Abstract
Infections produce severe respiratory muscle dysfunction. It is known that the proteasome proteolytic system is activated in skeletal muscle in sepsis, and it has been postulated that this degradative pathway is responsible for inducing skeletal muscle weakness and wasting. The objective of this study was to determine if administration of proteasomal inhibitors (MG132, epoxomicin, bortezomib) can prevent sepsis-induced diaphragm weakness. Rats were given either 1) saline (0.5 ml ip), 2) endotoxin (12 mg/kg ip), 3) endotoxin plus MG132 (2.5 mg/kg), 4) endotoxin plus epoxomicin (1 μmol/kg), or 5) endotoxin plus bortezomib (0.05 mg/kg). Animals were killed either 48 or 96 h after injections, and assessments were made of diaphragm proteolysis, force-frequency relationships, mass, protein content, and caspase activation. Endotoxin increased proteolysis (P <0.001). MG132, epoxomicin, and bortezomib each prevented the endotoxin-induced increase in proteolysis (P <0.01). Endotoxin induced severe reductions in diaphragm force generation by 48 h (P <0.01); none of the proteasomal inhibitors prevented loss of force. Endotoxin induced significant reductions in diaphragm mass and protein content by 96 h (P <0.01); neither MG132 nor epoxomicin prevented loss of mass or protein, but bortezomib attenuated the reduction in protein content (P <0.05). Endotoxin increased diaphragm caspase-3 activity (P <0.01); caspase-3 activity remained high when either MG132, epoxomicin, or bortezomib were given. These data suggest proteasomal inhibitors are not an adequate treatment to prevent endotoxin-induced diaphragmatic dysfunction.
Keywords: caspase, sepsis, skeletal muscle
recent work indicates that many hospitalized patients have severely weakened respiratory muscles (8, 25). Several factors are thought to contribute to the induction of muscle weakness in patients, including infection-induced alterations in muscle function, loss of muscle function due to inactivity, and contractile alterations related to the effects of hyperglycemia (1, 11, 23). Infections, in particular, have been shown in multiple human and animal studies to rapidly induce respiratory muscle weakness (1, 3, 12). The diaphragm appears to be especially susceptible to the effects of infection, and some animal models of infection, e.g., pneumonia, appear to preferentially induce diaphragmatic weakness (3).
The precise mechanisms by which infection induces respiratory and limb muscle weakness are incompletely understood. One pathway that has been postulated to play an important role in the induction of skeletal muscle weakness during infections is the proteasomal protein degradation system (16, 26). Supporting this concept, studies have shown that infections elicit increases in the activity of several components of the proteasomal system in skeletal muscle, including E3 ligases (16, 26). It has been suggested E3 ligase enzymes may directly associate with the contractile proteins, potentially marking these proteins for subsequent degradation by the main component of the proteasomal system, the multiunit 26s subunit (7). By this mechanism, it has been proposed that the proteasomal system induces reductions in skeletal muscle protein stores, mass, and force generating capacity in sepsis and other pathological states. In support of this concept, a recent study found that administration of bortezomib, a proteasomal inhibitor, preserved diaphragm force in an animal model of congestive heart failure (24).
Based on these considerations, several authorities have speculated that administration of inhibitors of the proteasomal degradation system may prevent infection-induced skeletal muscle protein degradation, loss of muscle mass, loss of muscle protein stores, and preservation of muscle strength (5, 24). If so, these agents could prove to be valuable therapeutic tools and could be used to prevent muscle atrophy and weakness in infected patients. The purpose of the present set of experiments, therefore, was to test the hypothesis that proteasome inhibitor administration would prevent sepsis-induced diaphragm weakness. We chose to use endotoxin administration as a means of simulating the effects of infection in rodents because this particular model has been well characterized and is known to produce a precise time- and dose-dependent reduction in diaphragm force generating capacity (20). We compared parameters of diaphragm atrophy (muscle mass and protein content), strength (measurement of the diaphragm force-frequency relationship), and proteolysis (the tyrosine release assay) between control animals, animals given endotoxin, and animals given endotoxin plus one of three different proteasomal inhibitors, i.e., MG132, epoxomicin, and bortezomib.
METHODS
Experimental protocol.
