Abstract
Decreases in fetal blood pressure stimulate homeostatic stress responses that help return blood pressure to normal levels. Fetal hypothalamus-pituitary-adrenal (HPA) axis responses to hypotension are mediated by chemoreceptor and baroreceptor reflexes and ischemia of the fetal central nervous system. Indomethacin, a nonselective inhibitor of prostaglandin endoperoxide synthase (PGHS)-1 and -2, attenuates the HPA response to hypotension in the fetus. The present study was designed to test the hypothesis that selective inhibition of PGHS-2 also inhibits the HPA response to cerebral hypoperfusion. We studied 13 chronically catheterized fetal sheep (126–136 days gestation). Five fetal sheep were subjected to intracerebroventricular infusion of nimesulide (0.01 mg/day), a specific inhibitor of PGHS-2, and eight were treated with vehicle (DMSO in water) for 5 days. Each fetus was subjected to a 10-min period of brachiocephalic occlusion, which decreased carotid arterial pressure ∼75% and reflexively increased fetal plasma concentrations of ACTH, POMC, cortisol, and femoral arterial pressure, and decreased fetal heart rate. Nimesulide significantly inhibited the ACTH response to the BCO, while significantly augmenting the reflex cardiovascular response and altering fetal heart rate variability consistent with increased sympathetic nervous system activity. The results of this study demonstrate that the activity of PGHS-2 in the brain is a necessary component of the fetal HPA response to cerebral hypoperfusion in the late-gestation fetal sheep. These results are consistent with those of recent study, in which we demonstrated that the preparturient increase in fetal ACTH secretion depends upon PGHS-2 activity within the fetal brain.
Keywords: adrenocorticotropin, cortisol, fetus, blood pressure
eicosanoids have been long recognized for their importance as signaling molecules in the fetus (7). Plasma concentrations of PGE2 are much higher in the late-gestation fetus than in the postnatal animal (7), and the concentrations increase prior to normal parturition in this species. The pattern of increase of fetal plasma PGE2, combined with the observation that exogenous PGE2 stimulates fetal hypothalamus-pituitary-adrenal (HPA) axis activity (7), led to the hypothesis that PGE2 secreted by the placenta acts as a hormone to stimulate fetal HPA axis activity prior to spontaneous parturition (34). Although the endogenous plasma concentrations of PGE2 are high compared with plasma concentrations postnatally, intracarotid arterial infusion of PGE2, producing plasma concentrations within the range of that occur endogenously prior to spontaneous parturition, failed to increase fetal HPA axis activity (11). From the results of that study, we concluded that plasma concentrations of PGE2 are not sufficient to stimulate fetal HPA axis activity (11). More recent evidence from this and other laboratories has demonstrated the presence in the fetal brain of prostaglandin endoperoxide synthases-1 and -2 (5, 6, 12, 42), consistent with the known ability of fetal brain tissue to synthesize prostanoids (19). Inhibition of prostaglandin endoperoxide synthase-2 (PGHS-2) in the fetal brain (but not in the placenta) blocks the ontogenetic increase in fetal plasma ACTH and proopiomelanocortin (POMC) concentrations and delays parturition slightly (17).
Fetal neuroendocrine responses to hypotension and cerebral hypoperfusion are, in part, modulated by prostanoids. We have reported that pretreatment of fetal sheep with indomethacin reduces the fetal hypothalamus-piuitary-adrenal axis (HPA) response to vena caval occlusion, a manipulation that causes arterial hypotension in the fetus (36). In our previous investigation of indomethacin and fetal HPA responses to hypotension, we infused indomethacin intravenously, using a body weight-adjusted dose that is similar to that used clinically in neonatal intensive care (26) but which also reduced prostanoid biosynthesis globally in the fetus (36). We hypothesized that a direct infusion of a PGHS-2 inhibitor into the brain of the fetus would inhibit the HPA response to cerebral hypoperfusion without altering circulating concentrations of PGE2. The studies presented in this report were designed to test this hypothesis.
MATERIALS AND METHODS
We studied 13 time-dated and chronically catheterized fetal sheep that were 126–136 days gestation at the time of study. These experiments were approved by the University of Florida Animal Care and Use Committee and were performed in accordance with the Guiding Principles for Use of Animals of the American Physiological Society.
Surgery.
