Abstract
Cigarette smoking is the major risk factor for developing chronic obstructive pulmonary disease, the fourth leading cause of deaths in the United States. Despite recent advances, the molecular mechanisms involved in the initiation and progression of this disease remain elusive. We used Affymetrix Gene Chip arrays to determine the temporal alterations in global gene expression during the progression of pulmonary emphysema in A/J mice. Chronic cigarette smoke (CS) exposure caused pulmonary emphysema in A/J mice, which was associated with pronounced bronchoalveolar inflammation, enhanced oxidative stress, and increased apoptosis of alveolar septal cells. Microarray analysis revealed the upregulation of 1,190, 715, 260, and 246 genes and the downregulation of 1,840, 730, 442, and 236 genes in the lungs of mice exposed to CS for 5 h, 8 days, and 1.5 and 6 mo, respectively. Most of the genes belong to the functional categories of phase I genes, Nrf2-regulated antioxidant and phase II genes, phase III detoxification genes, and others including immune/inflammatory response genes. Induction of the genes encoding multiple phase I enzymes was markedly higher in the emphysematous lungs, whereas reduced expression of various cytoprotective genes constituting ubiquitin-proteasome complex, cell survival pathways, solute carriers and transporters, transcription factors, and Nrf2-regulated antioxidant and phase II-responsive genes was noted. Our data indicate that the progression of CS-induced emphysema is associated with a steady decline in the expression of various genes involved in multiple pathways in the lungs of A/J mice. Many of the genes discovered in this study could rationally play an important role in the susceptibility to CS-induced emphysema.
Keywords: microarray, cigarette smoke exposure, genomics, lung cancer
chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States and is projected to be the number three cause of deaths globally by 2020 (1, 13). COPD is characterized by limitation of airflow that is not fully reversible, which usually progresses together with an abnormal inflammatory response to noxious particles or gases. Pulmonary emphysema is a major manifestation of COPD. The characteristic features of pulmonary emphysema in most patients with COPD are alveolar destruction and abnormal repair (23). A large number of COPD patients suffer from lung cancer (5). Cigarette smoking is the undisputed risk factor for the development of COPD and lung cancer. Despite the well-documented role of cigarette smoking in the etiopathogenesis of the disease, it is unclear what steps are common or different in the pathogenesis of COPD and lung cancer. It remains unclear why only 10–20% of smokers actually develop COPD (34).
Current hypotheses for the development of emphysema include an imbalance between protease and antiprotease activity, and oxidants/antioxidants that are interlinked to the inflammation in the pathogenesis of COPD (3, 7, 31, 44, 45, 58). Oxidant stress is believed to play an important role in the pathogenesis of cigarette smoke (CS)-induced emphysema by potentiating proteolytic damage, inducing endothelial and epithelial cell death, and inhibiting lung repair mechanisms (39, 58). The combined effect of enhanced proteolytic damage, increased cell death, and decreased lung repair leads to emphysematous lung (50).
Studies in animals have revealed the mechanisms by which cigarette smoking leads to the irreversible destruction of terminal air spaces of the lung. Transgenic and null-mutant mouse studies have identified a number of genes and pathways that when altered result in the morphological changes of emphysema (14, 32). These studies are limited to demonstrating the protective or destructive effects attributed to the abrogation or gross overexpression of a single gene. Many studies have aimed at defining the pathogenesis of emphysema using gene expression profiles of human emphysematous lung tissue (18, 20, 36, 38, 46, 47). However, to our knowledge, no studies exist that utilize temporal global gene expression profile of emphysematous lung tissues of chronic CS-exposed mice, through the progression of emphysema. The aim of this study was to determine the differential expression of genes during the progression of pulmonary emphysema in the A/J mice strain, which develop emphysema after chronic CS exposure.
MATERIALS AND METHODS
Antibodies and reagents.
Antibodies and reagents used were: InnoGenex Iso-IHC DAB kit (InnoGenex, San Ramon, CA); biotinylated anti-mouse IgG and peroxidase-conjugated streptavidin, Vectashield HardSet mounting medium, and Vector RTU HRP-avidin complex (Vector Laboratories, Burlingame, CA); rabbit anti-surfactant protein C (SPC) antibody (Chemicon International, Temecula, CA); anti-caspase-3 polyclonal antibody (Idun Pharmaceuticals, San Diego, CA); anti-rabbit Texas red antibody, streptavidin Texas red-conjugated complex, and DAPI (Molecular Probes, Eugene, OR); biotinylated rabbit anti-mouse secondary antibody (DakoCytomation, Carpinteria, CA); Fluorescein-FragEL DNA Fragmentation Detection Kit (Oncogene Research Products, San Diego, CA); Mouse Genome 430 2.0 GeneChip arrays (Affymetrix, Santa Clara, CA); Wright-Giemsa stain (Diff-Quik; Baxter Scientific Products, McGaw Park, IL); halothane (Halocarbon Laboratories, River Edge, NJ); QuickHyb solution (Stratagene, Carlsbad, CA); normal mouse IgG1 (Sigma-Aldrich, St. Louis, MO); anti-CD34 antibody (Zymed Laboratories, South San Francisco, CA).
Animals.
Male A/J mice (8 wk of age) were purchased from Jackson Laboratories (Bar Harbor, ME) and were housed in the animal facilities at Johns Hopkins University. Mice were fed AIN-76A diet and water ad libitum and housed under controlled conditions (23 ± 2°C; 12/12-h light/dark periods). Experiments on animals were conducted in compliance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions.
Exposure to CS.
A/J mice were divided into five groups: group I mice (n = 80) were kept in a filtered air environment, and group II, group III, group IV, and group V mice were exposed to 1-day, 8-day, 1.5-mo, and 6-mo CS (n = 35 mice/group), respectively, as previously described (39). The CS exposure protocol is described in detail in the materials and methods section of the online supplement.
Bronchoalveolar lavage, phenotyping, and localization of macrophages in lungs.
For bronchoalveolar lavage (BAL) and phenotyping, the mice (n = 7 per group) exposed to acute (1-day) or chronic (6-mo) CS were anesthetized, and differential count was performed as described in the materials and methods section of the online supplement.
The macrophages in the lung tissues (n = 5 mice/group) were stained using Griffonia (Bandeiraea) simplicifolia lectin I isolectin B4 (Vector Laboratories, Burlingame, CA) and quantified by immunohistochemistry using the procedure described in the materials and methods section of the online supplement.
Lung morphometric measurements.
CS-induced alveolar destruction in the lung was measured using computer-assisted topometric measurements (39). For lung morphometric measurements, the mice exposed to 1.5 and 6 mo of CS or filtered room air were anesthetized with halothane, and the lungs were immediately inflated with 0.5% low-melting agarose at a constant pressure of 25 cmH2O as previously described (39). The agarose-inflated lungs were then fixed in 10% buffered formalin and embedded in paraffin. Lung sections (5 μm) were stained with hematoxylin and eosin (H&E). The mean linear intercept (MLI) and the increase in alveolar diameter (AD) were determined by computer-assisted morphometry with Image Pro Plus software (Media Cybernetics, Silver Spring, MD) (39). The lung sections in each group were coded, and representative images (15/lung section) were acquired by an investigator blinded to the identity of the slides, using a Nikon E800 microscope using a ×10 lens.
Immunohistochemical detection of 8-oxo-dG.
The occurrence of oxidative stress in the lung sections of the CS-exposed (6 mo) or age-matched air-exposed A/J mice was assessed by measuring the level of the oxidative stress marker 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) using mouse anti-8-oxo-dG antibody (QED Bioscience, San Diego, CA) (39). The lung sections were then stained with InnoGenex Iso-IHC DAB kit. Normal mouse-IgG1 antibody was used as a negative control. The images of the lung sections were acquired with a Nikon E800 microscope using a ×20 lens. The 8-oxo-dG-positive cells were counted manually.
TUNEL assay.