Adult male Sprague-Dawley rats (initial weights ∼250–300 g) were used for experimentation. Animals were given food and water ad libitum and housed in university animal facilities. Experimental protocols were approved by the Institutional Animal Care and Use Committee.
Two groups of experiments were performed. In all studies, animals were weighed on the first day of study and on the day of death (see Table 1). The first set of experiments was designed to ascertain the time-dependent effects of endotoxin administration on diaphragm force generation, mass, and protein content. For this study, we examined saline-injected control rats (n = 7) and groups of animals killed 24, 48, and 96 h (n = 7, 6, and 7, respectively) after a single intraperitoneal injection of endotoxin (12 mg/kg, Escherichia coli lipopolysaccharide; Sigma Chemical, St. Louis, MO). To prevent dehydration, all animals were given subcutaneous injections of saline, 60 ml·kg−1·day−1. On the day of death, animals were anesthetized (pentobarbital, 50 mg/kg ip), the chest and abdomen were opened, and the diaphragm was excised and weighed. One diaphragm strip was dissected from the left costal diaphragm and mounted in an organ bath to assess the muscle force-frequency relationship. A second left costal strip was used for measurement of proteolysis using the tyrosine release assay. The right hemidiaphragm was frozen immediately after death, stored at −80°C, and later assayed for muscle protein content. Muscle protein levels were determined by homogenizing samples in buffer and using the Lowry method (DC Protein Assay kit; BioRad Laboratories, Hercules, CA); measurements were performed in triplicate, and values were averaged for a given sample.
Table 1.
Numbers of animals tested in experimental groups
| Groups | Animal Numbers |
|---|---|
| Time Course Experiment | |
| Control | 7 |
| Endotoxin, 24 h | 7 |
| Endotoxin, 48 h | 6 |
| Endotoxin, 96 h | 7 |
| Proteasome Inhibitor Testing | |
| Control | 5 |
| Endotoxin, 48 h | 5 |
| Endotoxin, 96 h | 7 |
| MG132 + endotoxin, 48 h | 4 |
| MG132 + endotoxin, 96 h | 4 |
| Epoxomicin + endotoxin, 48 h | 4 |
| Epoxomicin + endotoxin, 96 h | 4 |
| Bortezomib+ endotoxin, 48 h | 4 |
| Bortezomib + endotoxin, 96 h | 4 |
In a second group of experiments, we determined if administration of proteasome inhibitors, i.e., MG132, epoxomicin, and bortezomib, could prevent endotoxin-induced reductions in diaphragm force, mass, and protein content. The following groups were studied: 1) control, saline-injected animals (n = 5); 2) animals injected with endotoxin (given as an initial intraperitoneal injection, 12 mg/kg, on the first day of study) and killed at 48 h (n = 5); 3) animals given endotoxin and killed at 96 h (n = 7); 4) animals given both endotoxin and MG132, 2.5 mg·kg−1·day−1 intraperitoneally, and killed 48 h after endotoxin administration (n = 4); 5) animals given endotoxin and MG132 and killed 96 h after endotoxin administration (n = 4); 6) animals given endotoxin and epoxomicin (1.0 μmol/kg ip) and killed 48 h after endotoxin administration (n = 4); 7) animals given endotoxin and epoxomicin (1.0 μmol/kg) and killed 96 h after endotoxin administration (n = 4); 8) animals given endotoxin and bortezomib (0.05 mg/kg iv) and killed 48 h after endotoxin administration (n = 4); and 9) animals given endotoxin and bortezomib (0.05 mg/kg) and killed 96 h after endotoxin administration (n = 4). These particular time points for examining the effects of proteasome inhibition (i.e., 48 and 96 h) were chosen based on results from the first series of experiments. At the time of death, animals were anesthetized with pentobarbital, and diaphragms were removed. Force measurements and tyrosine release were assessed for strips from the left costal diaphragm, and the remaining diaphragm was frozen (−80°C) and later assayed for protein content and caspase activity.
The doses of proteasome inhibitors employed were chosen based on a series of preliminary experiments in which a range of doses of each these agents were used (i.e., doses of 2.5, 5.0, and 10.0 mg·kg−1·day−1 for MG132, doses of 1, 2.5, and 5 μmol/kg for epoxomicin, doses of 0.05, 0.10, 0.25, and 1.0 mg/kg for bortezomib). We found a high mortality when high doses of these inhibitors were administered in conjunction with endotoxin (i.e., all animals died when given both endotoxin and 10 mg·kg−1·day−1 of MG132, endotoxin, and either 2.5 or 5 μmol/kg epoxomicin, and endotoxin plus either 0.10, 0.25, or 1.0 mg/kg of bortezomib). The doses used for the present study were the highest that could be employed without inducing death.
