Later developmental time points were not examined. In contrast, as adults, lmx-1b−/− mice (missing nearly all brain stem 5-HT) have blunted respiratory responses to inhaled CO2 (19). It appears from these data that within the respiratory control system, the medullary 5-HT system could act in an age-dependent fashion.
Medullary 5-HT also impinges on cardiac control. In adult rats, 5-HT microinjections within the nucleus of the solitary tract (NTS) antagonize the bradycardias caused by chemoreceptor, pulmonary, and baroreceptor activation (6, 7). Activation of the raphé obscurus (ROb) is sufficient to reduce the bradycardia in response carotid body activation (8, 41). Overexpression of the 5HT1A receptor increases the risk of severe bradycardia, hypothermia, and death in mice between P25 and P80 (4). Thus, 5-HT from the medullary raphé appears to be important, at least in older animals, for maintaining heart rate and cardiac output during eupnea or in response to chemoreflex activation.
It seems possible that medullary 5-HT could have an important role during early postnatal life, when rodents (and human infants) are prone to cardiorespiratory instability. This study asks two questions. First, does medullary 5-HT reduce the magnitude of the bradycardias that commonly occur in early life? Second, is it important for breathing stability and the hypercapnic ventilatory response, as has been shown in adult animals? To address these questions, we induced lesions within the medullary 5-HT system of newborn rats. Using a pneumotach mask system that permitted the isolation of the airways from the body chamber, as well as the sudden (near square-wave) introduction of gases, we examined the heart rate and ventilatory responses of lesioned animals to the sudden activation reflexes both stimulatory (the chemoreflexes) and inhibitory [the Hering-Breuer Reflex (HBR)] to breathing.
MATERIALS AND METHODS
Animals
Rat pups were obtained from seven different litters. For each litter, equal number of male and female littermates were assigned to drug or vehicle groups. Pups were injected with either 5,7-dihydroxytryptamine (5,7-DHT) (Sigma Aldrich, St. Louis, MO) (n = 15) or vehicle (n = 16) on P2. Experiments were performed on each animal between P5 and P6 and again between P10 and P12 (P0 = day of birth). Dams were provided food and water ad libitum and were housed with a 12:120-h light-dark cycle at an ambient temperature of 21–23°C. All surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of Dartmouth College.
Surgery
P2 pups were cold anesthetized for 15 min within a small glass cylinder immersed in ice water. When the pup was motionless, it was removed from the bath and placed on a bed of crushed ice covered in aluminum foil to maintain deep hypothermia. While in the prone position, the head of the animal was placed over a small bar within a stereotactic setup. An ∼1-cm incision was then made in the back of the neck, and the muscle layer was displaced laterally to expose the section of thin membrane covering the entrance to the cisterna magna. The tip of a 30-gauge needle was inserted into the cisterna magna, and 1 μl (∼40 ng total) of 5,7-DHT or vehicle (0.1% ascorbic acid in saline) was injected, with the needle held in place for 10 min to allow for drug diffusion throughout the ventral medulla. The needle was then removed, and the muscle and epidermal layers sutured. Animals were then allowed to warm gradually at room temperature and behaviors were documented. Two of eighteen drug-injected animals did not display the characteristic features of 5,7-DHT injection (24) (e.g., neither repetitive limb extension immediately following injection nor reduced weight gain compared with littermates) and were therefore not used for experiments.
Experimental Setup
The experimental setup we used for measuring V̇e, V̇o2, and heart rate in newborn rats is similar to a head-out system described previously (15). The system has been modified for the near square-wave delivery of gases. This is necessary for the characterization of the effects of immediate (e.g., mediated peripherally) chemoreflex activation on both V̇e and heart rate. In addition, the system physically isolates the head from the body chamber to allow for manipulation of body pressure for HBR induction.
The animal chamber (volume, ∼40 ml) was constructed from a water-jacketed glass cylinder that, when perfused with heated water from a water bath (ThermoNeslab, Newlington, NH), allowed for precise control of ambient and thus body temperature. Body temperature was continually monitored with a fine rectal thermocouple (Omega Engineering, Stamford, CT) that was fed along with ECG leads into the body chamber through a small opening in a 50-ml syringe thermoplastic elastomer gasket (Terumo Medical, Somerset, NJ). The leads and thermocouple were sealed into the gasket using a removable polyether compound (Impregum F Polyether Impression material; 3M, St. Paul, MN). In the other port in the rear gasket we placed an 18-gauge needle attached to a vacuum line via a three-way tap. In conjunction with another port providing a small leak, this allowed us to reliably generate an instantaneous −10 mmHg pressure change to the body compartment, which inflated the lung and initiated the HBR.
The V̇e in all animals was measured with a mask and pneumotach (Fig. 1). The head chamber was fashioned by inserting the cut end of a 20-ml syringe tube (volume, ∼3 ml) into a rubber gasket ring that sealed a small two-ply section of vinyl in place over the open end of the syringe tube. A small hole was then cut in the vinyl through which the snout of the animal was placed and sealed with the polyether material. To allow for a seal in the P10–12 group, fur was first removed from the snout of the animal by using a commercially available hair removal product (Nair; Church and Dwight, Princeton, NJ). A pneumotach was connected to the low-resistance inlet port of the syringe, while a downstream pump (AEI Technologies, Naperville, IL) connected to the outlet port pulled gas through the mask at a flow of either 110 ml/min (P5–6) or 160 ml/min (P10–12). The flow rate was sufficient for rapid washout of gas from the small mask chamber (time from inlet to mask, <1 s; time constant, ∼ 1 s; time to 95% washout, ∼4 s; equivalent to ∼7 breaths). Inspired gases were altered by placing the inlet port of the small mask chamber into a larger-bore (50 cc) tube that delivered room temperature gases directly from premix gas cylinders into the immediate surrounds of the mask (to be pulled through the mask by the pump) with no change in pressure within the mask. Exposing animals suddenly to room air gas from tanks did not alter V̇e. Inspiratory and expiratory airflows were detected by connecting both side arms of the pneumotach to a differential pressure transducer (Validyne Engineering, Northridge, CA). Integration of the flow trace provided respiratory volume, calibrated by injecting and withdrawing known volumes of air at the end of each experiment. Gas was pulled through a small, vertical column of Drierite (W. A. Hammond Drierite, Xenia, OH) before being analyzed for the fractional concentrations of CO2 and O2 by gas analyzers (AEI Technologies).
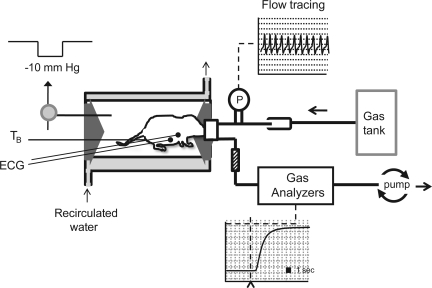
Fig. 1.
Experimental setup. Our system utilizes a pneumotach (P) and mask with a fast washout, as well as ECG, to measure the respiratory and heart rate (HR) responses to sudden changes in blood gases. Gas is delivered directly from gas tanks, flooding the surrounds of the mask, with gas being pulled through at the appropriate flow rate by a downstream pump. To measure metabolic rate, gases are fed through both O2 and CO2 analyzers after being pulled through a drying column. Because the head compartment is physically sealed from the body compartment, we were also able to generate a negative pressure (−10 mmHg) within the body compartment for the activation of the Hering-Breuer Reflex (HBR). Body temperature (TB) is regulated via a perfusable, jacketed glass chamber with temperature being continually measured with a rectal probe.
Heart rate data were obtained in most animals using standard ECGs. Conducting paste (Tenzo; D. O. Weaver, Aurora, CO) was applied to both the ventral surface of the animal (devoid of fur) as well as the ends of standard electrodes, which were fed through a small (4–5 cm) section of self-adhering, elastic tension bandage, which was then fastened around the body of the animal. In some animals, heart rate was obtained from a pulse oximeter (Starr Life Sciences, Oakmont, PA), which was adhered lightly to the tail with polyether material. All outputs were recorded at 1 kHz (PowerLab and Chart; ADInstruments, Colorado Springs, CO). Heart rate was also measured in some animals prior to being placed in the chamber and was found to be similar to that observed while on the mask.
