Abstract
Francisella tularensis, the causative agent of tularemia, is a highly virulent organism, especially when exposure occurs by inhalation. Recent data suggest that Francisella interacts directly with alveolar epithelial cells. Although F. tularensis causes septicemia and can live extracellularly in a murine infection model, there is little information about the role of the vascular endothelium in the host response. We hypothesized that F. tularensis would interact with pulmonary endothelial cells as a prerequisite to the clinically observed recruitment of neutrophils to the lung. Using an in vitro Transwell model system, we studied interactions between F. tularensis live vaccine strain (Ft LVS) and a pulmonary microvascular endothelial cell (PMVEC) monolayer. Organisms invaded the endothelium and were visualized within individual endothelial cells by confocal microscopy. Although these bacteria-endothelial cell interactions did not elicit production of the proinflammatory chemokines, polymorphonuclear leukocytes (PMN) were stimulated to transmigrate across the endothelium in response to Ft LVS. Moreover, transendothelial migration altered the phenotype of recruited PMN; i.e., the capacity of these PMN to activate NADPH oxidase and release elastase in response to subsequent stimulation was reduced compared with PMN that traversed PMVEC in response to Streptococcus pneumoniae. The blunting of PMN responsiveness required PMN transendothelial migration but did not require PMN uptake of Ft LVS, was not dependent on the presence of serum-derived factors, and was not reproduced by Ft LVS-conditioned medium. We speculate that the capacity of Ft LVS-stimulated PMVEC to support transendothelial migration of PMN without triggering release of IL-8 and monocyte chemotactic protein-1 and to suppress the responsiveness of transmigrated PMN to subsequent stimulation could contribute to the dramatic virulence during inhalational challenge with Francisella.
Keywords: tularemia, NADPH oxidase, pulmonary, polymorphonuclear leukocyte
its dramatic virulence and its potential for use as a biological weapon have increased recent interest in defining host responses to Francisella tularensis, a gram-negative bacterium that causes tularemia. Two major subspecies of Francisella are pathogenic for humans, including the most virulent strains F. tularensis subspecies tularensis (type A) and F. tularensis subspecies holarctica (type B), from which the attenuated live vaccine strain (Ft LVS) was derived. Because of the wide spectrum of disease that can be caused by individual strains of F. tularensis, it is likely that local host responses specific to the site or route of challenge contribute significantly to the clinical disease (for review see Ref. 19).
F. tularensis has been characterized as a facultative intracellular pathogen, and its survival and replication within macrophages have been identified as critical factors contributing to its virulence. Interactions of F. tularensis with host phagocytic cells have been intensively investigated, but more recent evidence suggests that this organism interacts directly with nonprofessional phagocytes, including cells of the airway epithelium (11, 16). Ft LVS invades and replicates in epithelial cells, eliciting an NF-κB-mediated inflammatory response. These investigations are particularly relevant to the study of an organism known to cause severe respiratory disease, with pneumonic tularemia initiated by as few as 10 organisms (5, 27). Moreover, recent work demonstrates that Ft LVS survives extracellularly in the bloodstream of experimentally infected mice (4, 10) after intranasal and intradermal inoculation, suggesting that invasion of airway epithelium, interaction with the pulmonary endothelium, and translocation into the vascular space occur in vivo.
On the basis of these findings, investigation of interactions between F. tularensis and the endothelium is necessary for achieving a better understanding of disease pathogenesis. Although few studies have focused attention on the role of the endothelium in tularemia, published data suggest that F. tularensis elicits a diminished proinflammatory response from human umbilical vein endothelial cells compared with other gram-negative organisms (9). In our studies of the interactions between Ft LVS and human microvascular endothelial cells of pulmonary and dermal origin, we found that Ft LVS invaded endothelial monolayers and concomitantly promoted transmigration of polymorphonuclear leukocytes (PMN), although less vigorously than did formylated peptide or pathogenic pneumococci. Despite mediating PMN transmigration, endothelium invaded by Ft LVS did not release detectable amounts of the chemoattractants IL-8 and monocyte chemotactic protein-1 (MCP-1). Furthermore, PMN that traversed the Ft LVS-stimulated endothelial monolayers were less responsive, with respect to NADPH oxidase activation and granule release, than were PMN that had transmigrated in response to other agonists. Thus the Ft LVS interacted with pulmonary microvascular endothelial cells (PMVEC) in a fashion that promoted PMN transmigration without eliciting proinflammatory cytokine release and resulted in a blunted inflammatory phenotype in the PMN that were stimulated to transmigrate. Taken together, changes in local host defenses may reflect the action(s) of bacterial virulence factors that contribute(s) to the remarkable infectivity of inhaled Francisella.
MATERIALS AND METHODS
Materials.
Endothelial cells and all tissue culture media were purchased from Clonetics (San Diego, CA), Transwell filters (12 mm, 3 μm pore) from Costar (Cambridge, MA), tryptic soy broth/agar and cysteine heart agar from Becton Dickinson (Sparks, MD), and defibrinated sheep blood from Colorado Serum (Denver, CO). Antibodies used for confocal microscopy studies include anti-platelet endothelial cell adhesion molecule (PECAM)-1 (clone hec 2.7; a generous gift from Dr. William Muller, Department of Pathology, Feinberg School of Medicine, Northwestern University) and rabbit anti-Ft LVS polyclonal antibodies (Becton Dickinson). All other chemicals were purchased from Sigma (St. Louis, MO). LPS was purified from F. tularensis as previously described (22). LPS-binding protein (LBP) was a generous gift from Dr. Jerrold Weiss (University of Iowa).
Human PMN purification.
Human PMN were isolated according to standard techniques from heparin-anticoagulated venous blood from healthy consenting adults in accordance with a protocol approved by the Institutional Review Board for Human Subjects at the University of Iowa. Briefly, PMN were isolated using dextran sedimentation and Hypaque-Ficoll density-gradient separation followed by hypotonic lysis of erythrocytes as previously described (2). Purified PMN (≥98% PMN) were resuspended in 0.9% saline before use in migration experiments.