Fetal surgery was performed as previously described (27). Ewes were fasted for 24 h before surgery. Before and during surgery, the ewe was anesthetized using 0.5–2% halothane or isoflurane in oxygen. Fetal hindlimbs were prepared with vascular catheters chronically implanted in the tibial arteries and saphenous veins, with catheter tips advanced to the subdiaphramatic aorta and inferior vena cava, respectively. After returning the fetal hindlimbs to the amniotic space, we delivered the head, and a single midline incision was made over the trachea at the level of the angle of the jaw. Both lingual arteries were exposed and catheterized (0.030 ID, 0.050 in OD), and the tips were advanced retrograde into the common carotid arteries. Through an incision in the fetal chest in the second intercostal space, an extravascular balloon occluder (8-mm diameter; In Vivo Metric, Healdsburg, CA) was placed around the brachiocephalic artery. After closing the incision in the fetal chest, an incision was made in the skin over the crown of the fetal head. A small hole was made in the skull 1 cm lateral to bregma; an 18-gauge hypodermic needle was used as a probe for identifying the depth of the lateral cerebral ventricle. A polyvinylchloride catheter (0.030“ ID, 0.050” OD) was cut to a length equal to the depth of the lateral cerebral ventricle relative to the surface of the skull and attached to an osmotic mini pump (model 2ML2, Alzet, Cupertino, CA) filled with nimesulide, then implanted subcutaneously in the neck. Incisions in the fetal skin and uterus were sutured. Nimesulide was purchased from Cayman Chemical (Ann Arbor, MI) and was infused at a rate of 10 μg/day icv in a 50%/50% mixture of water-DMSO vehicle. Corrected for differences in IC50 for inhibition of PGHS-2, this dose of nimesulide is equivalent to ∼10% of the clinical dose of indomethacin administered intravenously to reduce intraventricular hemorrhage in babies (22). We chose to scale the nimesulide dose at 10% to adjust for the percentage of fetal combined ventricular output that perfuses the head of the fetus, reasoning that the drug was being infused directly into the fetal brain (31, 38). Ampicillin (750 mg; Polyflex, Ft. Dodge Laboratories, Ft. Dodge, IA) was administered into the amniotic cavity before closure of the maternal linea alba and skin in separate layers. The catheters were routed subcutaneously to an incision in the maternal flank where they were protected within a fabric pocket that was kept in place underneath a commercial bandage wrapped around the ewe (Spandage, Medi-Tech International, Brooklyn, NY).
All ewes were treated with ampicillin (750 mg sc), and rectal temperatures and food consumption were recorded twice a day for 5 days following the surgery. The ewes were monitored for fever, anorexia, lethargy, and other signs of infection or distress. A minimum of 5 days were allowed between surgery and experimentation.
Experimental protocol.
Thirty minutes before the experiment, the pregnant ewe was moved to an experimental cart within the room in which it was housed and was allowed free access to food. Each fetus was subjected to one experiment. We connected one lingual, one aortic, and the amniotic fetal catheter to transducers (Cobe Instruments, Lakewood, CO) for measurement of fetal arterial and amniotic fluid pressures and heart rate from femoral arterial pressure pulse. Lingual arterial pressure was measured to verify occlusion. Pressures were measured using an online data acquisition system (Labview, National Instruments, Austin, TX). Lingual, femoral arterial, and amniotic fluid pressures were recorded at a sampling rate of 60 Hz for a total of 35 min. One-minute averages of each pressure and heart rate (calculated from the phasic pressure signal) were calculated off-line. Cerebral hypoperfusion was initiated by maximal inflation of the brachiocephalic occluder with 2 ml saline starting at 0 min and lasting 10 min. The second fetal femoral catheter was used to collect fetal arterial blood samples (5 ml) at 0, 5, 10, 20, and 30 min. The stimulus of brachiocephalic occlusion reproducibly reduces fetal cerebral blood flow and, when limited to 10 min duration, does not result in histologically discernable tissue damage (28).
Plasma analytes.
Blood samples were placed in chilled tubes containing K2EDTA (10.8 mg, Vacutainer, Becton Dickinson, Franklin Lakes, NJ). An additional 1.5 ml of blood was drawn anaerobically into syringes coated with heparin for measurement of blood gases using an ABL77 analyzer (Radiometer, Copenhagen, Denmark). Blood samples were kept on ice until centrifuged at 3,000 g for 20 min at 4°C (Sorvall RT 6000B; Dupont, Newton, CA). After centrifugation, the plasma was divided into aliquots, transferred to polypropylene tubes, and stored at −20°C until hormones were assayed.
Plasma ACTH concentrations were measured using a commercially available immunoradiometric assay (Diasorin, Stillwater, MN), according to the manufacturer's instructions. As characterized by Myers and colleagues (23), this assay measures only ACTH1–39. POMC/pro-ACTH concentrations were measured using a commercially available enzyme immunoassay kit (IDS, Boldon, UK), as per the manufacturer's instructions. This assay recognizes both POMC (31 kDa) and pro-ACTH (22 kDa). Plasma cortisol concentrations were measured using a commercially available enzyme immunoassay (EIA) kit (catalog number EA65; Oxford Biomedical Research, Oxford, MI), according to the manufacturer's instructions. Fetal cortisol was extracted from plasma (10 μl) after deproteinization in ethanol (1 ml) in borosilicate glass test tubes (12 × 75 mm).
Frequency analysis.
