Abstract
We investigated the influence of extracellular matrix on transport properties of mouse alveolar epithelial cell (AEC) monolayers (MAECM) and transdifferentiation of isolated mouse alveolar epithelial type II (AT2) cells into an alveolar epithelial type I (AT1) cell-like phenotype. Primary mouse AT2 cells plated on laminin 5-coated polycarbonate filters formed monolayers with transepithelial resistance (RT) and equivalent short-circuit current (IEQ) of 1.8 kΩ·cm2 and 5.3 μA/cm2, respectively, after 8 days in culture. Amiloride (10 μM), ouabain (0.1 mM), and pimozide (10 μM) decreased MAECM IEQ to 40%, 10%, and 65% of its initial value, respectively. Sequential addition of pimozide and amiloride, in either order, revealed that their inhibitory effects are additive, suggesting that cyclic nucleotide-gated channels contribute to amiloride-insensitive active ion transport across MAECM. Ussing chamber measurements of unidirectional ion fluxes across MAECM under short-circuit conditions indicated that net absorption of Na+ in the apical-to-basolateral direction is comparable to net ion flux calculated from the observed short-circuit current: 0.38 and 0.33 μeq·cm−2·h−1, respectively. Between days 1 and 9 in culture, AEC demonstrated increased expression of aquaporin-5 protein, an AT1 cell marker, and decreased expression of pro-surfactant protein-C protein, an AT2 cell marker, consistent with transition to an AT1 cell-like phenotype. These results demonstrate that AT1 cell-like MAECM grown on laminin 5-coated polycarbonate filters exhibit active and passive transport properties that likely reflect the properties of intact mouse alveolar epithelium. This mouse in vitro model will enhance the study of AEC derived from mutant strains of mice and help define important structure-function correlations.
Keywords: alveolar epithelium, ion transport, short-circuit current, transepithelial resistance, phenotypic transition
the mechanisms regulating active ion transport are of major importance for understanding alveolar fluid balance under physiological and pathophysiological conditions. The results of several in vivo studies have demonstrated that pharmacological inhibitors of Na+ transport reduce the rate of alveolar fluid clearance (AFC), whereas β-adrenergic agonists increase the rate of AFC in the lungs of several different species, including humans (35, 36). Studies using a model of confluent alveolar epithelial cell (AEC) monolayers derived from isolated rat type II (AT2) cells in primary culture have complemented in vivo studies and provided important mechanistic insights into ion transport across the alveolar epithelium (4, 9, 31, 34). Measurement of net ion fluxes under zero voltage gradient (i.e., short-circuit) conditions permits direct demonstration of active ion transport across AEC monolayers cultured on permeable supports. Results from in vivo and in vitro studies indicate that vectorial transport of salt, with water following passively, is the primary mechanism for fluid clearance from alveolar spaces (12, 35).
Although rat and human AEC monolayers (RAECM and HAECM, respectively) have been well characterized and are widely used as models to study epithelial barrier properties and regulation of AEC differentiation (3, 7, 9, 21, 22, 49), comparable progress in development of functional mouse AEC monolayers (MAECM) has not been accomplished. In an important early study, Corti et al. (11) reported a reliable method for isolating viable purified AT2 cells from mice. They cultured mouse AT2 cells on fibronectin-coated chamber slides and, after 5 days, reported that the cells attained a flattened polygonal morphology and lost their lamellar bodies, although transition toward the type I (AT1) cell phenotype, as reflected by expression of phenotype-specific markers, was not assessed. In a subsequent study, mouse AT2 cells cultured on plastic dishes coated with a two-component Matrigel-rat tail collagen matrix and maintained in medium supplemented with keratinocyte growth factor actively secreted and synthesized surfactant phospholipids for ≥7 days, consistent with retention of AT2 cell characteristics under these culture conditions (46). Studies using RAECM and HAECM have established that inclusion of keratinocyte growth factor in cell culture medium preserves the phenotype of isolated AT2 cells grown over time in culture (7, 49) and modulates the barrier properties of AEC monolayers (8). In addition to factors in culture medium, components of extracellular matrix have been shown to strongly influence AEC phenotype (25) and barrier properties (19, 34) of AEC monolayers.
Substantial advances in transgenic technology have provided powerful tools for study of the genetic basis of lung physiology and pathophysiology in mice (23). Development of in vitro systems that permit the study of AEC monolayers derived using AT2 cells from transgenic mouse lines or genetically defined strains of mice will strongly complement existing in vivo models and allow functional and mechanistic investigation of novel lung phenotypes. In the present study, we investigated the influence of extracellular matrix proteins on transport properties of primary mouse AEC and developed a model that supports the formation of high-resistance monolayers to investigate their transport properties and capacity for transdifferentiation. We demonstrate that mouse AT2 cells form functional monolayers that actively absorb Na+ but, in contrast to their rat and human counterparts, require laminin 5 extracellular matrix to achieve tight barrier properties. This mouse in vitro model system should be useful for functional characterization of AEC derived from genetically modified mice and facilitate important structure-function correlations.
METHODS
Mouse AT2 cell isolation and preparation of MAECM.