Endothelial cell culture.
PMVEC and human dermal microvascular endothelial cells (HMVEC-D) were purchased from Clonetics and cultured on collagen-coated flasks (type VI, human placental collagen, Sigma) using Endothelial Growth Medium-2 (Clonetics) to which bovine brain extract, VEGF, EGF, gentamicin, and hydrocortisone were added according to the manufacturer's specifications. Cells were obtained from Clonetics at passage 4 or 5 and used between passages 5 and 9. When the cells reached ∼70–80% confluency, they were passed from T-75 flasks into experimental plates. Cells were detached using trypsin-EDTA and cultured on collagen-coated Transwell (Costar) 12-mm filters for transmigration experiments and confocal microscopy. Cell monolayers on Transwell filters were monitored by measurement of resistance changes across the endothelial cell monolayer using an End Ohm Epithelial Voltohmeter (World Precision Instruments, Sarasota, FL). HMVEC-D monolayers reached an average peak resistance of 33 ± 1.0 Ω·cm2, whereas the average resistance across the PMVEC monolayers was 25 ± 0.94 Ω·cm2. Medium on the monolayers was changed every 48 h.
Bacterial cell cultures.
F. tularensis subspecies holarctica LVS (obtained from Dr. Michael Apicella, University of Iowa) was grown on 9% sheep blood-cysteine heart agar plates at 37°C in 5% CO2 for 36 h and then collected from the plates for use in the experiments. For some invasion assays, bacteria were grown overnight in modified Mueller-Hinton broth to mid-log phase. Bacteria were resuspended in endothelial basal medium (EBM; Clonetics) and quantified by measurement of absorbance at 600 nm. Streptococcus pneumoniae serotype 2 strain D39 (D39; a gift from Dr. Elaine Tuomanen, St. Jude Children's Research Hospital) was stored at −80°C, and an aliquot was streaked on blood agar plates 16 h before use. Bacteria were added to the lower chambers of the Transwell filters at a known optical density, and samples from 0 and 4 h (just before addition of PMN) were plated for colony-forming unit (CFU) determination of the growth of each bacterium during the initial 4-h bacteria/endothelial cell incubation. In experiments in which bacteria-conditioned medium was used, live bacteria were incubated in EBM as described above. In experiments where bacteria-endothelial cell-conditioned medium was used, live bacteria were incubated with endothelial monolayers. After these incubations, the bacteria-conditioned medium or bacteria-endothelial cell-conditioned medium was centrifuged and filtered to remove bacteria before incubation with PMN.
Bacterial invasion of the endothelial monolayer.
Transwell filters with attached PMVEC monolayers were transferred to clean 12-well plates and washed twice with HBSS. After HBSS was removed, EBM (400 μl) was added to the upper compartment of the Transwell filter. Ft LVS was added to the lower compartment in a total volume of 1 ml and incubated with the endothelial cells at 37°C. No bacteria were added to the upper chamber of the Transwell filter. At 3 and 6 h after the initial inoculum was added to the lower chamber, medium was removed and the monolayer was washed extensively before incubation with gentamicin (25 μg/ml) for 1 h. After gentamicin incubation, the monolayer was washed three times with HBSS to remove residual antibiotic and then lysed with 1% saponin for 5 min. Aliquots of the lysed monolayer were plated for enumeration of CFU. Endothelial monolayers grown to confluence on Transwell inserts were released from the Transwell filter by trypsin and counted for calculation of multiplicity of infection (MOI).
Confocal microscopy.
PMVEC were grown on Transwell 12-mm filters to peak resistance. Bacteria suspended at the described concentrations in EBM were added to the lower compartment in a total volume of 1.0 ml. Bacteria and endothelial cells were coincubated for 6 h at 37°C. Then bacterial suspensions were removed, and filters were washed twice with PBS, fixed with 2% paraformaldehyde at room temperature for 20 min, permeabilized with 0.1% Triton for 10 min at room temperature, and blocked overnight with PBS + 0.5% BSA. Primary antibodies [anti-PECAM (1:50 dilution) and anti-Ft LVS (1:10,000 dilution)] were applied to the luminal surface of the endothelial cells on the filter for 1 h at room temperature. After the filters were washed three times with PBS + 0.5% BSA, Texas Red- or FITC-conjugated secondary antibody (Molecular Probes, Eugene, OR) was applied (1:1,000 dilution) for 1 h at room temperature. Specificity of staining was assessed by use of isotype control mouse antibodies. Filters were washed and mounted on glass slides. Samples were viewed using a confocal microscope (model LSM-510, Carl Zeiss, Thornwood, NY) with accompanying software.
Analysis of chemokine expression.
Supernatants from the upper and lower chambers of bacterial invasion experiments were collected and filtered to remove bacteria before they were stored at −20°C. IL-8 and MCP-1 levels were assayed in the supernatant by ELISA. Briefly, 50-μl samples from the collected supernatant were added to 96-well plates, which were coated with a monoclonal antibody to the protein of interest (R & D Systems, Minneapolis, MN). The samples were probed sequentially with a biotinylated detection antibody and horseradish peroxidase-conjugated streptavidin (Pierce Chemicals) for colorimetric detection using the trimethoxybenzoate substrate (Sigma). Standard curves were made using recombinant protein for each of the proteins of interest, absorbance at 450 nm was measured for experimental samples, and the concentrations were determined by extrapolation from the standard curve. IL-8 expression was determined by coincubation of PMN with specified numbers of Ft LVS for 3 h. Suspensions of PMN and bacteria were centrifuged and filtered, and release of IL-8 was quantitated.
PMN transendothelial migration.
