Abstract
Marinobufagenin (MBG) is an endogenous mammalian cardiotonic steroid that is involved in the inhibition of the sodium pump Na+/K+-ATPase. Increased plasma levels of MBG have been reported in patients with preeclampsia. MBG increases microvascular barrier permeability in an animal model of preeclampsia. However, the mechanism by which MBG impairs endothelial permeability is unknown. We utilized rat lung microvascular endothelial cells (RLMEC) to examine alterations in MBG-induced monolayer permeability and the effect of MBG on the phosphorylation status of ERK1/2, Jnk, and p38. Apoptosis was evaluated by examining alterations in caspases 3/7, 8, and 9 and annexin-V staining. We also examined the effect of MBG on the endothelial adherens junctions of the RLMEC monolayer. MBG inhibited the proliferation, and increased the monolayer permeability, of RLMEC. These actions of MBG were attenuated by ERK, p38, and pan caspase inhibition. MBG significantly decreased the phosphorylation of ERK1/2 and activated the phosphorylation of Jnk and p38. MBG also significantly increased the expression of caspases 3/7, 8, and 9, indicating the activation of apoptosis. MBG-induced apoptosis signaling was not observed in cells pretreated with a p38 inhibitor. MBG treatment induced the disruption of endothelial cell junctions. This effect was prevented by a pan caspase inhibitor. In conclusion, 1) MBG induced an impairment of RLMEC proliferation; 2) the bufadienolide also caused endothelial hyperpermeability; and 3) these effects of MBG were mediated by the downregulation of ERK1/2, the upregulation of Jnk and p38, by the activation of apoptosis, and by the disruption of endothelial cell junctions.
Keywords: β-catenin, rat lung microvascular endothelial cells, MAP kinase, caspases, bufodienolide
marinobufagenin (mbg) is an endogenous mammalian cardiotonic bufodienolide that has vasoconstrictive properties and is involved in the regulation of the membrane-bound sodium pump Na+/K+-ATPase (1, 13, 29, 36). Increased plasma levels of MBG have been demonstrated in volume expansion-mediated hypertension, including renal failure, primary aldosteronism, congestive heart failure, and preeclampsia (16, 23, 46). The latter disorder is a pregnancy syndrome, which includes hypertension, proteinuria, and, often, intrauterine growth restriction. Its incidence varies between 3 and 10% of all pregnancies (30, 31). In an animal model of preeclampsia (19), we have shown that the urinary excretion of MBG is elevated “prior” to the development of hypertension and proteinuria. These data suggest that MBG may play a pathophysiological role in preeclampsia (46), a syndrome that is characterized by shallow placentation (14, 32). MBG administration to normal pregnant animals has been shown to result in the development of hypertension and proteinuria (19). Furthermore, the provision of antibodies to MBG corrects the hypertension (12, 46). We have recently demonstrated that MBG impairs both the proliferation and growth-factor induced migration of first trimester cytotrophoblast (CTB) cells, which are critical for placental development (22, 43). We have recently demonstrated that MBG alters vascular permeability in a rat model of preeclampsia (41). We have also demonstrated that MBG impairs CTB cell function by the modulation of MAPK and stimulation of apoptosis (42). However, there have been no studies of the mechanism(s) by which MBG might alter vascular permeability.
Increased vascular permeability is an underlying pathophysiological event in preeclampsia (33, 34). Disrupted and enlarged intercellular junctions between endothelial cells have been observed in myometrial vessels in women with preeclampsia (37). A transmembrane adhesive protein, vascular endothelial (VE)-cadherin forms endothelial adherens junctions that are involved in the regulation of paracellular permeability in microvascular endothelium (7, 15, 20). Endothelial permeability is affected by many agonists (7, 20, 48). Increased endothelial monolayer permeability is associated with alterations in the junctional adhesion molecule VE-cadherin (48).
Activation of MAPK signaling is known to play a critical role in the regulation of cellular proliferation and paracellular permeability in microvascular endothelium (4, 11, 15, 20, 27). Activated ERK1/2 controls cell proliferation and differentiation (4, 20). Stimulated Jnk and p38 pathways regulate cell proliferation, microvascular permeability, growth arrest, and apoptosis (4, 15, 20, 27). Several studies have implicated caspases as the molecular instigators of apoptosis (28, 38, 39). Recent studies have shown that the bufodienolide, bufalin, induces apoptosis in endometrial cells, which is associated with a downregulation of the cell cycle and the concomitant activation of Bax and caspase-9 (26).
Although increased vascular permeability is an important event in the pathogenesis of preeclampsia (34, 48), whether circulating factors elicit this endothelial barrier dysfunction is not known. In this study, we employed endothelial cells to determine whether MBG contributes to the increased vascular permeability in preeclampsia. Therefore, we examined the effects of MBG on ERK phosphorylation, as well as the proliferation and monolayer permeability of RLMEC. We evaluated the effects of MBG on Jnk and p38 phosphorylation, the early apoptotic marker caspases 3/7, 8, and 9, and annexin-V staining. We also studied the effect of MBG on the endothelial adherens junction of the RLMEC monolayer. In addition, we examined whether or not microvascular permeability could be prevented by the inhibition of caspases and whether apoptosis of RLMEC could be prevented by the inhibition of p38 and Jnk. We hypothesized that MBG increases endothelial cell permeability and that it does so by increasing p38 and caspase-mediated apoptosis and by disruption of endothelial cell junctions.
MATERIALS AND METHODS
Cell culture.
RLMEC (VEC Technologies, Rensselaer, NY) were maintained on fibronectin-coated dishes in complete MCDB-3 media supplemented with 10% FBS and used at passages 3–7. Cells were incubated at 37°C, 5% CO2, and 99% humidity (Isotemp CO2 incubator, Fisher Scientific, Pittsburgh, PA). The RLMEC exhibited properties characteristic of all endothelial cells, such as typical cobblestone morphology and incorporation of acetylated low-density lipoprotein (7, 40).