Isolation of AT2 cells from 129S6/SvEv mice (Taconic, Germantown, NY) was accomplished using a protocol of dispase (BD Biosciences, Bedford, MA) digestion-agar instillation modified from Corti et al. (11) and approved by the University of Southern California Institutional Animal Care and Use Committee. Mice were anesthetized with pentobarbital sodium (400 mg/kg ip; Ovation Pharmaceuticals, Deerfield, IL), the abdominal cavity was opened, and the renal artery was severed to exsanguinate the mouse. Mouse lungs were perfused and lavaged with PBS. Dispase, followed by 0.5 ml of 1% low-melting-point agarose (Sigma, St. Louis, MO), was injected into lungs via the trachea (cannulated with a 20-gauge barrel-tip needle). Lungs were excised and placed on ice for 2 min and then incubated in dispase for 45 min at room temperature. Lungs were dissected into wash medium containing a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 (DME/F-12; Sigma) supplemented with 0.01% DNase, 1 mM l-glutamine, 100 U/ml sodium penicillin G, and 100 μg/ml streptomycin. Lungs were chopped, and the resulting crude cell mixture was incubated for 10 min at room temperature. This crude cell mixture was passed through a series of Nitex filters (100, 40, and 8 μm; Tetko, Elmsford, NY) and then centrifuged at 300 g for 10 min at 4°C. Macrophages were removed by incubation with biotinylated anti-macrophage antibodies (anti-CD45, anti-Ter 119, and anti-CD16/32; BD Biosciences) and then subjected to magnetic selection with streptavidin-conjugated magnetic beads (Promega, Madison, WI). Cells were incubated on petri dishes precoated with mouse IgG for 2 h at 37°C. Nonadherent AEC were removed from IgG plates and resuspended in complete mouse medium (CMM) consisting of DME/F-12, 1 mM l-glutamine, 0.25% bovine serum albumin (BD Biosciences), 10 mM HEPES, 0.1 mM nonessential amino acids, 0.05% insulin-transferrin-sodium selenite (Roche, Basel, Switzerland), and 100 μg/ml Primocin (Invitrogen, Carlsbad, CA).
Transwell polycarbonate filters (0.4 μm pore size, 1.1 cm2; Corning Costar, Cambridge, MA) were precoated with 180 μl of Matrigel (BD Biosciences), 50 μg/ml laminin 1 (Trevigen, Helgerman, CT), 1 μg/ml laminin 5 (Chemicon, Billerica, MA), or 10 μg/ml type IV collagen (Trevigen) for 1 h at 37°C. Purified mouse AT2 cells in CMM plus 2% newborn bovine serum (Omega Scientific, Tarzana, CA) were plated onto these filters at 106 cells/cm2. Cell culture medium was replaced with serum-free CMM 3 days after plating and every other day thereafter. Monolayers were maintained in a humidified 5% CO2-95% air incubator at 37°C.
Measurement of MAECM bioelectric properties.
Transepithelial resistance (RT, kΩ·cm2) and spontaneous potential difference (PD, mV) were measured using a rapid screening device (MilliCell-ERS, Millipore, Bedford, MA) equipped with silver-silver chloride electrodes as previously described (2, 4, 13). Equivalent short-circuit current (IEQ, μA/cm2) was calculated from the following relation: IEQ = PD/RT. Measurements of RT and IEQ in response to the pharmacological agents amiloride (10 μM) and ouabain (0.1 mM) were performed on MAECM 4, 5, or 6 days after plating. For measurements of short-circuit current (ISC, μA/cm2) in response to terbutaline (10 μM) or sequential addition of pimozide (10 μM) and amiloride (10 μM), monolayers were mounted in modified Ussing chambers at 37°C and bathed on both sides with CMM. A stream of humidified 5% CO2 in air was continuously blown across the surfaces of the bathing fluids (9.5 ml) to maintain constant pH and agitate the bathing fluids. Monolayers were continuously short-circuited throughout the experimental period, except for brief interruptions to allow measurements of spontaneous PD and RT as previously described (31).
Unidirectional fluxes of Na+ and Cl−.
Unidirectional fluxes of Na+ and Cl− were determined using 22NaCl (Perkin Elmer, Boston, MA) and Na36Cl (American Radiolabeled Chemicals, St. Louis, MO). Unidirectional ion fluxes across the monolayers were determined under short-circuit conditions, as described previously (31), in the presence or absence of terbutaline (10 μM). Briefly, 22NaCl or Na36Cl was added to the apical or basolateral reservoir of the Ussing chamber to a specific activity of 2.0 or 1.0 μCi/ml, respectively. Downstream samples (0.1 ml) were taken at 30-min intervals for up to 120 min. Specific activity of the upstream fluid was determined 10 min after radioisotope addition and at the end of the experiment. Radioactive samples were mixed with 10 ml of Ecoscint (National Diagnostics, Manville, NJ) and assayed in a liquid scintillation counter (model LS 6000TA, Beckman, Fullerton, CA). Unidirectional ion fluxes were estimated from the rates of appearance of downstream radioactivity.
Immunofluorescence microscopy of isolated mouse AT2 cells and MAECM.
Purity was determined by immunofluorescence staining of freshly prepared cytospins of mouse AT2 cells using rabbit polyclonal anti-pro-surfactant protein C (pro-SPC; Seven Hills Bioreagents, Cincinnati, OH), rat anti-mouse CD45 (BD Biosciences), and rabbit polyclonal anti-aquaporin-5 (AQP5; Alomone, Jerusalem, Israel) antibodies. A rabbit polyclonal anti-claudin-18 antibody (Invitrogen) was used for immunofluorescence staining of monolayers on filters to delineate cell borders. Antibodies were detected using the following chromogens from Invitrogen: Alexa 488 anti-rabbit, Alexa 568 anti-mouse, and Alexa 488 anti-mouse antibodies.
Western analysis.