Protocols
After being instrumented for ECG and body temperature, animals were placed in the water-jacketed chamber. Body temperature was normally ∼31–33°C upon being placed in the chamber. After being placed on the mask, animals were allowed to settle until body temperature reached 35 ± 0.5°C (∼10–15 min). Most animals appeared to be minimally stressed by our system, resting with occasional involuntary limb movement, indicative of sleep. However, we did not attempt to record or document sleep state. When the target temperature was achieved, breathing and heart rate data were continuously recorded for 15 min. The breathing and heart rate of one litter (3 control and 3 treated animals) was measured for 30 min in room air with no subsequent gas or HBR challenge. For all other litters, either gas challenges or HBR testing were then given in a randomized fashion with littermates always receiving the same pattern. For HBR testing, five lung inflation tests were given to both control (n = 12) and lesioned (n = 13) littermates at P10–12, separated by 2 min of room air recovery. For gas challenges, P5–6 and P10–12 animals were given a 1-min exposure to moderate normoxic hypercapnia (5% CO2 in 21% O2, balance N2; lesioned: n = 8; control: n = 8), moderate hyperoxic hypercapnia (5% CO2 in 95% O2; lesioned: n = 9; control: n = 8), severe normoxic hypercapnia (8% CO2 in 21% O2 balance N2; lesioned: n = 10; control: n = 11), severe hyperoxic hypercapnia (8% CO2 in 92% O2; lesioned: n = 10; control: n = 11), hypoxia (10% O2, balance N2; lesioned: n = 14; control: n = 15), and asphyxia (8% CO2 in 10% O2; lesioned: n = 6; control: n = 7). Two minutes of room air recovery was given between each test. All animals receiving moderate hypercapnia at P5–6 (at both levels of O2) also received it at P10–12. The order of gas treatment was randomized between litters. While not all gases were given to all animals, all drug- or vehicle-treated members of any one litter received the same gases in the same order.
Immunohistochemical Analysis
After testing, rat pups (P12–13) were deeply anesthetized with an intraperitoneal injection of 10 mg/kg ketamine and xylazine. After confirming full anesthesia with a noxious tail pinch, pups were perfused through the ascending aorta with 5 ml chilled saline followed by ∼120 ml of chilled 4% paraformaldehyde Brains were removed, stored in 4% paraformaldehyde overnight, and then equilibrated in a solution of 25% sucrose in 0.1 M phosphate buffer. Brains were then frozen and sectioned 40-μm thick. Sections were stored in a cryoprotectant solution (30% sucrose, 30% ethylene glycol in phosphate buffer ) at −20°C until processing.
Every third section was processed free floating for immunofluorescence detection of 5-HT and tyrosine hydroxylase (TH). 5-HT was detected using a rabbit 5-HT antisera (Chemicon International, Temecula CA) diluted 1:10,000. TH was detected using a mouse TH antisera (Chemicon International) diluted 1:2,000. Both of these primary antisera were diluted together in 0.1 M PBS, 0.3% Triton X-100 (PBST), 0.04% BSA, and 0.1% sodium azide and incubated with the tissue for 2–3 days at 4°C. Secondary antisera were conjugated to CY3 (donkey anti-rabbit; Jackson Immunoresearch, West Grove, PA) or Alexa Fluor 488 (donkey anti-mouse, Invitrogen, Carlsbad, CA). Both secondary antisera were diluted 1:200 in PBST-BSA and incubated with the tissue for 60–90 min at room temperature. Between incubations sections were rinsed in excess PBS. All individuals from the same litter were processed at the same time using aliquots of the same reagents.
Following immunofluorescence labeling, sections were mounted on glass slides in rostral-to-caudal order using references from a stereotaxic atlas (33). Sections were sampled and analyzed while the researcher was blinded to treatment (5,7-DHT or vehicle-treated). Sections were photographed using conventional epifluorescence illumination for both fluorophores simultaneously, and images were pseudocolored and merged using Slidebook software (Intelligent Imaging Innovations, Denver, CO). For quantification of 5-HT and TH cell number, for each rat three representative sections were selected that bracketed the rostrocaudal midpoint of either the nucleus raphé magnus (RMg), ROb, raphé pallidus (RPa), or the A1/C1 adrenaline/nonadrenaline group. For RMg this was bregma −10.6 mm; for ROb, RPa, and A1/C1 this was bregma −12.7. On these sections, every cell residing in the cell group of interest was counted (33, 37). Thus for RMg, the triangular region from the midpoint of both pyramidal tracts to the apex distribution of 5-HT neurons was included. For ROb, paramedially dispersed cells dorsal to RPa neurons were counted. RPa neurons were distinguished from other groups of neurons by their propensity to cluster at the junction between the pyramidal tracts. These clustered neurons and contiguous midline ventromedullary surface cells were included in counts for RPa. For each area, the average number of cells per section was determined for each individual, which was then averaged across all individuals to generate group means.
Data and Statistical Analysis
Data are presented as means ± SE. Parameters reported are ventilatory frequency (fB, min−1), tidal volume (VT, ml/kg), and ventilation (V̇e = fB × VT, ml·min−1·kg−1). As a measure of breathing stability, we also report the coefficient of variation (%CV) in the breathing period during room air breathing. Metabolic O2 consumption (V̇o2) was calculated for all animals during room air breathing by using the difference in the fractional concentrations of each gas entering and leaving the mask chamber, multiplied by the flow (expressed as ml·min−1·kg−1). Heart rate is reported in beats/min.
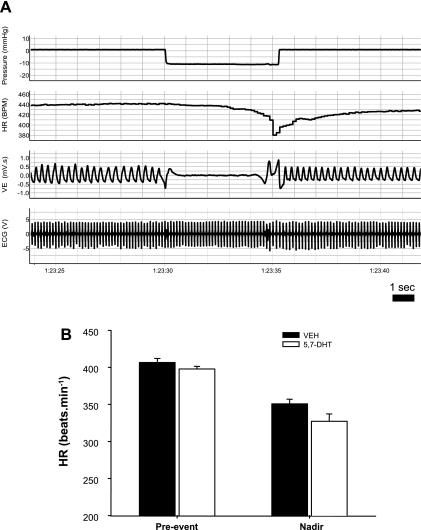
Mean ventilatory and heart rate data between lesioned and control animals during the 10 min of room air breathing were compared with Student's two-tailed t-test. The effect of drug treatment on the magnitude of spontaneous, room air bradycardias were assessed in both P5–6 and P10–12 groups during this 10-min period. A bradycardic event was counted if heart rate fell > 5% of the baseline value with a heart rate nadir being achieved in the period of a few breaths (i.e., 1–2 s). Nearly all bradycardias meeting these criteria were accompanied by a discernable change in V̇e (apnea and/or a disruption in the eupneic breathing pattern, see Fig. 4). The period of this disruption was measured from the beginning of hypoventilation until the resumption of normal breathing. The magnitude of bradycardic events was determined using the heart rate over ∼10 s prior to each event (baseline heart rate) and the minimum heart rate (regardless of its timing) that occurred within the event. The magnitude of bradycardias were compared between lesioned and control animals using a two-factor [factor 1: treatment; factor 2: time (heart rate pre- or during apnea)], repeated-measures ANOVA (2FRMA), using the average baseline heart rate and the average minimum heart rate achieved during the event. The heart rate and ventilatory responses to induction of the HBR were also quantified for both lesioned and control littermates. The magnitude and occurrence of bradycardias during HBR tests were most reliably determined in P10–12 animals, given the larger amplitude ECG signal and maintained signal-noise ratio during expansion of the thorax with HBR induction. Bradycardia magnitude was determined in the same fashion as spontaneous events, and drug effects were also determined using 2FRMA. One-factor ANOVA (1FA) was used to assess the effect of drug treatment on the duration of spontaneous breathing disruptions and induced apneas that were associated with bradycardic events, as well as on the average number of events experienced by each group.