Proliferation assay following MBG treatment.
Cell proliferation was measured as described previously (9, 22, 24, 43). RLMEC were seeded (7,000 cells/well) onto 96-well plates in complete medium and allowed to adhere overnight at 37°C. Cells were then serum starved in medium containing 0.5% FBS for 24 h, washed twice with 1× PBS, and eight replicates were subsequently treated with 10% FBS/MCDB-3 (to stimulate cell proliferation) containing DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG. Some cells were pretreated for 2 h with an ERK inhibitor (100 μM PD98059; Calbiochem-Novabiochem, San Diego, CA) before exposure to DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG. Cells were incubated for 48 h with the respective treatments, at which time 10 μl of cell titer 96 was added to each well. Absorbance at 490 nm was determined by a microtiter plate reader (Microplate Spectrophotometer, Spectra Max 3400 pc; Molecular Devices, Sunnyvale, CA). Absorbance is directly correlated with the number of viable cells (24).
Cell viability assay.
A cell viability assay was performed as described previously (43). The assay is based on the ability of living cells to convert a redox dye, resazurin into a fluorescent end product, resorufin. RLMEC were seeded onto 96-well plates in complete medium and allowed to adhere overnight at 37°C. Cells were then treated with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG. After incubation for 48 h with the respective treatments, 20 μl of Cell Titer-Blue reagent was added to each well. Absorbance at 520 nm was determined by a microtiter plate reader. The signal produced by conversion of resazurin to resorufin is directly proportional to the number of viable cells.
Monolayer permeability.
The monolayer permeability study was performed by a method described previously (7). RLMEC were maintained on fibronectin-coated dishes in complete MCDB-3 media supplemented with 10% FBS. They were later grown as monolayers on Costar Transwell membranes (Corning, Corning, NY) for 48 h. One hour prior to the start of the experiment, the cells were exposed to fresh media without phenol-red dye. Cells were treated with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG for 3 h. Some cells were pretreated for 2 h with an ERK inhibitor (100 μM PD98059, Calbiochem-Novabiochem, San Diego, CA), a p38 inhibitor (10 μM SB 202190, Sigma, St. Louis, MO), a Jnk inhibitor (20 μM SP 600125, Invitrogen, Carlsbad, CA), and a pan caspase inhibitor (20 μM Z-VAD-FMK, R&D Systems, Minneapolis, MN) before exposure to DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG. FITC-albumin (5 mg/ml final concentration) was added to the luminal chamber for 60 min, and samples were removed from both luminal and abluminal chambers. The samples (100 μl) collected from the abluminal chamber were analyzed using a fluorometric plate reader (excitation 400 nm/emission 505 nm). The readings were converted with the use of a standard curve to albumin concentration. These concentrations were then used in the following equation to determine the permeability coefficient of albumin (Pa): Pa = ([A]/t) × (1/A) × (V/[L]), where [A] is abluminal concentration, t is time in seconds, A is the area of the membrane in centimeters squared, V is the volume of the abluminal chamber, and [L] is luminal concentration.
Effect of MBG on ERK 1/2, Jnk, and p38 phosphorylation.
The effect of MBG on ERK 1/2, Jnk and p38 phosphorylation was evaluated by the cellular activation of signaling (CASE) ELISA kit (SuperArray, Frederick, MD) (45). The kit includes a complete antibody-based detection system for colorimetric quantification of the relative amount of phosphorylated protein and total target protein. For the CASE assay, RLMEC were seeded into 96-well plates and were stimulated with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG for 10, 30, 60, 120, or 240 min. Detection of total and phosphorylated protein expression was determined according to the manufacturer's protocol.
Immunoprecipitation and Western blot analysis.
RLMEC were treated with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG for 60 min. After each treatment, total cellular protein was obtained by lysis buffer containing 50 mm Tris at pH 7.4, 50 mm NaCl, 1% Triton X-100, 0.1% SDS, 0.3 mM Na-orthovanadate, 50 mM NaF, 1 mm DDT, 10 μg/ml leupeptin, and 5 μg/ml aprotinin. Protein concentrations were determined by BCA reagent (Pierce, Rockford, IL). An equal amount of protein in each sample was separated by 12% SDS/PAGE and transferred to nitrocellulose membrane. Membranes were blocked in 20 mM Tris (pH 7.6), 250 mM NaCl containing 5% BSA and probed with mouse anti-ERK or rabbit anti-Jnk or mouse anti-p38 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Following incubation with peroxidase- or alkaline phosphatase-conjugated donkey anti-mouse, or goat anti-rabbit secondary antibody (Santa Cruz Biotechnology), proteins were visualized with chemiluminence detection system (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The intensity of the bands was determined by scanning video densitometry using a phosphoimager (Storm 860 and the ImageQuant TL software version 2003.2; GE Healthcare).
Effect of MBG on caspase 3/7, 8, and caspase 9 activities.
The Apo-ONE homogeneous caspase 3/7, 8, and 9 assays were performed in 96-well formats after the RLMEC were incubated with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG for 3 h. Other cells were pretreated for 2 h with a p38 inhibitor (10 μM SB 202190; Sigma) or a Jnk inhibitor (20 μM SP 600125; Invitrogen) before exposure to DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG. One hundred microliters of Apo-ONE caspase 3, caspase 8, or caspase 9 (Promega, Madison, WI) reagent was added to each well. After a 1-h incubation at 37°C, the luminescence was measured by a luminometer (Fluoroskan Ascent FL, Thermo Labsystems, Franklin, MA), as described previously (21, 26, 28).
Annexin-V staining.