Protein was harvested from MAECM grown on laminin 5-coated filters 1–9 days after plating using 2% SDS sample buffer. Equal amounts of cell protein in sample buffer, as determined by the Bradford assay (Bio-Rad, Hercules, CA), were resolved by SDS-PAGE under reducing conditions using the buffer system of Laemmli (32) and transferred to Immobilon-P membranes (Millipore). Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20 for 60 min and incubated overnight with antibodies to pro-SPC and AQP5. After they were washed, the membranes were incubated with an anti-rabbit IgG antibody conjugated to horseradish peroxidase for 60 min, and antigen-antibody complexes were visualized by enhanced chemiluminescence (Pierce, Rockford, IL). Relative amounts of pro-SPC and AQP5 protein were determined using the FluorChem Imaging System (model 8900, Alpha Innotech, San Leandro, CA).
Statistical analysis.
Values are means ± SE. Significance (P < 0.05) was determined by one- or two-way analysis of variance followed by post hoc procedures based on modified Student-Newman-Keuls tests.
RESULTS
Characterization of purified murine AT2 cells and AEC in primary culture.
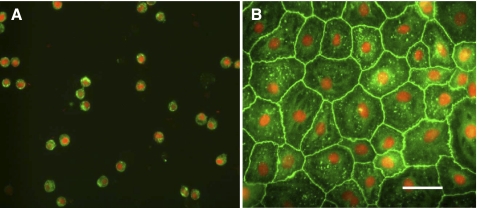
After dispase digestion and mechanical disaggregation of lungs from 129S6/SvEv mice, crude cell suspensions contained ∼25 × 106 cells per mouse (n = 24 preparations). Further enrichment using magnetic bead-based negative selection yielded suspensions containing ∼3.4 × 106 cells per mouse. Purity of final cell suspensions was >90%, as assessed by immunostaining for pro-SPC (Fig. 1A), and cell viability was >94%, as assessed by trypan blue dye exclusion. Cytospin preparations of final cell suspensions were evaluated for the presence of leukocytes (not including erythrocytes) and AT1 cells by immunostaining for CD45 and AQP5, respectively: 8.1% and 0.7% of the cells in suspension were immunoreactive for CD45 and AQP5, respectively, indicating minor leukocyte and minimal AT1 cell contamination in freshly isolated AT2 cell preparations. Mouse AT2 cells plated on laminin 5-coated polycarbonate filters and maintained in culture for 6 days formed confluent monolayers with a cobblestone-like morphology (Fig. 1B). Reactivity of claudin-18 [a tight junction protein expressed in lung epithelial cells (40)] was localized at cell-cell contacts in MAECM (Fig. 1B), consistent with the formation of tight junctions.
Fig. 1.
Cytological characterization of purified murine alveolar type II (AT2) cells. A: purities of freshly isolated AT2 cells from SvEv mice assessed by immunostaining for pro-surfactant protein C (SPC; green). Cytospin preparations reflect purities of 91 ± 1% AT2 cells. Nuclei were counterstained with propidium iodide (red). Results are representative of 24 independent experiments. B: immunoreactivity of claudin-18 (green) in mouse alveolar epithelial cell monolayers (MAECM) grown on a laminin 5-coated polycarbonate filter for 6 days. Nuclei were counterstained with propidium iodide (red). Scale bar, 50 μm. Original magnification ×40.
Phenotypic characterization of MAECM as a function of time.
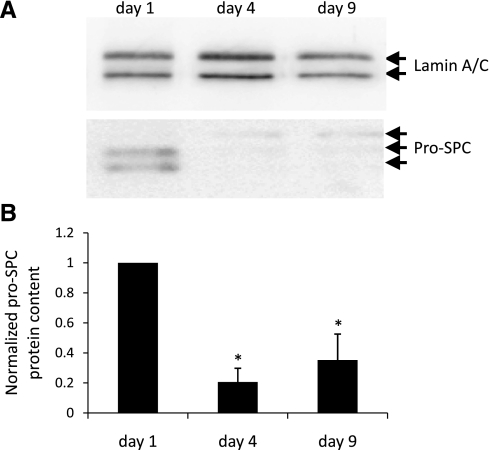
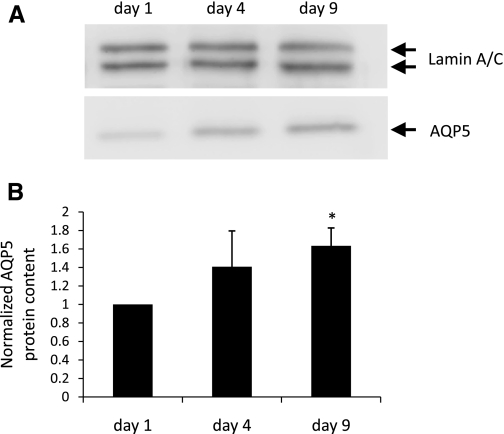
Rat AT2 cells grown in primary culture undergo transition to an AT1 cell-like morphology with time in culture (9). The cells also begin to express AT1 cell-specific markers, concurrent with the loss of lamellar bodies and other hallmarks of the AT2 cell phenotype (3, 14, 16, 38). To determine whether mouse AT2 cells similarly lose AT2 cell characteristics and undergo transition to an AT1 cell-like phenotype, pro-SPC expression was analyzed by Western blotting in MAECM plated on laminin 5-coated polycarbonate filters for 1, 4, and 9 days (Fig. 2A). Pro-SPC protein was significantly decreased after 4 days and remained significantly below day 1 levels after 9 days in culture (Fig. 2B). AQP5 is a useful marker of the AT1 cell phenotype in cultured rat (7) and human (49) AEC and in AT1 cells in situ (38). To investigate AQP5 expression in mouse AEC over time, protein lysates from MAECM were harvested after 1, 4, and 9 days and immunoblotted with an anti-AQP5 antibody (Fig. 3A). AQP5 protein was minimally detectable 1 day after plating and increased significantly after 9 days in culture (Fig. 3B). These results suggest that mouse AT2 cells transition toward an AT1 cell-like phenotype with time in culture.
