Abstract
To determine the relative contributions of Ca2+ signaling and Ca2+ sensitivity to the contractility of airway smooth muscle cells (SMCs), we compared the contractile responses of mouse and rat airways with the lung slice technique. Airway contraction was measured by monitoring changes in airway lumen area with phase-contrast microscopy, whereas changes in intracellular calcium concentration ([Ca2+]i) of the SMCs were recorded with laser scanning microscopy. In mice and rats, methacholine (MCh) or serotonin induced concentration-dependent airway contraction and Ca2+ oscillations in the SMCs. However, rat airways demonstrated greater contraction compared with mice, in response to agonist-induced Ca2+ oscillations of a similar frequency. Because this indicates that rat airway SMCs have a higher Ca2+ sensitivity compared with mice, we examined Ca2+ sensitivity with Ca2+-permeabilized airway SMCs in which the [Ca2+]i was experimentally controlled. In the absence of agonists, high [Ca2+]i induced a sustained contraction in rat airways but only a transient contraction in mouse airways. This sustained contraction of rat airways was relaxed by Y-23672, a Rho kinase inhibitor, but not affected by GF-109203X, a PKC inhibitor. The subsequent exposure of Ca2+-permeabilized airway SMCs, with high [Ca2+]i, to MCh elicited a further contraction of rat airways and initiated a sustained contraction of mouse airways, without changing the [Ca2+]i of the SMCs. Collectively, these results indicate that airway SMCs of rats have a substantially higher innate Ca2+ sensitivity than mice and that this strongly influences the transduction of the frequency of Ca2+ oscillations into the contractility of airway SMCs.
Keywords: lung slices, Ca2+ oscillations, confocal microscopy, asthma, hyperresponsiveness
asthma is characterized by airway inflammation and airway hyperresponsiveness to nonspecific stimulation. Although airway inflammation is known to result in airway remodeling, airway hyperresponsiveness occurs in the early stages of asthma when no obvious airway remodeling is discernable. Consequently, the fundamental mechanism leading to the excessive contraction of airway smooth muscle cells (SMCs) remains unknown. A straightforward hypothesis is that the innate contractility of the SMCs has been enhanced in asthmatic airways. However, the experimental evidence addressing the contractile responses of asthmatic SMCs in vitro is inconsistent. Increased (4, 6, 18, 21), normal (4, 5, 10, 34), and even reduced (12, 33, 37) airway constriction has been reported in human asthmatic or animal airways. These discrepancies may also result from the different protocols, methods, and parameters used to evaluate SMCs contraction.
To determine whether the contractile regulation of asthmatic airway SMCs is abnormal, it is necessary to first understand the regulation of normal airway contraction. Although airway SMCs contraction is determined by increases in intracellular calcium concentration ([Ca2+]i) linked with increases in Ca2+ sensitivity (30), the relative contribution of the Ca2+ signaling and Ca2+ sensitivity to contraction appears to be specific to the type of the SMC (31). For example, mouse airway SMCs seem to have a low Ca2+ sensitivity coupled with fast agonist-induced Ca2+ oscillations, whereas mouse pulmonary arteriole SMCs appear to have higher Ca2+ sensitivity coupled with slow agonist-induced Ca2+ oscillations (1, 25, 26). Similarly, agonist-induced airway contraction varies between species or different strains of the same species with certain strains of mice and rats displaying spontaneous, innate airway hyperresponsiveness (3, 9, 17, 19, 24, 35). Understanding how alterations in the regulatory mechanisms that are responsible for this physiological variation between species or strains is extremely valuable because it is possible that similar alterations may also account for enhanced airway contraction in asthma.
Because the relative contribution of the Ca2+ signaling and Ca2+ sensitivity to contractile regulation of various species or strains is not well-characterized, we compared the contractile response, Ca2+ sensitivity and Ca2+ signaling of airway SMCs in rats and mice. These species were initially selected because both have been used extensively as animal models (14, 20) with which to explore airway reactivity. In view of the strain-related differences in airway responses to agonists (19), we selected a mouse (BALB/c) and rat (Sprague-Dawley) strain with average airway responsiveness for the present study. BALB/c mice display an airway reactivity intermediate between A/J mice and C3H/HeJ, the most and least reactive strains, respectively (3, 9). Similarly, Sprague-Dawley rats displayed a midrange response compared with Flinders-sensitive rats and Flinders-resistant rats (23).
A similar concern related to airway responsiveness is the position of the airway within the respiratory tract. We (2) found in our previous studies that airway responsiveness was related to location and that agonist-induced airway contraction was weaker in the more proximal and distal airways of mice compared with midrange airways. Most other studies have only examined airway SMCs isolated or cultured from the trachea or large central airways, therefore their relative responsiveness is not addressable. However, in view of our data from mice, it is likely that large airway responses cannot be simply extrapolated to the small airways. Consequently, we used the lung slice technique, in combination with confocal or two-photon microscopy, to focus on the contractile and Ca2+ physiology of small intrapulmonary airways, a site commonly favored in the pathology of asthma (32).
By measuring the contractile responses of normal airway SMCs to contractile agonists [methacholine (MCh) and serotonin (5-HT)] in mice and rats (at both room temperature and 37°C), we found that although airway contraction was similar, agonist-induced Ca2+ oscillations were slower in rat airway SMCs. However, by clamping the intracellular Ca2+ level of the SMCs at a high level, we found that the Ca2+ sensitivity of the SMC was higher in rat airways compared with mice. These results demonstrate that Ca2+ sensitivity of the SMCs is extremely important in determining the magnitude of airway SMC contraction in response to the Ca2+ oscillations. An important implication of these studies is that airway hyperresponsiveness can readily result from a small increase in Ca2+ sensitivity of the SMCs.
METHODS
All the materials and methods have been previously described in detail (26); only a brief outline is given.
Materials.
Cell culture reagents were obtained from GIBCO-Invitrogen (Carlsbad, CA). Other reagents were obtained from Sigma-Aldrich (St. Louis, MO) or Calbiochem (La Jolla, CA). HBSS was supplemented with 20 mM HEPES buffer (sHBSS) and adjusted to pH 7.4. Isotonic high KCl solutions in sHBSS were made by adding 50 mM KCl and removing an equivalent amount of NaCl.
Lung slices.
Briefly, male BALB/c mice between 7 and 10 wk were killed by intraperitoneal injection of Nembutal. After opening the chest wall, the lungs were inflated with ∼1.3 ml of warm 2% agarose-sHBSS via a catheter inserted into the trachea. Subsequently, ∼0.2 ml of air was injected to flush the agarose-sHBSS out of the airways and into the distal alveoli. After the agarose-sHBSS was gelled by cooling to 4°C, a lung lobe was sectioned into lung slices of ∼140-μm thick with an EMS 4000 Vibratome. The slices were collected and maintained in DMEM at 37°C for up to 3 days.
Preparation of rat lung slices followed a similar procedure. Sprague-Dawley rats (∼200 g; Charles River Breeding Laboratories, Needham, MA) were initially anesthetized with an intraperitoneal injection of ∼0.6 ml Nembutal and killed by decapitation (as approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee). The trachea was cannulated with a catheter (20-gauge Intima; Becton Dickinson, Sandy, UT), which was secured with thread. After opening the chest wall, the lungs were inflated with ∼16 ml of warm 2% agarose-sHBSS followed by ∼1.0 ml of air to flush the agarose out of the airways. After cooling to 4°C to gel the agarose, a lung lobe was sectioned into lung slices as described.
Experiments were conducted at both room temperature (∼22°C) and 37°C. A custom-made Plexiglas chamber enclosed both the perfusion system and the microscope to form an isolated environment. The temperature inside the chamber was regulated at 37°C by a custom-built heater consisting of two air heater elements (AF20-300-120-1D-K-10-3.1; Farnam Custom Products, Philadelphia, PA) coupled with circulating fans (1976K37; McMaster-Carr Supply, Chicago, IL) and regulated by a temperature sensor (T thermocouple, HTTC36-T-14G-6; Omega Engineering, Stamford, CT) and a thermal controller (CC-A10; Farnam Custom Products).
Measurement of airway contraction.