In 7 control and in 5 nimesulide-treated fetuses, we quantified the power spectra of the variations in fetal heart rate, using methods similar to that reported by Kimura and coworkers (20) and later supported by studies by Li and coworkers (21). Five-minute epochs of fetal femoral or lingual arterial blood pressure, sampled at a rate of 60 Hz at the time of experimentation, were analyzed for heart period using LabView ver. 8.6 software. The resulting arrays of heart period were subjected to power spectral analysis after windowing using a Hanning window. The resulting power spectrum was quantified as ms2/(cycle/beat) as a function of (cycles/beat). Note that, for each animal, the frequency in Hertz (cycles/s) can be obtained by multiplying the frequency in cycles/beat by the heart rate in units of beats/s. The power spectra were further analyzed as the area under curve in the following frequency ranges: very low frequency (VL: 0.001–0.025 c/b), low frequency (L: 0.025–0.125 c/b), medium frequency (M: 0.125–0.2 c/b), and high frequency (H: 0.2–0.5 c/b). Areas under the curve were calculated using the trapezoidal rule of numerical integration. Kimura et al. (20), reported that the M frequency was reduced by alpha-adrenergic blockade, that the VL and L frequencies were reduced by beta-adrenergic blockade, and that the H and L frequencies were reduced by parasympathetic blockade (20). This analysis was performed in four time periods in each experiment: 1) during the 5-min baseline recording period, 2) 0–5 min immediately following the onset of BCO, 3) 5–10 min following the onset of BCO, and 4) 25–30 min following the onset of BCO (this corresponds to 15–20 min after the onset of recovery from BCO).
Statistical analysis.
Data are presented as mean values ± SE. Fetal lingual and femoral arterial blood pressures were corrected by subtraction of amniotic fluid pressure. Unless stated, plasma hormone, blood gas/pH, blood pressure, and heart rate data were analyzed by two-way ANOVA corrected for repeated measures in one dimension (time). Power spectra were analyzed by three-way ANOVA corrected for repeated measures in two dimensions (time and frequency). Power spectra calculated from recordings before BCO were analyzed by two-way ANOVA corrected for repeated measures in one dimension (frequency). Pairwise comparisons between groups were made using simple effects contrasts (16). All statistics were performed using SPSS ver. 17.0 for Windows (SPSS Inc., Chicago, IL).
RESULTS
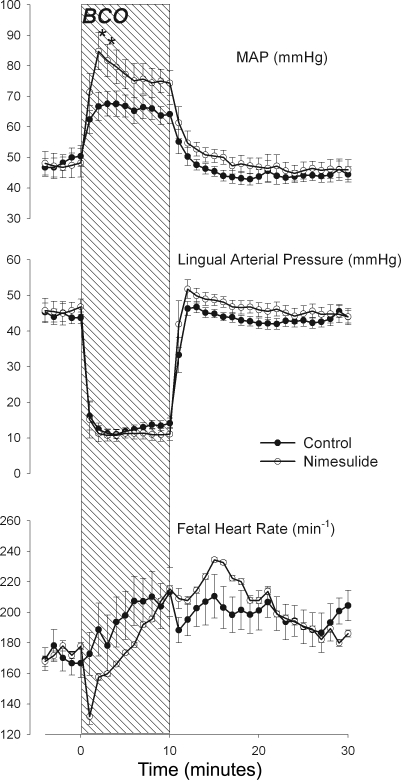
There were no group differences in fetal femoral arterial blood pressure, lingual arterial blood pressure, or heart rate, which were similar in the two groups (P = 0.193, P = 0.99, and P = 0. 539, respectively; Fig. 1). Brachiocephalic occlusion (BCO) reduced lingual arterial pressure to a similar extent in both groups (P = 0.571 for group × time interaction; Fig. 1, middle). Nimesulide significantly augmented the increase in systemic mean arterial blood pressure (Fig. 1, top, P = 0.007 for group × time interaction). Comparison of individual means between groups (using simple effects contrasts) revealed augmented increases in fetal blood pressure in the second and third minutes of the BCO. Fetal heart rate responses to BCO were not statistically significantly different between groups (P = 0.986 for group × time interaction), although there appeared to be a bradycardic response in the nimesulide-treated group at the onset of BCO.
Fig. 1.
One-minute averages of fetal femoral arterial blood pressure (MAP, top), lingual arterial blood pressure (Lingual Arterial Pressure, middle), and fetal heart rate (bottom) before, during, and after brachiocephalic occlusion (BCO, represented as hatched area). Data are reported from control (•) and nimesulide-treated (○) fetuses. Data are represented as mean values with vertical bars representing ± SE. *Statistically significant difference between groups.
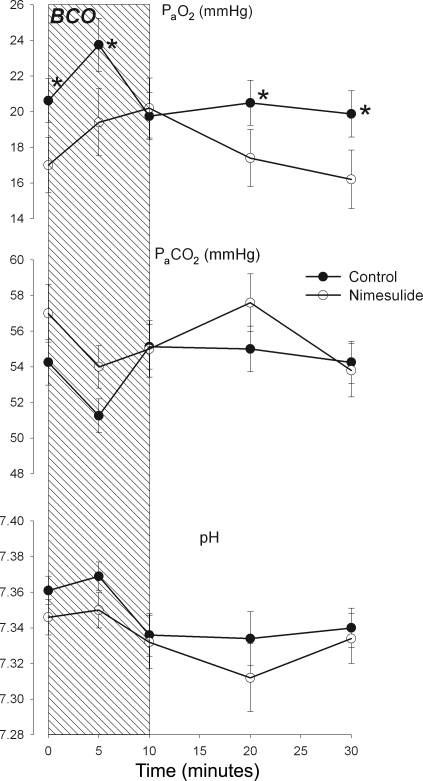
Blood gas and pH values (Fig. 2) were not significantly different between groups, although the apparent differences in PaO2 were nearly significant (P = 0.07). BCO increased PaO2 in both groups, but the timecourse of the increase was delayed in the nimesulide group (P = 0.025 for group × time interaction in ANOVA). Contrast of individual mean values between groups revealed that the PaO2 values were significantly different at 0, 5, and 30 min. PaCO2 and pHa (Fig. 2, top and middle, respectively) were decreased equally in both groups (P = 0.006 and P < 0.001, respectively, for the main effect of time and P = 0.227 and P = 0.424, respectively, for group × time interaction).