Determination of the diaphragm force-frequency relationship.
Diaphragm strips were mounted vertically in an organ bath containing Krebs-Henseleit (25°C, 50 mg/l curare, pH 7.40, 135 mM NaCl, 5 mM KCl, 11.1 mM dextrose, 2.5 mM CaCl2, 1 mM MgSO4, 14.9 mM NaHCO3, 1 mM NaHPO4, 50 U/l insulin, 95% O2/5% CO2, pH 7.4). One end was tied to the base of the organ bath and the other to a Grass FT10 transducer. After 15 min of equilibration, platinum electrodes were used to deliver supramaximal currents using a constant current amplifier attached to a Grass S48 stimulator. Muscle length was adjusted to length of maximal tension (Lo). Strips were stimulated with trains of 1, 10, 20, 50, and 80 Hz stimuli (pulse width 0.6 ms, train duration 800 ms, 30 s between adjacent trains), and force was recorded. Cross-sectional area was calculated as muscle strip weight times muscle density (1.06) divided by muscle length, and specific muscle force was calculated as raw force divided by cross-sectional area (2).
Assessment of proteolysis by the tyrosine release assay.
Rat diaphragm strips were preincubated at resting length for 30 min in a 37°C shaking water bath in 3 ml of Krebs buffer (bubbled with 95% O2/5% CO2, pH 7.4) containing 200 μM valine, 170 μM isoleucine, 100 μM leucine, 0.5 mM cycloheximide, and 0.1% BSA (15). Strips were then transferred to fresh media and incubated for an additional 2 h. The media was removed, trichloroacetic acid (20%) was added to precipitate proteins, and the media centrifuged (800 g, 10 min, 4°C). Subsequently, the supernatant (1.5 ml) was treated with 0.75 ml 0.1% nitrosonaphthol in 95% ethanol and 0.75 ml nitric acid (1:5 with 2.5% NaNO2 added). This mixture was incubated for 30 min at 55°C and allowed to cool. Excess nitrosonaphthol was extracted with 7.5 ml of dichloroethane. The aqueous layer was removed, and its fluorescence was read at excitation 450 nm, emission 550 nm, using a SpectroMax Gemini EM spectrofluorophotometer (Molecular Devices, Sunnydale, CA). Tyrosine standards were assayed in parallel with samples and used to calibrate fluorescence readings. Tyrosine release was normalized to muscle strip weight.
Assessment of proteasome activity.
Proteasome activity of diaphragm homogenates was measured using the Calbiochem kit assay. The manufacturer's protocol was followed, and AMC standards were used to calibrate fluorescent measurements of proteasomal activity.
Measurement of diaphragm caspase activity.
A modified BIOMOL assay (BIOMOL International, Plymouth Meeting, PA) was used to determine caspase activity; for this assay, muscle homogenates (100 μg of protein) were added to assay buffer and a caspase-3-specific fluorogenic substrate (30 μM N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin, Ac-DEVD-AMC). Duplicate determinations were made with muscle homogenate, assay buffer, Ac-DEVD-AMC, and a specific caspase-3 inhibitor (DEVD-CHO, 20 nM). Immediately after substrate was added, a baseline fluorescent measurement of AMC was performed using a Molecular Devices spectrofluorophotometer (excitation frequency of 360 nm and an emission frequency of 460 nm). This measurement was then repeated after 0.5 h of incubation at 30°C. AMC standards were used to quantitate substrate cleavage, with activity levels reported as nanomoles of AMC generated per minute per milligram of muscle homogenate protein.
Statistical analysis.
Because rats varied in body weight, we normalized muscle mass and total diaphragm protein content to initial body weights (i.e., before injections of saline and pharmacological agents) for comparison across groups. SigmaStat statistical software was employed for all statistical analyses. When data passed normality testing, one-way ANOVA was used for comparison of variables (e.g., protein levels and forces) across experimental groups. For these determinations, post hoc testing using Tukey's test was used to determine differences between individual groups. When data did not pass normality standards, ANOVA on ranks was used to compare data between different groups. A P value of less than 0.05 was taken as indicating statistical significance. Data are presented as means ± 1 SE.