Transendothelial migration assays were performed across PMVEC and HMVEC-D monolayers as previously described (20). Briefly, Transwell filters with attached endothelial cell monolayers were transferred to clean 12-well plates and washed twice with HBSS. After removal of HBSS, EBM (400 μl) was added to the Transwell filter compartment. Bacteria or purified LPS (in the presence of 100 ng/ml LBP) were added at the indicated concentrations to the lower compartment in a total volume of 1 ml and incubated with the endothelial cells for 4 h at 37°C. Subsequently, 10-μl samples were removed from the lower chamber for quantitation of the bacterial CFU, and 2 × 106 PMN in 100 μl were added to the upper chamber of the Transwell filter. PMN were allowed to migrate over a 3-h period at 37°C. Migrated cells were collected from the lower chamber and counted manually in a hemocytometer in triplicate. In preliminary experiments, PMN recovered from above and below the endothelial monolayer accounted for ∼90% of the initial number added (data not shown), indicating that the number of unrecovered tightly adherent PMN was not significant. Therefore, PMN were routinely counted from the lower chamber only.
Measurement of NADPH oxidase activity by lucigenin-enhanced chemiluminescence.
NADPH oxidase activity was assayed by lucigenin-enhanced chemiluminescence in a 96-well plate using the Lumistar Galaxy (BMG Technologies) or the Fluostar Omega (BMG Technologies). Briefly, PMN were collected after transendothelial migration in response to Ft LVS or D39. In parallel, PMN were maintained in the transendothelial migration buffer at 37°C for 3 h to control for potential cell activation unrelated to the migration process. A PMN suspension (200 μl) containing 2.5 × 106 PMN/ml in HBSS with 1% human serum albumin and 0.1% dextrose was added to each well, with a final lucigenin concentration of 100 μM. Cells were stimulated by addition of serum-opsonized zymosan (OpZ) at a 5:1 OpZ-to-PMN ratio or PMA at a final concentration of 10 ng/ml. Zymosan particles were opsonized with 100% autologous serum for 30 min at 37°C and centrifuged before use. Chemiluminescence was quantitated as relative luminescence units per minute using a kinetic assay, with readings obtained every minute for 1 h. In addition, aliquots of PMN that had transmigrated in response to Ft LVS were analyzed by microscopy to determine whether bacterial uptake had occurred.
Analysis of phagocytosis by flow cytometry.
Phagocytosis of opsonized particles by PMN after transendothelial migration in response to Ft LVS or D39 compared with control PMN (as described above) was quantitated by flow cytometry. Fluorescein-labeled zymosan (Molecular Probes) was opsonized with 100% serum for 30 min at 37°C, centrifuged, and washed. Transmigrated PMN were collected, counted, and suspended with OpZ for 30 min at an OpZ-to-PMN ratio of 1:1 or 5:1 at 37°C. After incubation, cells were washed twice to remove free particles and analyzed using the FACScalibur with gating by FITC intensity.
Elastase secretion.
Elastase secretion was measured in control PMN and in PMN after transendothelial migration elicited by F. tularensis or D39. Elastase activity was measured fluorometrically using aminomethyl coumarin as substrate (29). PMN were stimulated with 1 μM formyl-Met-Leu-Phe (fMLF) or 10 ng/ml PMA in triplicate. In addition, PMN from each experimental group were exposed to dihydrocytochalasin B (DHCB), a microfilament-disrupting agent (2.5 μg/ml), for 5 min before stimulation with fMLF to serve as a measurement of maximum elastase release from the primary granules. Fluorescence was quantitated using excitation at 342 nm and emission at 435 nm, with readings every 60 s for 1 h using the Spectramax Gemini fluorometer (Molecular Devices, Sunnyvale, CA) or the Fluostar Omega. Change in fluorescence over 60 min was calculated for each sample.
Statistical analysis.
Differences between two experimental groups with paired or unpaired comparisons composed of normally distributed data were analyzed for statistical significance by t-test. Differences among paired curves composed of multiple points were analyzed by 2-way ANOVA.
RESULTS
Invasion of Ft LVS into intact endothelial cell monolayers.
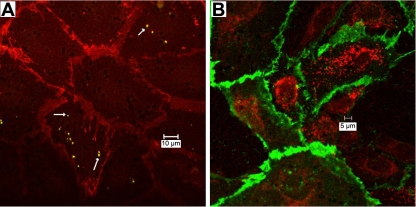
Previous reports suggest that F. tularensis elicits a diminished proinflammatory response from human umbilical vein endothelial cells compared with that triggered by other gram-negative organisms (9). To investigate the nature of the interaction between Ft LVS and the microvascular endothelium, we studied interactions of live organisms with an intact endothelial monolayer. Live Ft LVS were added at 5–500 MOI to the lower chamber of the Transwell apparatus and incubated for 3 or 6 h. At 3 and 6 h, lysis of the endothelial monolayer, after extensive washing of the monolayer followed by gentamicin treatment, demonstrated live organisms within the endothelium. Prompted by these data suggesting that Ft LVS invaded intact endothelial monolayers, we used confocal microscopy to determine whether bacteria were present within individual endothelial cells. Ft LVS was added at the abluminal surface of the PMVEC monolayer at 5 or 500 MOI and coincubated with the monolayer for 6 h. Although electrical resistance across the monolayer was unchanged (data not shown) and endothelial junctions were intact, as judged by PECAM staining (Fig. 1 ), bacteria were detected within a subset of endothelial cells. When the inoculum was increased 100-fold, the number of intracellular bacteria rose dramatically and the bacteria outlined the nucleus (Fig. 1B). Even at high inoculum, a significant number of cells did not appear to contain intracellular bacteria. The intracellular localization of the bacteria was confirmed using z-plane sectioning by confocal microscopy in monolayers stained for Ft LVS and PECAM.
Fig. 1.