Lung slices were placed on a large cover glass. A nylon mesh with a hole cut in the middle was placed on top of the slice to hold it in position and expose the airway of interest. A second smaller cover glass was placed over the slice and mesh and held in place by silicone grease to form a perfusion chamber. Perfusion of the lung slice was performed at a rate of ∼800 μl/min by a gravity driven perfusion system. Phase-contrast images were recorded with an inverted microscope and ×10 objective, a charge-coupled device (CCD) camera, and image acquisition software (Video Savant) in time-lapse mode (30 images per min). The area of the airway lumen in each image was measured by pixel summing with the use of custom-written software.
Measurement of intracellular Ca2+ signaling.
Approximately 15 lung slices were placed in 2 ml of sHBSS containing 20 μM Oregon Green 488 BAPTA-1 AM, 100 μM sulfobromophthalein (an inhibitor of anion exchanges to reduce dye extrusion), and 0.1% Pluronic F-127 and incubated in the dark for 1 h at 30°C. The slices were washed in sHBSS containing 100 μM sulfobromophthalein for an additional 30 min at 30°C to allow for dye deesterification. The lung slices were mounted in the perfusion chamber and examined with either a custom-built video-rate confocal or 2-photon scanning laser microscope. Images were recorded at rates of 15 or 30 images per second. Changes in fluorescence intensity of the SMCs that indicate changes in [Ca2+]i were determined with respect to time (image number) from the average gray value of user-selected regions of interest (ROI; 6 × 6 pixels) inside the cells with custom-written software. The relative fluorescence intensity was expressed as a fluorescence ratio (F/F0) normalized to the initial fluorescent intensity (F0).
Preparation of Ca2+-permeabilized lung slices.
The preparation of Ca2+-permeabilized cells/slices, which allow the [Ca2+]i to be clamped at a constant level, has been previously described in detail (1). Briefly, lung slices were exposed to sHBSS containing 20 mM caffeine and 50 μM ryanodine for 5 min. After the caffeine and ryanodine were washed away, the extracellular Ca2+ was manipulated with sHBSS containing 0 (containing 1 mM EGTA), 1.3, or 10 mM Ca2+ to regulate the [Ca2+]i.
Data analysis.
Data are expressed as means ± SE. The relationships between agonist concentration and contraction, agonist concentration and Ca2+ oscillation frequency, and Ca2+ signaling and contraction in rat and mouse airways were fitted using a nonlinear interactive fitting program (Boltzmann fit; Origin 7.0; Microcal Software).
RESULTS
Lung slices were cut from the single right lobe of the rat and mouse lung. For the most part, the lungs appeared similar in morphology. However, in addition to the obvious increase in the cross-section area of the larger airways, the rat lung slice was also characterized by larger alveoli and prominent connective tissue linking the airways to the surrounding lung parenchyma (Fig. 1A). The rat airway is also lined with a thicker pseudostratified epithelium compared with the cuboidal epithelium in similar-sized mouse airways. In addition, the rat epithelium had a higher density of ciliated cells. Because of these differences in rat and mice airway morphology, we carefully selected, for most of the comparative experimentation, rat and mice airways with a similar lumen diameter (mice: 176.2 ± 9.93 μm; rats: 183.09 ± 8.21 μm). Only airways with an intact epithelium, active cilia, and a lumen free of agarose were used.
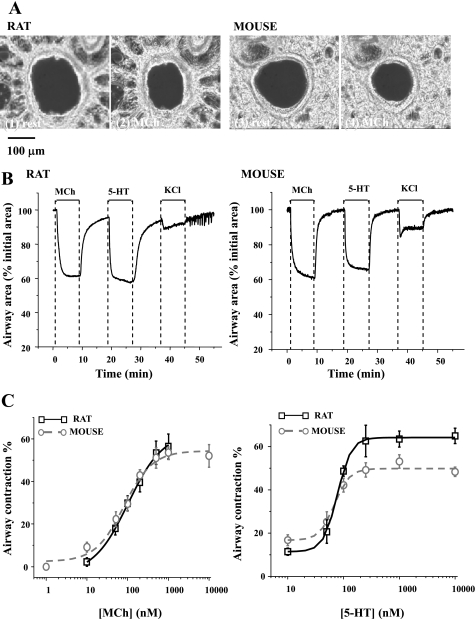
Fig. 1.
The contractile responses of rat and mouse airways to methacholine (MCh), serotonin (5-HT), and KCl. A: typical phase-contrast images showing lung slices cut from rat (1 and 2) and mouse (3 and 4) in a resting state (1 and 3) and after 5 min of exposure to 200 nM MCh (2 and 4). B: representative experiments showing the change in airway lumen area of rat and mouse with respect to time in response to 200 nM MCh, 100 nM 5-HT, and 50 mM KCl. C: summary of MCh- (left) and 5-HT- (right) induced airway contraction in rats (black squares fitted with black solid line) and mice (gray circles fitted with gray dotted line). Contraction was measured as the decrease in lumen area after 5 min of agonist exposure. Each point represents the mean ± SE from at least 7 experiments from different slices from at least 4 rats and 5 experiments from 3 mice.
Comparison of the contractile responses of rat and mice airways.
On exposure to the agonists, MCh or 5-HT, both the rat and mouse airways quickly displayed a strong contraction that reached a sustained steady-state (∼60% of the initial lumen area) within 2–3 min (Fig. 1B). However, membrane depolarization, induced by 50 mM KCl, resulted in a relatively weak contraction in rat (16.2 ± 3.3%, n = 5) and mouse (12.3 ± 0.7%, n = 5) airways. In contrast to the sustained contraction induced by agonists, the contraction induced by KCl was commonly associated with regional twitching of the airway wall.
When MCh and 5-HT were removed by washing with sHBSS, the rat and mouse airways quickly relaxed to approximately 80–85% of their initial size and thereafter continued to slowly relax for a further 10 min (Fig. 1B). However, the relaxation rate (increase in the percentage of airway size in 1 s) of the rat airway was slower than that of the mouse airway, irrespective of the contractile agonist. The maximal relaxation rate from contraction induced by 200 nM MCh was 0.25 ± 0.02% s−1 (5 airways from 3 rats) and 0.49 ± 0.05% s−1 (5 airways from 3 mice) for rat and mice airways, respectively. Similarly, the maximal relaxation rate from contraction induced by 100 nM 5-HT was 0.23 ± 0.05% s−1 (4 airways from 2 rats) and 0.46 ± 0.08% s−1 (5 airways from 3 mice) for rat and mice airways, respectively. These results suggest that the relaxation process from agonist stimulation within rat airway SMCs differs from that of mouse airway SMCs. There was no obvious difference in the relaxation response between the rat and mouse airways on KCl removal. However, it was commonly observed that the frequency of spontaneous, transient whole airway contractions increased in both rats and mice (e.g., Fig. 1B).
To compare the contractile sensitivity of the rat and mouse airways to agonist stimulation, we constructed an agonist concentration-contraction response curve using MCh and 5-HT (10 nM to 10 μM; Fig. 1C). In response to increasing concentrations of MCh, both the rat and mouse airways showed similar contractile responses and reached a maximum contraction of ∼55% in response to 1 μM MCh. By contrast, the rat displayed a substantially greater contraction (maximal response: ∼60%) than the mouse in response to 5-HT (Fig. 1C; maximal response: ∼40%); the maximal contraction was attained at ∼200 nM 5-HT.
Ca2+ signaling of rat and mouse airway SMCs.