TdT-mediated dUTP nick end labeling (TUNEL) kit (Oncogene Research Products, San Diego, CA) was used to detect the apoptotic cells in the agarose-inflated lung sections (n = 5/group) of the 6-mo CS-exposed and age-matched air-exposed A/J mice as described in our previous publication (39). The number of apoptotic cells was normalized by the total number of DAPI-positive cells.
Identification of alveolar apoptotic cell populations in the lungs.
The apoptotic type II epithelial cells and endothelial cells in the lungs were identified by incubating the TUNEL-labeled lung sections with anti-mouse SPC antibody and anti-mouse CD34 antibody. These procedures are described in the materials and methods section of the online supplement.
Immunohistochemical localization of active caspase-3 in the lungs.
Active caspase-3 in the lung sections from the 6-mo CS-exposed mice or age-matched air-exposed mice was localized by immunohistochemical staining using anti-active caspase-3 antibody by following our protocol described earlier (39). Images of the lung sections (10 fields/lung section) were captured using a Nikon Eclipse E800 microscope (Nikon) with a ×20 lens, and the number of active caspase-3-positive cells was counted manually.
Oligonucleotide microarray.
Total RNA was extracted from mice lungs using TRIzol reagent (Invitrogen, Carlsbad, CA). The extracted RNA was purified using the RNeasy mini kit (Qiagen, Valencia, CA). The quality of the RNA was assessed using the RNA 6000 nano assay kits (Agilent Technologies, Palo Alto, CA). The isolated RNA was applied to Mouse Genome 430 2.0 GeneChip arrays (Affymetrix, Santa Clara, CA) (n = 3/group) as described previously (39). Scanned output files were analyzed using Affymetrix GeneChip Operating Software (GCOS) version 1.3 and were independently normalized to an average intensity of 500. Analysis and classification of upregulated and downregulated genes in the lungs of A/J mice exposed to air or CS for various time points are explained in the materials and methods section of the online supplement.
Quantitative real-time RT-PCR.
We used quantitative real-time PCR (RT-PCR) to determine the transcriptional induction of antioxidant genes such as heme oxygenase 1 (HO-1), glutathione cysteine ligase catalytic (GCLc) subunit, and glutathione reductase (GSR) in the lungs of air- or CS-exposed mice. The reverse transcription reaction was performed by using the Superscript First-Strand Synthesis System (Invitrogen) as described previously (40). These analyses were performed using assay-on-demand primers and probe sets in the ABI 7000 Taqman system (Applied Biosystems, Foster City, CA). GAPDH was used for normalization, and all PCRs were assayed in triplicate.
Statistical analysis.
Statistical analysis was performed by multiple ANOVA using SigmaStat version 2.03, and differences between groups were determined by Student's t-test using the InStat program.
RESULTS
Histological and lung morphometric studies.
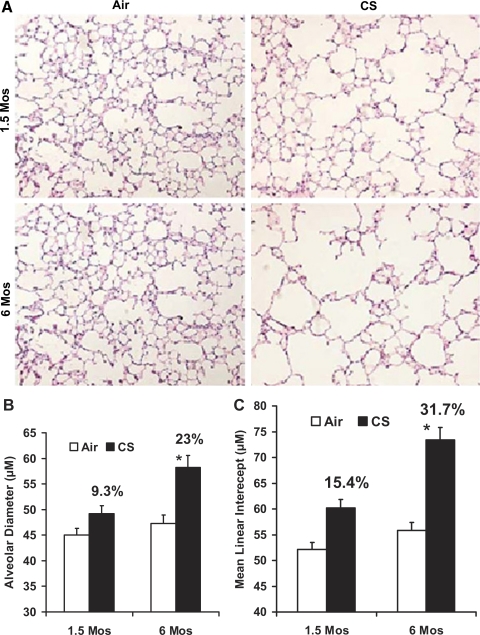
H&E staining of the lung sections from the air-exposed A/J mice showed normal alveolar structure (Fig. 1A). However, histological evaluation revealed enlargement of the air spaces accompanied by the destruction of the normal alveolar architecture in A/J mice after 6 mo of CS exposure (Fig. 1A). Lung morphometric measurements using Image Pro Plus software revealed a significant increase in the AD and MLI in 6-mo CS-exposed mice (AD = 58.2 ± 2.4 μm; MLI = 73.4 ± 3.9 μm) compared with the AD and MLI in the age-matched air-exposed mice (AD = 47.3 ± 1.6 μm; MLI = 55.8 ± 2.3 μm). On the contrary, the lungs from 1.5-mo CS-exposed mice showed minimal alveolar destruction. The AD (49.2 ± 1.6 μm) and MLI (60.2 ± 3.2 μm) in 1.5-mo CS-exposed mice were significantly lower than the AD and MLI in the 6-mo CS-exposed mice (Fig. 1, B and C). Histological examination of the lung sections did not reveal any tumors in the lungs of air or subacute/chronic CS-exposed A/J mice.
Fig. 1.
Increased air space enlargement in A/J mice exposed to chronic cigarette smoke (CS). A: H&E-stained lung sections from A/J mice exposed to room air or CS at the indicated time points. Lung sections from the 6-mo CS-exposed mice show increased alveolar destruction and air space enlargement compared with the lung sections from age-matched air-exposed A/J mice. Sections from the age-matched air-exposed mice show normal alveolar structure (n = 5 mice/group); original magnification, ×20. The images (15 fields/slide) of the H&E-stained lung sections from the air- and CS-exposed mice were acquired with a Nikon E800 microscope, and alveolar diameter (B) and mean linear intercepts (C) were determined by computer-assisted morphometry with the Image Pro Plus software. Six-month CS-exposed A/J mice show a significantly increased alveolar diameter (B) and mean linear intercept (C) compared with 1.5-mo CS-exposed mice. Data are means ± SE. *P ≤ 0.05.
Inflammatory cells in BAL fluid and lungs.
We analyzed the inflammatory cell population in the BAL fluid using Diff-quick reagent and tissue macrophages using lectin staining. The total number of inflammatory cells (predominantly macrophages) in the BAL fluid from 6-mo CS-exposed mice was significantly higher than the acute CS-exposed mice (see Supplemental Fig. 1, A and B, and the results section of the online supplement). Immunohistochemical staining revealed an increased infiltration of macrophages (see Supplemental Fig. 1, C and D, and the results section of the online supplement) in the emphysematous lungs (herein emphysematous lungs refer to the lungs from the mice exposed to 6 mo of CS) of A/J mice.
Increased markers of oxidative stress in the emphysematous lungs.
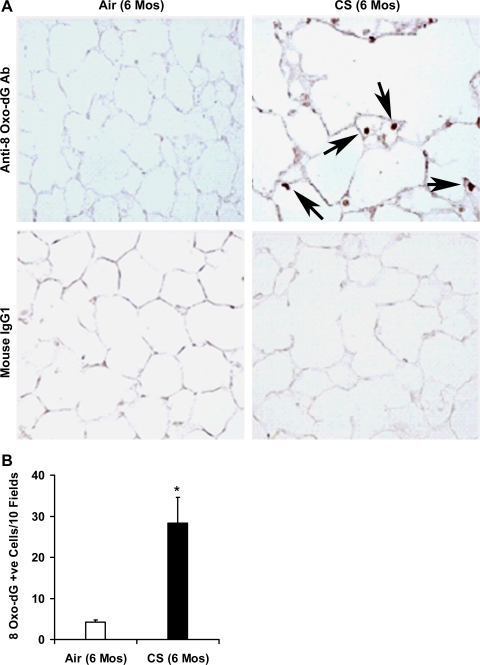
The occurrence of oxidative stress in the lung tissues from 6-mo CS-exposed mice was determined using anti-8-oxo-7,8-dihydro-2′-deoxyguanosine (anti-8-oxo-dG) antibody. Immunohistochemical staining revealed a significantly (P ≤ 0.05) increased number of 8-oxo-dG-positive cells in the lung sections from the mice exposed to 6 mo of CS (28.3 anti-8-oxo-dG-positive cells/10 fields) (Fig. 2, A and B). Lung sections from air-exposed mice showed few 8-oxo-dG-positive cells (4.2 anti-8-oxo-dG-positive cells/10 fields). Immunohistochemical staining with normal mouse IgG antibody did not show any IgG-reactive cells in the lungs of air- or CS-exposed mice. These results indicated that chronic CS-induced alveolar destruction was associated with enhanced oxidative stress in the lungs of the A/J mice.