Fig. 2.
Expression of pro-SPC in MAECM grown on laminin 5-coated polycarbonate filters. A: total lysates of MAECM were subjected to SDS-PAGE and immunoblotted with rabbit polyclonal anti-pro-SPC antibody. Lamin A/C was used as a loading control. B: relative densitometric values normalized to pro-SPC levels in monolayers grown in culture for 1 day. Values are means ± SE (n = 1 monolayer per day per preparation from 4 different cell preparations). *P < 0.05 vs. day 1.
Fig. 3.
Expression of aquaporin-5 (AQP5) in MAECM grown on laminin 5-coated polycarbonate filters. A: total lysates of MAECM were subjected to SDS-PAGE and immunoblotted with rabbit polyclonal anti-AQP5 antibody. Lamin A/C was used as a loading control. B: relative densitometric values normalized to AQP5 levels in monolayers grown in culture for 1 day. Values are means ± SE (n = 1 monolayer per day per preparation from 4 different cell preparations). *P < 0.05 vs. day 1.
Effects of extracellular matrix on bioelectric properties of MAECM.
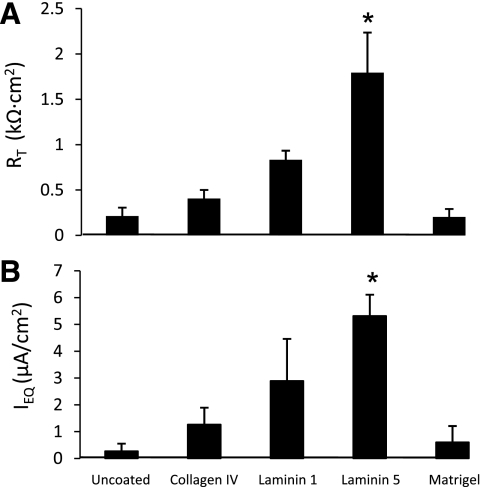
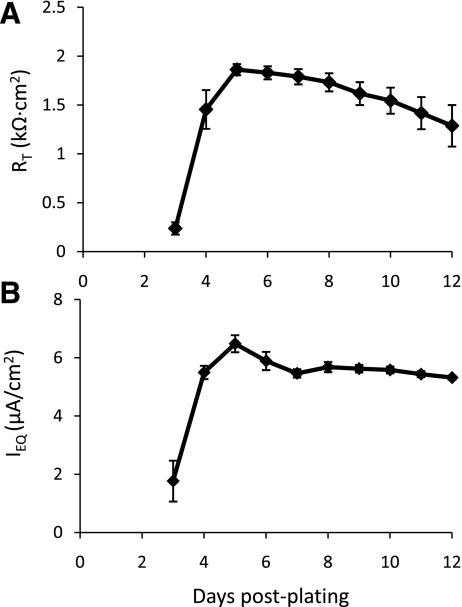
Previous studies in our laboratory (9, 31) showed that RAECM develop RT and IEQ in excess of 2.0 kΩ·cm2 and 4 μA/cm2, respectively, on uncoated polycarbonate filters 4–6 days after plating. In the present study, we investigated the influence of type IV collagen, laminins 1 and 5, and Matrigel on RT and IEQ exhibited by MAECM (Fig. 4). MAECM grown on uncoated polycarbonate filters developed marginal bioelectric properties, with RT only slightly above background. In contrast, MAECM grown on filters coated with type IV collagen, laminin 1, or laminin 5 developed significantly higher RT and IEQ than cells plated on uncoated filters and filters coated with Matrigel. Cells grown on filters coated with laminin 5, in particular, yielded RT and IEQ after 8 days in culture (1.8 ± 0.4 kΩ·cm2 and 5.3 ± 0.8 μA/cm2, respectively) that were significantly higher than those for MAECM grown on filters coated with the other extracellular matrix proteins. The time course of RT and IEQ for monolayers grown on laminin 5-coated filters over 12 days in culture is shown in Fig. 5. After 3 days, MAECM exhibited a brisk increase in the magnitude of RT, attaining maximum values 5 days after plating, with a gradual decline through day 12. These results indicate that, in contrast to RAECM, MAECM require exogenous extracellular matrix for establishment of confluent monolayers and that the substratum on which mouse AT2 cells are plated markedly influences their ability to form functional monolayers. Laminin 5 allows the formation of a tight epithelial barrier, which is necessary to maintain vectorial transport of Na+.
Fig. 4.
Effects of extracellular matrix components on bioelectric properties [transepithelial resistance (RT) and equivalent short-circuit current (IEQ)] of MAECM after 8 days in culture. Values are means ± SE (n = 1 monolayer per extracellular matrix component per preparation from 3 different cell preparations). *P < 0.05 vs. all other conditions.
Fig. 5.
Time course of RT (A) and IEQ (B) across MAECM grown on laminin 5-coated polycarbonate filters. Values are means ± SE (n = 4 total monolayers from 3 different cell preparations).
Effects of amiloride and ouabain on bioelectric properties of MAECM.