Fig. 2.
Arterial partial pressure of oxygen (PaO2, top) and carbon dioxide (PaCO2, middle), and arterial pH (bottom) before, during, and after brachiocephalic occlusion (BCO, represented as hatched area). Data are reported from control (•) and nimesulide-treated (○) fetuses. Data are represented as mean values with vertical bars representing ± SE. *Statistically significant difference between groups.
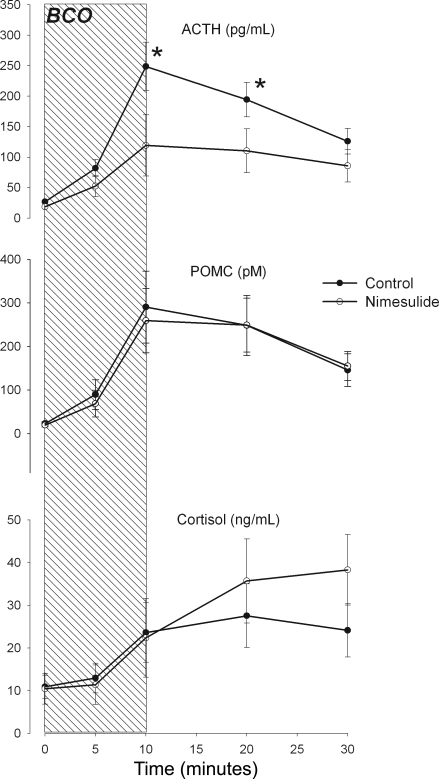
BCO stimulated robust increases in fetal plasma ACTH, POMC, and cortisol concentrations in both nimesulide- and vehicle-treated fetuses (Fig. 3). Nimesulide treatment significantly reduced the magnitude of the ACTH response to BCO (P = 0.04 for group × time interaction in ANOVA) but did not significantly alter the magnitude of the POMC response (P = 0.65). The fetal plasma cortisol response appeared to be moderately increased in the nimesulide-treated fetuses in the BCO recovery period, although this was not statistically significant (P = 0.06).
Fig. 3.
Plasma concentrations of adrenocorticotropin (ACTH; top), proopiomelanocortin and pro-ACTH (POMC, middle), and cortisol (bottom) before, during, and after brachiocephalic occlusion (BCO, represented as hatched area). Data are reported from control (•) and nimesulide-treated (○) fetuses. Data are represented as mean values with vertical bars representing ± SE. *Statistically significant difference between groups.
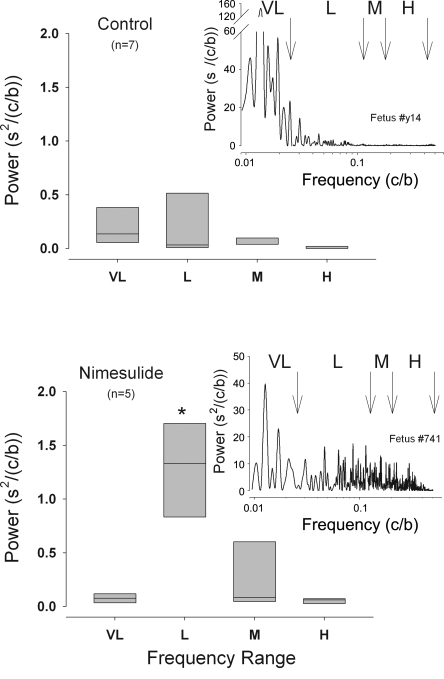
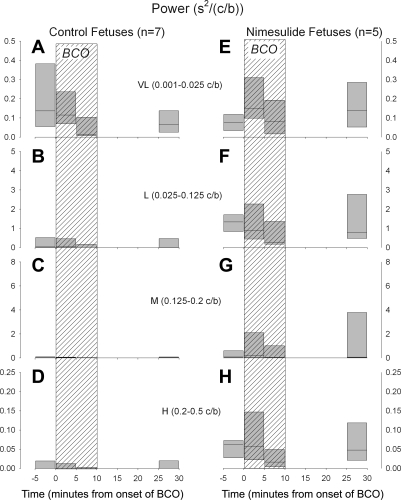
Power spectral analysis of the two groups of fetuses revealed that, in both groups, there was a high-amplitude peak of power in the VL range prestimulation. Examples of individual power spectra from each group are shown in Fig. 4. In baseline measurements made before BCO in the control fetuses, the majority of spectral power was in the L and VL ranges, while in the nimesulide-treated fetuses, there was a strong and statistically significant increased power in the L range compared with the control fetuses (Fig. 4). Analysis of the change in spectral power in the four ranges with brachiocephalic occlusion revealed a transient increase in power in the VL and M frequency ranges during BCO in the nimesulide-treated fetuses (Fig. 5, E and G). Overall, as shown in Fig. 5, there was greater spectral power in the nimesulide-treated fetuses, and the change in power with BCO in the nimesulide-treated fetuses differed significantly from that in the control fetuses (P = 0.021 and P = 0.011 for frequency × group and time × group two-way interactions in the ANOVA, respectively). There was a high degree of heterogeneity in the magnitude of responses that resulted in great variability in the summary data. Interestingly, the fetus with the lowest initial PaO2—this fetus was part of the nimesulide group—responded to the BCO with the largest increase in power in all frequency ranges.