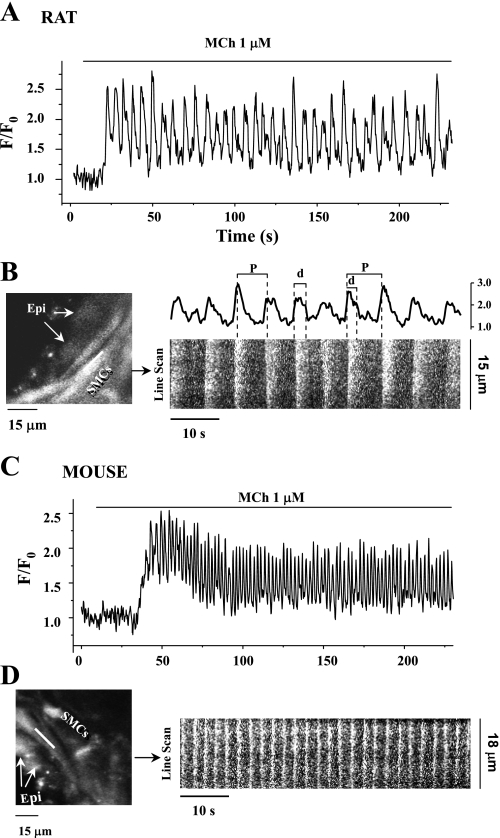
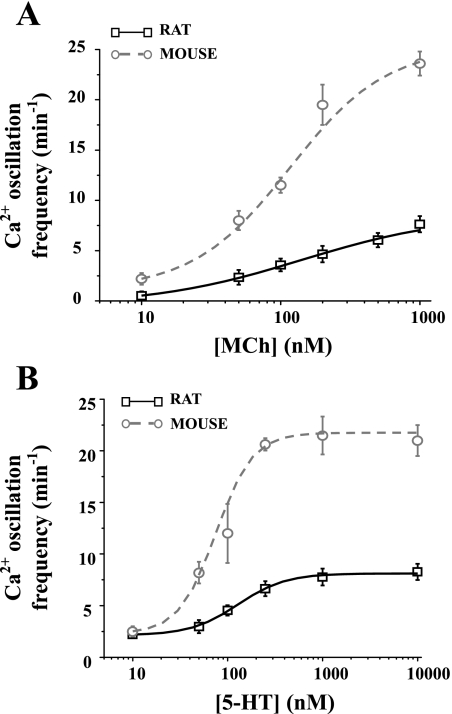
We next compared the intracellular Ca2+ signaling of the rat and mouse airway SMCs in response to MCh (Fig. 2) and 5-HT. In both species, MCh induced an initial increase in Ca2+ that was followed by regular oscillatory changes in [Ca2+]i while the MCh remained present. These Ca2+ oscillations were commonly initiated at one end of the cell and propagated as a Ca2+ wave along the length of the cell as indicated by the slight slope of the line-scan recording (Fig. 2, B and D). The contraction of both the rat and mouse SMCs was stable during the passage of these waves. However, the frequency of Ca2+ oscillations in response to the same concentration of MCh (1 μM) at room temperature was much slower in the rat airway SMCs (Fig. 2A) compared with the mouse airway SMCs (Fig. 2C). This difference in the frequency of the Ca2+ oscillations was maintained at all concentrations of MCh examined (10 nM to 1 μM; Fig. 3A). The Ca2+ oscillations of the rat SMCs ranged from 2.4 ± 0.4 to 7.7 ± 0.8 min−1 (6 cells from different airways of at least 3 rats), whereas the Ca2+ oscillations in the mouse SMCs ranged from 2.2 ± 0.7 to 23.6 ± 1.2 min−1 (5 cells from different airways of 2 mice) (Fig. 3A).
Fig. 2.
Oscillatory Ca2+ signaling of rat and mouse airway smooth muscle cells (SMCs) in response to MCh. Representative recordings are shown of the Ca2+ signaling in rat (A and B) and mouse (C and D) SMCs induced by 1 μM MCh. Changes in the fluorescence intensity representing changes in Ca2+ were collected either from regions of interest (ROI; 6 × 6 pixels) in single airway SMC and expressed as a fluorescence ratio (F/F0) (A and C) or from line scans along the SMCs with respect to time (B and D). Fluorescence confocal images showing part of rat (B, left) and mouse (D, left) airway indicate the position of the line scan (white line). Epi, epithelial cells. Line-scan plots were constructed by aligning the pixels along the white line from each fluorescence image from 120 to 180 s in rat (B, right, bottom) and mouse (D, right) airway SMCs. The Ca2+ oscillations of rat SMCs from ROI at right end of the scan line from 120 to 180 s are shown in B, right, top. The period (P) and duration (d) of Ca2+ oscillatory wave are shown. Ca2+ oscillation frequency = 1/period. Exposure to 1 μM MCh induced sustained Ca2+ oscillations in both the rat and mouse SMCs, but the Ca2+ oscillation frequency was higher in mouse (∼25 min−1) than in rat (∼10 min−1) airway SMCs. The duration of the Ca2+ oscillations was longer in the rat SMCs in response to 1 μM MCh.
Fig. 3.
Comparison of agonist-induced Ca2+ oscillations between rats and mice. The frequency of the Ca2+ oscillations after exposure for 5 min to MCh (A) or 5-HT (B) of rat (black squares fitted with black line) and mouse (gray circles fitted with gray line) airway SMCs. Rat airway SMCs displayed slower Ca2+ oscillations than those of mice. Each point represents the mean ± SE from at least 6 experiments from different slices from at least 3 rats and 5 experiments from at least 2 mice.
A similar difference between the Ca2+ signaling of the SMCs of rats and mice was also observed in response to 5-HT. Like MCh, 5-HT induced a series of Ca2+ oscillations in the rat and mice SMCs. These Ca2+ oscillations occurred at a slower frequency in the rat compared with the mice at all concentrations (Fig. 3B). In response to 1 μM 5-HT, the maximal frequency of the Ca2+ oscillations in rat SMCs was 8.3 ± 0.8 min−1 (6 cells from different airways of at least 3 rats), whereas in mouse SMCs, the maximal frequency was 21.5 ± 1.8 min−1 (5 cells from airways of 2 mice) (Fig. 3B).
To further characterize the dynamics of the increase in [Ca2+]i resulting from the Ca2+ oscillations, which mainly reflect the release and reuptake of Ca2+ by the sarcoplasmic reticulum (SR), the time duration (width of the waveform at half maximum) of the [Ca2+]i elevation in each individual Ca2+ oscillations was measured (Fig. 2B). In response to 200 nM MCh, the duration of elevated Ca2+ of the slow-frequency Ca2+ oscillations was longer in rat airway SMCs (3.38 ± 0.28 s, 5 cells from different airways of 3 rats) than that of the fast-frequency Ca2+ oscillations of mouse SMCs (1.18 ± 0.11 s, 4 cells from airways of 4 mice). However, the duration of the elevated Ca2+ of Ca2+ oscillations with a similar oscillatory frequency (∼7 min−1) induced by different agonist concentrations (1 μM MCh for rats or 50 nM MCh for mice) was not significantly different between species (2.1 ± 0.28 s from 4 cells from rat airways vs. 2.22 ± 0.27 s from 4 cells from mouse airways). This suggests that the duration of the Ca2+ oscillation is a function of the oscillation frequency.
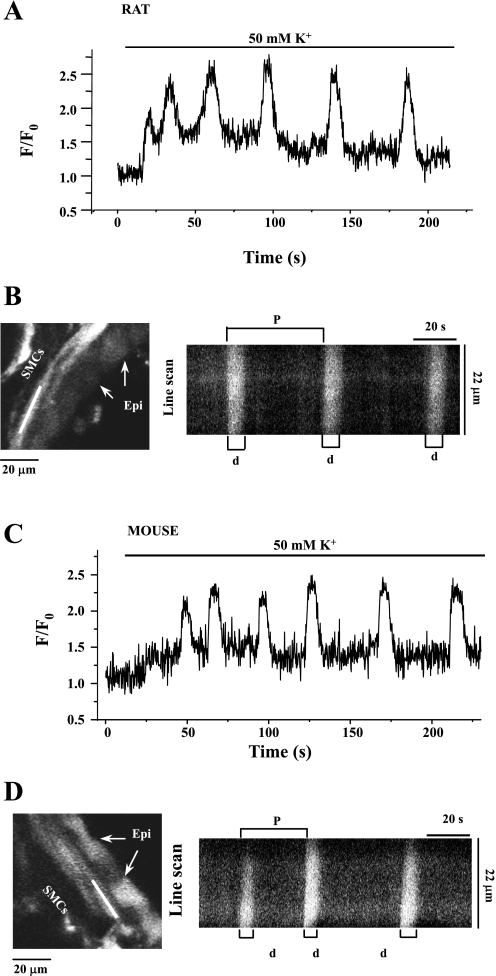
Membrane depolarization with 50 mM KCl also induced Ca2+ oscillations in rat and mice SMCs (Fig. 4). However, these Ca2+ oscillations were considerably slower than those induced by agonists and occurred with a frequency of 2–3 min−1 and a longer duration of elevated Ca2+ (8.11 ± 0.39 s for rat SMCs, n = 4; 7.4 ± 0.24 s for mouse SMCs, n = 5). These KCl-induced oscillations also propagated throughout the whole cell. However, there was no common site of origin of these Ca2+ oscillations. In addition, the SMC displayed a transient contraction that was associated with the passage of each KCl-induced Ca2+ oscillation.