RESULTS
Effects of endotoxin administration on diaphragm tyrosine release, protein content, weight, and force generation.
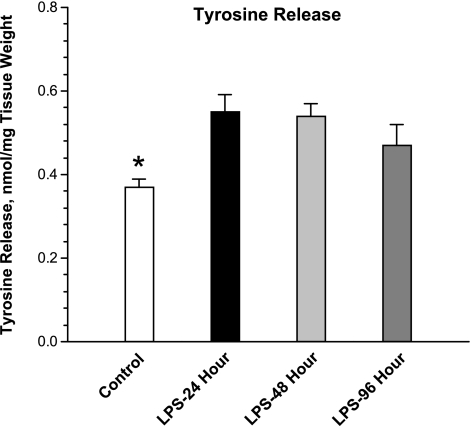
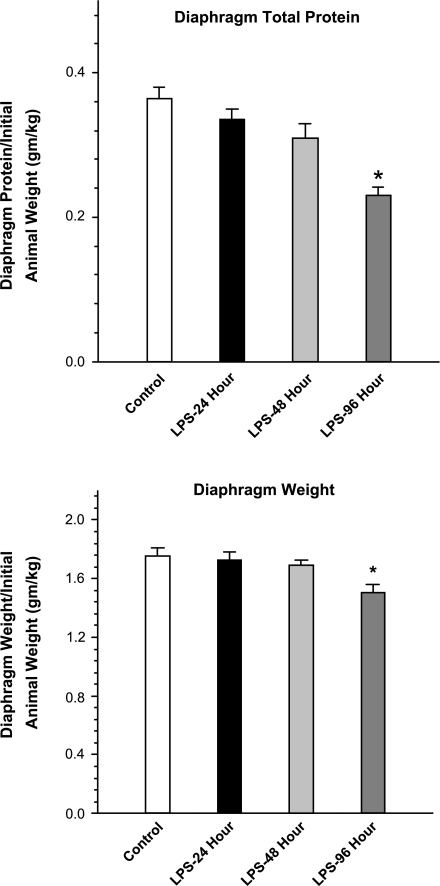
Endotoxin administration to rats induced a significant increase in diaphragm proteolysis, as judged by measurement of the tyrosine release assay. As shown in Fig. 1, this index of proteolysis increased to 150% of control levels by 48 h after the endotoxin injection and then began to return to control levels at the 96-h mark. As shown in Fig. 2, increased muscle proteolysis was followed by reductions in diaphragm muscle mass and total diaphragm protein content, which fell to levels significantly below baseline levels after endotoxin injections. These reductions in diaphragm mass (weight) and protein content were gradual, with little change in these indices from control levels by the 24- and 48-h time points after endotoxin but with significant reductions in both indices of muscle atrophy by the 96-h time point (P < 0.01 for comparison of control and 96-h time points for diaphragm muscle mass and P < 0.01 for comparison of control and 96-h time points for diaphragm protein levels).
Fig. 1.
Tyrosine release assay. Endotoxin (LPS) administration induced an increase in diaphragm tyrosine release, with higher levels of tyrosine release observed at both 24 h after endotoxin administration (black bar, LPS-24 h) and at 48 h after endotoxin injection (light gray bar, LPS-48 h) compared with levels for control samples (white bar). *Statistically significant difference between the control group and the LPS-24 h and LPS-48 h groups.
Fig. 2.
Diaphragm total protein and weight. Endotoxin (LPS) administration elicited a significant reduction in both diaphragm total protein stores (top) and diaphragm weight (bottom), with both indices significantly different at 96 h after endotoxin administration (LPS-96 h, dark gray bars) compared with values for the control group (control, white bars) (P < 0.01 for comparison of diaphragm protein and P < 0.01 for comparison of diaphragm weight). *Statistically significant difference from the control group.