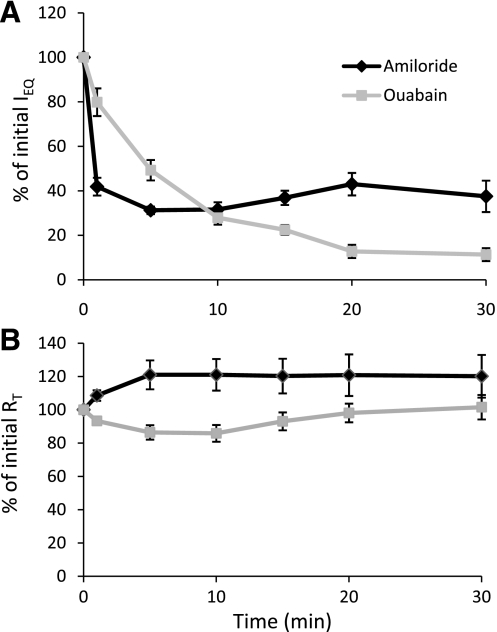
Unidirectional transport of Na+ is mediated by apical Na+ entry via amiloride-sensitive Na+ channels and basolateral Na+ extrusion by ouabain-sensitive Na+ pumps and accounts for the ISC across alveolar epithelium under baseline conditions in RAECM (9, 47). Bioelectric responses to amiloride and ouabain were measured in MAECM 5 days after plating on laminin 5-coated filters. Apical amiloride (10 μM) rapidly decreased IEQ to 40% of its initial value within 1 min and to 30% of its initial value within 5 min, after which there were no further decreases (Fig. 6A). RT, on the other hand, increased to 120% of its initial value after 5 min in response to apical amiloride (Fig. 6B). Addition of ouabain (0.1 mM) to the basolateral fluid of MAECM decreased IEQ to 10% of its initial value after 20 min (Fig. 6A), whereas RT was little changed (Fig. 6B). Addition of amiloride to the basolateral fluid or ouabain to the apical fluid did not appreciably affect IEQ (data not shown). These data indicate that the major pathways of apical Na+ entry and basolateral Na+ extrusion in MAECM are apical amiloride-sensitive Na+ channels and basolateral ouabain-sensitive Na+-K+-ATPase pumps, respectively.
Fig. 6.
Bioelectric responses (IEQ and RT) of MAECM grown on laminin 5-coated polycarbonate filters to amiloride or ouabain instilled into the apical fluid at time 0. Initial mean IEQ and RT for MAECM treated with amiloride (10 μM) were 8.4 ± 0.8 μA/cm2 and 1.9 ± 0.2 kΩ·cm2, respectively. Initial mean IEQ and RT for MAECM treated with ouabain (100 μM) were 7.9 ± 0.7 μA/cm2 and 1.8 ± 0.2 kΩ·cm2, respectively. Values are means ± SE (n = 8 total monolayers from 3 different cell preparations each for amiloride and ouabain).
Effect of pimozide on ISC of MAECM.
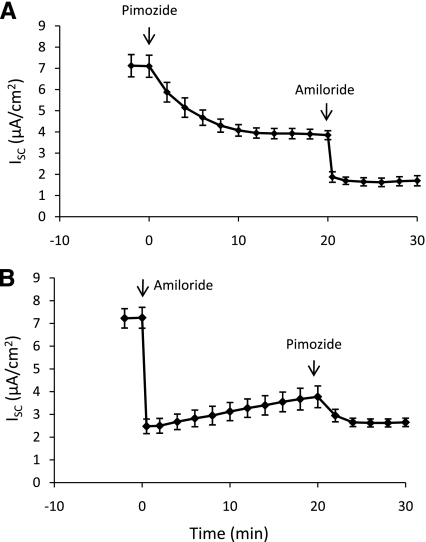
Prior studies suggest a potential role for amiloride-insensitive vectorial Na+ transport across alveolar epithelium via cyclic nucleotide-gated (CNG) nonselective cation channels (29, 30). In the present study, pimozide (10 μM), an inhibitor of CNG channels, was added to the apical fluid of MAECM mounted in Ussing chambers before or after the addition of amiloride (10 μM). As shown in Fig. 7A, pimozide inhibited 45% of basal ISC after 20 min. Subsequent addition of amiloride to the apical bathing solution blocked 58% of the remaining current after pimozide addition (at 30 min). Together, amiloride and pimozide inhibited 77% of the initial ISC. Addition of these inhibitors in the reverse order is shown in Fig. 7B. Addition of amiloride to MAECM inhibited 67% of basal current within 5 min; after 20 min, ISC slowly increased to 54% of its initial value. Subsequent addition of pimozide at 20 min blocked 32% of the remaining current after 10 min (at 30 min). Sequential addition of pimozide and amiloride, in either order, revealed an additive inhibitory effect on ISC and yielded approximately the same residual currents at 30 min. These data suggest that CNG channels contribute to the amiloride-insensitive component of ISC in MAECM.
Fig. 7.
Pimozide- and amiloride-sensitive short-circuit current (ISC) across MAECM grown on laminin 5-coated polycarbonate filters and mounted in Ussing chambers. Values are means ± SE (n = 4 total monolayers from 3 different cell preparations).
Unidirectional fluxes of Na+ and Cl− across MAECM.