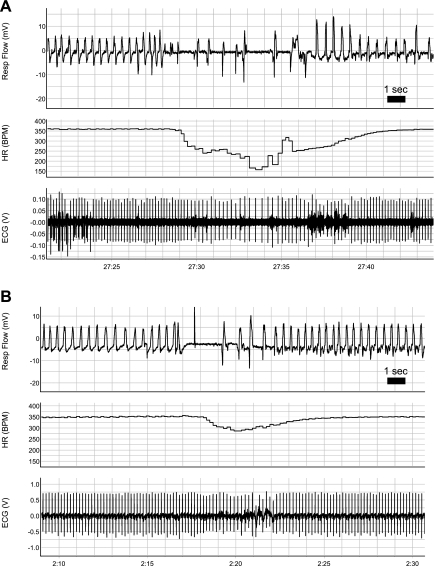
Fig. 4.
Examples of bradycardia associated with breathing disruptions in treated P5 neonates with medullary 5-HT lesions (A) and a control littermate (B). Note the more-pronounced fall in HR in the treated animal, associated with a longer disruption of eupnea, as well as the short delay (∼1 s) between the onset of hypopnea and the onset of bradycardia.
We compared the ventilatory responses of control and lesioned animals to 1 min of gas challenge using a 2FRMA (factor 1: inhaled gas; factor 2: lesion or control). In addition, when there was a significant difference identified between lesioned and control groups, we also determined whether the speed of the response differed between groups. We first smoothed the data using a moving average of three consecutive 2-s bins across the entire phase of the on-response. Subsequently, for each animal, the bin when the ventilatory response was 50% of the maximum response (Max50) was then determined, and values for Max50 were then compared between control and treated animals using a t-test.
With respect to the heart rate responses to chemo-challenge, similar to our analysis of spontaneous bradycardias, we wanted to determine whether reducing brain stem 5-HT had any effect on the minimum heart rates achieved during the 1-min challenge. This was assessed with a 2FRMA using the average heart rate for each animal 30 s prior to challenge and the minimum heart rate experienced over all of the 2-s bins of the challenge. Tukey's post hoc tests were performed when significant effects were found. Effects were considered significant at P < 0.05.
RESULTS
Effect of 5,7-DHT Treatment on Brain Stem 5-HT Neurons
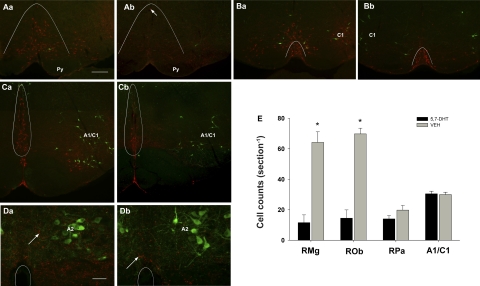
Brains from both control and 5,7-DHT-treated littermates were fixed, and sections from the brain stem and pons were immunostained for 5-HT and tyrosine hydroxylase. 5,7-DHT-treated animals had consistent abnormalities in brain stem raphé nuclei, while the rostral raphé appeared normal (not shown). Cell counts of lesioned (n = 10) and control (n = 10) animals (killed after testing at P12–13) confirmed that drug treatment significantly reduced the amount of 5-HT-positive neurons in the RMg and ROb (P < 0.001; Fig. 2). Remaining 5-HT neurons in the medulla were typically located in RPa; the number of 5-HT neurons in this region were not significantly different between groups (post hoc: P = 0.08). In addition, 5-HT axons throughout the medulla from treated rats were very sparse and dysmorphic, with swelling and intense 5-HT immunolabeling (Fig. 2). Catecholaminergic neurons throughout the medulla in 5,7-DHT-treated rats appeared identical to controls, and quantitative analysis of the A1/C1 region showed no change in cell number (P = 0.81; Fig. 2). Sectioning and staining of two P5-treated and control littermates also showed considerable lesioning within the RMg and ROb (not shown).
Fig. 2.
Intracisternal 5,7-dihydroxytryptamine (5,7-DHT)-altered cells and axons immunolabeled for serotonin (5-HT), but not tyrosine hydoxylase (TH) in the medulla. A: at bregma −10.6 mm, many 5-HT-immunolabeled neurons (red) are visible in raphé magnus (RMg) on the midline above the pyramidal tract (Py) in rats that received saline injections (Aa), but few are visible (arrow) in rats receiving 5,7-DHT (Ab). B: at bregma −11.3, TH-labeled cells (green, C1) are visible lateral to 5-HT cells (red) in raphé pallidus (RPa; under the curved line) in control (Ba) and 5,7-DHT-treated (Bb) rats. C: at bregma −12.7 mm, many 5-HT-labeled cells (red) in raphé obscurus (ROb, circled) are visible medial to A1/C1 catacholamine cells (green) in sections from control rats (Ca) and are reduced in 5,7-DHT-treated rats (Cb). D: at the level of the nucleus of the solitary tract (NTS) TH-labeled (green, A2) cells are innervated by 5-HT-immunolabeled axons (red, arrows). Circles designate the location of the central canal. Compared with control rats (Da), 5,7-DHT-treated rats (Db) have fewer 5-HT-labeled boutons (red) that tend to be large, while TH-immunolabeling appears equivalent. E: cell counts reveal 5,7-DHT produces a significant decrease in 5-HT cell number in RMg and ROb (P < 0.001), an insignificant reduction in RPa (P = 0.08) and no change in TH-immunolabeled cell number counted in the A1/C1 group. VEH, vehicle. A–C, same scale, scale bar in Aa = 250 microns; scale bar in Da = 50 microns.
Effect of Serotonergic Lesions on Body Weight and V̇o2
Animals with medullary 5-HT lesions weighed an average of 18.4% less by P5–6 compared with control littermates (P < 0.001; Table 1) and had a marginally (but not quite statistically significant) lower V̇o2 than treated animals (P = 0.09; Table 1). At P10–12, lesioned animals continued to lag behind controls in terms of body mass (P < 0.001; Table 2), but had a similar V̇o2 as controls (P = 0.42; Table 2).
Table 1.
Mean ventilatory, metabolic, and heart rate data from 5,7-DHT-treated, P5–6 rat pups and control littermates
| V̇e, ml·kg−1·min−1 | VT, ml/kg | fB, min−1 | CV, % | HR, min−1 | V̇o2, ml·kg−1·min− | V̇e/V̇o2 | Mass, g | |
|---|---|---|---|---|---|---|---|---|
| 5,7-DHT | 1368.3±70.6* | 10.5±0.4 | 130.8±4.4* | 16.8±1.7 | 366.0±8.6 | 58.0±3.5 | 24.3±1.2 | 9.5±0.4* |
| VEH | 1603.2±89.1 | 10.5±0.5 | 155.4±3.8 | 14.4±0.5 | 382.8±5.9 | 65.3±4.3 | 26.4±1.7 | 11.7±0.4 |
Data are means ± SE; 5,7-dihydroxytryptamine (5,7-DHT)-treated postnatal day 5–6 (P5–6) rat pups (n = 16) and vehicle (VEH) control littermates (n = 17). Animals with medullary 5-HT lesions had a significantly reduced respiratory frequency (fB) (*P < 0.001) and V̇e (*P = 0.04). No significant differences were observed between groups with respect to breathing stability as measured by the coefficient of period variability (CV), heart ate (HR), metabolic O2 consumption (V̇o2), or the ventilatory equivalent (V̇e/V̇o2). Lesioned animals weighed significantly less than controls (*P < 0.001).
Table 2.