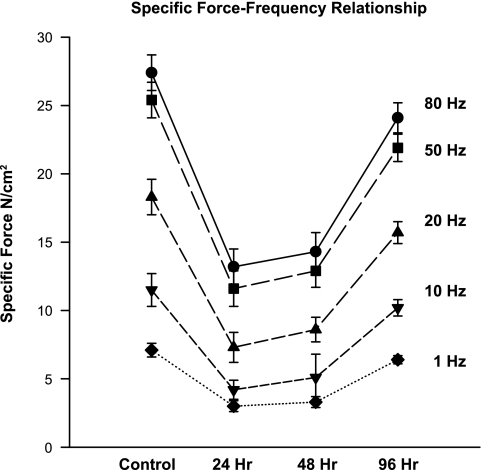
Diaphragm-specific force generation (force/cross-sectional area) fell after endotoxin administration (Fig. 3), but unlike reductions in diaphragm mass and protein content, reductions in specific force occurred early. Specific force declined significantly by 24 h after endotoxin administration, remained low at 48 h (48% below control levels for force in response to 80 Hz stimulation frequencies, P < 0.01), and began to recover towards control values by the 96-h time point. A direct comparison of the time courses of alterations in indices of diaphragm atrophy and specific force generation is provided in Fig. 4. As shown in this figure, reductions in force were marked before any reduction in total protein was observed (i.e., over the first 48 h after endotoxin administration). Paradoxically, as specific force began to recover between 48 and 96 h, total diaphragm protein content fell.
Fig. 3.
Specific force-frequency relationship. Shown is the effect of endotoxin administration on the diaphragm force-frequency relationship. Force generation in response to all stimulation frequencies tested (1–80 Hz) was significantly lower for diaphragms tested at 24 h after endotoxin (see x-axis) compared with the forces generated by diaphragms from the control group (symbols at far left) (P < 0.01 for comparison of force at all frequencies between control and 24-h time points). Force remained lower than control levels at the 48-h time point (P < 0.01 for comparison of force at all frequencies between control and 48-h time points) but then began to recover, increasing between 48 and 96 h after endotoxin administration.
Fig. 4.
Comparison of diaphragm-specific force and protein over time. Shown is the contrast of the time course of alterations in specific force (circles) and diaphragm total protein content (squares) after endotoxin administration. Specific force declines early (by 24 h) and begins to rise between 48 and 96 h, whereas total protein content falls slowly for the first 48 h and then decreases more rapidly between 48 and 96 h after endotoxin administration.
Effects of proteasomal inhibitors on diaphragm atrophy and force generation.
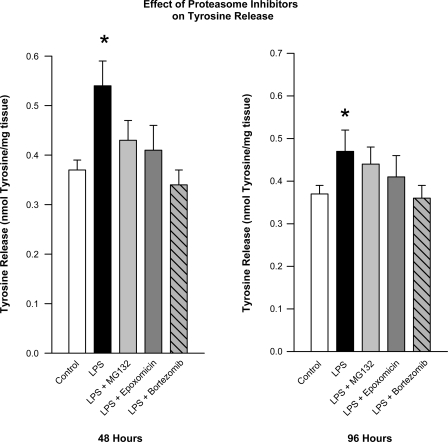
Administration of all three proteasomal inhibitors reduced tyrosine release by incubated diaphragm muscle, consistent with an effect of these agents to reduce proteolysis. This was best demonstrated for diaphragm muscle samples studied at 48 h after administration of endotoxin with and without proteasomal inhibitors, as shown in Fig. 5. Compared with tyrosine release levels obtained for diaphragm samples taken from animals administered endotoxin 48 h before, tyrosine release was reduced by 21% for animals given both endotoxin and MG132 (P < 0.05), was reduced by 25% for animals given both endotoxin and epoxomicin (P < 0.05), and was reduced by 37% for animals given both endotoxin and bortezomib (P < 0.01). Tyrosine release for comparisons made at 96 h (shown in Fig. 5) was also lower for diaphragm sample from animals given both proteasomal inhibitors and endotoxin compared with samples from animals given endotoxin alone.
Fig. 5.
Effect of proteasome inhibitors on tyrosine release. Tyrosine release assay data is compared between different experimental groups at 48 h after endotoxin administration (left) and at 96 h after endotoxin administration (right). Tyrosine release was higher than control for animals given endotoxin alone (black bars, P < 0.05). Administration of either MG132, epoxomicin, or bortezomib reduced the endotoxin-mediated increase in diaphragm tyrosine release (P < 0.05 for comparison of LPS alone to LPS + MG132, LPS + epoxomicin, or LPS + bortezomib at 48 h). *Value statistically different from control.