Fig. 2.
Increased oxidative stress in the lungs of chronic CS-exposed A/J mice. A: the occurrence of oxidative stress in the lungs of 6-mo CS-exposed mice was determined by immunohistochemistry with anti-8-oxo-dG antibody. Lung sections from the CS-exposed A/J mice show increased staining for 8-oxo-dG (indicated by arrows) compared with lung sections from the age-matched air-exposed control mice. Normal mouse IgG1 was used as a negative control (magnification, ×20). B: quantification of 8-oxo-dG-positive cells in lungs after 6 mo of CS exposure. Increased number (28.3 anti-8-oxo-dG-positive cells/10 fields) of 8-oxo-dG-positive cells was detected in the lungs of CS-exposed mice. Few (4.2 anti-8-oxo-dG-positive cells/10 fields) 8-oxo-dG-positive cells were detected in the lung tissues of air-exposed mice (n = 5 mice/group). Values (positive cells/10 fields) are represented as means ± SE, *P ≤ 0.05 vs. air-exposed A/J mice.
Increased apoptosis of lung cells due to chronic CS exposure.
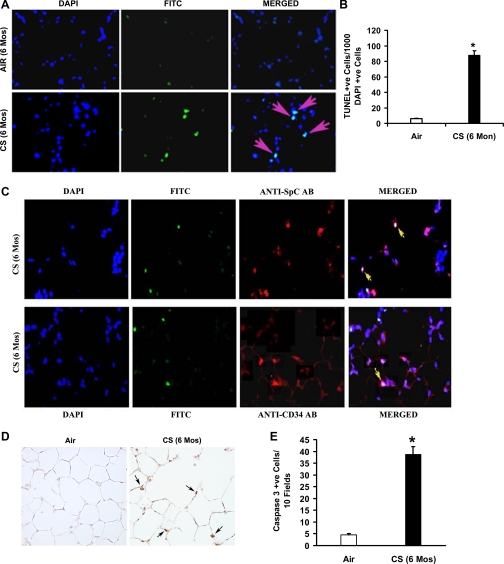
Oxidants are key mediators of apoptosis, and apoptosis is thought to contribute to the development of emphysema in animal models and in humans (39, 50). Therefore, we evaluated chronic CS-exposed (6-mo) mice for DNA strand breaks in situ by fluorescent TUNEL staining (Fig. 3, A and B) and found an increased number of TUNEL-positive cells in the lungs of chronic CS-exposed mice (87.6 ± 6.3 TUNEL-positive cells/1,000 DAPI-positive cells) compared with the lungs of age-matched air-exposed mice (6.2 ± 0.48 TUNEL-positive cells/1,000 DAPI-positive cells). Double staining of the TUNEL-labeled lung sections with anti-SPC (for type II epithelial cells) and anti-CD34 (for endothelial cells) antibodies revealed that apoptosis of these cells occurred in the emphysematous lungs of A/J mice (Fig. 3C).
Fig. 3.
Chronic CS exposure causes alveolar cell apoptosis in A/J mice. A: lung sections (n = 5) from air- or CS-exposed (6-mo) mice were subjected to TUNEL (middle) and DAPI (left) staining. Merged images are shown at right. Overlapping DAPI in blue and FITC in green create a magenta, apoptotic-positive signal. B: CS-exposed mice (6-mo) showed abundant TUNEL-positive alveolar septal cells compared with air-exposed mice (n = 5 mice/group). Values are represented as means ± SE. P ≤ 0.05. C: identification of apoptotic type II epithelial cells (top) and endothelial cells (bottom) in the lungs of CS-exposed mice (6-mo). Type II epithelial cells and endothelial cells were detected using anti-SPC and anti-CD34 antibodies, respectively. Nuclei were detected with DAPI (blue). The merged images with colocalization of cell-specific markers (cytoplasmic red signal) with apoptosis (green FITC + blue DAPI) signal, resulting in lavender-like signal (yellow arrows), are shown. D: active caspase-3 expression in lung sections from chronic CS-exposed (6-mo) mice. CS-exposed A/J mice show increased numbers of caspase-3-positive cells (indicated by arrows) in the lungs (n = 5/group) (magnification, ×20). E: number of caspase-3-positive cells in the lungs of age-matched air- or CS-exposed mice. Caspase-3-positive cells were significantly (*P ≤ 0.05) higher in the lungs of 6-mo CS-exposed mice than the air-exposed mice. Values are represented as means ± SE.
The occurrence of apoptosis in the emphysematous lungs was further demonstrated by staining the lung sections using anti-active caspase-3 antibody (Fig. 3, D and E). Immunohistochemical analysis showed a significantly (P ≤ 0.05) higher number of caspase-3-positive cells in the alveolar septa of 6-mo CS-exposed mice (38.7 ± 3.4 active caspase-3-positive cells/10 field) than in the age-matched air-exposed mice (4.5 ± 0.5 active caspase-3-positive cells/10 fields).
Microarray analysis during the course of development of CS-induced emphysema.
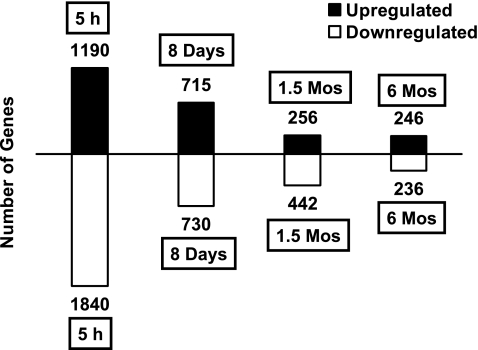
It is clearly evident from Fig. 4 that there is a steady temporal decline in the numbers of genes induced or repressed at each progressive time point (per length of exposure). In addition, the numbers of genes upregulated (see Supplemental Table 1) and downregulated (see Supplemental Table 2) for each individual time point seem to be in the same order of magnitude.
Fig. 4.
Graph depicting the numbers of differentially expressed genes (upregulated or downregulated) at each exposure time point. Solid black bars above the horizontal line represent the number of upregulated genes at each time point. Similarly, white bars below the horizontal line represent the number of downregulated genes at the same time points.