Fig. 4.
Spectral power of fetal heart rate variability in four ranges of frequency (VL, 0.001–0.025 cycles/beat; L, 0.025–0.125 cycles/beat; M, 0.125–0.2 cycles/beat; H, 0.2–0.5 cycles/beat) in control (top) and nimesulide-treated (bottom) fetuses prior to BCO. Power spectra for representative individual fetuses in each group are shown as insets (nimesulide fetus #741, top; control fetus #y14 bottom). Summary data are represented as box plots with outer edges representing 95th and 5th percentile and horizontal lines representing median.
Fig. 5.
Spectral power of fetal heart rate variability before, during, and after brachiocephalic occlusion (BCO, hatched areas) in control (left) and nimesulide-treated (right) fetuses. Summary results are shown for very low (VL, A and E), low (L, B and F), middle (M, C and G), and high (H, D and H) frequency ranges. Summary data are represented as box plots with outer edges representing 95th and 5th percentile and horizontal lines representing median.
DISCUSSION
Prostaglandins generated within the fetal central nervous system (CNS) are integral to the fetal HPA axis response to hypotension. In a previous work, we reported that indomethacin, the nonselective PGHS-1/PGHS-2 (COX-1/COX-2) inhibitor that is used commonly in neonatal intensive care units for the prevention of intraventricular hemorrhage (33), partially inhibited the fetal ACTH response to hypotension that was, in turn, caused by vena caval obstruction (36). In that study, we administered the indomethacin intravenously, using a standard clinical dose. We could not, therefore, distinguish peripheral from CNS actions of the drug. In a more recent study, we chronically infused nimesulide (a specific PGHS-2 inhibitor) at a rate of 1 mg/day intracerebroventricularly into chronically catheterized fetal sheep. We found that the nimesulide was infused at a rate too low to significantly alter plasma concentrations of PGE2 and therefore too low to significantly inhibit placental PGHS-2. In that study, the nimesulide prevented the spontaneous late-gestation rise in fetal ACTH secretion that heralds parturition and delayed the timing of parturition slightly (17). In the present study, a lower rate of infusion of nimesulide (0.01 mg/day icv) partially inhibited the fetal ACTH response to brachiocephalic occlusion. Together, these studies demonstrate the importance of fetal CNS prostaglandins as modulators of fetal ACTH secretion. The neuroanatomical substrate for this influence of prostaglandins on fetal ACTH secretion is unknown, although we do know that both PGHS-1 and PGHS-2 are present at the mRNA and protein levels in hypothalamus, hippocampus, medulla, cerebellum, and cerebral cortex. Expression of the enzyme and tissue concentrations of PGE2 appears to be greatest in cerebral cortex and hippocampus (5, 6, 17, 42).
Because of the design of the study, we were unable to assess whether the blockade of PGHS-2 was complete or only partial. In a previous study, we demonstrated that brain interstitial PGE2 concentrations (measured by microdialysis) increased during brachiocephalic occlusion (37). We have also reported that 1 mg/day nimesulide infused intracerebroventricularly did reduce tissue content of PGE2 in fetal brain (17). What we do not know is the degree to which the current dose of nimesulide blocks the increase in PGE2 tissue production in response to the BCO; these are data that would be difficult to obtain in a chronically catheterized fetal sheep preparation.