Annexin-V staining was performed by a method described previously (42, 44). RLMEC were incubated with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG for 24 h. Some cells were pretreated for 2 h with a p38 inhibitor (10 μM SB 202190; Sigma) or a Jnk inhibitor (20 μM SP 600125; Invitrogen) before treatment with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG for 24 h. Some cells were treated only with either a p38 inhibitor (10 μM SB 202190; Sigma) or a Jnk inhibitor (20 μM SP 600125; Invitrogen). Cells were then incubated for 30 min in 1:50 dilution of biotinylated annexin-V (Roche Diagnostics, Indianapolis, IN) in incubation buffer. After incubation, cells were fixed in 4% paraformaldehyde (PFA) for 10 min at room temperature. The cells were then incubated with a 1:200 dilution of Cy3-labeled streptavidin (GE Healthcare) for 30–60 min at room temperature. After incubation, cells were mounted by coverslips onto microscope slides along with the nuclear marker 4′, 6′ diamidino-2-phenylindole (DAPI, Invitrogen, Eugene, OR). Cells were visualized using an Olympus FluoView FV 300 confocal laser-scanning microscope.
Effect of MBG on endothelial adherens junctions.
RLMEC were grown on fibronectin-coated glass chamber slides. The cells were treated with 0.1, 1, 10, or 100 nM MBG for 24 h. Other cells were pretreated for 2 h with a 20 μM concentration of a pan caspase inhibitor (Z-VAD-FMK, R&D Systems, Minneapolis, MN) before treatment with 0.1, 1, 10, or 100 nM MBG. The control group of cells was treated with DMSO (vehicle). In separate experiments, cells were treated only with the pan caspase inhibitor. After MBG treatment, cells were washed in PBS and fixed in 4% paraformaldehyde. After repeated washing steps, Triton X-100 treatment, and blocking for nonspecific binding, cells were incubated with a primary antibody for β-catenin (1:100) raised in the rabbit (Santa Cruz Biotechnology) at 4°C overnight. Cells were washed in PBS and exposed to an FITC-conjugated secondary antibody for 1 h. After repeated washing steps, the cells were mounted in an antifade mounting medium that contained the nuclear stain DAPI (Invitrogen, Eugene, OR). Cells were observed under an Olympus FluoView FV 300 confocal laser-scanning microscope with appropriate filters for visualizing FITC and DAPI.
Statistical analysis.
Data (presented as means ± SE) from MBG-treated groups were compared with vehicle (DMSO)-treated groups. Statistical comparisons for multiple determinations were performed using a one-way ANOVA with Tukey's post hoc test. A P value of less than 0.05 was considered significant.
RESULTS
MBG inhibited RLMEC proliferation.
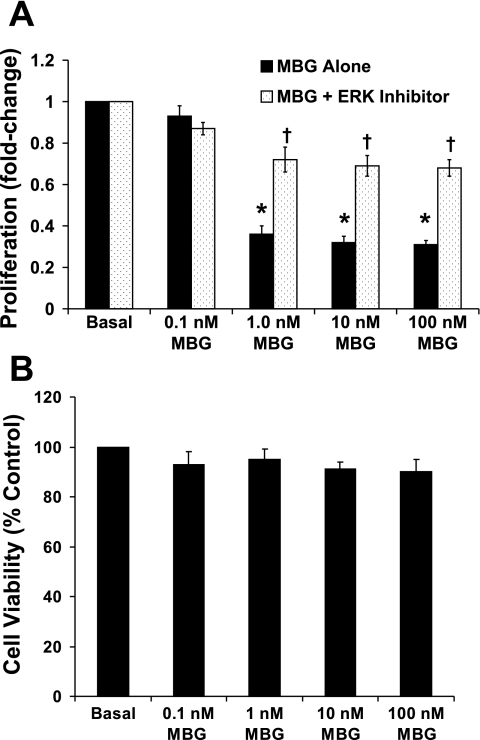
As shown in Fig. 1A, MBG treatment significantly inhibited RLMEC proliferation compared with DMSO (vehicle)-treated cells (66%, 70%, and 70% inhibition by 1, 10, and 100 nM MBG, respectively; P < 0.001 for each). The 0.1 nM MBG treatment had no effect on RLMEC cell proliferation. MBG-induced inhibition of RLMEC proliferation was significantly attenuated by 100 μM ERK inhibitor (P < 0.001 for each) (Fig. 1A). These results indicate that MBG has an antiproliferative effect on RLMEC, which was attenuated by ERK inhibition. The antiproliferative capacity of MBG on the cells was not due to a cytotoxic effect of the steroid, as evaluated by cell viability assays (Fig. 1B). As shown in Fig. 1B, RLMEC viability was not affected by MBG treatment.
Fig. 1.
A: inhibition of cell proliferation by marinobufagenin (MBG). Cell proliferation (ordinate) is expressed as the fold-change compared with basal. Cell proliferation was significantly inhibited in 1, 10, and 100 nM MBG-treated cells compared with DMSO-treated groups (*P < 0.001 for all groups). MBG-induced inhibition of rat lung microvascular endothelial cells (RLMEC) proliferation was significantly attenuated by the ERK inhibitor in a concentration of 100 μM (†P < 0.001 in each case). The 0.1 nM MBG treatment had no effect. The results are presented as the means ± SE (n = 5, 8 replicates each). B: MBG, at concentrations from 0.1 to 100 nM had no effect on RLMEC viability. Cell viability is expressed as the percentage of basal.
MBG increased monolayer permeability.