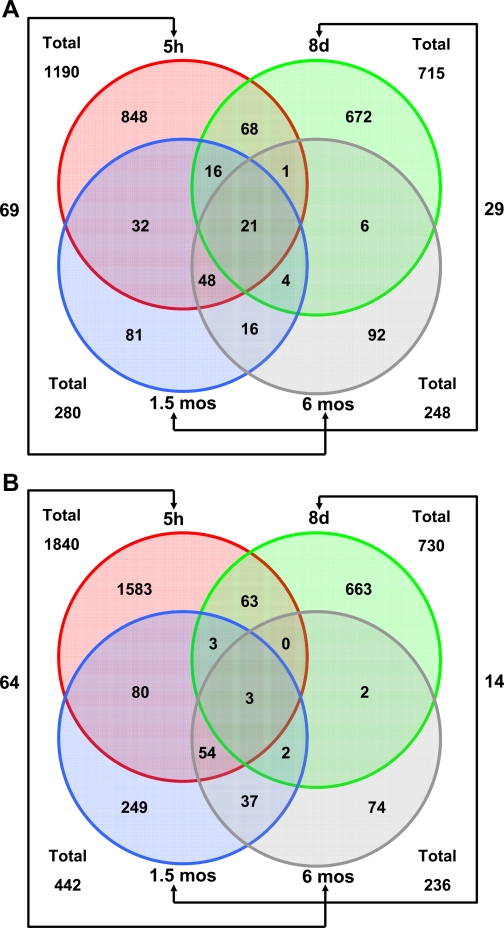
Figure 5A shows the upregulation of 1,190, 715, 260, and 246 genes in the lungs of A/J mice exposed to CS for 5 h, 8 days, 1.5 mo, and 6 mo, respectively. Twenty-one genes were commonly upregulated in the lungs during all time points of CS exposure (see Supplemental Table 3). The commonly upregulated genes include three antioxidant genes (thioredoxin reductase 1, transcobalamin 2, and selenium binding protein 1), various phase I genes [cytochrome P-450 (CYP) 1A1, CYP1B1, carbonyl reductase 3, aldehyde dehydrogenase family 3, subfamily A1, and alcohol dehydrogenase 7], transcription factor forkhead box O3a, ATP-binding cassette (subfamily A), and semaphorin 7A. Figure 5B shows the downregulation of 1,840, 730, 442, and 236 genes in the lungs of A/J mice exposed to CS for 5 h, 8 days, 1.5 mo, and 6 mo, respectively.
Fig. 5.
Venn diagrams depicting the extent of overlap between the gene expression profiles from the lungs of mice exposed to CS for various time periods. Groups were compared, and genes that satisfied the criteria of fold change ≥1.5 and P ≤ 0.05 were designated as significantly changed genes. The number of genes summarized in the Venn plot are as follows: circles shaded red, green, blue, and gray represent the total numbers of genes upregulated (A) or downregulated (B) with CS exposure at 5 h, 8 days, 1.5 mo, and 6 mo, respectively.
Pathway changes in response to cigarette smoke.
We have classified the upregulated (see Supplemental Table 1) and downregulated (see Supplemental Table 2) genes into different categories based on their functions. The total number of upregulated or downregulated genes in each functional category is represented in Supplemental Table 4. Cluster analysis of the expression of phase I genes, antioxidant and phase II detoxification genes, phase III genes, growth factors, genes constituting the ubiquitin-proteasome complex, and genes involved in cell survival, apoptosis, and extracellular matrix maintenance are represented in Fig. 6. Genes constituting phase I detoxification pathways remained upregulated during the course of the study (see Supplemental Table 4). However, by day 8 of CS exposure, the expression of more than 39% of the genes constituting multiple cellular pathways was significantly reduced. After 1.5 and 6 mo of CS exposure, the expression of genes constituting different biological pathways was markedly reduced (see Supplemental Table 4).
Fig. 6.
Pulmonary gene expression profiles of A/J mice exposed to CS. Cluster analysis shows the expression of phase I genes, antioxidant and phase II detoxification genes, phase III genes, growth factors, genes constituting the ubiquitin-proteasome complex, and genes involved in cell survival, apoptosis, and extracellular matrix maintenance. The genes are visualized by Treeview. Green indicates upregulated genes, and black represents no significant change in expression. Gene symbols are provided.
Comparison with previously published studies of genome-wide transcriptional analysis of human COPD (18, 36) and rat (48) showed little overlap for differentially expressed genes (see Supplemental Table 5). Downregulated genes in the lungs of A/J mice exposed to CS whose deletion or overexpression has been shown to contribute to the pathogenesis of spontaneous emphysema in various mice models are listed in Supplemental Table 6.
Validation of antioxidant gene expression by real-time RT-PCR.
We used real-time RT-PCR analysis to confirm the relative expression of three classic antioxidant and phase II detoxification genes such as HO-1, GCLc, and GSR. Real-time RT-PCR results revealed a significantly (P ≤ 0.05) reduced expression of these three antioxidant and phase II detoxification genes in the lungs of the mice exposed to CS for 8 days, 1.5 mo, and 6 mo compared with the lungs of the mice exposed to CS for 1 day (see Supplemental Fig. 2) and were in agreement with the microarray results (see Supplemental Table 1).
DISCUSSION
Due to the poor understanding of pathogenesis of COPD, effective therapies are not available, and there is an urgent need to develop new therapeutic targets for treatment of COPD. The aim of this study was to identify molecular pathways that could be responsible for the damaging consequences of CS exposure in the lungs of A/J mice (susceptible to lung cancer) for various time periods using microarray analysis.
The current hypothesis of emphysema disease pathogenesis suggests that inflammation is a contributing factor for the genesis of pulmonary emphysema, which is associated with a chronic inflammatory response predominantly in small airways and lung parenchyma and is characterized by an increased number of macrophages, neutrophils, and T lymphocytes. Many inflammatory proteases, peptides, chemokines, cytokines, lipid mediators, reactive oxygen and nitrogen species, and growth factors are involved in orchestrating the complex inflammatory process that results in alveolar destruction (44, 58). Analysis of differential cell counts in the BAL fluid revealed a significant increase in the number of total inflammatory cells, and macrophages were predominantly higher in the lungs of 6-mo CS-exposed mice compared with age-matched air-exposed mice. These BAL alterations in response to chronic CS exposure were associated with an increased number of macrophages in the lung tissues.
CS-induced inflammatory response was associated with the expression of more than 56 and 23 inflammatory genes in the lungs of acute and 6-mo CS-exposed mice, respectively. Multiple cytokines/chemokines and their receptors [such as monocyte chemoattractant protein 1 (MCP-1 or CCL2), chemokine (C-C motif) ligand 5 (CCL5), CCL6, chemokine (C-C motif) receptor 2 (CCR2)], growth factors PDGF-D and VEGF, and endothelin were induced by exposure to CS. All these genes have been shown to be involved in the trafficking/accumulation of macrophages. PDGF-D attracts macrophages (51) and so are the chemokines like MCP-1 (12), CCL5 (52), CCL6 (29), and endothelin (2, 4). MCP-1 and CCR2 were involved in the recruitment of macrophages and mast cells into the airway epithelium in COPD (12). Microarray analysis revealed the expression of endothelin in the lungs of day 1 and 1.5-mo CS-exposed mice and expression of CCL6 in the lungs of 6-mo CS-exposed mice (see Supplemental Table 1). Intraperitoneal injection of thioglycollate increased peritoneal CCL6, and neutralization of CCL6 significantly inhibited the macrophage infiltration in a murine model of acute peritonitis (29). Endothelin plays a critical role in the recruitment of monocyte/macrophages (4, 15) and neutrophils (4) in animal models. Both matrix metalloproteinase-12 (MMP-12) and elastin fragments generated by MMP-12 have been shown to contribute to the accumulation of macrophages and the pathogenesis of CS-induced emphysema in mice (21, 25). However, CS did not upregulate or downregulate the expression of MMP-12 in the lungs of A/J mice.