Mean ventilatory, metabolic, and heart rate data from 5,7-DHT treated, P10–12 rat pups and control littermates
| V̇e, ml·kg−1·min−1 | VT, ml/kg | fB, min−1 | CV, % | HR, min−1 | V̇o2, ml·kg−1·min− | V̇e/V̇o2 | Mass, g | |
|---|---|---|---|---|---|---|---|---|
| 5,7-DHT | 1576.3±81.2 | 9.80±0.5 | 164.3±6.5 | 12.5±0.9* | 410.1±6.2 | 66.5±4.2 | 24.8±1.5 | 17.2±0.8* |
| VEH | 1527.3±72.4 | 9.51±0.4 | 162.0±5.1 | 10.4±0.5 | 407.4±8.8 | 62.6±3.9 | 25.5±1.6 | 24.7±0.6 |
Data are means ± SE; 5,7-DHT treated P10–12 rat pups (n = 16) and control littermates (n = 17). Animals with medullary 5-HT lesions had recovered a normal fB and V̇e but developed a significantly more unstable respiratory pattern as measured by the CV (*P = 0.04). No significant differences were observed between groups with respect to HR, V̇o2, or the ventilatory equivalent (V̇e/Vo2≈). Lesioned animals remained significantly lighter than controls (*P < 0.001).
Effect of Serotonergic Lesions on Cardiorespiratory Parameters During Room Air Breathing
The V̇e and heart rate at P5–6 and P10–12 were examined in lesioned and control littermates while they were breathing room air. At P5–6, lesioned animals had a significant reduction in fB, with an average decrease of ∼25 respiratory events per minute compared with control littermates (P < 0.001; Table 1). This reduced breathing frequency resulted in a reduced V̇e (∼15%) in lesioned animals compared with controls (P = 0.04; Table 1). However, the matching of V̇e to V̇o2 was not different between lesioned and control animals (t-test comparing V̇e with V̇o2: P = 0.36). At P5–6, neither heart rate nor breathing stability were different between control and lesioned littermates (heart rate: P = 0.10; CV%: P = 0.09; Table 1).
By P10–12, lesioned animals had a normal breathing frequency and overall V̇e (Table 2), but had more unstable breathing. The CV% of the breathing period was ∼20% greater in 5-HT depleted animals compared with controls (P = 0.04; Table 2).
Effect of Brain Stem Serotonergic Lesions on Spontaneous Bradycardic Events
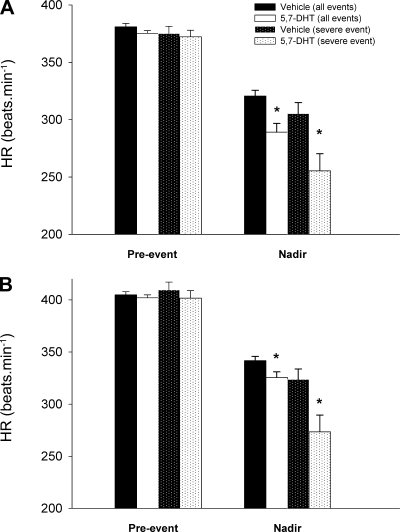
For both lesioned and control littermates breathing room air, we quantified the occurrence and magnitude of bradycardias associated with disruptions to eupnea. The number of bradycardias experienced for each animal within each group was the same (P = 0.68; Table 3). The number of bradycardic events was significantly greater at P10–12 compared with P5–6 (time effect: P = 0.007; Table 3). For each bradycardic event, we quantified the pre-event heart rate and the minimum heart rate during the bradycardia. In the P5–6 group, the bradycardias of P5–6 lesioned animals are on average ∼50% greater than the bradycardias from control animals (drug: 375.3 ± 2.4 beats/min to 289.1 ± 7.6 beats/min; control: 380.9 ± 2.9 beats/min to 320.6 ± 4.9 beats/min; 2FRMA interaction effect: P = 0.002; Fig. 3A). Furthermore, lesioned animals experience on average a 70% greater fall in heart rate during the most severe bradycardias (drug: 372.3 ± 5.7 beats/min to 255.4 ± 14.9 beats/min; control: 375.0 ± 6.3 beats/min to 341.7 ± 9.6 beats/min; 2FRMA interaction effect: P = 0.004; Fig. 3A).
Table 3.
Bradycardia occurrence and duration of associated breathing disruptions in 5,7-DHT-treated rat pups and control littermates
| Age | Treatment | No. Bradycardias | Bradycardias/Animal | Hypopnea Duration, s |
|---|---|---|---|---|
| P5–6 | Vehicle | 39 | 2.4±0.5 | 3.4±0.4 |
| 5,7-DHT | 42 | 2.7±0.6 | 4.3±0.5 | |
| P10–12 | Vehicle | 74 | 4.6±1.0* | 2.3±0.3 |
| 5,7-DHT | 68 | 5.0±1.0* | 3.2±0.3† |
Lesioning the medullary 5-HT system had no effect on the occurrence of bradycardias at either age. Bradycardic events occurred more frequently during the 15 min of room air breathing in P10–12 animals compared with P5–6 littermates (*P = 0.007 compared with P5–6). Note however that disruptions to eupnea tend to be longer in lesioned animals at both ages. In P10–12 animals, this difference is statistically significant (†P = 0.04 compared with control).
Fig. 3.
Average effect of lesioning the medullary 5-HT system on spontaneous, room air (RA) bradycardias. Mean data comparing the severity of bradycardias in control animals (n = 15) and littermates with 5,7-DHT-induced medullary 5-HT lesions (n = 16) at P5–6 (A) and P10–12 (B). Shown are mean values for HR just before the bradycardic episode (Pre-event HR), as well as for the minimum HR (Nadir) achieved across all bradycardias and across only the most severe event in each animal. *Significantly lower HR experienced by drug-treated animals, especially with respect to the most severe event in each animal (P5–6: P = 0.004; P10–12: P = 0.03).
At P10–12, the bradycardias of lesioned animals continued to be more pronounced, with an average 22% greater decrease in heart rate (drug: 402.0 ± 2.9 beats/min to 325.6 ± 5.4 beats/min; control: 404.7 ± 3.1 beats/min to 341.7 ± 4.1 beats/min; 2FRMA interaction effect: P = 0.018; Fig. 3B). The susceptibility of lesioned animals was most evident during severe events; with respect to the largest bradycardia in each animal, heart rate fell on average 50% more in lesioned animals than control littermates (drug: 401.7 ± 7.2 beats/min to 273.4 ± 16.1 beats/min; control: 409.6 ± 7.4 beats/min to 323.7 ± 10.1 beats/min; 2FRMA interaction effect: P = 0.03; Fig. 3B). In fact, the average heart rate decline among the largest bradycardic events in control animals was similar to the average heart rate decline across all bradycardic events in animals with lesions in the 5-HT system (Fig. 3B). An example of a bradycardic event from both a lesioned and control animal are shown in Fig. 4. Note that the drop in heart rate can occur very soon (∼1–2 s) after the onset of the breathing disruption. As suggested by Fig. 4, disruptions to eupnea that are associated with bradycardias are slightly longer in duration in 5-HT-lesioned animals compared with their control siblings (P = 0.04; Table 3).
These data indicate that while 5-HT-lesioned and control animals experience the same number of bradycardias during normal breathing, throughout the first two postnatal weeks lesioned animals experience more pronounced declines in heart rate during disruptions to eupnea.
Effect of Lesioning the Medullary 5-HT System on the Cardiorespiratory Response to HBR Activation
We utilized our system to systematically examine the cardiac response of lesioned and control animals to HBR-induced apnea. An example trace is shown in Fig. 5A. The magnitude of the bradycardic responses to HBR activation was not affected by lesions to the 5-HT system (2FRMA, P = 0.19; Fig. 5B). Furthermore, although there was variable occurrence of bradycardia across the five HBR tests within each animal, overall there was no significant effect of the lesion on the incidence of bradycardia (control: 2.8 ± 0.5 episodes; lesioned: 3.9 ± 0.5 episodes; 1FA: P = 0.11) or on the duration of apnea between control and lesioned groups (control: 1.9 ± 0.4 s; lesioned: 2.8 ± 0.6 s; P = 0.36).
Fig. 5.