To further verify that the doses of proteasome inhibitors were adequate to impact protein degradation pathways, we also assayed diaphragm homogenates for proteasomal activity. We found that proteasomal activity was increased at 48 h after endotoxin administration (6 ± 1 and 19 ± 3 pmol AMC·h−1·100 μg−1 for control and endotoxin samples, respectively, P < 0.001) and that proteasomal activity was reduced by all three proteasomal inhibitors (11 ± 2 pmol AMC·h−1·100 μg−1 for endotoxin plus MG132, 8 ± 2 pmol AMC·h−1·100 μg−1 for endotoxin plus epoxomicin, and 5 ± 1 pmol AMC·h−1·100 μg−1 for endotoxin plus bortezomib, P < 0.03 for each comparison to endotoxin alone).
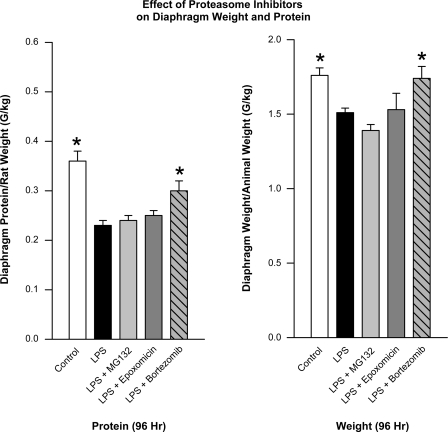
The effects of proteasomal inhibitors on diaphragm weight and protein content were inconsistent. Neither MG132 nor epoxomicin prevented endotoxin-induced reductions in diaphragm weight and protein levels, as shown in Fig. 6. On the other hand, bortezomib significantly diminished endotoxin-induced alterations in diaphragm protein content (P < 0.05, Fig. 6).
Fig. 6.
Effect of proteasome inhibitors on diaphragm weight and protein content. Diaphragm protein content (left) and diaphragm weight (right) is compared between control animal groups (white bars) and groups given either LPS alone (black bars), LPS + MG132 (light gray bars), LPS + epoxomicin (dark gray bars), and LPS + bortezomib (hatched bars). Data are shown for animals killed 96 h after endotoxin administration. LPS significantly decreased protein levels and diaphragm weight compared with controls (P < 0.01 for both comparisons). Neither MG132 nor epoxomicin prevented these endotoxin-induced reductions in diaphragm protein content and weight. Bortezomib partially prevented the endotoxin-induced reduction in diaphragm protein content (*P < 0.05).
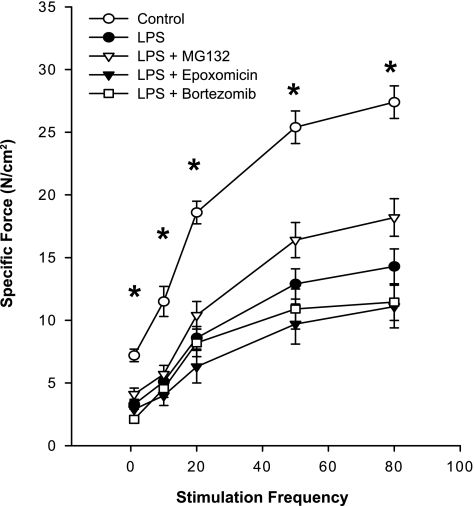
It is important to note, however, that none of the proteasomal inhibitors prevented endotoxin-induced reductions in diaphragm-specific force generation, as shown in Fig. 7. At all stimulation frequencies tested, specific diaphragm force generation for samples from groups given endotoxin plus either MG132, epoxomicin, or bortezomib were similar to values obtained for the endotoxin-treated group and were markedly reduced compared with the diaphragm force generated by muscles from saline-treated control animals (P < 0.02, 0.01, and 0.01, for comparison of force in response to 80 Hz stimulation between controls, and, respectively, endotoxin + MG132, endotoxin + epoxomicin, and endotoxin + bortezomib groups).
Fig. 7.