Even though acute CS exposure induced the expression of more than 50 genes concerned with inflammatory processes, surprisingly only 50% of these genes were upregulated in the emphysematous lung tissues of A/J mice. Reduction in the differential expression of these genes could be due to the loss of lung tissues or due to the immunosuppressive effects caused by chronic CS. The following genes were upregulated more than fivefold in response to acute CS: IL-6, IL-1 receptor (type II), double C2 (β), chemokine (C-X-C motif) ligand 5 (CXCL5), suppressor of cytokine signaling 3 (SOCS3), calcitonin/calcitonin-related polypeptide (α), cathelicidin antimicrobial peptide, pentaxin-related gene, prostaglandin-endoperoxide synthase 2, and nuclear factor, IL-3, regulated gene. CXCL5 was the predominantly expressed inflammatory gene in both acute and 6-mo CS-exposed lungs. Other inflammatory genes expressed in the emphysematous lung tissues include pre-B cell colony-enhancing factor 1 (PBEF), inhibitor of κB kinase γ, CXCL 5, the RANTES receptor [chemokine (C-C motif) receptor 1], ankyrin repeat and SOCs box-containing protein 5, SOCS3, CD163 antigen, and CD14 antigen. PBEF is a novel candidate gene induced in mechanical stress-induced acute lung injury (57). The inhibitor of κB kinase γ is the regulatory subunit of IκB kinase (IKK) complex, which serves as the master regulator for the activation of NF-κB by various stimuli (43). SOCS3 is an important negative regulator of IL-6. Mice in which the SOCS3 gene was deleted in all hematopoietic cells developed neutrophilia and a variety of inflammatory pathologies (59). CD163 is a member of the macrophage scavenger receptors and is expressed on most subpopulations of mature tissue macrophages. CD14 is a well-characterized pattern recognition receptor that binds to LPS and other bacterial-derived components and plays a significant role in the development of Th1 response (60). CXCL5 is an epithelial cell-derived neutrophil-activating peptide (ENA-78) and has been shown to activate neutrophils and possesses angiogenic properties (28, 49). CS exposure significantly inhibited the expression of various genes involved in humoral immune responses, different B cell, T cell, and dendritic cell receptors/cell surface antigens, and various inflammatory cytokines and chemokines. CS exposure also downregulated more than 20 interferon-regulated genes, Toll-like receptor (Tlr)1, Tlr3, and Tlr4, which play a key role in the innate immune system (see GEO acc. no. GSE8790). Mice deficient in Tlr4 develop spontaneous emphysema (63).
Emphysema associated with the adult lung is thought to result from the progressive proteolytic destruction of extracellular matrix without adequate repair, occurring through an imbalance in proteinase-antiproteinase activity (23, 24). Studies using knockout and transgenic animal models have revealed contribution of neutrophil elastase, macrophage metalloproteinases, and cysteine proteinases in the development of CS-induced emphysema (23, 45, 58). Acute CS exposure induced the expression of 30 genes involved in extracellular matrix homeostasis. The genes that were upregulated both in the acute and chronic CS-exposed lung tissues included a disintegrin and metalloprotease domain 8, ADAM metallopeptidase with thrombospondin type 1 motif, 15 (Adamts15), elastin, and serine proteinase inhibitor, clade A, lysyl oxidase, and naked cuticle 1 homolog. Cathepsin K and fibrinogen γ polypeptide were uniquely expressed in the emphysematous lung tissues. Cathepsin K is the most potent mammalian elastase yet described (9, 17). Cathepsin K also possesses a unique collagenolytic activity and has been shown to cleave both type I and type II collagen (9, 17). A recent study (6) using cathepsin K knockout mice demonstrated the pivotal role of cathepsin K in collagen metabolism and lung matrix homeostasis. Chronic CS exposure also resulted in the downregulation of 51 genes involved in the maintenance of the lung extracellular matrix. The genes downregulated in the emphysematous lungs include various types of collagen genes; elastin microfibril interface 1; transforming growth factor-β2; latent transforming growth factor-β binding protein 4; matrix metalloproteinases 11 (Mmp11) and Mmp15; serine (or cysteine) proteinase inhibitor, clade H, member 1; a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 1 (Adamts1); and lysyl oxidase-like 1 gene (see GEO acc. no. GSE8790). Mice lacking the protein lysyl oxidase-like 1 do not deposit normal elastic fibers and develop air space enlargement in the lungs (30).
Oxidative stress plays an important role in the pathogenesis of pulmonary emphysema. Like protease/antiprotease and inflammation, oxidant/antioxidant imbalance is central to the process of tissue destruction and apoptosis in CS-induced emphysema (39, 58). There is overwhelming evidence of the presence of markers of oxidative stress in a smoker's lung, which is caused by chronic inflammation and oxidants in the smoke (31). Oxidative stress enhances inflammation, inactivates critical antiproteinase inhibitors such as AAT, and enhances apoptosis of alveolar cells (58). The transcription factor Nrf2 plays a critical role in protecting the lungs against CS-induced inflammation and oxidative stress and alveolar cell apoptosis by upregulating multiple antioxidant phase II and phase III detoxification genes. The importance of antioxidants in emphysema susceptibility was supported by our recent work using the mice deficient in transcription factor Nrf2 (39). We observed an increased expression of 8-oxo-dG, the marker of oxidative stress, in the emphysematous lung tissues of chronic CS-exposed mice. The increased oxidative stress in the emphysematous lung tissues of susceptible A/J mice was associated with reduced differential expression of various Nrf2-regulated pulmonary antioxidant genes, phase II and phase III detoxification genes, as well as various Nrf2-binding partners. In response to acute CS exposure, 27 antioxidant and phase II detoxification genes were upregulated in the lung tissues of A/J mice, and the majority of these genes were regulated by Nrf2 (see Supplemental Table 1). Of these 27 genes, 11 genes were upregulated only in the 8-day CS-exposed lungs, and there was a significantly reduced expression of 6 Nrf2-regulated genes [heme oxygenase 1, glutathione S-transferase, α2 (GSTα2), metallothionein 1, metallothionein 2, thioredoxin reductase 1, and glutathione reductase 1] in the emphysematous lung tissues compared with the acute CS-exposed lungs. This reduced expression was consistent with the low-level induction of the v-maf musculoaponeurotic fibrosarcoma oncogene family, protein F (MafF), the positive regulator of Nrf2, as well as reduced differential expression of Nrf2-phosphorylating casein kinases and other regulators of Nrf2 such as MafG, MafK, CCAAT/enhancer binding protein (C/EBPβ), C/EBPδ, and activating transcription factor IV. Among the phase II detoxification genes, NAD(P)H dehydrogenase quinone 1 (Nqo1) was the only gene with significantly higher expression in the emphysematous lung tissues compared with the acute CS-exposed lungs. The genes upregulated only in the emphysematous lung tissues were ferritin light chain 2, ceruloplasmin 1, uridine 5′-diphospho (UDP)-glucuronosyltransferase 1 family, member 2, and 4 glutathione S-transferases (GSTp2, GSTμ1, GSTα3, and microsomal GSTα2).
Similar to antioxidant and phase II genes, acute CS induced the expression of more than 13 phase I detoxification genes in the lungs. The oxidative metabolism process in the phase I system is mainly mediated by the CYP family or flavin function oxidases. Among the phase I genes, CYP1A1, CYP1B1, and aldehyde dehydrogenase family 3 were highly expressed in the lungs of acute and 6-mo CS-exposed mice. In contrast to classic Nrf2-regulated antioxidant and phase II detoxification genes, the expression of phase I genes such as CYP1A1, CYP1B1, carboxyl esterase 1, and alcohol dehydrogenase 7, was significantly increased in the emphysematous lungs. Numerous carcinogens such as polycyclic aromatic hydrocarbons and nitrosamines are metabolized by CYP enzymes. Among all upregulated genes, CYP1A1 is the predominant gene with more than 100-fold expression in the emphysematous lungs. However, microarray analysis revealed inhibition of aryl hydrocarbon receptor, reduced expression of aryl hydrocarbon receptor nuclear translocator, and higher expression (30-fold) of aryl hydrocarbon receptor repressor in the emphysematous lung tissues. These results suggest the possible role of other transcription factor(s) in the transcriptional induction of CYP1A1 and 1B1 genes and warrant further investigation.
In response to acute CS exposure, 40 genes constituting solute carrier and channel proteins and phase III multidrug transporters were upregulated in the lungs of A/J mice compared with the upregulation of only 7 such genes in the emphysematous lungs. Acute CS exposure also downregulated 9 antioxidant and phase II detoxification genes, 27 phase I genes, and 98 solute carrier and phase III genes. Several ABC transporter proteins are believed to play an important role in preventing the accumulation of potentially harmful xenobiotics in the lung (42). Even though potassium channel openers (37) and inhibitors of calcium-activated chloride channels (41) are being developed for the treatment of COPD, very little is known about the role of solute carrier and channel proteins in the pathogenesis of CS-induced emphysema.