Induction of the HBR in animals with medullary 5-HT lesions and control littermates at P10–12. A: example showing the apnea and bradycardia resulting from a drop (−10 mmHg) in pressure within the body compartment. V̇e, ventilation. B: there was no significant difference in the magnitude of bradycardia between control (n = 12) and lesioned (n = 13) littermates (P = 0.19).
Effect Medullary 5-HT Lesions on the Heart Rate Responses to Hypoxia, Hypercapnia, and Asphyxia
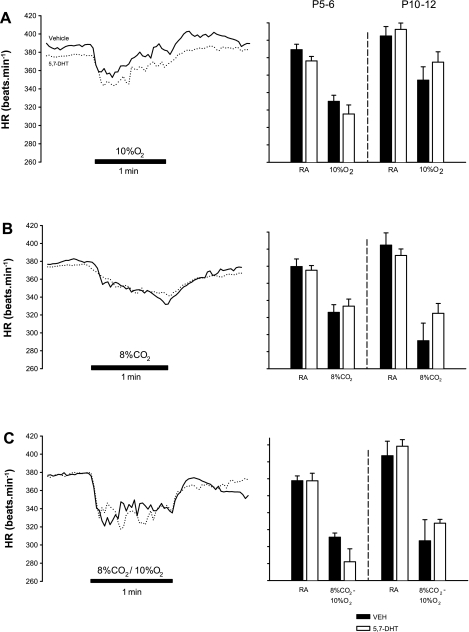
In adults, brain stem 5-HT is an important regulator of heart rate responses to peripheral chemoreceptor activation. Accordingly, we measured the heart rate responses to near square-wave hypoxia, hypercapnia, and asphyxia; this enables the resolution of the heart rate responses to both peripheral and total chemoreflex activation. Heart rate responses to 5% CO2 were minimal (<10%) and are therefore not shown. The responses to hypoxia, severe hypercapnia, and asphyxia are shown in Fig. 6, A–C, respectively. The heart rate response to hypoxia was biphasic, displaying an initial bradycardia followed by a return to baseline levels (Fig. 6A, left). At both ages, reducing the number of 5-HT neurons had no significant effect on the magnitude of the change in heart rate during hypoxia (P5–6: control: −15.1 ± 1.8%; lesioned: −16.2 ± 2.5%; P = 0.73; P10–12: control: −12.6 ± 2.8%; lesion: −9.2 ± 2.3%; 2FRMA: P = 0.72; Fig. 6A, right). The heart rate decline with 8% CO2 was gradual and monophasic (Fig. 6B, left) and was also the same between control and 5-HT-lesioned animals (P5–6: control: −13.9 ± 2.4%; lesioned: −11.2 ± 2.1%; 2FRMA: P = 0.33; P10–12: control: −27.5 ± 5.4%; lesioned: −17.1 ± 3.2%; 2FRMA: P = 0.08; Fig. 6B, right). The heart rate responses to asphyxia were the most abrupt and most severe (Fig. 6C, left), but overall there was no effect of lesioning on the magnitude of the bradycardia (P5–6: control: −17.6 ± 2.1%; lesioned: −25.0 ± 5.0%; 2FRMA: P = 0.18; P10–12: control: −23.6 ± 7.6%; lesioned: −21.5 ± 2.3%; 2FRMA: P = 0.18; Fig. 6C, right).
Fig. 6.
Changes in HR associated with sudden changes in inspired gases in animals with medullary 5-HT lesions and control littermates. Example traces are shown (left), demonstrating the typical fall in HR associated with hypoxia (A; 10% O2), hypercapnia (B; 8% CO2), and asphyxia (C; 10% O2 with 8% CO2) in both vehicle controls (solid lines) and 5,7-DHT-treated littermates (dotted lines). The most dramatic and abrupt fall in HR occurred with asphyxia. Mean data for HR in RA and the minimum HR achieved during gas challenge are shown (right) for both P5–6 and P10–12 vehicle and 5,7-DHT-treated animals. Although at P5–6-treated animals tended to have more severe bradycardias during asphyxia (C) than control animals, for all gases there were no significant differences observed with respect to the minimum HR achieved during the challenge.
Effect of medullary 5-HT lesions on the ventilatory responses to hypoxia, hypercapnia and asphyxia
Ventilatory responses to normoxic hypercapnia.
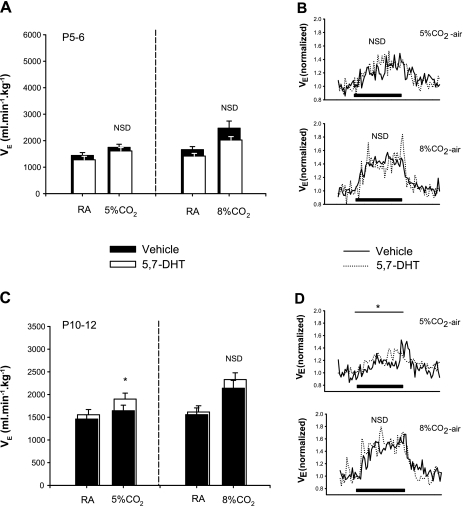
At P5–6, both control and lesioned animals had significant ventilatory responses to moderate and severe normoxic hypercapnia (P < 0.001 for both levels). Lesioning the 5-HT system had no effect on the magnitude of the response. During a 1-min exposure to moderate hypercapnia, control and lesioned animals increased their V̇e by 22.0 ± 5.5% and 28.6 ± 7.1%, respectively, relative to room air (2FRMA comparing gas × treatment interaction: P = 0.76; Fig. 7A, left). During a 1-min exposure to severe hypercapnia, the V̇e of control and lesioned animals increased by 40.1 ± 4.6% and 38.0 ± 6.2%, respectively, relative to room air (2FRMA comparing gas × treatment interaction: 0.35; Fig. 7A, right).
Fig. 7.
Ventilatory response to normoxic hypercapnia in animals with medullary 5-HT lesions and control littermates. Average responses over 1 min of normoxic hypercapnia are shown for 5.7-DHT-treated and control littermates at P5–6 (A and B) and P10–12 (C and D). Average data for breathing during RA and during the challenge are summarized in (A) and (C) for moderate (5% CO2-21% O2; control: n = 8; treated: n = 8; left) and severe (8% CO2-21% O2; control: n = 10; treated: n = 11; right) normoxic hypercapnia, while (B) and (D) show the ventilatory dynamics in response to moderate (B and D, top) and severe (B and D, bottom) hypercapnia. Note that while there is no significant difference (NSD) in the ventilatory responses of treated and control animals at P5–6 (A), treated animals have a significantly greater response to moderate CO2 at P10–12 (*P = 0.007). NSD existed with respect to the ventilatory response to severe CO2 challenge (C, right and D, bottom). NSD existed with respect to the rate of increase in breathing during hypercapnia for vehicle control (solid line) and 5,7-DHT-treated littermates (dotted line) (as measured by Max50; see text). Black bar indicates the 1-min period of CO2 challenge.
At P10–12, both control and lesioned animals experienced a significant increase in V̇e over the course of 1 min of either moderate or severe hypercapnia (P < 0.001 for both levels). However, lesioned animals had a significantly augmented ventilatory response to moderate hypercapnia: lesioned and control animals increased their V̇e by 21.4% and 11.4 ± 2.3%, respectively, relative to room air (2FRMA comparing gas × treatment interaction: P = 0.007; Fig. 7C, left). Despite this increased sensitivity, the rate of the response was not different between groups, with control and lesioned animals having Max50 values of 23.4 ± 4.8 s and 18.2 ± 3.4 s, respectively (compare on-response in Fig. 7D, top; P = 0.35). In response to severe hypercapnia, the ventilatory responses of each group was similar: lesioned and control animals increased their V̇e by 50.0 ± 14.2% and 41.2 ± 8.5%, respectively, relative to room air (2FRMA comparing gas × treatment interaction: P = 0.22; Fig. 7C, right). Again, as is evident in Fig. 7D, bottom, the rate of the ventilatory increase in response to severe hypercapnia was the same: control and lesioned animals Max50 was 17.8 ± 3.2 s and 16.8 ± 3.8 s, respectively (t-test: P = 0.83).