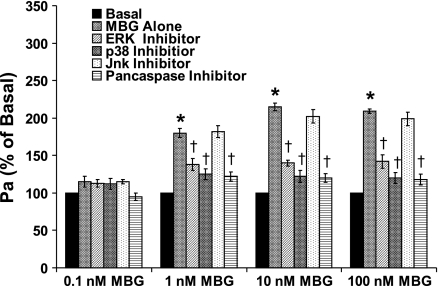
RLMEC monolayer permeability was significantly increased (∼2-fold) by 1, 10, and 100 nM MBG treatment for 3 h (P < 0.001, in each case) (Fig. 2). The 0.1 nM MBG treatment had no effect on the RLMEC monolayer permeability. To test whether the increased hyperpermeability was downstream of ERK, Jnk, p38, and caspases, RLMEC were pretreated with an ERK, a Jnk, a p38 or a pan caspase inhibitor before treatment with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG. The ERK, p38, and pan caspase inhibitors each significantly attenuated the hyperpermeability induced by 1, 10, or 100 nM MBG (P < 0.001, in each case, Fig. 2), while the Jnk inhibitor did not do so.
Fig. 2.
The effect of MBG on the permeability of RLMEC monolayers. Plotted on the y-axis is the change in permeability coefficient of albumin (Pa) expressed as a percentage of the basal value. The 0.1, 10, and 100 nM MBG treatments significantly increased the monolayer permeability of RLMEC within 3 h of treatment (*P < 0.001 in each case). The 0.1 nM MBG treatment had no effect. Pretreatment with an ERK, a p38 or a pan caspase inhibitor significantly attenuated, but did not completely prevent, the MBG-induced hyperpermeability of the RLMEC (†P <0.001 in each case).
MBG downregulated ERK 1/2 phosphorylation.
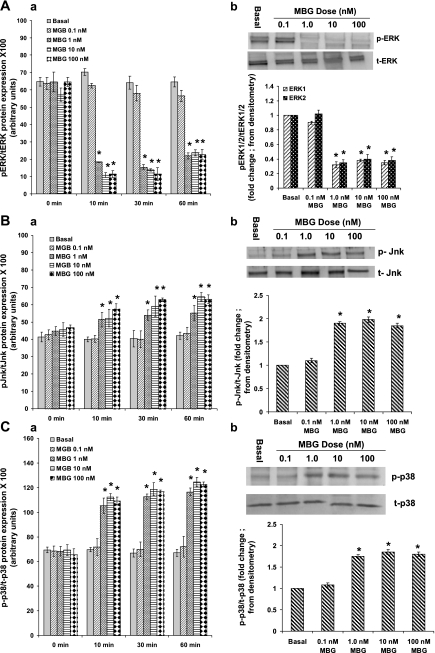
To determine whether the decreased proliferation and increased monolayer permeability were due to reduced ERK1/2 activity, we measured ERK1/2 phosphorylation in RLMEC. As shown in Fig. 3A, a, 1, 10, and 100 nM MBG caused a significant decrease in the ratio of phosphorylated ERK1/2 to total ERK1/2 in RLMEC compared with controls [80%, 80%, and 72% decrease in phosphorylation at 10, 30, and 60 min, respectively; P < 0.001 for DMSO (vehicle) vs. 1, 10, or 100 nM MBG treatment]. Ratios of phosphoprotein to total protein at 2 h and 4 h time points were not statistically different compared with 1 h for all measures (data not shown). MBG had no effect on total ERK protein expression, demonstrating that the change in the ratio was due to increased phosphorylation.
Fig. 3.
A: effect of MBG on ERK phosphorylation in RLMEC. a: phosphorylation of ERK1/2 is presented as the ratio of phosphorylated to total ERK. ERK1/2 phosphorylation in the RLMEC was significantly decreased in MBG-treated cells compared with the DMSO-treated cells at all time points. (*P < 0.001, basal vs. 1, 10, and 100 nM treated cells). The 0.1 nM MBG treatment had no effect. The results are presented as the means ± SE (n = 6, 4 replicates each). b: Western blot analysis of ERK1/2 phosphorylation. After each treatment, cell lysates were analyzed by Western blot for the phosphorylated and total ERK1/2. ERK1/2 phosphorylation was significantly downregulated by 1, 10, and 100 nM MBG (*P < 0.001 for DMSO vs. 1, 10, and 100 nM MBG treatment). The data are presented as means ± SE for three experiments. The 0.1 nM MBG treatment had no effect. B: effect of MBG on Jnk phosphorylation in RLMEC. a: ratio of phosphorylated to total Jnk protein expression was significantly increased in MBG-treated cells compared with the DMSO-treated cells at all time points for 1, 10, and 100 nM MBG (*P < 0.001, basal vs. 1, 10, and 100 nM-treated cells for 10, 30, or 60 min). The results are presented as the means ± SE (n = 6, 4 replicates each). b: Western blot analysis of Jnk phosphorylation. Cell lysates were analyzed by Western blot for phosphorylated and total Jnk. Jnk phosphorylation was significantly upregulated by 1, 10, and 100 nM MBG (*P < 0.001 for DMSO vs. 1, 10, and 100 nM MBG treatment). The data are presented as means ± SE for three experiments. The 0.1 nM MBG treatment had no effect on Jnk phosphorylation in RLMEC. C, a: p38 phosphorylation was significantly increased in MBG-treated RLMEC compared with the DMSO-treated cells at all time points for 1, 10, and 100 nM MBG (*P < 0.001, basal vs. 1, 10, and 100 nM treated cells for 10, 30, or 60 min). The results are presented as the means ± SE (n = 6, 4 replicates each). b: Western blot analysis of p38 phosphorylation. Cell lysates were analyzed by Western blot for the phosphorylated and total p38. p38 phosphorylation was significantly upregulated by 1, 10, and 100 nM MBG (*P < 0.001 for DMSO vs. 1, 10, and 100 nM MBG treatment). The data are presented as means ± SE for three experiments. The 0.1 nM concentration of MBG had no effect.