Pathogenesis of lung diseases such as COPD and lung cancer is tightly linked to environmental chemicals, most notably tobacco smoke. Many of the compounds associated with these diseases require phase I enzymatic activation to exert their deleterious effects on pulmonary cells. These activated hydrophobic xenobiotics are converted into hydrophilic forms via conjugation reactions catalyzed by phase II enzymes. The metabolites generated by phase I and phase II reactions are excreted from the body with the aid of phase III detoxification systems. The results of the present study revealed that the pathogenesis of pulmonary emphysema in A/J mice is clearly associated with increased expression of major phase I genes and reduced expression of various phase III and Nrf2-regulated antioxidant and phase II detoxification genes in the emphysematous lung tissues. Our findings are similar to the findings in the recent study in humans (38), which revealed the upregulation of CYP1A1 and CYP1B1 genes in both comparisons (COPD vs. heavy smokers and heavy smokers vs. nonsmokers). The expression of a number of genes involved in oxidant stress responses were increased in heavy smokers compared with nonsmokers. This expression of oxidant response genes further increased in individuals with chronic productive cough, but no significant airflow limitation (i.e., COPD stage 0) with even a further increase in stage 1 COPD was noted. In more severe COPD, there was a fall in mean expression levels noted in stage 0.
Apoptosis plays an important role in the pathophysiology of lung diseases (22, 58). Gene expression profiling revealed the expression of various genes constituting both the death receptor-induced extrinsic apoptotic pathway and the mitochondrial-regulated intrinsic apoptotic pathway in the lungs after CS exposure. CS induced the expression of 13 and 7 genes in the lungs of acute and chronic CS-exposed mice, respectively. Death-associated protein kinase 1 and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly(ADP-ribose) polymerase were expressed in the lungs of the mice exposed to CS for 1 day, 1.5 mo, and 6 mo. Death-associated protein kinase 1 is a calcium/calmodulin-dependent serine/threonine kinase and functions as a positive mediator of apoptosis induced by a variety of stimuli, such as Fas, TNFα, TGF-β, ceramide, and cellular myelocytomatosis viral oncogene (c-myc). The apoptotic genes uniquely upregulated in the emphysematous lungs were Bcl-2/adenovirus E1B 19 kDa-interacting protein 1, NIP3 (BNIP3), p53 apoptosis effector related to PMP-22 (PERP)-TP53 apoptosis effector, cytochrome c oxidase, subunit VIIIb, cell death-inducing DNA fragmentation factor, α-subunit-like effector A (CIDE-A), and cell death-inducing DFFA-like effector c. BNIP3, a proapoptotic protein in the Bcl-2 family, is a central regulator of mitochondrial membrane permeability (61). PERP is an apoptosis-associated target of the p53 tumor suppressor gene and is involved in DNA damage-induced apoptosis (26). CIDE-A is a 40-kDa caspase-3-activated nuclease that is associated with the regulation of the apoptosis/DNA fragmentation pathway (27). Forkhead box O3 is implicated in the regulation of a variety of cellular processes, including cell cycle, p53-dependent apoptosis, DNA repair, and stress resistance (16). Chronic exposure to CS resulted in increased apoptosis of alveolar septal cells in the lungs of A/J mice. Staining of the TUNEL-labeled lung sections with anti-SPC and anti-CD34 antibodies revealed the apoptosis of type II epithelial and endothelial cells, respectively, in the emphysematous lungs. Alveolar cell apoptosis has been recognized as a critically important mechanism of alveolar septal destruction in emphysema. The presence of enhanced apoptosis in the lungs of chronic CS-exposed mice might be related to enhanced oxidative stress, inflammation, or excessive lung proteolysis (58). Apoptosis in the emphysematous lung tissues was consistent with reduced differential expression of various genes constituting the cell survival pathways. Microarray analysis revealed the upregulation of 43 and 5 cell survival genes in the lungs from acute and 6-mo CS-exposed mice, respectively. Most of the genes upregulated in the acute CS-exposed lungs constitute the cell survival pathways mediated by phosphoinositide 3 kinase (PI3K)/Akt (protein kinase B). Endothelial cell survival by Akt is driven by the modulation of a series of intrinsic cellular pathways that include protein tyrosine kinase and the G protein-coupled receptor, PI3K, the receptors for VEGF such as FMS-like tyrosine kinase 1 (Flt 1) and FMS-like tyrosine kinase 4 (Flt 4), Ras/mitogen-activated protein kinases, endothelial nitric oxide synthase 3, and Bcl-2. Several growth factors, such as epidermal growth factor and basic fibroblast growth factor, which were induced in the acute CS-exposed lungs, are known to provide endothelial cell survival by inhibiting endothelial cell apoptosis. These growth factors inhibit endothelial cell apoptosis by upregulating the expression of the antiapoptotic proteins Bcl-2 and endothelial nitric oxide synthase by activating PI3/Akt pathways (10). Furthermore, the interaction of cells with the extracellular matrix via integrin β1 also provides a potent survival signal (35). Association of the α1β1-integrin with the adaptor protein Shc can regulate cell survival and cell cycle progression via the Ras/MAPK/extracellular signal-regulated kinase pathway (10). The cell survival genes upregulated in the emphysematous lung tissues were fibroblast growth factor binding protein 1, fibroblast growth factor 13, angiopoietin-like 4, mitogen-activated protein kinase kinase kinase 6, and forkhead box O3a.
Strain A/J mice have a relatively high spontaneous adenoma/adenocarcinoma incidence, and following exposure to a carcinogen, readily develop lung tumors (33). Even though CS induced the expression of more than 54 cancer-associated genes in the acute CS-exposed lungs and 5 oncogenes in the emphysematous lungs of A/J mice (see Supplemental Table 1), histological examination did not reveal any tumors in the lungs of CS/age-matched air-exposed control mice. Recent studies (53, 56) indicate that ETS is a lung carcinogen in A/J mice. However, following ETS exposure, a recovery period of air is necessary to fully reveal the tumorigenic action of cigarette smoke in A/J mice (56).
The net result of alveolar cell death on alveolar structure depends on the lung's ability to undergo cell proliferation, which is pivotal for the maintenance of normal tissue homeostasis. The data on the balance of apoptosis vs. cell proliferation have been discrepant (58). We observed the expression of 52 genes involved in cell adhesion and cell cycle/proliferation in acute CS-exposed lungs compared with the expression of 7 genes in the emphysematous lungs. The cell cycle regulator cyclin-dependent kinase inhibitor 1A (p21) gene is predominantly expressed in both acute (∼46-fold) and 6-mo (∼9-fold) CS-exposed lungs of A/J mice. Progression through the cell cycle is regulated by cyclins and cyclin-dependent kinases. The cyclin kinase inhibitor p21 can induce G1 arrest and senescence in a variety of cell types and has been shown to augment TGF-β1-induced alveolar destruction (54) and protect against fMLP-mediated air space enlargement (55). Microarray analysis also showed reduced differential expression of various growth factors, ubiquitin-proteasome complex, heat shock proteins (see discussion in online supplement), transcription factors and multiple genes involved in signal transduction, cytoskeleton reorganization, and lipid metabolism in the emphysematous lungs compared with the lungs of the acute CS-exposed mice (see Supplemental Table 1).