Invasion of Francisella tularensis live vaccine strain (Ft LVS) into an intact pulmonary microvascular endothelial cell (PMVEC) monolayer. Ft LVS were incubated at the abluminal surface of the PMVEC monolayer [5 or 500 multiplicity of infection (MOI)] for 6 h; then monolayers were collected and fixed for microscopy. A: at ∼5 MOI, staining with rabbit anti-Ft LVS antibody (arrows) and secondary FITC reveals individual Ft LVS and staining with anti-platelet endothelial cell adhesion molecule (PECAM) antibody and secondary Texas Red reveals intercellular junctions. B: at 500 MOI, staining with rabbit anti-Ft LVS antibody and secondary Texas Red and staining with anti-PECAM antibody and secondary FITC reveal numerous intracellular bacteria. Images represent sections from 4 experiments.
Chemokine production in response to F. tularensis-endothelial cell-PMN interactions.
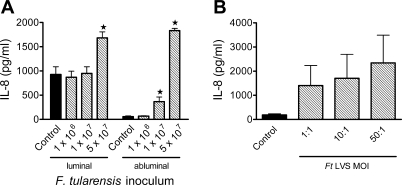
To determine whether the presence of Ft LVS within individual endothelial cells activated the monolayer, we assessed secretion of the chemokine IL-8 in response to Ft LVS as an indicator of the endothelial inflammatory response to bacteria, as we previously demonstrated in response to other pulmonary pathogens (20). Bacteria were added to the abluminal chamber at 1 × 106–5 × 107 CPU (corresponding to 5–250 MOI) and coincubated with the endothelium for 6 h. In transmigration experiments, medium was collected after 7 h of bacteria-endothelial cell interactions, with PMN present during the final 3 h of this coincubation. In the absence of PMN, IL-8 secretion did not increase above baseline levels in response to any of the concentrations of bacteria tested in the luminal or abluminal compartment of the monolayer (data not shown). In addition, F. tularensis LVS did not stimulate MCP-1 secretion by the endothelium. In PMN transmigration experiments, levels of IL-8 measured in the luminal chamber were similar to those from unstimulated monolayers and monolayers stimulated with Ft LVS at ≤1 × 107 organisms. In response to 5 × 107 bacteria, a significant increase in IL-8 was detected in the luminal and abluminal chambers (Fig. 2A). Regardless of the presence of Ft LVS, IL-8 levels were higher in both compartments when PMN were present, suggesting that PMN are required for IL-8 release. PMN incubated with unopsonized Ft LVS alone at 1–50 MOI for 1–3 h did elicit release of IL-8 (Fig. 2B). These data suggest that Ft LVS directly invaded endothelial cells without triggering the anticipated release of proinflammatory chemokine from the endothelial monolayer.
Fig. 2.
IL-8 production elicited by Ft LVS-endothelial cell-PMN interactions. A: inocula of Ft LVS or no bacteria (control) were incubated at the abluminal surface of a PMVEC monolayer for 4 h; then PMN were added to the luminal surface, and medium was collected after 3 h (7 h after addition of bacteria) for determination of IL-8 levels. More IL-8 was secreted at the luminal than at the abluminal surface of the monolayer, consistent with the presence of PMN on that side of the monolayer. There was no significant increase in luminal levels of IL-8 above control levels in monolayers stimulated with up to 1 × 107 Ft LVS. Coincubation of the monolayer with 5 × 107 Ft LVS elicited a significant increase in IL-8 production above baseline levels in the luminal compartment. In the abluminal chamber, IL-8 levels were greater than control with inoculum of ≥1 × 107/ml. Values are means ± SE (n = 5). *P < 0.05 vs. control for that compartment. B: PMN were incubated with Ft LVS at 1–50 MOI for 3 h before collection of supernatant for detection of IL-8 levels. Control PMN were incubated in the same medium; incubation of PMN with purified LPS from Escherichia coli was used as a positive control for IL-8 release. Ft LVS elicited release of IL-8 from PMN, although there was wide variation in the amount released. Values are means ± SE (n = 4).
PMN transendothelial migration in response to F. tularensis.
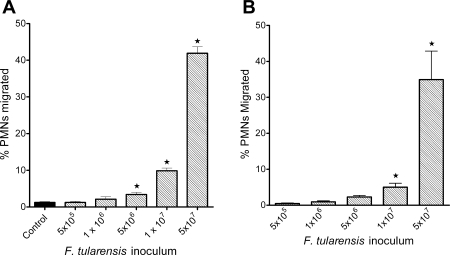
Previously published data suggest that only prolonged stimulation of endothelial monolayers with Francisella elicits PMN transmigration (9). We studied monolayers of microvascular endothelial cells from two different tissues, lung and skin, reasoning that they would be relevant to naturally occurring infection via aerosol and skin inoculation, respectively. Live Ft LVS elicited PMN transmigration across PMVEC (Fig. 3A) and HMVEC-D (Fig. 3B) monolayers. Although as few as 5 × 106 bacteria in the lower chamber elicited significant migration above baseline levels, the extent of transmigration was relatively low until the bacterial inoculum reached 5 × 107. Heat-killed Ft LVS, even at very high inoculum (5 × 108/ml in the lower chamber), elicited <1% PMN migration (data not shown), demonstrating that recruitment of PMN requires the interaction of live bacteria with the endothelial monolayer.
Fig. 3.
PMN transendothelial migration in response to Ft LVS. Ft LVS elicited migration of PMN across PMVEC (A) and human dermal microvascular endothelial cells (HMVEC-D; B) in a concentration-dependent manner. Inocula of ≥5 × 106 elicited PMN transmigration above baseline levels of migration in control, unstimulated monolayers (A). Values are means ± SE of 7 experiments done in triplicate. *P < 0.05 vs. control.