A most interesting aspect of this and previous studies is the apparent dissociation of fetal ACTH and cortisol. In our previous study, we found that intracerebroventricular infusion of nimesulide was ineffective in blocking the preparturient rise in fetal plasma cortisol, while it completely blocked the rise in fetal ACTH and pro-ACTH/proopiomelanocortin (17). This observation essentially reproduced an earlier report by Jacobs and colleagues (18), in which ontogenetic increases in fetal plasma cortisol were measured in fetal sheep that had been hypophysectomized and infused with exogenous ACTH. In the present study, the partial inhibition of fetal ACTH responses to BCO was not accompanied by partial inhibition of the fetal cortisol responses. In fact, the fetal cortisol response to the BCO had the appearance of being augmented, not decreased (although this was not statistically significant). Although it is well established that increases in fetal plasma ACTH concentration increase fetal adrenal secretion of cortisol, there appears to be a dynamic regulation of adrenal sensitivity to ACTH (24). One possible explanation for this is a change in the activity of sympathetic efferent nerves that innervate the adrenal cortex. Myers and colleagues (24), for example, found that ablation of the splanchnic nerves in a fetal sheep reduced the fetal plasma cortisol response to hypoxia, while not altering the fetal plasma ACTH response. This concept of adrenal cortical control by adrenal nerves is similar to that demonstrated by Edwards and coworkers (14, 15). In the fetal sheep, it is possible that the splanchnic innervation of the adrenal cortex dynamically regulates adrenal sensitivity to ACTH. If so, it is the ACTH that is modulated by CNS prostaglandin, not the adrenal sensitivity. On the other hand, it is possible that the elevation in sympathetic nervous system activity that we identified using power analysis of fetal heart rate variability has produced an increase in adrenal sensitivity (secondary to increased splanchnic nerve activity) that has allowed relatively normal looking fetal plasma cortisol responses to BCO in the face of reduced ACTH responses.
Another interesting aspect of this study is the dissociation of ACTH and POMC/proACTH. We have, in other studies, demonstrated similar dissociation. Intracerebroventricular infusion of ICI 182,781—an estrogen receptor antagonist—inhibited the preparturient rise in POMC/proACTH but did not decrease the preparturient rise in ACTH (32). Conversely, intracerebroventricular infusion of nimesulide (at a dose higher than that used in the present study) blocked the preparturient rise in both ACTH and POMC/proACTH (17). It has become apparent from studies like these that plasma POMC and ACTH1–39 do not always increase in parallel, suggesting either extrapituitary sites of POMC secretion or altered processing of POMC in the pituitary. Supporting this idea is the observation that not all corticotropes in the anterior pituitary express the prohormone convertase, which is needed to process POMC to ACTH (4). Also supporting this idea is the observation that the fetal lung expresses and secretes POMC abundantly, but it does not appear to process the peptide to ACTH (3, 9, 10, 40).
PGHS-2 is often thought of as an inducible enzyme, especially in inflammatory responses in peripheral tissues and in the brain after cerebral ischemia (13, 25, 35, 37). However, PGHS-2 also appears to be constitutively expressed in the adult and fetal brain (5, 17, 35). Little is known about the mechanism of this so-called constitutive expression, with the exception that the expression of the enzyme is in part dependent upon circulating estrogen in the fetus (32) and that it can be upregulated in brain stem and cerebellum by further increases in fetal plasma estrogen concentrations (42). However, it is likely that other factors are important in determining the abundance of PGHS-2 in fetal brain. We suggest, for example, that the activity of the baroreceptor and chemoreceptor reflex pathways might, themselves, be partial determinants of the expression level of PGHS-2. This might result from glutamatergic stimulation of PGHS-2 expression (2).
Power analysis of fetal heart rate variability suggests that chronic treatment with nimesulide increases autonomic activity. Power within the L frequency range was increased significantly in the nimesulide-treated fetuses. Power in the low-frequency range is influenced both by sympathetic and parasympathetic activity, although previous experiments by Kimura et al. (20), suggest that beta-adrenergic blockade in the fetal sheep had a greater influence on L power than did cholinergic blockade (20). In that study, beta blockade reduced L power 78%, while cholinergic blockade reduced power in this frequency range 61% (20). An increase in autonomic activity in the present study would be consistent with the results of our own previous experiments, in which we found that acute administration of nimesulide increased fetal blood pressure and produced an apparent baroreceptor reflex-mediated reduction in fetal heart rate (30). The present results are consistent with the possibility that chronic nimesulide produces a chronic elevation in sympathetic efferent activity. If so, it is possible that fetal hemodynamics (e.g., change in the distribution of combined ventricular output) might also be chronically influenced by the nimesulide. The difference in PaO2 tensions with respect to time between the control and nimesulide groups in the present study are consistent with this possibility. And as previously discussed, an increased sympathetic tone might chronically elevate adrenal sensitivity by increasing splanchnic nerve activity.
With respect to the inhibition of fetal ACTH secretion, intracerebroventricular infusion had a different effect than intracerebroventricular injection of nimesulide (29). In a previous study, we found that acute intracerebroventricular injection of 0.1 mg nimesulide increased fetal blood pressure and plasma concentrations of ACTH 30 min after injection (29). Interestingly, this acute injection of nimesulide did not alter the magnitude of ACTH response to BCO. We concluded that brain PGHS-2 inhibited fetal HPA axis activity, essentially the opposite of our conclusions in this and another recent study (17). The difference in our conclusions resulted from a difference in experimental design: acute injection vs. chronic infusion of nimesulide intracerebroventricularly. Chronic infusion of the drug intracerebroventricularly (for days) has a dramatic effect of reducing fetal HPA axis activity, now demonstrated in two studies that were performed independently of each other. It is interesting but at present unexplained why both acute and chronic nimesulide appear to increase autonomic nervous system activity but that only chronic nimesulide inhibits HPA axis activity.