We compared the differentially expressed genes in the emphysematous lungs of AJ mice with the gene expression profile of lung tissues from human COPD (18, 36) and pulmonary gene expression profile of chronic CS-exposed rats (48). In a report using serial analysis of gene expression (SAGE) and microarray analysis, Ning et al. (36) have identified several genes, and many of them have not been previously associated with COPD. The 11 commonly expressed genes reported by Ning et al. and the present study were as follows: cyclin-dependent kinase inhibitor 1A (P21), fibrinogen, γ-polypeptide, heme oxygenase (decycling) 1, lectin, galactose binding, soluble 3, phosphogluconate dehydrogenase, protein tyrosine phosphatase 4a1, receptor (calcitonin) activity modifying protein 2, receptor (calcitonin) activity modifying protein 3, secretoglobin, family 3A, member 1, selenium binding protein 1, and solute carrier family 40 (iron-regulated transporter), member 1. Early growth response 1 (Egr-1) gene has been shown to be expressed in lung tissues from patients with late-stage emphysema (62). Egr-1 plays an important role in the regulation of matrix metalloproteinase-9 (MMP-9) (36) and CS-induced autophagy (11). Acute CS exposure significantly induced the expression of Egr-1 in the lungs of A/J mice. However, Egr-1 was not induced in the lungs from the mice exposed to subacute and chronic CS exposure. There was no commonality in the genes (upregulated) reported in this study and reported by Golpon et al. (18). Comparison of microarray data identified 11 genes commonly expressed in the emphysematous lungs of A/J mice and in the lungs of chronic CS-exposed rats (48). The commonly expressed genes were aldehyde dehydrogenase family 3 (subfamily A1), CYP1B1, GCLc, GCLm, glutamate-ammonia ligase, hexokinase 2, interleukin 1 receptor (type II), metallothionein 1, NAD(P)H dehydrogenase, quinone 1, and thioredoxin reductase 1 components of the cigarette smoke. These results clearly suggest the need for caution when extrapolating data on emphysema development in one species of rodents to another and also for its implications in humans.
Mice strains differ in their susceptibility to CS-induced emphysema. The role of strain difference in the response to cigarette smoke was investigated in various mice models (8, 19). Mice of the strains C57BL/6J, SJ/L, and A/J were moderately susceptible to CS-mediated emphysema, and AKR/J mice were supersusceptible to pulmonary emphysema. However, NZWLac/J and ICR did not develop emphysema after chronic CS exposure. The factor(s) that determines the resistance to CS-emphysema was largely not known. In our previous study (39) using ICR mice deficient in the transcription factor Nrf2, we showed the importance of intrinsic antioxidant defenses in protecting the lungs against CS-induced emphysema. Disruption of the Nrf2 gene in the emphysema-resistant ICR mice strain led to earlier-onset and more extensive CS-induced emphysema after chronic CS exposure. Microarray analysis revealed the expression of as many as 120 genes, and most of them are antioxidant and phase II detoxification genes in the lungs of ICR mice after acute CS exposure. In contrast, acute CS induced the expression of more than 1,190 genes in the lungs of emphysema-susceptible A/J mice strain. These results clearly suggest the marked difference in the gene expression pattern in the lungs of emphysema-resistant and emphysema-susceptible mice strains. Similar to the upregulated genes, microarray analysis revealed a steady decline in the number of downregulated genes during the progression of CS-induced pulmonary emphysema. We have classified the downregulated genes into different functional categories. Some of the downregulated genes whose deletion or overexpression has been shown to cause spontaneous/age-dependant emphysema are listed in Supplemental Table 6. Future studies are needed to delineate the molecular pathways that are altered in different emphysema-resistant and -susceptible mice strains using microarray and proteomic technologies.
Animal models play an important role in the understanding of the pathogenesis COPD. However, both transgenic and gene-targeted models suffer limitations, and their applicability to COPD in humans may depend on several factors, including the disease model and similarities in mouse structure and function between species. Mouse and human clearly share many basic physiological processes, but the details of how gas exchange is achieved will determine how closely findings in mice can be applied to humans (2). Hence, careful translation of the findings from studies in mouse to human is required.
Conclusions.
In conclusion, CS exposure significantly influenced the expression of numerous genes in the lungs of A/J mice. Importantly, the progression of pulmonary emphysema is associated with a steady decline in the differential of expression of various genes constituting multiple pathways, speculatively participating in the pathogenesis of COPD. Acute CS exposure induced the expression of more than 1,000 genes in the lungs. A decline in the differential expression of 40% of genes in the lungs after subacute CS exposure and more than 80% of genes in the lungs after subchronic and chronic CS exposure was noted. The decline in the expression of genes during subacute to chronic CS exposure is consistent with the reduction in the differential expression of more than 75–90% transcription factors in lungs. Further investigation is needed to delineate the role of other factors contributing to the reduced differential expression of genes due to exposure to CS.
The expression of genes constituting cell survival pathways, ubiquitin-proteasome complex, and various heat shock proteins was significantly affected as early as during subacute CS exposure. However, the expression of the majority of growth factors, solute carrier, and phase III genes, inflammatory genes; genes involved in cell adhesion/cell cycle/cell proliferation; and genes constituting signal transduction pathways were affected during subchronic and chronic CS exposure. In contrast, CS did not significantly affect the expression of multiple genes constituting phase I detoxification pathway, and Cyp1A1 was predominantly expressed in the emphysematous lungs of A/J mice. The strength of expression of multiple antioxidant and phase II detoxification genes in the emphysematous lungs was reduced after chronic CS exposure, which is consistent with the enhanced expression of marker of oxidative stress, increased infiltration of inflammatory cells, and apoptosis of alveolar septal cells. Exposure of the mice to CS also resulted in the downregulation of multiple key genes such as lysyl oxidase-like 1, macrophage/colony-stimulating factor, platelet-derived growth factor-α, latent transforming growth factor-β binding protein 4, integrin β6, Toll-like receptor 4, interleukin 1β, elastin, and fibrillin 1 in the lungs. Deletion or overexpression of these downregulated genes has been shown to contribute to the pathogenesis of pulmonary emphysema in various mice models. Our data indicate that the pathogenesis of emphysema in susceptible mice strain is unlikely entirely caused by inflammation or deletion of a single gene. Instead, reduction in the differential expression of various genes constituting cell survival pathways, ubiquitin-proteasome complex, heat shock proteins, and multiple transcription factors during subacute CS exposure along with alterations in the expression of various genes involved in cell adhesion/cell cycle/cell proliferation, cytoskeletal reorganization, extracellular matrix production, solute carrier, and phase III genes, and phase II detoxification and antioxidant genes during subchronic and chronic CS exposure are likely to be involved in the pathogenesis of pulmonary emphysema. Results of the present study not only support the possible involvement of some of the previously reported genes and pathways in the pathogenesis of the disease, but also suggest the alterations of some novel potential pathways during the progression of CS-mediated pulmonary emphysema.
GRANTS
This work was supported by National Institutes of Health Grants HL-081205 (S. Biswal), COPD SCCOR P50-HL-084945 (S. Biswal and R. M. Tuder), Children Asthma Center P50-ES-015903 (S. Biswal), HL-66554, P30-ES-03819, AG-21057 (C. G. Tankersley), and HL-010342 (C. G. Tankersley), a grant from Maryland Cigarette Restitution Fund and Flight Attendant Medical Research Institute (S. Biswal), and Alpha 1 Foundation research grant (R. M. Tuder).
Supplementary Material
Acknowledgments
Present address of R. M. Tuder: Program in Translational Lung Research, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Colorado, Denver, CO 80262.