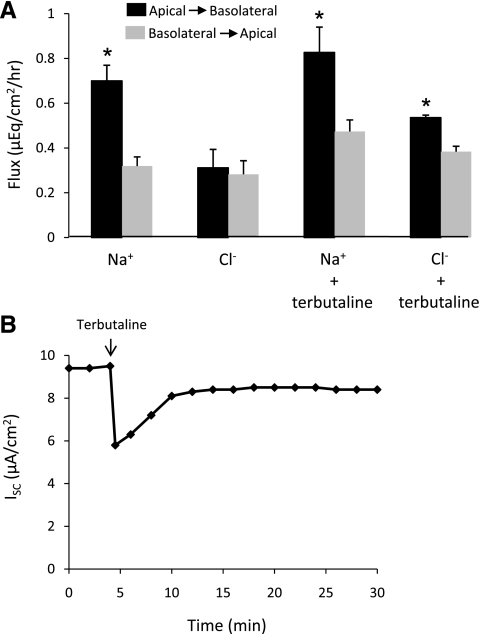
To directly demonstrate active ion transport across MAECM, unidirectional fluxes of Na+ and Cl− were determined under short-circuit conditions from the linear time courses of 22Na and 36Cl accumulation in downstream basolateral and apical reservoirs. Accumulation of 22Na was greater in the downstream basolateral reservoir than in the downstream apical reservoir, indicating that MAECM actively remove Na+ from the apical fluid. Apical-to-basolateral Na+ flux was significantly greater than Na+ flux measured in the opposite direction: 0.70 ± 0.07 vs. 0.32 ± 0.04 μeq·cm−2·h−1 (Fig. 8A). Net absorption of Na+ in the apical-to-basolateral direction was comparable to net ion flux calculated from ISC during the Na+ flux measurements: 0.38 ± 0.05 and 0.33 ± 0.03 μeq·cm−2·h−1, respectively. Apical-to-basolateral Cl− flux was not significantly different from basolateral-to-apical Cl− flux, indicating that Cl− transport across MAECM is passive under baseline conditions.
Fig. 8.
Effects of terbutaline on Na+ and Cl− flux and ISC across MAECM grown on laminin 5-coated polycarbonate filters and mounted in Ussing chambers. A: Na+ flux was significantly greater in the apical-to-basolateral direction in the presence and absence of terbutaline (10 μM). Terbutaline stimulated net absorption of Cl−, although no net transport of Cl− was observed in the absence of terbutaline. Values are means ± SE (n = 1 monolayer per condition per preparation from 4 different cell preparations). *P < 0.05 vs. respective flux in the basolateral-to-apical direction. B: representative response of ISC to terbutaline. Mean basal ISC and peak decrease due to terbutaline exposure were 7.4 ± 0.9 and 2.6 ± 0.3 μA/cm2, respectively, for 14 total monolayers from 4 different cell preparations.
Effects of terbutaline on unidirectional fluxes of Na+ and Cl− across MAECM.
It is well known that β-adrenergic agonists stimulate fluid and electrolyte absorption from the alveolar space in fetal (41, 44) and adult (1, 48) lungs. In the present study, the influence of terbutaline on ISC and unidirectional fluxes of Na+ and Cl− was determined. Instillation of 10 μM terbutaline into the basolateral fluid of MAECM caused a rapid decrease in ISC, followed by a recovery toward the initial current (Fig. 8B). The minimum ISC obtained 30 s after terbutaline addition was 62% of the initial current, with recovery to 92% of the initial current after 30 min. Terbutaline exposure did not alter net absorption of Na+ across MAECM: 0.38 ± 0.05 vs. 0.35 ± 0.07 μeq·cm−2·h−1 in untreated and terbutaline-treated monolayers, respectively. Net Cl− absorption of 0.15 ± 0.03 μeq·cm−2·h−1 was observed (Fig. 8A), indicating that MAECM actively remove Cl− from the apical fluid in response to terbutaline.
DISCUSSION
On the basis of in vivo and in vitro studies, it has been known for some time that extracellular matrices influence the morphology and function of epithelial and endothelial cell populations, including AT2 cells. Alveolar epithelial basement membrane components include, but are not limited to, laminins, type IV collagen, and fibronectin (17). One or more of these extracellular matrix components may influence the formation of functional MAECM and the transition of mouse AT2 cells to AT1-like cells. In this study, we report that laminin 5-coated Transwell polycarbonate filters permitted the formation of high-resistance monolayers that exhibit active Na+ absorption. In addition, the expression of AQP5, a specific marker for AT1 cells in situ, increases, while the expression of pro-SPC, a specific marker for AT2 cells, decreases, as a function of time in mouse AT2 cells cultured on laminin 5. These results suggest that the extracellular matrix is an important determinant of mouse AT2 cell transdifferentiation and transport properties of MAECM.
Rat AT2 cells plated on uncoated polycarbonate filters form confluent monolayers that exhibit high RT and acquire AT1 cell-like characteristics with time in culture (3, 7, 10). Similarly, rat AT2 cells maintained on uncoated plastic surfaces lose typical lamellar bodies and assume many AT1-like characteristics over time (15, 16), whereas mouse AT2 cells have been found to adhere to fibronectin-coated, but not uncoated, plastic surfaces (11). In the present study, expression of AQP5 in MAECM plated on laminin 5-coated filters increases with time in culture, similar to the time courses of AQP5 and other well-established markers of the AT1 cell phenotype (i.e., VIIIB2 epitope and T1α) in RAECM (3, 5, 7). Furthermore, in MAECM, expression of pro-SPC decreases with time in culture, as has been observed in RAECM and HAECM (7, 49). Interestingly, in rat AT2 cell cultures, laminin extracellular matrix proteins have been shown to preserve AT2 cell morphology for longer periods of time in culture than fibronectin or plastic surfaces; in general, laminin or laminin-rich complex surfaces favor retention of differentiated AT2 cell characteristics in the rat, whereas fibronectin matrices accelerate loss of these characteristics (45). The phenotypic transition of RAECM cultured on uncoated filters occurs over a time course similar to that observed in MAECM cultured on laminin 5-coated filters.