Fig. 4.
Comparison of KCl (50 mM)-induced Ca2+ oscillations in rat (A) and mouse (C) airway SMCs. Fluorescence images showing part of the rat (B, left) and mouse (D, left) airway and the line-scan plots (right) constructed by aligning pixels indicated by the white line along the SMCs from successive fluorescence images from 75 to 200 s. In both rats and mice, the Ca2+ oscillations induced by KCl were very slow (∼2 min−1) and had a long duration of Ca2+ elevation.
Calcium-contraction coupling.
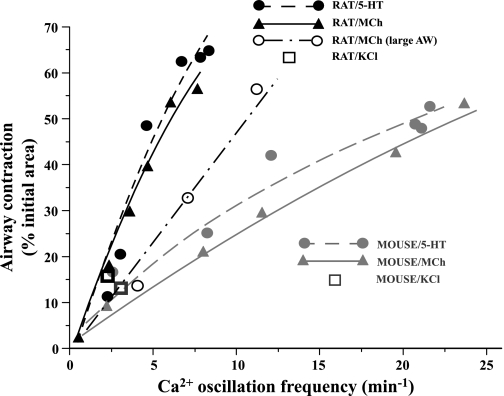
To examine the relationship between the Ca2+ signaling and contractility of airway SMCs, a summary plot of agonist-induced contraction with respect to frequency of the underlying Ca2+ oscillations in both species is presented in Fig. 5. In both rats and mice, a greater airway contraction was associated with faster Ca2+ oscillations. However, the gradient of this contraction-frequency relationship is substantially greater in rats compared with mice. A Ca2+ oscillation frequency of ∼5 min−1 induced ∼50% contraction in rats compared with ∼15% contraction in mice. Similarly, the gradient of the contraction-frequency relationship was influenced by the stimulating agonist (Fig. 5). In both rats and mice, 5-HT induced a greater contraction than MCh for a given Ca2+ oscillation frequency (significant difference detected at the frequency of ∼12 min−1 in rats). The contractile response to the slow Ca2+ oscillation induced by KCl was small and similar for both rats and mice (Fig. 5). These data clearly indicate that an increase in Ca2+ oscillation frequency correlates with an increase in contractility. However, these data also indicate that sensitivity of this relationship is determined by both the agonist and species.
Fig. 5.
Relationship between Ca2+ oscillation frequency and airway contraction in mouse and rat. Contractile responses of rat airway (black symbols) and mouse airway (gray symbols) of a similar size in response to MCh (triangles, solid line), 5-HT (circles, dashed line), and 50 mM KCl (squares) or larger rat airways (AW) in response to MCh (black open circle, dot-dash line) were plotted with respect to the corresponding Ca2+ oscillation frequency and fitted with logistic functions. These data indicated that the same frequency of Ca2+ oscillations induced greater contraction in rat than in mouse airways irrespective of the airway generation.
In previous experiments, we (2) established that the responsiveness of mouse airway SMCs varied in different airway generations and that the sensitivity of the relationship between contraction and Ca2+ oscillations was decreased in larger airways. Because we have compared similar-sized airways from rat and mice, it is possible that the difference we observe between similar-sized mouse and rat airways is the result of a comparison of airways from different airway generations. To test this idea, we compared the responses of larger airways (diameter: 401.1 ± 26.6 μm) from rats to a range of MCh concentrations to determine how the relationship between contractility and Ca2+ signaling varies with airway size; the larger rat airway serves as a comparison of rat and mice airways from similar generations. We found that larger rat airways displayed a decrease in the sensitivity of the relationship between contraction and Ca2+ oscillation frequency (decreased slope, Fig. 5). In response to 50 nM to 1 μM MCh, the contraction of larger rat airway increased from 13.7 ± 4.8 to 56.2 ± 3.4% (n = 8 airways from 3 rats), and this was associated with an increase in the Ca2+ oscillation frequency from 4.0 ± 1.2 to 11.1 ± 2.2 (n = 9 airways from 3 rats). We also examined the response of smaller rat airways (diameter: 98.1 ± 12.7 μm) and found that they did not alter their behavior. These data indicate the contraction-Ca2+ signaling relationship in rats is also influenced by the airway position within the rat intrapulmonary tree. However, even with this comparison of the responses of airways that are more closely matched with respect to airway generation, a major difference between the sensitivity of the airway contraction-Ca2+ signaling relationships of mice and rats remains. These results suggest that this difference in sensitivity is related to the species, and we have investigated this hypothesis with Ca2+-permeabilized lung slices.
Comparison of the Ca2+ sensitivity of rat and mouse airway SMCs.
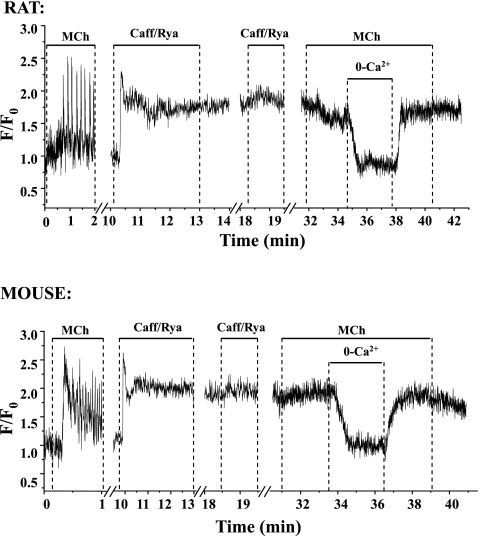
To investigate how the Ca2+ sensitivity varies with agonist or species, we treated the lung slices with 20 mM caffeine and 50 μM ryanodine (as previously described) to specifically permeabilize the airway SMCs to Ca2+ so that the [Ca2+]i could be experimentally controlled. The response of both rat and mouse airway SMCs to this caffeine/ryanodine treatment was nearly identical (Fig. 6). Before caffeine/ryanodine treatment, the viability of the lung slice was confirmed by exposure to 200 nM MCh. As expected, MCh induced Ca2+ oscillations in the normal SMCs. Subsequent exposure of the lung slice to caffeine/ryanodine induced a transient Ca2+ release (peak value F/F0 = 2.0–2.5) that progressed into a sustained uniform increase in [Ca2+]i, which persisted when the caffeine/ryanodine was removed. Reexposure of the lung slice to caffeine/ryanodine or MCh or their subsequent removal did not significantly change the [Ca2+]i. We believe the [Ca2+]i is maintained at a sustained high level in these Ca2+-permeabilized airway SMCs by 1) Ca2+ release from the SR into the cytoplasm via ryanodine receptors irreversibly locked in the open state by ryanodine, and 2) a Ca2+ influx into the cytosol from the extracellular space via store-operated channels that open in response to the emptying of the SR. The [Ca2+]i of Ca2+-permeabilized airway SMCs could be manipulated by changing the extracellular Ca2+ concentration ([Ca2+]o) (Fig. 6). When the [Ca2+]o was zero, the [Ca2+]i fell to zero. When the [Ca2+]o was increased to 1.3 mM Ca2+, the [Ca2+]i increased back to the original elevated level (Fig. 6). This approach was used to examine the Ca2+ sensitivity of the rat and mice airway SMCs.
Fig. 6.