We speculated that a secreted or released bacterial product might be responsible for eliciting transmigration of PMN. In view of our previous data demonstrating that LPS from gram-negative organisms elicits PMN transmigration (20), we investigated transendothelial migration of PMN elicited by LPS purified from F. tularensis. PMN did not migrate across endothelium stimulated with LPS from F. tularensis at concentrations up to 1,000 ng/ml in the abluminal compartment (data not shown).
NADPH oxidase activity of transmigrated PMN.
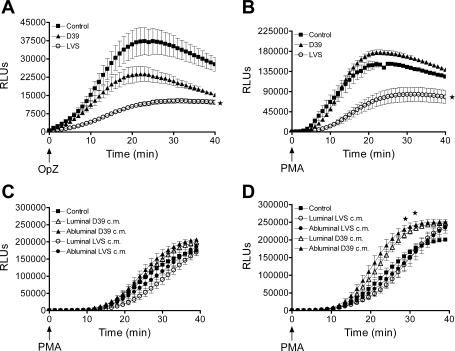
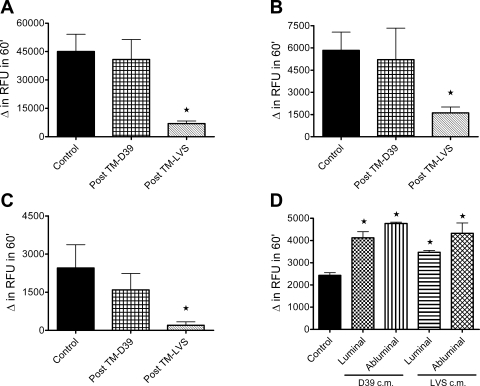
Although PMN migration in response to F. tularensis in vitro was less robust than that previously documented in response to other pathogens (20), the recruitment of even a small number of PMN to the air space would likely provoke an inflammatory response. However, the early pulmonary inflammatory response to F. tularensis is minimal in vivo (5), raising the possibility that PMN recruited to the airway by Francisella may not be fully effective. Accordingly, we reasoned that the function of such PMN, including the capacity to activate NADPH oxidase, might be compromised. To test this hypothesis, we compared the NADPH oxidase activity of PMN after transendothelial migration in response to Ft LVS with that of PMN that had migrated in response to a virulent S. pneumoniae strain. As an additional cell for comparison, we studied control PMN that were maintained in buffer at 37°C for 3 h during the transmigration period to mimic exposure to buffer and exposure to other PMN. The bacteria that elicited transmigration in these assays were not serum opsonized, and no serum was present in the transmigration system. Consequently, the bacteria were not ingested by the migrating PMN, as confirmed by microscopy (data not shown). As measured by lucigenin-enhanced chemiluminescence, NADPH oxidase activity of PMN that migrated in response to Ft LVS was significantly diminished in response to PMA (10 ng/ml) and OpZ (5:1 particle-to-cell ratio; Fig. 4, A and B ) compared with that of control PMN and PMN that transmigrated in response to live S. pneumoniae (D39). These data suggest that migration across the Ft LVS-stimulated endothelium downregulated responsiveness of the PMN to subsequent activation. To provide further evidence for a specific requirement for transendothelial migration in the blunting of PMN NADPH oxidase activity, we studied PMN incubated with bacteria-endothelial cell-conditioned medium (after filtration to remove intact bacteria). Interestingly, in PMN incubated for 1 h (Fig. 4C) or 3 h (Fig. 4D) with conditioned medium from the luminal or abluminal surface of the Ft LVS-treated endothelium, reactive oxygen species (ROS) generation in response to PMA (Fig. 4, C and D) and OpZ (data not shown) was normal. NADPH oxidase activity was not significantly increased in PMN incubated with D39-endothelial cell-conditioned medium for 3 h (Fig. 4D). To determine whether a secreted or shed microbial product altered the phenotype of the PMN in the lower chamber of the Transwell filter, independent of their migration across the endothelium, we incubated PMN with EBM-conditioned medium, generated by incubation of the live bacteria under conditions identical to the transmigration assay without endothelial cells. After 60 min of incubation, PMN NADPH oxidase activity in response to PMA and OpZ was unchanged from control (data not shown). The 60-min incubation was selected for exposure of PMN to conditioned medium, because the transmigrated PMN were generally exposed in the lower compartment for ≤1 h, since most PMN migrate across the endothelium during the final hour after addition to the endothelial monolayer. Taken together, these data suggest that the endothelial monolayer was modulated by interaction with live Francisella and altered PMN inflammatory responsiveness during transmigration.
Fig. 4.
NADPH oxidase activity of transmigrated PMN as measured by lucigenin-enhanced chemiluminescence in relative luminescence units (RLUs). A: after transendothelial migration in response to Ft LVS, NADPH oxidase activity in PMN was significantly depressed in response to the phagocytic stimulus opsonized zymosan (OpZ) compared with control PMN or PMN that migrated in response to Streptococcus pneumoniae strain D39. Values are means ± SE (n = 5–8). *P < 0.05 vs. control and D39 (by 2-way ANOVA). B: response to PMA (10 ng/ml) was significantly increased in control PMN and PMN that migrated in response to D39 compared with PMN that migrated in response to Ft LVS. Values are means ± SE (n = 6). *P < 0.05 vs. control and D39. C and D: PMN incubated with bacteria-endothelial cell-conditioned medium for 1 and 3 h, respectively. There was no alteration in PMN NADPH oxidase activity in response to PMA after 1 h of incubation with bacteria-endothelial cell-conditioned medium from the luminal or abluminal compartment of monolayers exposed to Ft LVS (LVS) or S. pneumoniae (D39). After 3 h of incubation, NADPH oxidase activity was significantly enhanced in PMN exposed to D39-endothelial cell-conditioned medium compared with control PMN or PMN exposed to Ft LVS-endothelial cell-conditioned medium. Values are means ± SE (n = 3). *P < 0.05 vs. control and LVS.
Phagocytosis of opsonized particles by PMN after migration.