To confirm the CASE data for the phosphorylation status of ERK1/2, we treated RLMEC with DMSO (vehicle) or 0.1, 1, 10, or 100 nM MBG for 60 min. Cell lysates were analyzed by Western blot analysis for phosphorylated and total ERK1/2. As shown in Fig. 3A, b, ERK1/2 phosphorylation was significantly downregulated by 1, 10, and 100 nM MBG [P < 0.001 for DMSO (vehicle) vs. 1, 10, and 100 nM MBG treatment]. The 0.1 nM MBG treatment had no effect.
MBG increased phosphorylation of Jnk and p38.
A significant increase in the ratio of phosphorylated Jnk to total Jnk was observed in 1, 10, and 100 nM MBG-treated RLMEC compared with basal [50%, 56%, and 56% increase at 10, 30, and 60 min, respectively; P < 0.001 for DMSO (vehicle) vs. 1, 10, and 100 nM MBG treatment] (Fig. 3B, a). Similarly, 1, 10, and 100 nM MBG significantly increased the ratio of phosphorylated p38 to total p38 in RLMEC compared with basal [70%, 71%, and 71% increase at 10, 30, and 60 min, respectively; P < 0.001 for DMSO (vehicle) vs. 1, 10, and 100 nM MBG treatment] (Fig. 3C, a). Ratios of phosphoprotein to total protein at 2 h and 4 h time points were not statistically different compared with 1 h for all measures (data not shown). MBG had no effect on total Jnk and p38 protein expression demonstrating that the changes in the ratio were due to increased phosphorylation.
To confirm the CASE data for the phosphorylation status of Jnk and p38, we treated RLMEC with DMSO (vehicle) or 0.1, 1, 10, or 10 nM MBG for 60 min. After each treatment, cell lysates were analyzed by Western blot for phosphorylated and total Jnk and p38. As shown in Fig. 3B, b and 3C, b, Jnk and p38 phosphorylation were significantly upregulated by 1, 10, and 100 nM MBG (P < 0.001 for DMSO vs. 1, 10, and 100 nM MBG treatment, in each case). The 0.1 nM MBG treatment had no effect on either Jnk or p38 phosphorylation.
Activation of apoptosis by MBG in RLME cells was attenuated by p38 inhibition.
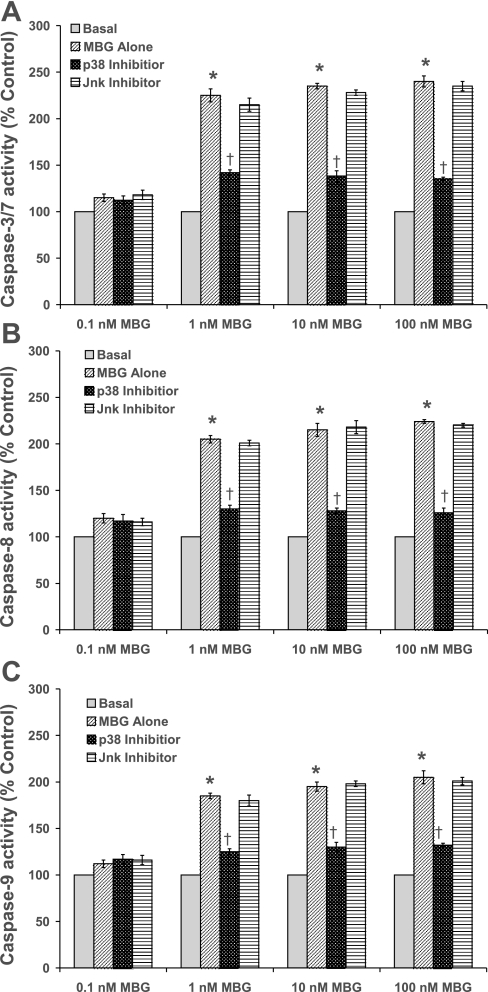
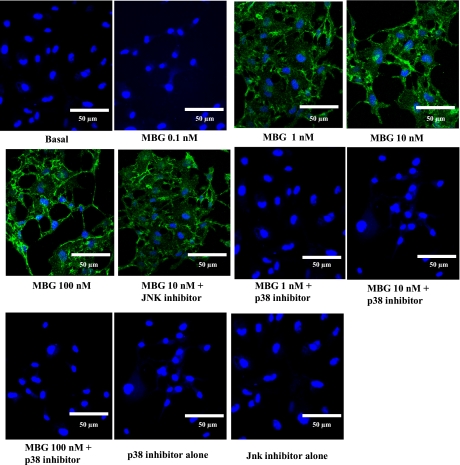
Apoptosis was evaluated by caspase 3/7, 8 and 9 activation and Annexin-V staining. As shown in Figs. 4, A–C, 1, 10, and 100 nM MBG significantly (∼2.0-fold) activated caspase 3/7, 8, and 9 activities, respectively, in RLMEC compared with basal at 3 h [P < 0.001 for DMSO (vehicle) vs. 1, 10, or 100 nM MBG treatment]. The 0.1 nM MBG treatment had no effect on the activation of caspase 3/7, 8, or 9 activities (Fig. 4, A–C). Cells pretreated with the p38 inhibitor prevented the activation of caspase 3/7, 8, and 9 activities (P < 0.001), while the Jnk inhibitor did not alter MBG-induced activation of the caspases (Figs. 4, A–C). Staining with annexin-V revealed apoptotic cells in the RLMEC treated with 1, 10, and 100 nM MBG. However, 0.1 nM MBG had no such effect. Contrarily, apoptosis was not observed in cells treated with either p38 or Jnk inhibitors alone. These data indicate that apoptosis was activated by the upregulation of the p38 pathway (Fig. 5).
Fig. 4.