MCh-induced Ca2+ signaling in rat and mouse airway SMCs before and after treatment with caffeine and ryanodine (Caff/Rya). These representative traces show that MCh induced Ca2+ oscillations before caffeine/ryanodine treatment. Exposure to caffeine/ryanodine to create a Ca2+-permeabilized lung slice induced a fast elevation of intracellular calcium concentration ([Ca2+]i) in the SMCs, which subsequently stabilized and remained at a high level when caffeine/ryanodine was removed. A 2nd exposure to caffeine/ryanodine or MCh had no further effect on the [Ca2+]i. However, [Ca2+]i dropped significantly when the extracellular Ca2+ was removed and was elevated to the previous level when extracellular Ca2+ was replaced. The responses were similar in rat and mouse airway SMCs.
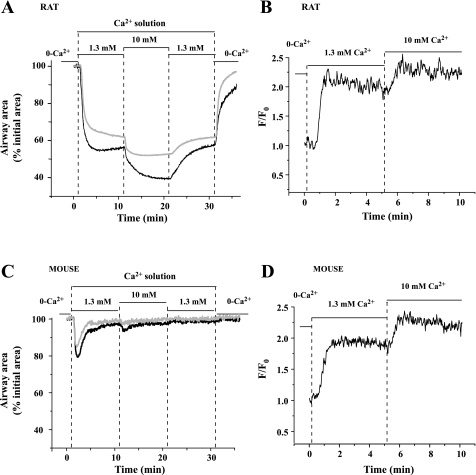
With the [Ca2+]o set at zero, both rat and mice airways were relaxed, and the [Ca2+]i of their SMCs was low (Fig. 7). On exposure to 1.3 mM Ca2+, the [Ca2+]i of the rat airway SMC increased and was sustained at a high level (Fig. 7B). This correlated with a contraction of 33.4 ± 2.7% of the rat airway that remained relatively unchanged after 10 min (33.2 ± 3.3%, from 5 airways of 3 rats; Fig. 7A). By contrast, in response to the same treatment with 1.3 mM [Ca2+]o, the mouse airway only displayed a transient contraction (Fig. 7C) even though the [Ca2+]i remained at a high level (Fig. 7D). When the [Ca2+]o was further increased to 10 mM, the [Ca2+]i of the rat and mouse SMCs was also elevated to a higher level (Fig. 7, B and D), and the contraction of the rat airway increased further. However, the mouse airway only displayed a smaller transient contraction to the increased [Ca2+]i. These responses were reversible when the [Ca2+]o was decreased (Fig. 7, A and C). These results indicate that the rat airway SMCs are inherently very sensitive to, and contracted by, increases in [Ca2+]i, whereas the same sustained increases in [Ca2+]i leads to the desensitization and relaxation of the mouse airway SMCs.
Fig. 7.
The contraction and Ca2+ signaling of Ca2+-permeabilized rat and mouse airway SMCs in response to various extracellular Ca2+ concentration ([Ca2+]o). Representative traces show that in rat airway SMCs (top, black line), 1.3 mM Ca2+ [in HBSS supplemented with 20 mM HEPES buffer (sHBSS)] induced a sustained contractile response (A) and increase in [Ca2+]i (B). When the [Ca2+]o was increased to 10 mM, a greater contractile response (A) and higher level of [Ca2+]i (B) were observed. Ca2+-permeabilized large rat airways (top, gray line) had similar responses to the elevation of [Ca2+]o. In mouse airway SMCs (bottom, black line), 1.3 mM Ca2+ followed by 10 mM Ca2+ induced similar changes in [Ca2+]i (D) compared with the rat airway SMCs, but the contractile response consisted of a transient contraction followed by relaxation (C). The elevation of [Ca2+]i to 10 mM did not induce a further significant airway contraction in the mouse (C). Similar responses were observed in Ca2+-permeabilized large mouse airways (diameter: ∼220 μm; bottom, gray line).
To determine whether airway generation influenced this response to Ca2+, we examined the response of larger rat airways in Ca2+-permeabilized lung slices. The responses of the larger airways were similar in form to those of the smaller rat airway (Fig. 7A). The larger rat airway responded with a sustained contraction in response to elevated Ca2+. Different-sized mouse airways responded to elevated Ca2+ with a transient contraction (Fig. 7C). These data indicate the fundamental Ca2+ sensitivity responses of rat and mice airway SMCs to Ca2+ are different irrespective of their airway position.
Modulation of Ca2+ sensitivity by agonist.
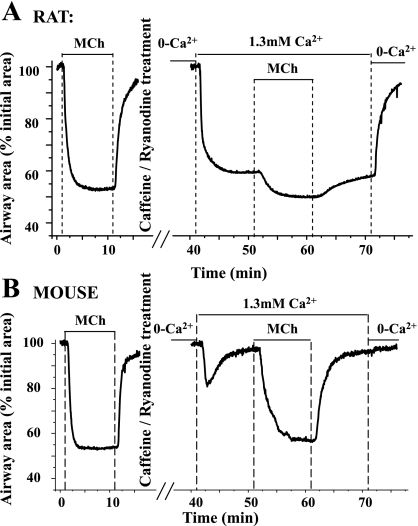
To understand how agonists modulate the Ca2+ sensitivity of airway SMCs, we examined the effects of MCh (200 nM) on Ca2+-permeabilized SMCs. As described above, the addition of 1.3 mM Ca2+ induced the rat airways to partially contracted (lumen size = ∼67%), whereas mouse airways were relaxed following the transient contraction (lumen size = 95.2 ± 1.6%, 8 airways of 4 mice; Fig. 8). The subsequent exposure of the SMCs to 1 μM MCh induced a further ∼18% contraction of rat airway, whereas this same treatment induced a near full contraction (∼40%) in the mouse airway. When MCh was removed, these airway responses were reversed (Fig. 8).
Fig. 8.
Airway contraction in response to an elevation of [Ca2+]i, with and without agonist, in rat and mouse airways. MCh (200 nM) induced contractile responses (∼40%) in rat (top, left) and mouse (bottom, left). After Ca2+ permeabilization by caffeine/ryanodine treatment and exposure to 0 [Ca2+]o, the [Ca2+]i was low. Exposure to 1.3 mM Ca2+ induced ∼40% contraction in rat airways but only a transient contraction in mouse airways. Exposure to MCh induced an additional contraction in rat airway but a full contraction in the mouse airway. Compared with normal airways, 1 μM MCh induced ∼5% more contraction in the Ca2+-permeabilized rat airway but similar contraction in the Ca2+-permeabilized mouse airway.
It is interesting to note that the same concentration of MCh induced a similar-sized contraction in the normal (39.2 ± 5.6%, 6 airways of 4 mice) and Ca2+-permeabilized (37.2 ± 3.8%) mouse airways. The final contraction induced by agonist in Ca2+-permeabilized rat airways was slightly greater (58.3 ± 5.3%) than normal (48.6 ± 4.1%, 5 airways of 3 rats) rat airways (Fig. 8). Together, these results indicate that MCh increases the Ca2+ sensitivity of both the rat and mouse airway SMCs. Similarly responses were induced by 5-HT (data not shown).
Influence of temperature on the relationship between contraction and Ca2+ signaling.
Because the contraction of airway SMCs normally occurs in vivo at ∼37°C, room temperature may affect enzyme activity or other mechanisms sufficiently to alter the responses of the SMCs. Consequently, we compared the airway contraction and Ca2+ signaling of SMCs at 37°C and room temperature (∼22°C) in both species.
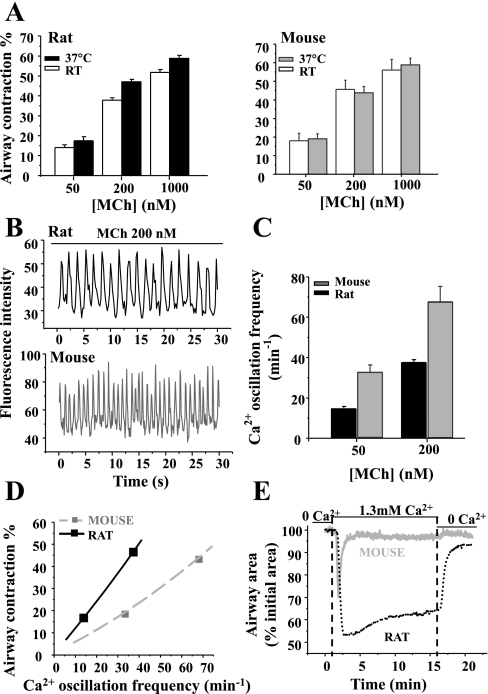
In the rat, MCh (50 nM to 1 μM) elicited slightly more contraction (P < 0.05) at 37°C (from 17.4 ± 1.7 to 58.8 ± 1.2%) than at room temperature (from 14.0 ± 1.0 to 51.8 ± 1.4%). In the mouse, MCh (50 nM to 1 μM) induced a similar concentration-dependent contraction at 37°C (from 19.0 ± 2.7 to 58.8 ± 3.5%, paired data from same airway) as it did at room temperature (from 17.9 ± 4.1 to 56.1 ± 5.6%; Fig. 9A). However, the airway contraction in response to all concentration of MCh was not significantly different between mice and rats at 37°C (Fig. 9A).