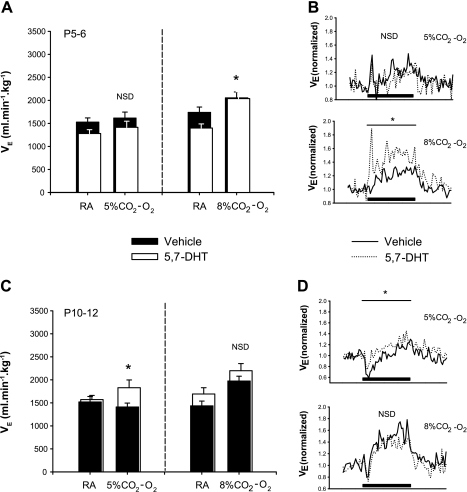
Ventilatory responses to hyperoxic hypercapnia.
At P5–6, hyperoxia effectively blunted the ventilatory response to moderate CO2 (2FRMA, overall CO2 effect: P = 0.12), and there was no significant effect of the lesion on either the magnitude of the response (2FRMA comparing gas × treatment interaction: P = 0.74; Fig. 8A, left) or on the speed of the on-response, which tended to be rather variable [Max50: 16.8 ± 6.8 s (control); 12.0 ± 4.2 s (lesioned); P = 0.54; compare ramping phase, Fig. 8B, top]. Hyperoxia did not completely abolish the ventilatory response to severe hypercapnia (2FRMA, overall CO2 effect: P < 0.001), and lesioned animals had a greater response: lesioned and control animals increased their V̇e by 47.3 ± 8.8% and 19.0 ± 3.1%, respectively, over room air levels (2FRMA comparing gas × treatment interaction: P = 0.012; Fig. 8A, right). Although the on-response tended to be faster in lesioned animals compared with controls, this difference did not reach statistical significance [P = 0.20; Max50: 19.0 ± 3.6 s (control) vs. 13.6 ± 3.0 s (lesioned); Fig. 8B, top].
Fig. 8.
Ventilatory responses to hyperoxic hypercapnia in animals with medullary 5-HT lesions and control littermates. Average responses over 1-min challenge are shown for 5.7-DHT-treated and control littermates at P5–6 (A and B) and P10–12 (C and D). Average data for breathing during RA and during 1-min challenge are summarized in (A) and (C) for moderate (5% CO2–95% O2; control: n = 8; treated: n = 9; left) and severe (8% CO2-92% O2; control: n = 11; treated: n = 10; right) hyperoxic hypercapnia, while B and D show the ventilatory dynamics in response to moderate (B and D, top) and severe (B and D, bottom) hypercapnia. Note that at P5–6 (A and B), while there is no differences between groups with respect to moderate hyperoxic challenge, animals with medullary 5-HT lesions have an enhanced response to severe hyperoxic CO2 relative to controls (A, right and in B, bottom: * P = 0.012). By P10–12, similar to the effects of moderate normoxic hypercapnia, lesioned animals also have an increased ventilatory sensitivity to moderate hyperoxic hypercapnia (*C, left and in D, top), with no difference in the response to severe hyperoxic hypercapnia (C, right and D, bottom: NSD). No differences existed with respect to the rate of increase in breathing during hypercapnia for vehicle control and 5,7-DHT-treated littermates (as measured by Max50; see text). Black bar indicates the 1-min period of CO2 challenge.
Similar to the response at P5–6, hyperoxia blunted the ventilatory response to moderate hypercapnia in P10–12 animals (2FRMA, overall CO2 effect: P = 0.35). The V̇e of lesioned animals, however, was significantly greater over the 1 min of moderate hyperoxic hypercapnia compared with control animals (2FRMA comparing gas × treatment interaction: P = 0.03; Fig. 8C, left and 8D, top). Again, like P5–6 animals, P10–12 animals had a significant ventilatory response to severe hyperoxic hypercapnia (2FRMA, overall CO2 effect: P < 0.001) with no effect of reducing 5-HT neurons on the magnitude of the response: the V̇e of lesioned and control animals increased by 29.9 ± 5.2 and 37.4 ± 6.5%, respectively (2FRMA comparing gas × treatment interaction: P = 0.47; Fig. 8C, right). There was also no effect of lesioning on the speed of the on-response [Fig. 8D, bottom; Max50: 25.8 ± 3.2 s (control); bin 26.8 ± 4.4 (lesioned); P = 0.83].
Taken together, reducing brain stem 5-HT content in P5–6 animals has no effect on the ventilatory responses to a moderate level of inhaled (and systemic) Pco2, regardless of the background Po2. By P10–12, lesioned animals have an enhanced ventilatory response to moderate hypercapnia, regardless of background PO2.
Effect of Medullary 5-HT Lesions on the Hypoxic and Asphyxic Ventilatory Responses
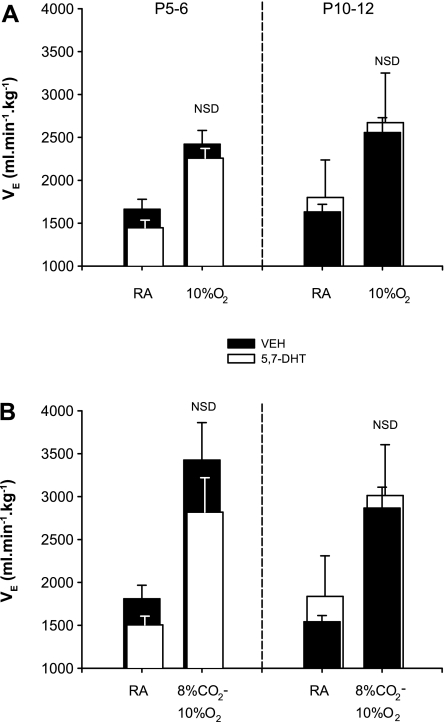
We also exposed both lesioned and control littermates to a near square-wave of 10% O2. Hypoxia stimulated V̇e in both groups and at both ages (P < 0.001); however, there was no effect of lesioning on the magnitude of the response at either age. In control and lesioned animals, breathing increased by 59.5 ± 9.6% and 45.1 ± 4.7%, respectively, at P5–6 (2FRMA: drug × gas interaction: P = 0.48; Fig. 9A, left), and by 51.6 ± 7.2% and 54.8 ± 8.8%, respectively, at P10–12 (2FRMA: drug × gas interaction: P = 0.74; Fig. 9A, left).
Fig. 9.
Ventilatory responses over 1 min of (A) hypoxia and (B) asphyxia in 5.7-DHT-treated and control littermates. Average data for breathing during RA and during hypoxia (10% O2; control: n = 15; treated: n = 14) and asphyxia (8% CO2, 10% O2; control: n = 7; treated: n = 6) are indicated in for P5–6 (left) and P10–12 (right) littermates. There was no effect of lesioning on the magnitude of ventilatory responses to either hypoxia (P5–6: P = 0.48; P10–12: P = 0.74) or asphyxia (P5–6: P = 0.48; P10–12).
Similarly, 1 min of asphyxia (8% CO2, 10% O2, balance N2) had similar effects on control and lesioned animals. Relative to baseline, V̇e increased 85.3 ± 23.1% and 76.2 ± 8.2% in P5–6 lesioned and control animals, respectively, and at P10–12, V̇e increased 78.0 ± 11.1% and 84.7 ± 11.8%, respectively [2FRMA, drug × gas interaction: P = 0.53 (P5–6); P = 0.58 (P10–12); Fig. 9B].