Effect of MBG on the activation of caspase 3/7 (A), caspase 8 (B), and caspase 9 (C) activities in RLMEC. On the ordinate are plotted the percentages of caspase activity compared with basal. Caspases 3/7, 8 and 9 activities were significantly increased in MBG-treated cells compared with DMSO-treated cells (*P < 0.001 for basal vs. 1, 10, and 100 nM MBG treatment). The results are presented as the means ± SE (n = 5, 8 replicates each). The 0.1 nM MBG treatment had no effect. Pretreatment with a p38 inhibitor attenuated the activation of caspase 3/7, 8, and 9 activities (†P < 0.001), while the Jnk inhibitor had no effect on MBG-induced caspase activation.
Fig. 5.
Evaluation of apoptosis by Annexin-V staining. Green staining is Annexin-V positive (apoptotic cell). Blue staining is DAPI, which stains the nucleus. Images were taken at ×60. The 1, 10, and 100 nM MBG-treated cells showed apoptosis, while 0.1 nM MBG had no effect. Apoptosis was prevented in cells pretreated with a p38 inhibitor, but not by a Jnk inhibitor. Jnk, p38, and pan caspase inhibitors alone had no effect.
MBG-induced disruption of endothelial cell adherens junctions was attenuated by a pan caspase inhibitor.
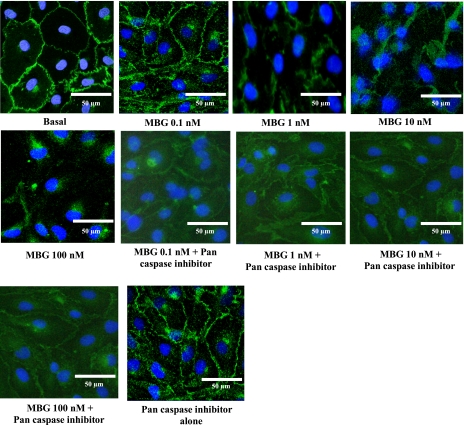
RLMEC treated with DMSO showed strong and continuous β-catenin immunofluorescence at the endothelial cell-cell junctions (Fig. 6), indicating an intact cell barrier. Treatment with 1, 10, and 100 nM MBG induced disruption of endothelial cell junctions, as evidenced by irregular and scattered β-catenin fluorescence (Fig. 6). Cells treated with 0.1 nM MBG did not demonstrate this effect (Fig. 6). Cells pretreated with a pan caspase inhibitor prior to 1, 10, or 100 nM MBG demonstrated attenuated β-catenin immunofluorescence at the endothelial cell-cell junctions (Fig. 6). The cells treated with the pan caspase inhibitor alone did not show any disruption of the endothelial cell barrier (Fig. 6).
Fig. 6.
Immunofluorescence images of endothelial cell adherens junction protein β-catenin in RLMEC. MBG disrupted endothelial junctions. Control (DMSO-treated) cells show intact junctions evidenced by the strong and continuous presence of β-catenin at the junctions. The 1, 10, and 100 nM MBG-treated cells show disruption of the junctions evidenced by scattered β-catenin fluorescence. Pretreatment with the pan caspase inhibitor eliminated the effect of MBG. The pan caspase inhibitor alone had no effect.
DISCUSSION
Preeclampsia is a hypertensive disorder which develops de novo in women after 20 wk of gestation (30, 31). In normal pregnancy, expansion of the extracellular fluid volume appears to be distributed rather evenly between the intravascular and interstitial compartments (5). In preeclampsia, however, the hematocrit rises above that seen in normal pregnancy. Although the patient is volume expanded, the fluid seems to be redistributed more extensively to the interstitial compartment than is seen in normal pregnancy (5, 18). Accordingly, the preeclamptic patient has been suspected of demonstrating increased vascular permeability or “capillary leak” syndrome. A rat model that has many of the phenotypic characteristics of human preeclampsia has been developed in our laboratory (19). Recently, we demonstrated an alteration in vascular permeability in this model (41). We also reported that MBG is involved in the development of the vascular leakage (41).
To determine the cellular and molecular mechanisms underlying the process of altered vascular permeability, we examined the effect of MBG on RLMEC. We demonstrated that MBG treatment inhibited the proliferative capacity of RLMEC, similar to its effects on CTB cell proliferation (22, 43). We also demonstrated that MBG increased the monolayer permeability of RLMEC. We endeavored to directly examine the intrinsic apoptotic-signaling cascade as a potential contributor to MBG-induced hyperpermeability. Therefore, following exposure to MBG, we blocked caspase activation and demonstrated a marked attenuation of microvascular hyperpermeability (Fig. 2). Hemorrhagic shock following trauma has been shown by several investigators to activate mediators of apoptosis, including caspases (7, 10, 49). In our study, we found that MBG induced RLMEC apoptosis. This was demonstrated by the activation of caspases 3/7, 8, and 9 (Fig. 4, A–C) and also by positive annexin-V staining (Fig. 5). These effects of MBG were similar to those reported previously in CTB cells (42).
MBG-induced downregulation of ERK 1/2 suggests that this cardiotonic steroid leads to a decrease in cell cycle progression. In ovarian endometric cyst stromal cells bufalin downregulates the expression of cyclin A, which is important for DNA replication (26). Thus, MBG may inhibit RLMEC proliferation and increase monolayer permeability by modulating ERK1/2 activation, as well as by activation of apoptosis. In a recent study, we demonstrated that MBG-induced impairment of CTB cell function was associated with decreased ERK1/2 activity (43). Furthermore, it has been reported that inhibition of ERK activity is associated with apoptosis and the activation of Jnk and p38 phosphorylation (2, 8). To determine whether MBG-induced apoptosis might contribute to RLMEC dysfunction, we examined the effects of MBG on Jnk and p38 activity in RLMEC. We found that MBG upregulated Jnk and p38 phosphorylation. To examine the involvement of p38 in the activation of apoptosis, we blocked MBG-induced apoptotic signaling with a p38 inhibitor. We demonstrated that a marked attenuation of apoptotic signaling resulted. Jnk inhibition had no such effect. Similar findings were reported by Chuang et al. (8) in CL13 human lung adenocarcinoma cells by treatment with cadmium. Apoptotic signaling cascades eventually lead to a common effector phase of apoptosis characterized by the activation of caspases and p38 or Jnk signaling pathways (2, 8, 35).