Fig. 9.
The contractile and Ca2+ responses of airway SMCs at 37°C. A: summary of MCh-induced (50, 200, and 1,000 nM) contraction in rats (left) and mice (right) at room temperature (RT; white bars) and 37°C (filled bars) in the same airway. Each column represents the mean ± SE from 5 paired airways of 3 rats and mice. B: representative experiments showing the Ca2+ oscillations of rat (top) or mouse (bottom) airway SMCs in response to 200 nM MCh at 37°C. These oscillations are faster than those observed at RT. C: summary data of the frequency of the airway SMC Ca2+ oscillations after 5-min exposure to 50 and 200 nM MCh in rats (black bar) and mice (gray bar) at 37°C. Each column represents the mean ± SE from at least 5 experiments from different slices from 3 rats and 3 mice. D: MCh-induced airway contraction as a function of Ca2+ oscillation frequency of rat (black solid line) and mouse (gray dashed line) at 37°C. Although the slope of the relationship is reduced with increased temperature, an equivalent contraction of the rat and mouse airway is achieved by slower Ca2+ oscillations in the rat SMCs. E: representative traces showing the contractile responses of Ca2+-permeabilized rat (black dotted line) and mouse (gray solid line) airway SMCs to the elevation of [Ca2+]o in absence of agonists at 37°C; 1.3 mM Ca2+ induced a sustained contraction of rat airways and a transient contraction of mouse airways. These responses are similar to those obtained at RT.
When the temperature was elevated from 22 to 37°C, the frequency of the MCh-induced Ca2+ oscillations substantially increased in both rats and mice (Fig. 9, B and C). However, at each concentration of MCh (50 or 200 nM) tested at 37°C, the Ca2+ oscillations in mouse airway SMCs were faster (32.7 ± 3.6; 67.6 ± 7.7 min−1) than those in rats (14.5 ± 1.0; 37.5 ± 1.2 min−1), respectively (Fig. 9C).
The contraction-Ca2+ signaling relationship at 37°C for mice and rat airways is plotted in Fig. 9D and demonstrated that increased temperature decreased the percent contraction related to the Ca2+ oscillation frequency (compare with Fig. 5). However, slower Ca2+ oscillations of SMCs were still associated with greater contraction in rat airways at 37°C. This, again, indicated a temperature-independent difference in the regulation of Ca2+ sensitivity between rats and mice. Consequently, we used Ca2+-permeable lung slices to reexamine the Ca2+ sensitivity of rat and mice airway SMCs at 37°C (Fig. 9E). The elevation of [Ca2+]i, by increasing [Ca2+]o from 0 to 1.3 mM, induced a similar transient contraction in mouse airways as observed at room temperature (Fig. 7C) except that the airway relaxation rate was faster at 37°C (Fig. 9E). By comparison, high [Ca2+]i induced a relatively stable contraction of rat airway SMCs at 37°C (49.9 ± 7.7% after 2 min and 38.9 ± 9.1% after 10 min, n = 6 airways from 3 different rats). These results indicate that rat airway SMCs have a high inherent Ca2+ sensitivity and that mouse airway SMCs display a Ca2+-induced Ca2+ desensitization and that both of these properties are not the result of a temperature change.
The role of Rho kinase and PKC in Ca2+ sensitivity.
In view of our conclusion that the intrinsic Ca2+ sensitivity of rat airway SMCs, independent of the presence of agonists, was higher than mouse SMCs, we hypothesized that the high Ca2+ sensitivity of rat airway SMCs was associated with low activity of myosin light chain phosphatase (MLCP). Consequently, we examined the possibility that increased activity of Rho kinase or PKC was responsible for the inhibition of MLCP activity.
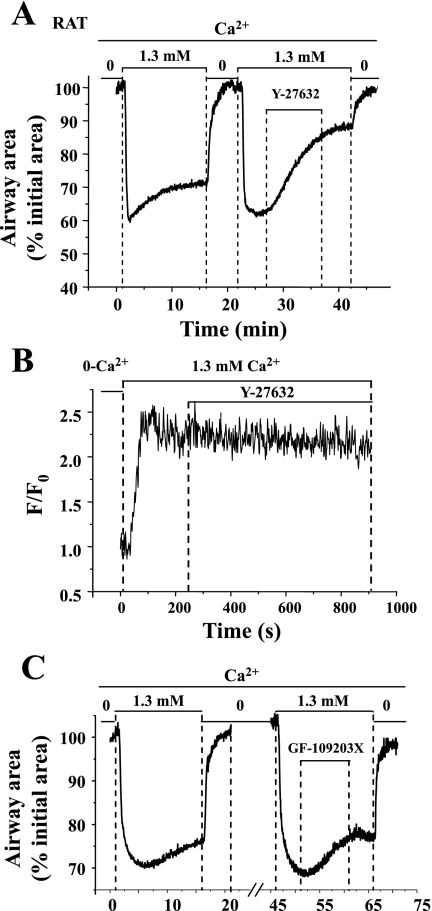
We found that Y-27632 (10 μM), a selective inhibitor of Rho kinase, relaxed (63.0 ± 1.9% after 10 min of exposure) the sustained contraction induced by 1.3 mM [Ca2+]o in Ca2+-permeabilized rat airways (Fig. 10A). During this relaxation, there was no obvious change in the [Ca2+]i (Fig. 10B). In our previous study (1), we also found that Y-27632 induced airway relaxation in Ca2+-permeabilized mouse slices contracted with MCh and high Ca2+. These results indicate that the inherent Ca2+ sensitivity of rat airway SMCs and the agonist-induced Ca2+ sensitivity of mouse airway SMCs can be reduced by inhibiting Rho kinase activity. Ca2+-permeabilized rat airways contracted with Ca2+ were also exposed to 2 μM GF-109203X, an inhibitor of PKC, but there was little effect on contraction (Fig. 10C). These results indicate that the PKC has little effect on the inherent Ca2+ sensitivity of rat airways. By contrast, our previous studies (1) indicated that agonist-induced Ca2+ sensitivity of mouse airways was partially reduced by GF-109203X.
Fig. 10.
The contraction and intracellular Ca2+ signaling of Ca2+-permeabilized rat airway SMCs in response to Y-27632, a Rho kinase inhibitor, and GF-109203X, a PKC inhibitor. A: a representative trace showing the relaxant effect of 10 μM Y-27632 on the Ca2+-induced contraction in the Ca2+-permeabilized rat airway SMCs. This relaxant response was not associated with significant change in [Ca2+]i (B). In comparison, 2 μM GF-109203X (C) had little effect on the Ca2+-permeabilized rat airway contraction induced by 1.3 mM Ca2+.
DISCUSSION
Intracellular Ca2+ signaling and Ca2+ sensitivity of airway SMCs are fundamental regulators of SMC contraction. Consequently, the characteristics and relative contribution of these two mechanisms determines the innate contractile property of airway SMCs. Airway contractility in response to specific agonists can vary from species to species (27), and this has commonly been ascribed to variations in structure (19), such as receptor distribution and density or airway morphology. However, in asthmatic hyperreactive airways, a comparable, but excessive, contraction can be induced by a variety of stimuli or agonists. This hyperresponsiveness does not appear to be correlated with a specific structural change, although smooth muscle mass is usually increased in the later stages of asthma. In addition, the idea that hyperresponsiveness is associated with a structural difference assumes that the underlying Ca2+-dependent signaling mechanisms in each SMC remains identical. However, our investigations have revealed that the extent of airway contraction varies at different airway locations and that this correlates with different frequencies of Ca2+ oscillations within SMCs (2). Consequently, the focus of this study was to determine the importance of the contribution of the Ca2+ signaling and Ca2+ sensitivity to airway SMCs contractility by comparing these two processes in rats and mice. Furthermore, in view of the fact that rodents are frequently used as experimental animals for the study of asthma, it is essential to characterize the airway responses of these animals to agonists, to determine whether it is appropriate to extrapolate data to human airways.