REFERENCES
- 1.Anderson RN, Smith BL. Deaths: leading causes for 2001. Natl Vital Stat Rep 52: 1–85, 2003. [PubMed] [Google Scholar]
- 2.Babaei S, Picard P, Ravandi A, Monge JC, Lee TC, Cernacek P, Stewart DJ. Blockade of endothelin receptors markedly reduces atherosclerosis in LDL receptor deficient mice: role of endothelin in macrophage foam cell formation. Cardiovasc Res 48: 158–167, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 22: 672–688, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bhavsar TM, Liu X, Cerreta JM, Liu M, Cantor JO. Endothelin-1 potentiates smoke-induced acute lung inflammation. Exp Lung Res 34: 707–716, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Brody JS, Spira A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc Am Thorac Soc 3: 535–537, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Buhling F, Rocken C, Brasch F, Hartig R, Yasuda Y, Saftig P, Bromme D, Welte T. Pivotal role of cathepsin K in lung fibrosis. Am J Pathol 164: 2203–2216, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantin A, Crystal RG. Oxidants, antioxidants and the pathogenesis of emphysema. Eur J Respir Dis Suppl 139: 7–17, 1985. [PubMed] [Google Scholar]
- 8.Cavarra E, Bartalesi B, Lucattelli M, Fineschi S, Lunghi B, Gambelli F, Ortiz LA, Martorana PA, Lungarella G. Effects of cigarette smoke in mice with different levels of alpha(1)-proteinase inhibitor and sensitivity to oxidants. Am J Respir Crit Care Med 164: 886–890, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol 59: 63–88, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol 22: 887–893, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, Nakahira K, Pilewski JM, Lee JS, Zhang Y, Ryter SW, Choi AM. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS ONE 3: e3316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Boer WI, Sont JK, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Monocyte chemoattractant protein 1, interleukin 8, chronic airway inflammation in COPD. J Pathol 190: 619–626, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet 362: 847–852, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Fehrenbach H Animal models of chronic obstructive pulmonary disease: some critical remarks. Pathobiology 70: 277–283, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Forbes JM, Leaker B, Hewitson TD, Becker GJ, Jones CL. Macrophage and myofibroblast involvement in ischemic acute renal failure is attenuated by endothelin receptor antagonists. Kidney Int 55: 198–208, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal 7: 752–760, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaisse JM. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 273: 32347–32352, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Golpon HA, Coldren CD, Zamora MR, Cosgrove GP, Moore MD, Tuder RM, Geraci MW, Voelkel NF. Emphysema lung tissue gene expression profiling. Am J Respir Cell Mol Biol 31: 595–600, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hackett NR, Heguy A, Harvey BG, O'Connor TP, Luettich K, Flieder DB, Kaplan R, Crystal RG. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol 29: 331–343, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277: 2002–2004, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc 3: 713–717, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hogg JC, Senior RM. Chronic obstructive pulmonary disease - part 2: pathology and biochemistry of emphysema. Thorax 57: 830–834, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 116: 753–759, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, Mills AA, Attardi LD. Perp is a p63-regulated gene essential for epithelial integrity. Cell 120: 843–856, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Inohara N, Koseki T, Chen S, Benedict MA, Nunez G. Identification of regulatory and catalytic domains in the apoptosis nuclease DFF40/CAD. J Biol Chem 274: 270–274, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Koch AE, Volin MV, Woods JM, Kunkel SL, Connors MA, Harlow LA, Woodruff DC, Burdick MD, Strieter RM. Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil activating peptide 78 in the rheumatoid joint. Arthritis Rheum 44: 31–40, 2001. [DOI] [PubMed] [Google Scholar]
- 29.LaFleur AM, Lukacs NW, Kunkel SL, Matsukawa A. Role of CC chemokine CCL6/C10 as a monocyte chemoattractant in a murine acute peritonitis. Mediators Inflamm 13: 349–355, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36: 178–182, 2004. [DOI] [PubMed] [Google Scholar]
- 31.MacNee W, Selby C. New perspectives on basic mechanisms in lung disease. 2. Neutrophil traffic in the lungs: role of haemodynamics, cell adhesion, and deformability. Thorax 48: 79–88, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahadeva R, Shapiro SD. Chronic obstructive pulmonary disease * 3: experimental animal models of pulmonary emphysema. Thorax 57: 908–914, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malkinson AM Molecular comparison of human and mouse pulmonary adenocarcinomas. Exp Lung Res 24: 541–555, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Mannino DM Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care 48: 1185–1191; discussion 1191–1193, 2003. [PubMed] [Google Scholar]
- 35.Meredith JE, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell 4: 953–961, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, Pinsky DJ, Watkins SC, Pilewski JM, Sciurba FC, Peters DG, Hogg JC, Choi AM. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 101: 14895–14900, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelaia G, Gallelli L, Vatrella A, Grembiale RD, Maselli R, De Sarro GB, Marsico SA. Potential role of potassium channel openers in the treatment of asthma and chronic obstructive pulmonary disease. Life Sci 70: 977–990, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Pierrou S, Broberg P, O'Donnell RA, Pawlowski K, Virtala R, Lindqvist E, Richter A, Wilson SJ, Angco G, Moller S, Bergstrand H, Koopmann W, Wieslander E, Stromstedt PE, Holgate ST, Davies DE, Lund J, Djukanovic R. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175: 577–586, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202: 47–59, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med 38: 116–125, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Scheffer GL, Pijnenborg AC, Smit EF, Muller M, Postma DS, Timens W, van der Valk P, de Vries EG, Scheper RJ. Multidrug resistance related molecules in human and murine lung. J Clin Pathol 55: 332–339, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shambharkar PB, Blonska M, Pappu BP, Li H, You Y, Sakurai H, Darnay BG, Hara H, Penninger J, Lin X. Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways. EMBO J 26: 1794–1805, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro SD The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: S29–S32, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro SD Proteinases in chronic obstructive pulmonary disease. Biochem Soc Trans 30: 98–102, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Spira A, Beane J, Pinto-Plata V, Kadar A, Liu G, Shah V, Celli B, Brody JS. Gene expression profiling of human lung tissue from smokers with severe emphysema. Am J Respir Cell Mol Biol 31: 601–610, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA 101: 10143–10148, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevenson CS, Docx C, Webster R, Battram C, Hynx D, Giddings J, Cooper PR, Chakravarty P, Rahman I, Marwick JA, Kirkham PA, Charman C, Richardson DL, Nirmala NR, Whittaker P, Butler K. Comprehensive gene expression profiling of rat lung reveals distinct acute and chronic responses to cigarette smoke inhalation. Am J Physiol Lung Cell Mol Physiol 293: L1183–L1193, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 270: 27348–27357, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 28: 551–554, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Uutela M, Wirzenius M, Paavonen K, Rajantie I, He Y, Karpanen T, Lohela M, Wiig H, Salven P, Pajusola K, Eriksson U, Alitalo K. PDGF-D induces macrophage recruitment, increased interstitial pressure and blood vessel maturation during angio-genesis. Blood 104: 3198–3204, 2004. [DOI] [PubMed] [Google Scholar]
- 52.William GG, Michael TL, William AK, Thomas EL. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology 288: 8–17, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE. The carcinogenicity of environmental tobacco smoke. Carcinogenesis 18: 575–586, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki M, Kang HR, Homer RJ, Chapoval SP, Cho SJ, Lee BJ, Elias JA, Lee CG. P21 regulates TGF-beta1-induced pulmonary responses via a TNF-alpha-signaling pathway. Am J Respir Cell Mol Biol 38: 346–353, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, O'Reilly MA, Rahman I. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am J Respir Cell Mol Biol 39: 7–18, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao R, Wang Y, D'Agostini F, Izzotti A, Lubet RA, You M, De Flora S. K-ras mutations in lung tumors from p53 mutant mice exposed to cigarette smoke. Exp Lung Res 31: 271–281, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, Easley RB, McVerry BJ, Tuder RM, Standiford T, Brower RG, Barnes KC, Garcia JG. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med 171: 361–370, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 87: 1047–1082, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res Ther 7: 100–110, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zdolsek HA, Jenmalm MC. Reduced levels of soluble CD14 in atopic children. Clin Exp Allergy 34: 532–539, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Zhang HM, Cheung P, Yanagawa B, McManus BM, Yang DC. BNips: a group of pro-apoptotic proteins in the Bcl-2 family. Apoptosis 8: 229–236, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Zhang W, Yan SD, Zhu A, Zou YS, Williams M, Godman GC, Thomashow BM, Ginsburg ME, Stern DM, Yan SF. Expression of Egr-1 in late stage emphysema. Am J Pathol 157: 1311–1320, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest 116: 3050–3059, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.