It is well documented that vectorial fluid clearance is dependent in part on active Na+ transport across the adult mammalian alveolar epithelium (6, 12, 36). Na+ ions diffuse passively into AEC through amiloride-sensitive and -insensitive cation channels in the apical cell plasma membrane and are extruded across the basolateral cell plasma membrane by ouabain-sensitive Na+-K+-ATPase (9, 47). In the present study, ∼65% of basal IEQ of MAECM was inhibited by apical addition of amiloride, and 90% of IEQ was inhibited by basolateral addition of ouabain, consistent with active Na+ transport and previous results in our laboratory using cultured adult rat AT2 cells (9, 31). Furthermore, accumulation of 22Na was greater in the downstream basolateral reservoir than in the downstream apical reservoir under short-circuit conditions, directly demonstrating active Na+ reabsorption across MAECM. Cl− flux in the apical-to-basolateral direction was identical to Cl− flux in the opposite direction, demonstrating no net Cl− absorption across MAECM under basal conditions. The magnitude of net Na+ flux is consistent with net ion flux calculated from ISC determined during the Na+ flux measurements (0.38 ± 0.05 and 0.33 ± 0.03 μeq·cm−2·h−1, respectively). These data indicate that mouse AT2 cells in primary culture form tight monolayers that actively transport Na+ in the apical-to-basolateral direction.
Amiloride inhibits fluid reabsorption in the lung shortly after birth (42, 44), and neonatal transgenic mice that lack the gene encoding the α-subunit of the epithelial Na+ channel die within 40 h of birth from failure to clear fluid from their lungs (24), supporting an important role for amiloride-sensitive pathways during the perinatal period. In the mature lung, however, a significant portion, if not the majority, of AFC is insensitive to amiloride in a variety of mammals. Investigators have reported that up to 40% of AFC in mice is amiloride insensitive (18, 20, 37). In postnatal sheep in situ, CNG channels were found to be responsible for the amiloride-insensitive fraction of AFC (29), whereas AFC in fetal sheep was unaffected by inhibitors of CNG channels (28). Previous studies in our laboratory showed that pimozide, a specific inhibitor of CNG channels, inhibited whole cell cation conductance of isolated rat AT2 cells by ∼55%, whereas amiloride decreased cation conductance by ∼25% (30). Similarly, pimozide has been shown to block amiloride-sensitive nonselective Na+ channels in AT1 cells grown on glass or permeable supports (27). In the present study, pimozide blocked a significant portion of amiloride-insensitive current across MAECM, suggesting that CNG channels also contribute to AFC in the adult mouse lung.
Terbutaline, a β-adrenergic agonist, has been reported to rapidly decrease ISC and, subsequently, induce a slow recovery toward or above initial baseline values (9, 26) and to increase net absorption of Na+ and Cl− across RAECM under short-circuit conditions (31). In the present study, in MAECM on laminin 5-coated filters, terbutaline induced an immediate reduction in ISC, which recovered to near-basal values after 30 min. The initial decrease in ISC is similar to findings in RAECM (9, 26) and suggests that terbutaline increases Cl− transport. Our radionuclide transport studies across short-circuited MAECM directly demonstrate that terbutaline stimulates active Cl− reabsorption, whereas active Na+ reabsorption remains relatively unchanged compared with baseline. These data are consistent with previous reports that terbutaline activates Cl− channels in the apical cell membrane (26, 43). Other studies have reported that terbutaline elicits a transient decrease in ISC followed by a gradual increase to steady-state levels above baseline in RAECM (9) and rabbit AEC monolayers (39), suggesting that, in addition to activation of Cl− transport, terbutaline stimulates Na+ conductive pathways. Controversy remains as to the relative contributions of various ion transport pathways (e.g., Na+-K+-ATPase and/or ion channels) and passive transport driven by increased Cl− conductance to the net transport of Na+ induced by terbutaline (33). Potential reasons for the different steady-state responses of ISC observed after terbutaline exposure in MAECM vs. RAECM include different experimental conditions (e.g., cell purity, enzymes used to dissociate AT2 cells, and culture media) and/or different animal species/strains. Although additional studies are required to further delineate molecular mechanisms of β-agonist effects on alveolar ion transport, it is clear that terbutaline increases net absorption of NaCl, thereby increasing the osmotic gradient that drives increased AFC in adult mice and rats.
In summary, isolated mouse AEC form tight, polarized monolayers when plated on laminin 5-coated polycarbonate permeable supports. The effects of ouabain and amiloride on IEQ, together with the 22Na and 36Cl transport under short-circuit conditions, indicate that mouse AT2 cells in primary culture actively transport Na+ in the apical-to-basolateral direction, characteristic of intact alveolar epithelium. The presence of CNG channels and terbutaline-stimulated active reabsorption of Cl− are also characteristic of MAECM. Furthermore, as in other in vitro models of alveolar epithelium, mouse AT2 cells transdifferentiate into AT1-like cells with time in culture. We expect that use of this in vitro model of MAECM, in conjunction with the development of knockout and transgenic mice, will provide new approaches for determination of the functional characteristics of wild-type and mutant mouse AEC phenotypes.
GRANTS
This work was supported in part by the Hastings Foundation, the Whittier Foundation, and the Alan A. and Edith L. Wolff Charitable Trust/Barnes-Jewish Hospital Foundation (R. M. Senior), National Institutes of Health Grants EY-11386, EY-17923, ES-07048, HL-38578, HL-38621, HL-38658, HL-62569, HL-64365, and HL-029594, and American Heart Association Grant 0730280N. E. D. Crandall is Hastings Professor and Kenneth T. Norris Jr. Chair of Medicine. Z. Borok is Ralph Edgington Chair in Medicine.
Acknowledgments
The authors appreciate the statistical assistance provided by Nazanin Yacobi.