We initially compared the responses of rat and mice airways to the G protein-coupled receptor agonists, MCh and 5-HT. The effects of different agonists were examined to separate agonist-specific effects from fundamental differences in the relationship between contraction and Ca2+ signaling in airway SMCs of different species. However, it is important to point out that whereas 5-HT acts on rodent airways, this agonist has no effect on airways of some other species, such as guinea pig and human. The opposite scenario applies to histamine.
We also decided to initially compare airways with a similar size from each species to minimize the variation in structure and recoil forces from surrounding alveoli tissue. On the other hand, a comparison of airways at equivalent positions of the respiratory tract might be more appropriate. Although, we favor the comparison of equal-sized airways because the biophysics of each airway, and therefore the local physiological responses, are likely to be similar, we also examined the contractile responses of more distal and proximal airways to determine whether the differences we observed in similar-sized airways were specific to the species or the airway generation.
Both MCh and 5-HT induced substantial contraction of mouse and rat airways. In view of the previously reported variability in contractility to agonists, we were surprised to find that a concentration range of agonists from 10 to 500 nM induced a similar contraction in rat and mouse airways. Only at high concentrations of 5-HT (>500 nM) was there any observable difference where rat airways displayed a stronger sustained contraction (by ∼10%). In contrast to agonist-induced contraction, membrane depolarization induced with KCl only induced a small airway contraction in both rats and mice that were not significantly different.
The similarity of the airway responses of rats and mice suggests that the contractility of individual SMCs, the number of SMCs in the luminal wall, the resistance of the airway wall, surrounding connective tissue, and the agarose-filled alveoli were all equivalent in the rat and mouse lung slices, and this is consistent with the comparison of similar-sized airways. We also confirmed, by loading the SMCs with Ca2+ fluorescent dye, that the airways of each species had similar amounts of SMCs surrounding each airway.
In view of the similar contractile responses at room temperature, we expected to find an equivalent underlying Ca2+ signaling mechanism. Therefore, it was extremely interesting to find that the rat airway SMCs displayed substantially slower Ca2+ oscillations in response to all concentrations of MCh or 5-HT. In both the rat and mouse SMCs, these Ca2+ oscillations propagated along the SMCs as Ca2+ waves with similar velocities. In addition to a slower frequency, it was clear that each Ca2+ oscillation in rat SMCs had an extended duration of elevated Ca2+. This feature of the Ca2+ oscillation may reflect a prolonged Ca2+ release process from the SR via inositol trisphosphate (IP3) or ryanodine receptors or a slower reuptake of Ca2+ into the SR or extracellular space via SR or plasma membrane Ca2+ pumps. However, this extended period of Ca2+ elevation was not specific to rats because it could also be observed in mouse airway SMCs when the Ca2+ oscillations were induced with low agonist concentrations and had a similar slow frequency. These results indicate that the dynamics of the Ca2+ oscillations (or waves) are a function of the Ca2+ oscillation frequency, and this, in turn, implies that the organization and regulation of the Ca2+ release mechanisms underlying the Ca2+ oscillations is common to both rats and mice. In this case, it seems likely that the different frequencies of Ca2+ oscillations induced by similar concentrations of agonist in rat and mouse SMCs results from a differential activation of the G protein receptors responsible for the production of IP3.
At 37°C, the frequency of the Ca2+ oscillations of the SMCs was significantly increased in both rats and mice. However, the extent of the airway contraction in either the rat or mouse was changed very little. This indicates that the increased Ca2+ signaling is not effectively translated into increased contraction and underscores the idea that SMC contraction is not a simple function of [Ca2+]i. One possible explanation for this result is that the SMCs are unable to generate additional force to overcome the local tethering forces. However, this effect extends over the range of the concentration-response curve where the SMCs are not producing maximal force. An alternative idea is that the increased temperature has also increased the antagonistic activity of the enzyme MLCP to offset the action of increased MLC kinase activity. The increased airway relaxation rate observed in Ca2+-permeabilized mouse lung slices at 37°C in response to high [Ca2+]i is consistent with this idea of increased MLCP activity.
Although the frequency of the Ca2+ oscillations was increased in both rats and mice by increased temperature, a similar airway contraction was again achieved by slower Ca2+ oscillations in rat SMCs. In more proximal or distal rat airways, we found that the same concentration of MCh induced Ca2+ oscillations of a similar frequency, but the bigger proximal airways displayed less contraction. This is probably due to the changes in the structure of airway walls and surrounding tissues (11, 16). These results suggest that the difference in the correlation between Ca2+ signaling and contractile response of airway SMCs is species-specific and not a function of temperature or airway region. Moreover, in the mouse, the relationship of the Ca2+ oscillation frequency to airway contraction determined with 5-HT was left-shifted compared with that determined with MCh. This difference also reveals the existence of an agonist-specific influence. In the rat, this agonist-specific difference was less obvious. This can be explained by our findings that agonist-induced Ca2+ sensitization has a much smaller influence on SMC contraction in rat airway than in mouse airway.
Moreover, when Ca2+ oscillations, induced by KCl or low concentrations of agonist, were very slow, the airway contractile responses were similar in rats and mice. These responses are highlighted by the convergence of the Ca2+ oscillation-contraction relationships (i.e., Ca2+ sensitivity) for rat and mice with KCl or agonists (Fig. 5). This convergence, rather than a sustained difference in Ca2+ oscillation-contraction relationships, indicates that the difference in Ca2+ sensitivity between rat and mouse airway SMCs is proportional to the agonist concentration. This behavior suggests a difference in the mechanism of agonist- or Ca2+-dependent Ca2+ sensitization of rat and mouse airway SMCs.
To examine this Ca2+ sensitivity of rat and mice airway SMCs, we permeabilized the SMCs to Ca2+ by treating the lung slices with caffeine and ryanodine, and this allowed us to clamp the [Ca2+]i of the SMCs by varying the [Ca2+]o. A major advantage of this method for Ca2+ permeabilization is that the cell membranes are preserved intact, therefore any relevant molecules inside the cell or on the membrane, such as agonist receptors, are retained. In all cases, the exposure of Ca2+-permeabilized lung slices to agonists (contractile or relaxant) or KCl had no effect on the [Ca2+]i. We found that in response to a sustained high [Ca2+]i in the absence of agonists, the Ca2+-permeabilized mouse airway displayed a time-dependent decrease in Ca2+ sensitivity; the airway initially contracted as the [Ca2+]i was elevated but relaxed during the sustained increase of [Ca2+]i. By contrast, sustained [Ca2+]i induced Ca2+-permeabilized rat airways to display a substantial and stable contraction. When the [Ca2+]i was further increased, the mouse airway responded with a second, smaller transient contraction but essentially remained relaxed, whereas the rat airway increased its contracted state. These responses of Ca2+-permeabilized mouse and rat airways to high [Ca2+]i are virtually unchanged at 37°C. These results suggest that the contraction of the rat airway is primarily a function of increasing [Ca2+]i. By contrast, mouse airways display a complex response of Ca2+-dependent Ca2+ desensitization.
Although MCh had no effect on the [Ca2+]i of Ca2+-permeabilized airways of either rats or mice, MCh restored the contractile response of the mouse airway and induced an additional contraction of the rat airways. These results indicate that MCh increases the Ca2+ sensitivity of both rat and mouse SMCs (similar results were obtained with 5-HT). However, these results also indicate that the Ca2+ sensitivity of mouse airways is suppressed by [Ca2+]i. Consequently, in mouse airways, the activity of MLCP is antagonistically regulated by agonist concentration and [Ca2+]i, whereas in rat airways, MLCP activity appears to be regulated by only agonist concentration. Therefore, Sprague-Dawley rat airway SMCs have a higher Ca2+ sensitivity than BALB/c mice at an equivalent [Ca2+]i. We have found fast Ca2+ oscillations and a similar Ca2+-induced Ca2+ desensitization in another mouse strain (B6129SF2/J) used for genetic manipulation (Y. Bai, A. J. Halayko, and M. J. Sanderson, unpublished observations). Likewise, human airway SMCs display slow Ca2+ oscillations and Ca2+ sensitivity responses similar to the rat (P. Delmotte, A. Ressmeyer, and M. J. Sanderson, unpublished data).