DISCUSSION
We assessed the heart rate and breathing of neonatal rat pups with a pharmacologically induced reduction of medullary 5-HT neurons during room air breathing and during the sudden activation of the chemoreflexes (excitatory to breathing) and the HBR (inhibitory to breathing). Within the first two postnatal weeks, rat pups with lesions suffer more severe spontaneous bradycardia; however, this effect cannot be fully explained by an altered sensitivity of the heart to either sudden chemoreflex activation or activation of the HBR. In addition, by the second postnatal week animals with a reduced number of 5-HT neurons have more unstable breathing and have enhanced respiratory responses to elevated Pco2. These findings support the hypothesis that 5-HT is important for cardiorespiratory homeostasis in the early postnatal period. A reduction in medullary 5-HT neurons or abnormal 5-HT signaling may, therefore, make neonates (including human infants) more susceptible to life-threatening cardiorespiratory events.
Methodological Considerations
Accurately determining VT and V̇e in small newborn mammals is not trivial. Methods commonly employed for studying breathing in adult animals (e.g., the barometric method) are not suited for measuring V̇e in neonates. This is owed in part to error introduced by the small difference between ambient and body temperature, but particularly troublesome is the contamination imparted on the respiratory signal by a signal caused by the rarefaction and compression of gases within the airways (29). We have measured V̇e in our animals using a head-out system based on one described previously (15). This system directly measures the flow of air produced by breathing (thus giving an accurate measure of VT and V̇e), and has been modified for the near square-wave delivery of gases and the measurement of ECG. Near square-wave delivery of gases are crucial for resolving the changes in V̇e and heart rate that are owing to the immediate activation of the chemoreceptors. Furthermore, because the head chamber is physically isolated from the body chamber, the pressure in the body chamber can be manipulated for the induction of the HBR. This avoids the potentially confounding effects of changes in airway pressure on breathing. To our knowledge, no other preparation exists that permits the direct measurement of the V̇e and heart rate responses of unanesthetized, intact neonatal mammals to induced apnea (i.e., the HBR) or to near square-wave delivery of gases.
5,7-DHT can, in certain circumstances, be toxic to catecholaminergic neurons. However, a normal number of catecholaminergic neurones were present in the A1/C1 region of the caudal medulla, and, although not quantitatively examined, all other catecholamine nuclei including the A2 region of the NTS appeared normal. In contrast, there was a considerable reduction (∼80%) in the number of neurons within the RMg and ROb, as well as a considerable number of dysmorphic serotonergic axons throughout the medulla. Thus, it is extremely unlikely that the effects we describe herein are owing to an effect on catecholaminergic neurons and not serotonergic neurons. The number of neurons in RPa was not significantly affected (P = 0.08). Although we have no obvious explanation for this, the high density of cells in this region and our use of traditional epifluorscence (and not confocal) microscopy may have increased the error in cell counts.
Animals with 5-HT lesions are significantly smaller than control littermates at both ages, raising the possibility that the cardiorespiratory phenotypes observed in lesioned animals may be secondary to changes in development and/or metabolism. Lmx1b−/− mice, missing nearly all 5-HT neurons, are also smaller as adults and have a relatively reduced V̇o2 during mild cold stress compared with wild-type littermates (18). Although we have not systematically studied the thermogenic ability of our lesioned animals (all experiments were done with body temperature held constant at 35°C), our data indicate that there were no differences in V̇o2 between treated and control animals. However, this does not preclude the possibility that a developmental, rather than physiological, defect underlies the described phenotype of lesioned animals.
Role for Medullary 5-HT in Room Air Breathing
Compared to controls, neonates with fewer medullary 5-HT neurons also have a reduced breathing frequency and V̇e at P5–6 and a decrease in breathing stability by P10–12. Serotonergic projections impinge on almost all respiratory-related brain stem nuclei, and there is evidence that 5-HT and its agonists, when either bath or focally applied to neonatal rodent brain stem slice preparations, can increase the frequency of respiratory motoneuron activity (2, 36). Our data, as well as those obtained from neonatal Pet-1−/− mice and adult lmx1b−/− mice, support these in vitro findings (13, 19). That being said, lesioned animals are probably not hypoxic or hypercapnic to any great degree because their V̇e/V̇o2 is similar to control littermates (Table 1).
Associated with the decrease in stability during the second postnatal week was an increased ventilatory response to moderate CO2. Although we have no direct evidence, an increase in the gain of chemoreceptive elements with fewer medullary 5-HT systems (thus increasing total loop gain of the control system) could contribute to the decrease in stability, as has been previously described (9).
Medullary 5-HT and Bradycardic Events
Our results suggest that during the first two weeks of life, brain stem 5-HT plays a crucial role in maintaining heart rate during brief disruptions to eupnea. But where is 5-HT exerting its influence and in what physiological context? 5-HT-positive neurons residing in the medullary raphé are certainly well positioned to influence the control of heart rate in early postnatal life, sending projections to the NTS and the nucleus ambiguous, where a large proportion of preganglionic cardiac vagal neurons (CVPNs) reside (21, 38). These neurons are influenced by inputs arising from both rhythm-generating neurons and the chemoreceptors (23). During normal breathing, phasic inhibition of CVPNs by GABAergic and glycinergic inhibitory neurons (mediated by respiratory rhythm-generating neurons) contributes to the well-described respiratory sinus arrhythmia. The incidence of overt bradycardia is lessened by the presence of these inhibitory inputs to CVPNs (31). In contrast, during apnea or hypopnea the activity of CVPNs is relatively enhanced due to 1) a decrease in GABAergic and glycinergic inputs (if apnea is of central origin), 2) peripheral chemoreceptor inputs to CVPNs via the NTS, and 3) decreased inputs from pulmonary stretch receptors (3, 5, 42). In adult rats, carotid body stimulation with concurrent activation of the ROb results in a more subtle bradycardia than with carotid body stimulation alone (41). With this in mind, the enhanced bradycardia during brief disruptions to eupnea in pups missing medullary 5-HT neurons may be the result of a lack of GABAergic and/or glycinergic “braking” of CVPN activity during peripheral chemoreceptor activation. This effect could be mediated through 5-HT receptors located either within the NTS or the nucleus ambiguous.
With our novel system, we assessed differences between control and lesioned animals with respect to the heart rate response to near square-wave gas challenges. This method should minimize the interference from central chemoreceptors on the response of heart rate to peripheral chemoreceptor inputs. We found that the bradycardias of lesioned animals were of the same magnitude as controls during sudden changes in blood gases. However, this experimental paradigm probably does not mimic perfectly the influence of hypoxia and/or hypercapnia on heart rate during apnea; the hyperventilation in response to hypoxia and/or hypercapnia likely leads to a maintenance or increase of heart rate through both central (e.g., GABAergic and glycinergic modulation of CVPNs) and peripheral (feedback from lung stretch receptors) mechanisms. We also cannot be sure that any of our inhaled gas challenges led to changes in blood gases akin to those occurring during spontaneous apnea. Furthermore, the periods of respiratory disruption were significantly longer in lesioned animals, so we cannot discount the possibility that a larger peripheral chemoreceptor response (impinging on CVPNs via the NTS) was responsible for the more profound bradycardias in lesioned animals.
We hypothesized that a stronger HBR, a prominent reflex in neonates inhibitory to breathing, might underlie both the prolonged apnea and augmented bradycardia in lesioned animals. However, neither the duration of HBR-induced apnea nor the occurrence or magnitude of the resulting bradycardias was significantly different compared with control littermates. Thus, it is unlikely that serotonergic projections originating in the ROb or magnus exert any significant physiological role with respect to ventilatory or heart rate responses to lung inflation.