REFERENCES
- 1.Berthiaume Y, Broaddus VC, Gropper MA, Tanita T, Matthay MA. Alveolar liquid and protein clearance from normal dog lungs. J Appl Physiol 65: 585–593, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Borok Z, Danto SI, Dimen LL, Zhang XL, Lubman RL. Na+-K+-ATPase expression in alveolar epithelial cells: upregulation of active ion transport by KGF. Am J Physiol Lung Cell Mol Physiol 274: L149–L158, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of T1α expression with alveolar epithelial cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 275: L155–L164, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Borok Z, Hami A, Danto SI, Lubman RL, Kim KJ, Crandall ED. Effects of EGF on alveolar epithelial junctional permeability and active sodium transport. Am J Physiol Lung Cell Mol Physiol 270: L559–L565, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol 12: 50–55, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol 282: L599–L608, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol 18: 554–561, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Borok Z, Mihyu S, Fernandes VF, Zhang XL, Kim KJ, Lubman RL. KGF prevents hyperoxia-induced reduction of active ion transport in alveolar epithelial cells. Am J Physiol Cell Physiol 276: C1352–C1360, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Cheek JM, Kim KJ, Crandall ED. Tight monolayers of rat alveolar epithelial cells: bioelectric properties and active sodium transport. Am J Physiol Cell Physiol 256: C688–C693, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, Ann DK, Crandall ED, Borok Z. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol 98: 322–328, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 14: 309–315, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med 163: 1021–1029, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Danto SI, Borok Z, Zhang XL, Lopez MZ, Patel P, Crandall ED, Lubman RL. Mechanisms of EGF-induced stimulation of sodium reabsorption by alveolar epithelial cells. Am J Physiol Cell Physiol 275: C82–C92, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 12: 497–502, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Danto SI, Zabski SM, Crandall ED. Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies. Am J Respir Cell Mol Biol 6: 296–306, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Dobbs LG, Williams MC, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta 970: 146–156, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Dunsmore SE, Rannels DE. Extracellular matrix biology in the lung. Am J Physiol Lung Cell Mol Physiol 270: L3–L27, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Egli M, Duplain H, Lepori M, Cook S, Nicod P, Hummler E, Sartori C, Scherrer U. Defective respiratory amiloride-sensitive sodium transport predisposes to pulmonary oedema and delays its resolution in mice. J Physiol 560: 857–865, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbert KJ, Schafer UF, Schafers HJ, Kim KJ, Lee VH, Lehr CM. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharm Res 16: 601–608, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 119: 199–207, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Goodman BE, Crandall ED. Dome formation in primary cultured monolayers of alveolar epithelial cells. Am J Physiol Cell Physiol 243: C96–C100, 1982. [DOI] [PubMed] [Google Scholar]
- 23.Ho YS Transgenic models for the study of lung biology and disease. Am J Physiol Lung Cell Mol Physiol 266: L319–L353, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in α-ENaC-deficient mice. Nat Genet 12: 325–328, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Isakson BE, Lubman RL, Seedorf GJ, Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am J Physiol Cell Physiol 281: C1291–C1299, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Ingbar DH, O'Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl− channels. Am J Physiol Cell Physiol 275: C1610–C1620, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junor RW, Benjamin AR, Alexandrou D, Guggino SE, Walters DV. Lack of a role for cyclic nucleotide gated cation channels in lung liquid absorption in fetal sheep. J Physiol 523: 493–502, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junor RW, Benjamin AR, Alexandrou D, Guggino SE, Walters DV. A novel role for cyclic nucleotide-gated cation channels in lung liquid homeostasis in sheep. J Physiol 520: 255–260, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp PJ, Kim KJ, Borok Z, Crandall ED. Re-evaluating the Na+ conductance of adult rat alveolar type II pneumocytes: evidence for the involvement of cGMP-activated cation channels. J Physiol 536: 693–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KJ, Cheek JM, Crandall ED. Contribution of active Na+ and Cl− fluxes to net ion transport by alveolar epithelium. Respir Physiol 85: 245–256, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 33.Lazrak A, Nielsen VG, Matalon S. Mechanisms of increased Na+ transport in ATII cells by cAMP: we agree to disagree and do more experiments. Am J Physiol Lung Cell Mol Physiol 278: L233–L238, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Mason RJ, Williams MC, Widdicombe JH, Sanders MJ, Misfeldt DS, Berry LC Jr. Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci USA 79: 6033–6037, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2: 206–213, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, Matalon S, Hollenberg S, Factor P. Upregulation of alveolar epithelial active Na+ transport is dependent on β2-adrenergic receptor signaling. Circ Res 94: 1091–1100, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol Cell Physiol 273: C1549–C1561, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen VG, Duvall MD, Baird MS, Matalon S. cAMP activation of chloride and fluid secretion across the rabbit alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 275: L1127–L1133, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, Kimura S. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 21: 7380–7390, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Brodovich H Epithelial ion transport in the fetal and perinatal lung. Am J Physiol Cell Physiol 261: C555–C564, 1991. [DOI] [PubMed] [Google Scholar]
- 42.O'Brodovich H, Hannam V, Seear M, Mullen JB. Amiloride impairs lung water clearance in newborn guinea pigs. J Appl Physiol 68: 1758–1762, 1990. [DOI] [PubMed] [Google Scholar]
- 43.O'Grady SM, Jiang X, Ingbar DH. Cl- channel activation is necessary for stimulation of Na transport in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L239–L244, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. J Physiol 376: 321–340, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rannels DE, Rannels SR. Influence of the extracellular matrix on type 2 cell differentiation. Chest 96: 165–173, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 283: L256–L264, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Russo RM, Lubman RL, Crandall ED. Evidence for amiloride-sensitive sodium channels in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 262: L405–L411, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med 150: 305–310, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 36: 661–668, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]