Effect of proteasome inhibitors on the diaphragm force-frequency relationship. Data are shown for diaphragm force-frequency curves assessed at 48 h after endotoxin administration. LPS significantly reduced force generation at all stimulation frequencies (P < 0.01). None of the proteasome inhibitors prevented this LPS-induced reduction in specific force, with the forces generated for diaphragms from groups given either LPS + MG132, LPS + epoxomicin, or LPS + bortezomib statistically similar to the forces generated by diaphragms from animals given LPS alone. *Statistical difference between control and the other groups.
Effects of proteasomal inhibitors on diaphragm caspase activation.
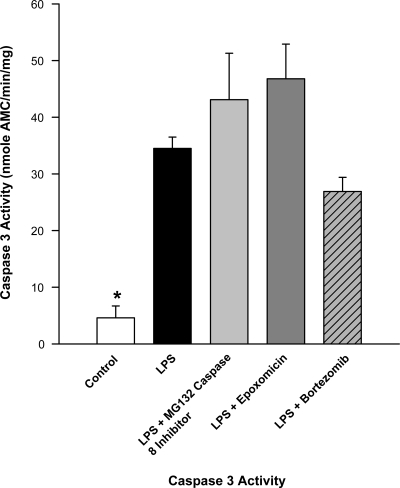
Endotoxin elicited a significant increase in diaphragm caspase activity, as shown in Fig. 8 (P < 0.01). Caspase activity levels were also higher than control levels for groups given combinations of either endotoxin + MG132, endotoxin + epoxomicin, or endotoxin + bortezomib (P < 0.01, 0.01, and 0.01, respectively, for these comparisons).
Fig. 8.
Effect of proteasome inhibitors on diaphragm caspase activity. Diaphragms from animals given LPS alone (black bar) had caspase activity levels much higher than controls (white bar, P < 0.01 for this comparison). Caspase activity levels were also high for groups given either LPS + MG132, LPS + epoxomicin, or LPS + bortezomib (P < 0.01 for comparison of these 3 groups to controls). *Statistical difference between control and the other groups.
DISCUSSION
In this study, we found that endotoxin produced a significant reduction in diaphragm force, muscle mass, and protein content. Losses in specific force preceded reductions in muscle mass and protein levels, with specific force reaching its nadir between 24 and 48 h after endotoxin administration, whereas protein and muscle mass did not significantly fall until 96 h after endotoxin administration. We also found that none of the proteasomal inhibitors tested prevented reductions in diaphragm-specific force generation. In fact, there was a trend for two of the three proteasomal inhibitors (i.e., MG132 and bortezomib) to reduce force beyond the reductions observed in response to endotoxin treatment alone. We also found that two of these inhibitors failed to prevent endotoxin-induced reductions in diaphragm muscle mass and protein content. The third inhibitor, bortezomib, had a small effect to blunt the endotoxin-induced reduction in diaphragm protein content but did not restore protein levels to control levels.
Infection-induced respiratory skeletal muscle dysfunction.
Recent studies indicate that many hospitalized patients have profound diaphragm weakness (8, 25). In particular, the role of infection in producing respiratory muscle dysfunction has been extensively studied, and it is known that even relatively trivial infections can rapidly produce severe reductions in respiratory muscle function (1, 3, 12). It is also known that infections elicit a marked increase in muscle proteolysis, and it is commonly thought that enhanced proteolysis is the major mechanism by which infections produce reductions in muscle mass and protein content (5). Moreover, the major proteolytic enzyme system commonly thought to contribute to infection-induced muscle proteolysis is the proteasomal protein degradation pathway (16, 19, 26).
In keeping with the possibility that enhanced activation of the proteasomal system is responsible for muscle wasting with infection, a number of publications have shown increases in levels of many of the components of the proteasomal degradation system in skeletal muscle in animal models of infection, including increases in ubiquitin, the 20S proteasome component, and several E3 ligases (atrogin and MuRF1, i.e., muscle ring finger 1) (16, 26). Work has also suggested that cytokines may be key modulators of infection-induced upregulation or proteasomal components in muscle (9, 10).