Medullary 5-HT and the Sensitivity of Breathing to CO2
Our data indicate that, relative to control littermates, animals with fewer 5-HT neurons develop an enhanced ventilatory response to moderate hypercapnia by the second postnatal week. This result contrasts with those obtained from experiments using unanesthetized adult animals where the raphé are either excited or inhibited focally (25, 28, 30, 39), as well as those obtained from genetically-manipulated adult animals nearly devoid of 5-HT (19). Both suggest a stimulatory role for the raphé in the hypercapnic ventilatory response. There are a number of possibilities that could explain the current findings. The most obvious is that the raphé nuclei themselves are inhibitory to the hypercapnic response during the postnatal period, either directly as chemosensitive nuclei or indirectly by inhibiting other chemosensitive neurons. An alternative hypothesis is that 5-HT from the raphé nuclei increases the reactivity of the cerebral vasculature to hypercapnia: an absence of 5-HT reduces the magnitude of the cerebrovascular response to increasing arterial PCO2, thus presenting a larger stimulus of central chemoreceptive neurons (34). Notably, our current results are supported by our previous finding that focal application of 8-hydroxy-2-(di-N-propylamino) tetralin (an agonist for the inhibitory 5-HT1A receptor) to the caudal raphé augments the CO2 response of younger piglets (<P8) and only becomes inhibitory later in development (27). Pet-1−/− neonatal mice (missing ∼70% of their brain stem 5-HT) have a normal ventilatory response to moderate hypercapnia in the first postnatal week (13). Older individuals were not tested. Thus, data from in vivo experiments suggest that the role of serotonergic neurons in ventilatory response to CO2 is age-dependent. These neurons are either unnecessary or inhibitory for the response in early postnatal life (with perhaps substance P or catecholamines playing a dominant stimulatory role) and take on a stimulatory role during adulthood. This idea is congruous with the observation that cultured 5-HT neurons are not chemosensitive until P12 (40).
Whether an enhanced hypercapnic chemoresponse contributes to the augmented bradycardias in lesioned animals remains to be proven. Although we did not find exacerbated heart rate responses to hypercapnia or asphyxia in the present studies, neither of the paradigms used to assess between-group differences with respect to chemoreflex responses (heart rate and ventilatory responses to inhaled gases) mimic perfectly the stimulatory and inhibitory inputs to cardiorespiratory control centers that would occur during spontaneous apnea.
Perspectives and Significance
We have shown that during the first 2 wk of postnatal life, the medullary 5-HT system: 1) mitigates acute bradycardias experienced during spontaneous disruptions to eupnea, 2) stabilizes the breathing pattern, and 3) dampens the hypercapnic ventilatory response. These observations support the hypothesis that medullary 5-HT, in addition to its role in adults, is important to cardiorespiratory homeostasis during the neonatal period.
Animals with medullary 5-HT lesions sometimes experienced severe events, with heart rate falling ∼70% more than controls. Although, in our hands, lesioned animals did not die during bradycardic episodes, our data suggest that defects within the medullary 5-HT system create an inherent susceptibility within an infant that could be exploited by exogenous stressors (e.g., temperature, cigarette smoke) to further increase the chances of a life-threatening cardiorespiratory event. Notably, some of the extreme bradycardias in lesioned animals (e.g., Fig. 4) are of similar magnitude as bradycardic episodes previously documented in SIDS infants immediately prior to death (26). With this in mind, future experiments exploring the interaction between the medullary 5-HT system and exogenous stressors should benefit our understanding of the etiology of SIDS, a disease that has been linked to both environmental triggers and 5-HT defects (14).
GRANTS
This work was supported by National Institutes of Health Grants HL-28066 and HD-36379. Support for K. J. Cummings was generously provided by the Parker B. Francis Foundation.
REFERENCES
- 1.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what's new? Pediatr Pulmonol 43: 937–944, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflügers Arch 431: 942–949, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Angell-James JE, Daly MD. The effects of artificial lung inflation on reflexly induced bradycardia associated with apnoea in the dog. J Physiol 274: 349–366, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science 321: 130–133, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bonsignore MR, Marrone O, Insalaco G, Bonsignore G. The cardiovascular effects of obstructive sleep apnoeas: analysis of pathogenic mechanisms. Eur Respir J 7: 786–805, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Callera JC, Bonagamba LG, Sevoz C, Laguzzi R, Machado BH. Cardiovascular effects of microinjection of low doses of serotonin into the NTS of unanesthetized rats. Am J Physiol Regul Integr Comp Physiol 272: R1135–R1142, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Callera JC, Sevoz C, Laguzzi R, Machado BH. Microinjection of a serotonin 3 receptor agonist into the NTS of unanesthetized rats inhibits the bradycardia evoked by activation of the baro- and chemoreflexes. J Auton Nerv Syst 63: 127–136, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Damaso EL, Bonagamba LG, Kellett DO, Jordan D, Ramage AG, Machado BH. Involvement of central 5-HT7 receptors in modulation of cardiovascular reflexes in awake rats. Brain Res 1144: 82–90, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey JA, Smith CA, Przybylowski T, Chenuel B, Xie A, Nakayama H, Skatrud JB. The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep. J Physiol 560: 1–11, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dergacheva O, Griffioen KJ, Wang X, Kamendi H, Gorini C, Mendelowitz D. 5-HT2 receptor subtypes mediate different long-term changes in GABAergic activity to parasympathetic cardiac vagal neurons in the nucleus ambiguus. Neuroscience 149: 696–705, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dergacheva O, Wang X, Kamendi H, Cheng Q, Pinol RM, Jameson H, Gorini C, Mendelowitz D. 5HT2 receptor activation facilitates P2X receptor mediated excitatory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuropharmacology 54: 1095–1102, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emancipator JL, Storfer-Isser A, Taylor HG, Rosen CL, Kirchner HL, Johnson NL, Zambito AM, Redline S. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med 160: 203–210, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol 159: 85–101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate 65: 194–197, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Frappell PB, Mortola JP. Respiratory function in a newborn marsupial with skin gas exchange. Respir Physiol 120: 35–45, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphé. J Appl Physiol 96: 1815–1824, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphé area of awake goats. J Appl Physiol 97: 2236–2247, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol 164: 350–357, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoppenbrouwers T, Hodgman JE, Harper RM, Hofmann E, Sterman MB, McGinty DJ. Polygraphic studies of normal infants during the first six months of life: III. Incidence of apnea and periodic breathing. Pediatrics 60: 418–425, 1977. [PubMed] [Google Scholar]
- 21.Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurones in the rat: immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J Comp Neurol 327: 572–583, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol 24: 763–768, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Jordan D Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol 90: 175–181, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Knuth ED, Etgen AM. Neural and hormonal consequences of neonatal 5,7-dihydroxytryptamine may not be associated with serotonin depletion. Brain Res Dev Brain Res 151: 203–208, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphé and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol 577: 307–318, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics 93: 44–49, 1994. [PubMed] [Google Scholar]
- 27.Messier ML, Li A, Nattie EE. Inhibition of medullary raphé serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol 96: 1909–1919, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Messier ML, Li A, Nattie EE. Muscimol inhibition of medullary raphé neurons decreases the CO2 response and alters sleep in newborn piglets. Respir Physiol Neurobiol 133: 197–214, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Canadian J Physiol Pharmacol 76: 937–944, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Nattie EE, Li A. CO2 dialysis in the medullary raphé of the rat increases ventilation in sleep. J Appl Physiol 90: 1247–1257, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res 93: 565–572, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998.
- 34.Peebles K, Celi L, McGrattan K, Murrell C, Thomas K, Ainslie PN. Human cerebrovascular and ventilatory CO2 reactivity to end-tidal, arterial and internal jugular vein Pco2. J Physiol 584: 347–357, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45: 350–354, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience 115: 1247–1259, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Steinbusch H Serotonin-immunoreactive neurons and their projections in the CNS. In: Handbook of Chemical Neuroanatomy, edited by Bjorklund A, Hokfelt T, and Kuhar M. Amsterdam: Elsevier Science, 1984, p. 68–121.
- 38.Steinbusch HW Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557–618, 1981. [DOI] [PubMed] [Google Scholar]
- 39.Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566: 543–557, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphé neurons. Neuroscience 90: 1001–1011, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Weissheimer KV, Machado BH. Inhibitory modulation of chemoreflex bradycardia by stimulation of the nucleus raphé obscurus is mediated by 5-HT3 receptors in the NTS of awake rats. Auton Neurosci 132: 27–36, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Zwillich C, Devlin T, White D, Douglas N, Weil J, Martin R. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest 69: 1286–1292, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]