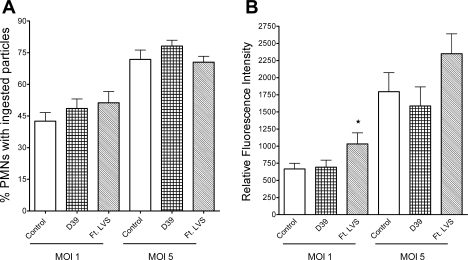
To determine whether the differences in OpZ-stimulated NADPH oxidase activity were secondary to depressed uptake of OpZ, we quantitated phagocytosis by PMN that transmigrated the endothelial monolayer. PMN that were not exposed to endothelial cells and PMN that migrated in response to Ft LVS or S. pneumoniae were incubated for 10 min with fluorescent OpZ at 1:1 and 5:1 OpZ-to-PMN ratio. PMN in all groups ingested OpZ, with enhanced uptake at the higher particle-to-cell ratio, as expected. There were no significant differences among the groups in the percentage of PMN that displayed cell-associated or phagocytosed particles at either MOI (Fig. 5A). In addition, the number of phagocytosed or cell-associated particles in the PMN that transmigrated in response to Ft LVS, as judged by the mean fluorescence intensity of the cell population, was enhanced compared with control PMN (Fig. 5B). Thus the phagocytic capability of PMN that traversed the endothelial monolayer stimulated by Ft LVS was not reduced, and the reduction in ROS generated in response to a particulate stimulus resulted from an impairment of the respiratory burst, rather than depressed particle uptake.
Fig. 5.
Phagocytosis of OpZ by PMN after transendothelial migration. A: phagocytosis in control PMN and PMN after transendothelial migration in response to S. pneumoniae strain D39 or Ft LVS. At 1 and 5 MOI, percentage of PMN with ingested particles was similar in all 3 groups. B: mean fluorescence intensity was similar in control PMN and PMN after transendothelial migration in response to D39 when incubated with serum-opsonized zymosan particles at 1 and 5 MOI. Flow cytometry was used to determine mean fluorescence intensity per cell as a measure of the number of phagocytosed particles. Fluorescence was slightly enhanced in PMN after migration in response to Ft LVS at 1 MOI. Values are means ± SE (n = 5–6). *P < 0.05 vs. control.
Elastase release.
Because PMN activate NADPH oxidase and degranulate in response to microbial stimulation, we quantitated elastase release from PMN that transmigrated in response to Ft LVS or S. pneumoniae with control PMN that had not traversed an endothelial monolayer. Minimal elastase was secreted by control PMN or PMN that transmigrated in response to Ft LVS or S. pneumoniae in the absence of other added agonists (data not shown). Control PMN pretreated with the microfilament-disrupting agent DHCB and then stimulated with fMLF displayed maximal elastase release, and stimulation of PMN following transendothelial migration in response to S. pneumoniae resulted in similar levels of elastase release. However, elastase release was more than sixfold less after fMLF challenge in PMN that migrated in response to Ft LVS than in control PMN (Fig. 6A). In response to fMLF alone (no DHCB) or PMA (10 ng/ml), elastase secretion was above background levels. Moreover, elastase release was significantly diminished in response to fMLF (Fig. 6B) or PMA (Fig. 6C) in PMN that migrated in response to Ft LVS compared with control PMN or PMN that migrated in response to S. pneumoniae. To investigate whether this impairment in granule content secretion after transmigration was dependent on transendothelial migration, rather than an indirect effect of a secreted or released endothelial or bacterial product, we studied PMN incubated with filtered bacteria-endothelial cell-conditioned medium. Neither 1 nor 3 h of incubation with conditioned medium derived from either surface of the endothelial monolayer blunted agonist-dependent elastase release from PMN. In fact, PMN coincubated with Ft LVS-endothelial cell-conditioned medium or S. pneumoniae-conditioned medium for 3 h exhibited enhanced elastase secretion when stimulated with fMLF (Fig. 6D). As observed in the controls for the NADPH oxidase activity assays, coincubation of PMN with Ft LVS-conditioned medium had no effect on elastase release in response to any of the agonists (data not shown). Similar to the blunted activation of NADPH oxidase, transendothelial migration of PMN in response to Ft LVS inhibited the ability of PMN to degranulate in response to subsequent stimulation.
Fig. 6.
Elastase release from PMN after transendothelial migration (post TM). A–C: fluorometric analysis of elastase release from control PMN and PMN after transendothelial migration in response to S. pneumoniae strain D39 or Ft LVS. Elastase release after pretreatment with dihydrocytochalasin B followed by stimulation with bacterial chemoattractant formyl-Met-Leu-Phe (fMLF; A), stimulation with fMLF alone (B), or stimulation with PMA (C) was significantly inhibited in PMN that migrated across the endothelium in response to Ft LVS compared with control PMN or PMN after migration in response to D39. Values are means ± SE (n = 5). *P < 0.05 vs. control. D: elastase release in response to fMLF alone was enhanced in PMN incubated with bacteria-endothelial cell-conditioned medium (cm) for 3 h. This significant increase in elastase release occurred in response to D39- and Ft LVS-endothelial cell-conditioned medium from luminal and abluminal surfaces of the endothelial monolayer. Values are means ± SE (n = 4). *P < 0.05 vs. control.
DISCUSSION
Because of the remarkable virulence of inhaled F. tularensis and the potential exploitation of this property for biological warfare, a better understanding of interactions between Francisella and host cells within the lung is a high priority. Recent studies have confirmed that Francisella invades and grows within human bronchial epithelial cells and A549 cells (16) and stimulates NF-κB-mediated cytokine production by primary alveolar type II cells (11). In addition, mice that are infected intranasally with Francisella develop bacteremia following airway inoculation (4, 10), confirming that the organism crosses the alveolar epithelium and interacts with vascular endothelial cells before it enters the bloodstream. Relatively little is known about interactions between Francisella and pulmonary endothelial cells.