Our results show that the disruption of β-catenin/endothelial cell adherens junctions occurs after treatment of cells with MBG (Fig. 6). These results support the view that endothelial cell hyperpermeability occurs because of the activation of caspase-3 and subsequent cleavage of β-catenin resulting in cell detachment. Caspase-3 cleaves a variety of cell adhesion proteins (39). The caspase-3-dependent cleavage of β-catenin occurs during apoptosis (3, 17, 25). Our results also demonstrate that the MBG-induced disruption of β-catenin is attenuated by a pan caspase inhibitor (Fig. 6). These observations are in agreement with previous studies (3, 17, 25). Our results support the involvement of caspases in the cleavage of endothelial cell adherens junction protein β-catenin in the MBG-induced hyperpermeability of RLMEC monolayers (Fig. 6). Previously, Childs et al. (7) have demonstrated that hemorrhagic shock serum induces disruption of endothelial cell adherens junctions.
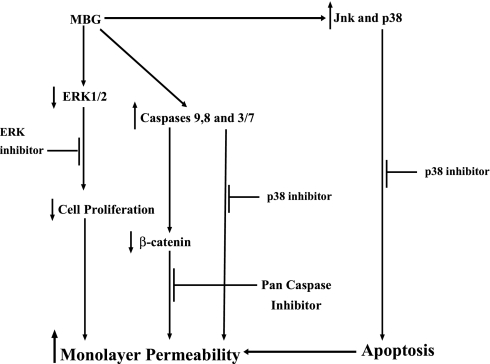
In Fig. 7 is presented a schematic diagram of the mechanisms involved in MBG-induced RLMEC monolayer hyperpermeability.
Fig. 7.
Working model for MBG-induced hyperpermeability and apoptosis signaling mechanisms in RLMEC. MBG downregulates ERK1/2 phosphorylation, inhibits proliferation and triggers endothelial hyperpermeability. The bufadienolide also activates Jnk and p38 leading to RLMEC apoptosis. Inhibition of ERK, p38, and pan caspase prevents endothelial hyperpermeability.
Perspectives and Significance
Multiple etiologic factors have been proposed in the etiology of preeclampsia (30, 31). We have proposed that MBG plays a role, perhaps a major one, in the development of leak from the vascular tree into the interstitium in preeclampsia (41). To test this hypothesis in vitro, we utilized RLMEC to determine whether alterations in monolayer permeability occurred as the result of treatment of these cells with MBG. We also examined changes in MBG-induced cellular signaling. We found that MBG downregulated phosphorylation of ERK1/2 but upregulated phosphorylation of p38. Furthermore, MBG-induced hypermeability of RLMEC monolayers and apoptotic signaling. The latter effect was attenuated by p38, but not Jnk inhibition. In addition, MBG caused disruption of endothelial cell junction integrity, which was attenuated by a pan caspase inhibitor. This communication provides the first evidence that MBG increases vascular permeability in endothelial cells. Furthermore, these data provide confirmatory evidence for the potential importance of MBG as an etiologic factor in the alteration in vascular permeability seen in our rat model of human preeclampsia. These observations may have important implications with regard to the involvement of MBG in the causation of an abnormal vascular leak in preeclampsia and perhaps, other syndromes characterized by “capillary leak.”
GRANTS
These studies were supported, in part, by a research grant-in-aid from Dialysis Clinic, and by the Department of Medicine, Texas A&M College of Medicine and the Scott & White Memorial Hospital, Temple, TX and by a grant from the National Heart, Lung, and Blood Institute (1K01-HL-07815-01A1; EW Childs).
Acknowledgments
We acknowledge the assistance of the Texas A&M University Health Science Center, Integrated Microscopy/Imaging Laboratory. We thank Dr. G. R. Pettit (Arizona State University) for his gift of the marinobufagenin. We thank Dr. Binu Tharakan for the gift of the rat lung microvascular endothelial cells.