A confounding variable in the present study is the difference in blood gas status between the two groups. The nimesulide group had a lower PaO2 compared with the control group. The difference in blood gases could be either the cause or the result of increased sympathetic efferent tone in the treated fetuses: the research design does not allow us to determine cause-and-effect. Although the blood gas status confounds our interpretation of sympathetic tone, we believe that it does not confound our interpretation of the HPA response to the BCO. Long-term hypoxia (LTH) increases the magnitude of the ACTH response to hypotension (1). Acute acidemia is a stimulus to fetal HPA axis activity (8, 41). Chronic stress in adult rats also causes activation of the HPA axis (39). In the present study, the expected activation of the fetal HPA axis by the concomitant arterial hypoxia and acidemia would have biased the fetal ACTH concentrations in the nimesulide group to higher levels. The inhibition of fetal ACTH by nimesulide suggests that the drug does inhibit the axis and that the degree of inhibition might have been underestimated by the counfounding influence of hypoxia and/or acidemia.
We conclude that the activity of PGHS-2 in the fetal brain is an important component of the fetal ACTH response to cerebral hypoperfusion. By analogy, this influence of PGHS-2 might be important in the ACTH responses to other stressors, as well, although at present, this idea is untested. Blocking this enzyme pharmacologically reduces fetal ACTH secretion and appears to increase autonomic tone. We further conclude that partial inhibition of the ACTH response to BCO with nimesulide is not reflected in partial inhibition of the adrenal cortisol response, suggesting possible alterations in adrenal sensitivity. The results of this and previous studies in our laboratory suggest that PGHS-2 in the fetal brain is a critical component of neuroendocrine development and reflex responsiveness in the late-gestation sheep fetus.
Acknowledgments
This work was supported by HD33053 and HD42135 to CEW, as well as by predoctoral fellowship grants from the American Heart Association, Florida-Puerto Rico Affiliate, to M. J. Powers Fraites. We thank Dr. Yun-Ju He for his expert technical help in performing endocrine assays, Ms. Whitney Hartz, Mr. Jarret McCartney, and Mr. Jared Winikor for their technical help with the animal care aspects of this project.
REFERENCES
- 1.Adachi K, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters ovine fetal endocrine and physiological responses to hypotension. Am J Physiol Regul Integr Comp Physiol 287: R209–R217, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Adams J, Collaco-Moraes Y, de BJ. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J Neurochem 66: 6–13, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Ali NS, Keller-Wood M, Wood CE. Ontogenetic changes in the extra-pituitary expression of pro-opiomelanocortin in the developing ovine fetus. Peptides 26: 301–306, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bell ME, Myers TR, Myers DA. Expression of proopiomelanocortin and prohormone convertase-1 and -2 in the late gestation fetal sheep pituitary. Endocrinology 139: 5135–5143, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Breder CD, DeWitt DL, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 355: 296–315, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, Saper CB. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J Comp Neurol 322: 409–438, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Challis JR, Hart I, Louis TM, Mitchell MD, Jenkin G, Robinson JS, Thorburn GD. Prostaglandins in the sheep fetus: implications for fetal function. Adv Prostaglandin Thromboxane Res 4: 115–132, 1978. [PubMed] [Google Scholar]
- 8.Chen HG, Wood CE. The adrenocorticotropic hormone and arginine vasopressin responses to hypercapnia in fetal and maternal sheep. Am J Physiol Regul Integr Comp Physiol 264: R324–R330, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Cudd TA, Castro MI, Wood CE. Content, in vivo release, and bioactivity of fetal pulmonary immunoreactive adrenocorticotropin. Am J Physiol Endocrinol Metab 265: E667–E672, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Cudd TA, Wood CE. Secretion and clearance of immunoreactive ACTH by fetal lung. Am J Physiol Endocrinol Metab 268: E845–E848, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Cudd TA, Wood CE. Prostaglandin E2 releases ovine fetal ACTH from a site not perfused by the carotid vasculature. Am J Physiol Regul Integr Comp Physiol 263: R136–R140, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Deauseault D, Giroux D, Wood CE. Ontogeny of immunoreactive prostaglandin endoperoxide synthase isoforms in ovine fetal pituitary, hypothalamus, and brainstem. Neuroendocrinology 71: 287–291, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Domoki F, Veltkamp R, Thrikawala N, Robins G, Bari F, Louis TM, Busija DW. Ischemia-reperfusion rapidly increases COX-2 expression in piglet cerebral arteries. Am J Physiol Heart Circ Physiol 277: H1207–H1214, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Edwards AV, Jones CT. The effect of splanchnic nerve section on the sensitivity of the adrenal cortex to adrenocorticotrophin in the calf. J Physiol 390: 23–31, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards AV, Jones CT, Bloom SR. Reduced adrenal cortical sensitivity to ACTH in lambs with cut splanchnic nerves. J Endocrinol 110: 81–85, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Field A Discovering Statistics Using SPSS. London: Sage Publications, 2005.