In our in vitro Transwell system, Ft LVS and PMN were added to opposite sides of endothelial monolayers to mimic the relative orientation of organisms and phagocytes at the onset of an aerosolized infectious challenge. Using this experimental model system, we made three novel observations pertinent to pulmonary infection with Francisella. 1) Ft LVS interacted with and invaded PMVEC when presented at the abluminal surface of the endothelium, as would occur after an organism had traversed the alveolar epithelium en route to the bloodstream. To our knowledge, this is the first demonstration that Francisella can enter microvascular endothelial cells, behavior similar to that of another nonmotile organism, S. pneumoniae (21). Ft LVS alone did not elicit secretion of IL-8 or MCP-1 from PMVEC. 2) Ft LVS directly promoted PMN transmigration across endothelial monolayers. Although our data did not elucidate the mechanism(s) responsible for PMN translocation, LPS purified from Francisella did not support PMN transmigration. In contrast to our findings, a previous report found that 4 h of stimulation of the endothelium with Ft LVS failed to stimulate neutrophil transmigration (9). However, the endothelial monolayer and the experimental design employed in the previous study differed those used in the present study. Whereas the previous study utilized human umbilical vein endothelial cells, we have exclusively employed microvascular endothelial cell monolayers in our model. In addition, in the previous study, bacteria and PMN were applied to the same luminal surface, whereas our system distributed the organisms and PMN in an orientation that modeled the interactions in the lung, whereby live bacteria would approach the endothelium at the opposite interface (abluminal) from where PMN would interact (luminal). 3) PMN that successfully traversed the Ft LVS-stimulated microvascular endothelial monolayer exhibited blunted responses to subsequent stimulation, in terms of NADPH oxidase activity and azurophilic granule release.
Several previous studies noted that ingested Francisella disrupt normal PMN oxidative function (17, 23). Furthermore, inhibition of the respiratory burst includes not only the response to ingested Francisella but also the response to heterologous stimuli delivered after ingestion of Francisella (18). However, our findings of altered proinflammatory responses in transmigrated PMN differ from each of those previous reports in which oxidase and degranulation were suppressed: 1) PMN in our studies were not resting before the challenge but had traversed stimulated endothelial monolayers and 2) the Ft LVS were not serum opsonized and were not ingested by PMN. Although the mechanism by which Francisella disabled the normal inflammatory phenotype of the transmigrated PMN is not defined by our studies, the failure of Francisella-conditioned medium and Francisella-endothelial cell-conditioned medium to alter PMN function suggests that 1) the endothelium was a required intermediary in these events and 2) the process of PMN migration was necessary to affect these changes.
As a working hypothesis, we propose that Ft LVS directly alters the phenotype of the endothelial monolayer, which subsequently downregulates the proinflammatory potential of migrating PMN. There are numerous examples in the literature of endothelial modulation of the phenotype of migrating cells. These include modulation of apoptosis of migrating eosinophils (8), alteration of PMN phenotype after reverse endothelial migration (3), changes in T lymphocyte transendothelial migration by PMN elastase deactivation of endothelial factors (24), and inhibition of PMN apoptosis following migration across an endothelial-epithelial bilayer (12). In addition, there is significant evidence that host endothelial cell-mediated signaling can be altered by bacterial (25, 26, 30, 32) and viral (14, 15) pathogens.
We speculate that the capacity of Francisella to modulate endothelial inflammatory signaling serves as a virulence factor in infection, by downregulating the host response to the pathogen in the lung. Consequently, fewer PMN than other lung pathogens are recruited, and the phenotype of the few recruited PMN is compromised. Analogous microbial tactics to alter the host immune response via modulation of inflammatory endothelial cell signaling have also been described (7, 31). Potential neutrophil targets that could alter NADPH oxidase activation and degranulation include effects on class IB phosphoinositide 3-kinase pathway signaling (1).
The role of neutrophils in host defense against primary infection with F. tularensis appears to be related to the route of infection. Granulocyte depletion experiments demonstrated a critical role for PMN in a murine model of intradermal or intravenous infection with Francisella (28). However, animals that were exposed via an aerosol route did not require PMN for protection (6). One possible mechanism to account for this difference would be tissue-specific differences in the vascular endothelium leading to altered PMN-endothelial cell interactions. Although we did not find quantitative differences in PMN transendothelial migration across dermal vs. pulmonary microvascular endothelial monolayers, there is evidence of altered permeability of the microvascular endothelium in the lung, which has a crucial role in barrier function, compared with larger conduit vessels (13). The specific bacterial factors responsible for altering the endothelial monolayer remain to be determined. Francisella, however, has not been reported to secrete any specific toxins that might damage the monolayer (19), and we did not observe evidence of monolayer disruption.
In conclusion, we demonstrate that Ft LVS directly interacted with and invaded intact microvascular endothelial cells without compromising the integrity of the monolayer. This interaction was sufficient to elicit transendothelial migration of PMN in the opposite direction by an unknown mechanism that was independent of endothelial release of IL-8 or MCP-1 and, in fact, occurs against the IL-8 concentration gradient. The migrated PMN were less responsive to subsequent stimulation than either control PMN or PMN migrating in response to another pulmonary pathogen. Future studies should focus on dissecting the alterations in endothelial signaling pathways elicited by Francisella that might affect migrating PMN to more precisely define the role of the vascular endothelium in the pathogenesis of Francisella infection. Such studies may identify elements of the PMN-endothelial cell interaction that are critical for clearance of aerosolized bacterial pathogens in general.
GRANTS
This work was supported by National Institute of Allergy and Infectious Diseases Grants AI-067533 (to J. G. Moreland), AI-34879-19 (to W. M. Nauseef), and P01 AI-04462 (to J. G. Moreland and W. M. Nauseef), funding from the American Lung Association (to J. G. Moreland), and a Merit Review Grant (to W. M. Nauseef).