REFERENCES
- 1.Bagrov AY, Fedorova OV. Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+, K+-pump in human mesenteric arteries. J Hypertens 16: 1953–1958, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Berra E, Diaz-Meco MT, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem 273: 10792–10797, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Cervello M, Giannitrapani L, La Rosa M, Notarbartolo M, Labozzetta M, Poma P, Montalto G, D'Alessandro N. Induction of apoptosis by the proteasome inhibitor MG132 in human HCC cells: possible correlation with specific caspase-dependent cleavage of beta-catenin and inhibition of beta-catenin-mediated transactivation. Int J Mol Med 13: 741–748, 2004. [PubMed] [Google Scholar]
- 4.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature 410: 37–40, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Chesley LC Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol 112: 440–450, 1972. [DOI] [PubMed] [Google Scholar]
- 6.Childs EW, Tharakan B, Byrge N, Tinsley JH, Hunter FA, Smythe WR. Angiopoietin-1 inhibits intrinsic apoptotic signaling and vascular hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol 294: H2285–H2295, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol 292: H3179–H3189, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chuang SM, Wang IC, Yang JL. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis 21: 1423–1432, 2000. [PubMed] [Google Scholar]
- 9.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazen assay for cell growth assays in culture. Cancer Commun 3: 207–212, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Davidson MT, Deitch EA, Lu Q, Hasko G, Abungu B, Nemeth ZH, Zaets SB, Gaspers LD, Thomas AP, Xu DZ. Trauma-hemorrhagic shock mesenteric lymph induces endothelial apoptosis that involves both caspase-dependent and caspase-independent mechanisms. Ann Surg 240: 123–131, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis RJ Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Durst J, Vu HV, Ianosi-Irimie MR, Pridjian CA, Bagrov A, Fedorova OV, Pridjian G, Puschett JB. Antibodies to marinobufagenin reduce blood pressure in a rat model of preeclampsia. J Investig Med 52: S318, 2004. [Google Scholar]
- 13.Fedorova OV, Kolodkin NI, Agalakova NI, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension 37: 462–466, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Fisher SJ The placental problem: Linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol 2: 53, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via beta (2) integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med 191: 1829–1839, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin Exp Hypertens 20: 617–627, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Gregorc U, Ivanova S, Thomas M, Turk V, Banks L, Turk B. hDLG/SAP97, a member of the MAGUK protein family, is a novel caspase target during cell-cell detachment in apoptosis. Biol Chem 386: 705–710, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hays PM, Cruikshank DP, Dunn LJ. Plasma volume determination in normal and preeclamptic pregnancies. Am J Obstet Gynecol 151: 958–966, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Ianosi-Irimie MR, Vu HV, Whitbred JM, Pridjian CA, Nadig JD, Williams MY, Wrenn DC, Pridjian G, Puschett JB. A rat model of preeclampsia. Clin Exp Hypertens 8: 605–617, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol 18: 227–234, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kavinen J, Hurkainen P, Gopalakrishnan S, Burns D, Warrior U, Hemmila I. Homogeneous time-resolved fluorescence quenching assay (LANCE) for caspase-3. J Biomol Screen 7: 223–231, 2002. [DOI] [PubMed] [Google Scholar]
- 22.LaMarca HL, Morris CA, Pettit GR, Nagowa T, Puschett JB. Marinobufagenin impairs first trimester cytotrophoblast differentiation. Placenta 27: 984–988, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, Bagrov AY. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens 17: 1179–1187, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Nakamoto K, Kuratsu J, Ozawa M. Beta-catenin cleavage in non-apoptotic cells with reduced cell adhesion activity. Int J Mol Med 15: 973–979, 2005. [PubMed] [Google Scholar]
- 26.Nasu K, Nishida M, Ueda T, Takai N, Bing S, Narahara H, Miyakawa I. Bufalin induces apoptosis and the G0/G1 cell cycle arrest of endometriotic stromal cells: a promising agent for the treatment of endometriosis. Mol Hum Reprod 11: 817–823, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem Sci 25: 257–260, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Pamnani MB, Chen S, Yuan CM, Haddy FJ. Chronic blood pressure effects of bufalin, a sodium-potassium ATPase inhibitor, in rats. Hypertension 23: I106–109, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Pridjian G, Puschett JB. Preeclampsia Part 1: Clinical and pathophysiological considerations. Obstet Gynecol Surv 57: 598–618, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Pridjian G, Puschett JB. Preeclampsia Part 2: Experimental and genetic considerations. Obstet Gynecol Surv 57: 619–640, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Readline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol 26: 594–600, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 341: 1447–1451, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 161: 1200–1204, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res 153: 245–257, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Schoner W Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem 269: 2440–2448, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Svedas E, Islam KB, Nisell H, Kublickiene KR. Vascular endothelial growth factor induced functional and morphologic signs of endothelial dysfunction in isolated arteries from normal pregnant women. Am J Obstet Gynecol 188: 168–176, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly (ADP-ribose) polymerase. Cell 81: 801–809, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 281: 1312–1316, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Tinsley JH, Wu MH, Ma W, Taulman AC, Yuan SY. Activated neutrophils induce hyperpermeability and phosphorylation of adherens junction proteins in coronary venular endothelial cells. J Biol Chem 274: 24930–24934, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Uddin MN, McLean LB, Hunter FA, Horvat D, Severson J, Tharakan B, Childs EW, Puschett JB. Vascular leak in a rat model of preeclampsia. Am J Nephrol 30: 26–33, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uddin MN, Horvat D, Glaser SS, Mitchell BM, Puschett JB. Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J Biol Chem 283: 17946–17953, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Uddin MN, Horvat D, Glaser SS, Danchuk S, Mitchell BM, Sullivan DE, Morris CA, Puschett JB. Marinobufagenin inhibits proliferation and migration of cytotrophoblast and CHO cells. Placenta 29: 266–273, 2008. [DOI] [PubMed] [Google Scholar]
- 44.van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 24: 131–139, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Versteeg HH, Nijhuis E, van den Brink GR, Evertzen M, Pynaert GN, van Deventer SJ, Coffer PJ, Peppelenbosch MP. A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B, and cAMP-response-element-binding protein. Biochem J 350: 717–722, 2000. [PMC free article] [PubMed] [Google Scholar]
- 46.Vu HV, Ianosi-Irimie MR, Pridjian CA, Whitbred JM, Durst JM, Bagrov AY, Fedorova OV, Pridjian G, Puschett JB. The involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol 25: 520–528, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Adair CD, Weeks JW, Lewis DF, Alexander JS. Increased neutrophil-endothelial adhesion induced by placental factors is mediated by platelet-activating factor in preeclampsia. J Soc Gynecol Investig 6: 136–141, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Gu Y, Granger DN, Roberts JM, Alexander JS. Endothelial junctional protein redistribution and increased monolayer permeability in human umbilical vein endothelial cells isolated during preeclampsia. Am J Obstet Gynecol 186: 214–220, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Watts JA, Grattan RM, Whitlow BS, Kline JA. Activation of poly (ADP-ribose) polymerase in severe hemorrhagic shock and resuscitation. Am J Physiol Gastrointest Liver Physiol 281: G498–G506, 2001. [DOI] [PubMed] [Google Scholar]