- 17.Gersting J, Schaub CE, Keller-Wood M, Wood CE. Inhibition of brain prostaglandin endoperoxide synthase-2 prevents the preparturient increase in fetal adrenocorticotropin secretion in the sheep fetus. Endocrinology 149: 4128–4136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs RA, Young IR, Hollingworth SA, Thorburn GD. Chronic administration of low doses of adrenocorticotropin to hypophysectomized fetal sheep leads to normal term labor. Endocrinology 134: 1389–1394, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Jones SA, Adamson SL, Engelberts D, Bishai I, Norton J, Coceani F. PGE2 in the perinatal brain: local synthesis and transfer across the blood-brain barrier. J Lipid Mediat 6: 487–492, 1993. [PubMed] [Google Scholar]
- 20.Kimura Y, Okamura K, Watanabe T, Murotsuki J, Suzuki T, Yano M, Yajima A. Power spectral analysis for autonomic influences in heart rate and blood pressure variability in fetal lambs. Am J Physiol Heart Circ Physiol 271: H1333–H1339, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Tang D, Zhou S, Zhou G, Wang C, Zhuang Y, Wu G, Shen L. Redistribution of power spectrum of heart rate variability during acute umbilical artery embolism and hypoxemia in late-gestation fetal sheep. Eur J Obstet Gynecol Reprod Biol 114: 137–143, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr BR, Allen W, Duncan CC, Scott DT, Taylor KJW, Katz KH, Schneider KC, Makuch RW. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics 93: 543–550, 1994. [PubMed] [Google Scholar]
- 23.Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 288: R1178–R1184, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Myers DA, Robertshaw D, Nathanielsz PW. Effect of bilateral splanchnic nerve section on adrenal function in the ovine fetus. Endocrinology 127: 2328–2335, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Needleman P, Isakson PC. The discovery and function of COX-2. J Rheumatol 24 Suppl 49: 6–8, 1997. [PubMed] [Google Scholar]
- 26.Ohlsson A, Roberts RS, Schmidt B, Davis P, Moddeman D, Saigal S, Solimano A, Vincer M, Wright L. Male/female differences in indomethacin effects in preterm infants. J Pediatr 147: 860–862, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Powers MJ, Wood CE. Ketamine inhibits fetal ACTH responses to cerebral hypoperfusion. Am J Physiol Regul Integr Comp Physiol 292: R1542–R1549, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purinton SC, Wood CE. Oestrogen augments the fetal ovine hypothalamus-pituitary-adrenal axis in response to hypotension. J Physiol 544: 919–929, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimsnider S, Wood CE. Differential modulation of ovine fetal ACTH secretion by PGHS-1 and PGHS-2. Neuroendocrinology 83: 4–11, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Reimsnider SK, Wood CE. Does reduction of circulating prostaglandin E2 reduce fetal hypothalamic-pituitary-adrenal axis activity? J Soc Gynecol Investig 12: e13-e19, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph AM Congenital diseases of the heart. Chicago: Year Book Medical Publishers, 1974.
- 32.Schaub CE, Keller-Wood M, Wood CE. Blockade of estrogen receptors decreases CNS and pituitary prostaglandin synthase expression in fetal sheep. Neuroendocrinology 87: 121–128, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalak L, Perlman JM. Hemorrhagic-ischemic cerebral injury in the preterm infant: current concepts. Clin Perinatol 29: 745–763, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Thorburn GD, Hollingworth SA, Hooper SB. The trigger for parturition in sheep: fetal hypothalamus or placenta? J Dev Physiol 15: 71–79, 1991. [PubMed] [Google Scholar]
- 35.Tong H, Dhillon H, Wood CE. Induction of PGHS-2 mRNA in response to cerebral hypoperfusion in late-gestation fetal sheep. Prostaglandins Other Lipid Mediat 62: 165–172, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 275: R735–R741, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Tong H, Richards E, Wood CE. Prostaglandin endoperoxide synthase-2 abundance is increased in brain tissues of late-gestation fetal sheep in response to cerebral hypoperfusion. J Soc Gynecol Investig 6: 127–135, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Tong H, Wood CE. Indomethacin attenuates the cerebral blood flow response to hypotension in late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 277: R1268–R1273, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Vernikos J, Dallman MF, Bonner C, Katzen A, Shinsako J. Pituitary-adrenal function in rats chronically exposed to cold. Endocrinology 110: 413–420, 1982. [DOI] [PubMed] [Google Scholar]
- 40.Wood CE, Barkoe D, The A, Newman H, Cudd TA, Purinton S, Castro MI. Fetal pulmonary immunoreactive adrenocorticotropin: molecular weight and cellular localization. Regul Pept 73: 191–196, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Wood CE, Chen HG. Acidemia stimulates ACTH, vasopressin, and heart rate responses in fetal sheep. Am J Physiol Regul Integr Comp Physiol 257: R344–R349, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Wood CE, Giroux D. Central nervous system prostaglandin endoperoxide synthase-1 and -2 responses to oestradiol and cerebral hypoperfusion in late-gestation fetal sheep. J Physiol 549: 573–581, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]