REFERENCES
- 1.Andrews S, Stephens LR, Hawkins PT. PI3K class IB pathway in neutrophils. Sci STKE 2007: CM3, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Boyum A Isolation of mononuclear cells and granulocytes from human blood. J Clin Lab Invest 21: 77–89, 1968. [PubMed] [Google Scholar]
- 3.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, Nash GB, Rainger GE. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 79: 303–311, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chiavolini D, Alroy J, King CA, Jorth P, Weir S, Madico G, Murphy JR, Wetzler LM. Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia. Infect Immun 76: 486–496, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan JW, Chen W, Shen H, Webb A, KuoLee R. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb Pathog 34: 239–248, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Conlan JW, KuoLee R, Shen H, Webb A. Different host defenses are required to protect mice from primary systemic vs. pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb Pathog 32: 127–134, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Doran KS, Liu GY, Nizet V. Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest 112: 736–744, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farahi N, Cowburn AS, Upton PD, Deighton J, Sobolewski A, Gherardi E, Morrell NW, Chilvers ER. Eotaxin-1/CC chemokine ligand 11: a novel eosinophil survival factor secreted by human pulmonary artery endothelial cells. J Immunol 179: 1264–1273, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Forestal CA, Benach JL, Carbonara C, Italo JK, Lisinski TJ, Furie MB. Francisella tularensis selectively induces proinflammatory changes in endothelial cells. J Immunol 171: 2563–2570, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Forestal CA, Malik M, Catlett SV, Savitt AG, Benach JL, Sellati TJ, Furie MB. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis 196: 134–137, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Gentry M, Taormina J, Pyles RB, Yeager L, Kirtley M, Popov VL, Klimpel G, Eaves-Pyles T. Role of primary human alveolar epithelial cells in host defense against Francisella tularensis infection. Infect Immun 75: 3969–3978, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu M, Lin X, Du Q, Miller EJ, Wang P, Simms HH. Regulation of polymorphonuclear leukocyte apoptosis: role of lung endothelium-epithelium bilayer transmigration. Am J Physiol Lung Cell Mol Physiol 288: L266–L274, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Kelly J, Moore T, Babal P, Diwan A, Stevens T, Thompson W. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol Lung Cell Mol Physiol 274: L810–L819, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan HH, Sharma-Walia N, Streblow DN, Naranatt PP, Chandran B. Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J Virol 80: 1167–1180, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagos D, Vart RJ, Gratrix F, Westrop SJ, Emuss V, Wong PP, Robey R, Imami N, Bower M, Gotch F, Boshoff C. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe 4: 470–483, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemann SR, McLendon MK, Apicella MA, Jones BD. An in vitro model system used to study adherence and invasion of Francisella tularensis live vaccine strain in nonphagocytic cells. Infect Immun 75: 3178–3182, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lofgren S, Tarnvik A, Bloom GD, Sjoberg W. Phagocytosis and killing of Francisella tularensis by human polymorphonuclear leukocytes. Infect Immun 39: 715–720, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol 80: 1224–1230, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLendon MK, Apicella MA, Allen LA. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol 60: 167–185, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreland J, Bailey G, Nauseef WM, Weiss JP. Organism-specific neutrophil-endothelial cell interactions in response to E. coli, S. pneumoniae, and S. aureus. J Immunol 172: 476–432, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Moreland JG, Bailey G. Neutrophil transendothelial migration in vitro to Streptococcus pneumoniae is pneumolysin dependent. Am J Physiol Lung Cell Mol Physiol 290: L833–L840, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. Novel modification of lipid A of Francisella tularensis. Infect Immun 72: 5340–5348, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proctor RA, White JD, Ayala E, Canonico PG. Phagocytosis of Francisella tularensis by rhesus monkey peripheral leukocytes. Infect Immun 11: 146–151, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao RM, Betz TV, Lamont DJ, Kim MB, Shaw SK, Froio RM, Baleux F, Arenzana-Seisdedos F, Alon R, Luscinskas FW. Elastase release by transmigrating neutrophils deactivates endothelial-bound SDF-1α and attenuates subsequent T lymphocyte transendothelial migration. J Exp Med 200: 713–724, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rydkina E, Sahni A, Baggs RB, Silverman DJ, Sahni SK. Infection of human endothelial cells with spotted fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins. Infect Immun 74: 5067–5074, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rydkina E, Silverman DJ, Sahni SK. Similarities and differences in host cell signaling following infection with different Rickettsia species. Ann NY Acad Sci 1063: 203–206, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med 107: 702–714, 1961. [DOI] [PubMed] [Google Scholar]
- 28.Sjostedt A, Conlan JW, North RJ. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun 62: 2779–2783, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sklar LA, McNeil VM, Jesaitis AJ, Painter RG, Cochrane CG. A continuous, spectroscopic analysis of the kinetics of elastase secretion by neutrophils. The dependence of secretion upon receptor occupancy. J Biol Chem 257: 5471–5475, 1982. [PubMed] [Google Scholar]
- 30.Sylte MJ, Kuckleburg CJ, Atapattu D, Leite FP, McClenahan D, Inzana TJ, Czuprynski CJ. Signaling through interleukin-1 type 1 receptor diminishes Haemophilus somnus lipooligosaccharide-mediated apoptosis of endothelial cells. Microb Pathog 39: 121–130, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Tajima A, Iwase T, Shinji H, Seki K, Mizunoe Y. Inhibition of endothelial interleukin-8 production and neutrophil transmigration by Staphylococcus aureus β-hemolysin. Infect Immun 77: 327–334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Sorge NM, Ebrahimi CM, McGillivray SM, Quach D, Sabet M, Guiney DG, Doran KS. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PLoS ONE 3: e2964, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]