In contrast to mice airways, the Ca2+ sensitivity of rat airways may be also increased by [Ca2+]i, (Ca2+-dependent Ca2+ sensitization). A similar idea has been proposed for vascular SMCs (13) where Ca2+-dependent activation of RhoA (22, 28, 29) by phosphatidylinositol 3-kinase (PI3K-C2α; Refs. 36, 39) or PKC (8) serves to inactivate MLCP via the phosphorylation of its regulatory proteins myosin phosphatase targeting subunit 1 (MYPT1) or PKC-potentiated inhibitory protein of 17 kDa (CPI-17). We found that Y-27632, an inhibitor of Rho kinase, relaxed Ca2+-permeabilized rat airways without significantly changing the [Ca2+]i, whereas GF-109203X, a PKC inhibitor, had little effect. These results suggest that Rho kinase but not PKC downregulates the activity of rat MLCP under conditions of high [Ca2+]i. The minimal involvement of PKC may result from the low expression level of CPI-17, which largely varies depending on the type of SMCs and animal species (15, 38). However, it remains to be determined whether the high Ca2+ sensitivity associated with high [Ca2+]i was mediated by the activation of RhoA/Rho kinase pathway to inactivate MLCP or whether the activity of rat MLCP is inherently low.
In conclusion, we have demonstrated that rat airway SMCs possess a higher Ca2+ sensitivity than mice and that this leads to major differences in the relationship between contraction and intracellular Ca2+ signaling of airway SMCs between the rats and mice. These findings show that the differences in Ca2+ sensitivity have a strong influence on the resulting contraction of airway SMCs in response to different agonists, a scenario reflected by the hyperresponsiveness of asthmatic airways. Furthermore, this variation in the innate regulation of SMC Ca2+ sensitivity between species requires caution in the extrapolation of mouse airway data as a model for asthmatic airway behavior.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-71930 and HL-087401 to M. J. Sanderson.
REFERENCES
- 1.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 291: L208–L221, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol 36: 122–130, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergner A, Sanderson MJ. Airway contractility and smooth muscle Ca2+ signaling in lung slices from different mouse strains. J Appl Physiol 95: 1325–1332; discussion 1314, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Björck T, Gustafsson LE, Dahlén SE. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis 145: 1087–1091, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Black JL Pharmacology of airway smooth muscle in chronic obstructive pulmonary disease and in asthma. Am Rev Respir Dis 143: 1177–1181, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Chiba Y, Ueno A, Sakai H, Misawa M. Hyperresponsiveness of bronchial but not tracheal smooth muscle in a murine model of allergic bronchial asthma. Inflamm Res 53: 636–642, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res 100: 121–129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duguet A, Biyah K, Minshall E, Gomes R, Wang CG, Taoudi-Benchekroun M, Bates JH, Eidelman DH. Bronchial responsiveness among inbred mouse strains. Role of airway smooth-muscle shortening velocity. Am J Respir Crit Care Med 161: 839–848, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Dulin NO, Fernandes DJ, Dowell M, Bellam S, McConville J, Lakser O, Mitchell R, Camoretti-Mercado B, Kogut P, Solway J. What evidence implicates airway smooth muscle in the cause of BHR? Clin Rev Allergy Immunol 24: 73–84, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Ebina M, Yaegashi H, Takahashi T, Motomiya M, Tanemura M. Distribution of smooth muscles along the bronchial tree. A morphometric study of ordinary autopsy lungs. Am Rev Respir Dis 141: 1322–1326, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Goldie RG, Spina D, Henry PJ, Lulich KM, Paterson JW. In vitro responsiveness of human asthmatic bronchus to carbachol, histamine, beta-adrenoceptor agonists and theophylline. Br J Clin Pharmacol 22: 669–676, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano K Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharm Sci 104: 109–115, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, Kemeny DM, Lotvall J, Pauwels RA, Plopper CG, Schmidt D, Sterk PJ, Van Oosterhout AJ, Vargaftig BB, Chung KF. Murine models of asthma. Eur Respir J 22: 374–382, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Kitazawa T, Polzin AN, Eto M. CPI-17-deficient smooth muscle of chicken. J Physiol 557: 515–528, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kott KS, Pinkerton KE, Bric JM, Plopper CG, Avadhanam KP, Joad JP. Methacholine responsiveness of proximal and distal airways of monkeys and rats using videomicrometry. J Appl Physiol 92: 989–996, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Levitt RC, Mitzner W. Expression of airway hyperreactivity to acetylcholine as a simple autosomal recessive trait in mice. FASEB J 2: 2605–2608, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol 283: L1181–L1189, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Martin JG, Jo T. Genetic differences in airway smooth muscle function. Proc Am Thorac Soc 5: 73–79, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Martin JG, Tamaoka M. Rat models of asthma and chronic obstructive lung disease. Pulm Pharmacol Ther 19: 377–385, 2006. [DOI] [PubMed] [Google Scholar]
- 21.McParland BE, Tait RR, Pare PD, Seow CY. The role of airway smooth muscle during an attack of asthma simulated in vitro. Am J Respir Cell Mol Biol 33: 500–504, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Mita M, Yanagihara H, Hishinuma S, Saito M, Walsh MP. Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J 364: 431–440, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overstreet DH, Djuric V. A genetic rat model of cholinergic hypersensitivity: implications for chemical intolerance, chronic fatigue, and asthma. Ann NY Acad Sci 933: 92–102, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels R, Van Der Straeten M, Weyne J, Bazin H. Genetic factors in non-specific bronchial reactivity in rats. Eur J Respir Dis 66: 98–104, 1985. [PubMed] [Google Scholar]
- 25.Perez JF, Sanderson MJ. The contraction of smooth muscle cells of intrapulmonary arterioles is determined by the frequency of Ca2+ oscillations induced by 5-HT and KCl. J Gen Physiol 125: 555–567, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 125: 535–553, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ressmeyer AR, Larsson AK, Vollmer E, Dahlèn SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J 28: 603–611, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto K, Hori M, Izumi M, Oka T, Kohama K, Ozaki H, Karaki H. Inhibition of high K+-induced contraction by the ROCKs inhibitor Y-27632 in vascular smooth muscle: possible involvement of ROCKs in a signal transduction pathway. J Pharm Sci 92: 56–69, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 93: 548–556, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc 5: 23–31, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Tulic MK, Hamid Q. New insights into the pathophysiology of the small airways in asthma. Clin Chest Med 27: 41–52, vi, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Turner DJ, Noble PB, Lucas MP, Mitchell HW. Decreased airway narrowing and smooth muscle contraction in hyperresponsive pigs. J Appl Physiol 93: 1296–1300, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol 96: 2019–2027, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Wang CG, Almirall JJ, Dolman CS, Dandurand RJ, Eidelman DH. In vitro bronchial responsiveness in two highly inbred rat strains. J Appl Physiol 82: 1445–1452, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Yoshioka K, Azam MA, Takuwa N, Sakurada S, Kayaba Y, Sugimoto N, Inoki I, Kimura T, Kuwaki T, Takuwa Y. Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem J 394: 581–592, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whicker SD, Armour CL, Black JL. Responsiveness of bronchial smooth muscle from asthmatic patients to relaxant and contractile agonists. Pulm Pharmacol 1: 25–31, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshioka K, Sugimoto N, Takuwa N, Takuwa Y. Essential role for class II phosphoinositide 3-kinase alpha-isoform in Ca2+-induced, Rho- and Rho kinase-dependent regulation of myosin phosphatase and contraction in isolated vascular smooth muscle cells. Mol Pharmacol 71: 912–920, 2007. [DOI] [PubMed] [Google Scholar]