While these previous studies have made a convincing argument that cytokines can induce upregulation of proteasomal components in isolated muscle cells and that systemic models of infection (e.g., endotoxin administration, peritonitis) result in upregulation of proteasomal components in skeletal muscle in vivo, there is less evidence linking activation of proteasomal components to actual reductions in muscle function during infections. The only previous study, of which we are aware, that has directly examined the effect of administration of proteasomal inhibitors in a animal model of infection is the work of Kadlcikova et al. (6). These authors found that administration of several proteasome inhibitors (i.e., MG132, ZL3VS, and AdaAhx3L3VS) in an animal model of sepsis resulted in a marked reduction in protein degradation, as judged by use of the tyrosine release assay (6). This finding is important because the tyrosine release assay is a highly reliable measure of total protein degradation and does not just reflect protein degradation related to one specific proteolytic pathway. This assay is also extremely sensitive, capable of measuring small differences in protein turnover that are not detectable using other techniques. Based on these findings, the authors of this previous study could conclude that the proteasome system is largely responsible for infection-induced muscle proteolysis. This previous investigation, however, did not measure muscle force generation or determine if these inhibitors had any affect to preserve muscle strength during infection.
The current experiments extend these previous findings, examining several important outcome parameters (muscle mass, muscle protein content, muscle-specific force generation) that have not been previously evaluated in experiments examining the effects of proteasomal inhibition on skeletal muscle during infection. As in the previous work by Kadlcikova et al. (6), we found that proteasomal inhibitors potently blocked the effect of endotoxin to increase muscle proteolysis, as judged from measurement of muscle tyrosine release. Proteasomal inhibition, however, failed to preserve diaphragm force generation in the present study. In addition, two of the three inhibitors tested (MG132 and epoxomicin) did not preserve muscle mass or protein concentrations, and the third (bortezomib) incompletely prevented endotoxin-induced reductions in protein levels.
One possible explanation for the failure of the agents tested to produce their anticipated effects could be that we employed insufficient dosages to adequately prevent proteasomal activity. As indicated in methods, however, we found that doses of proteasomal inhibitors that were higher than those employed in the present work were extremely toxic, resulting in a high mortality when administered in combination with endotoxin. Moreover, the doses employed were sufficient to suppress proteolytic activity, as judged by the tyrosine release assay, arguing that these doses should have also been capable of preserving force and muscle protein levels if proteasomal activation was the only factor causing reductions in muscle function following endotoxin administration.
The simplest alternative explanation for our findings would be that factors other than proteasomal activation may be primarily responsible for causing diaphragm dysfunction during infections. Recent papers have suggested, in fact, that other proteolytic pathways, such as caspase and calpain, may need to be initially activated in skeletal muscle to break down the contractile protein lattice under catabolic conditions, allowing contractile protein elements to be released and subsequently targeted by the proteasomal system (4, 18). According to this theory, caspase- and calpain-mediated disruption should initially produce a reduction in muscle-specific force, as contractile elements are released from the lattice, with subsequent breakdown of these proteins by the proteasomal system leading to reductions in muscle mass and protein content. This possibility is supported by the results of the present study, in which sepsis induced an initial reduction in muscle-specific force generation followed by a subsequent reduction in muscle mass and protein content. This theory also accounts for the failure of proteasomal inhibitors to alter specific force in the current study, since force loss should result from lattice degradation, and the proteasome does not appear capable of degrading the intact myofibrillar complex (14, 17).
Our data also suggest that proteasomal inhibition may paradoxically increase muscle caspase activation. This finding is in keeping with previous studies showing that some proteasomal inhibitors, such as bortezomib, increase tissue caspase activation and promote cellular apoptosis (22). Activation of caspase by proteasomal inhibitors would be expected to further reduce muscle force generation, as caspase activation can directly degrade contractile proteins, reducing muscle force generation. Another reason why proteasomal inhibition may have had limited effectiveness in reducing muscle protein loss in the present study is that protein synthesis is also reduced in sepsis, and inhibition of muscle proteolysis alone would not be expected to prevent the atrophic effects of reduced protein synthesis (21).
Clinical implications.
The present study indicates that sepsis enhances diaphragm proteolytic degradation, but inhibition of proteasomally mediated proteolysis alone with currently available inhibitors does not prevent reductions in diaphragm force generation, and, as a result, administration of currently available proteasomal inhibitors is unlikely to provide a clinical benefit in septic patients. It could be argued that development of a proteasomal inhibitor without proapoptotic effects could circumvent this problem, but such a possibility seems unlikely. Activation of NF-κB during inflammation requires an active proteasomal system (13), and nonspecific inhibition of the proteasomal pathway should invariably block NF-κB activation. NF-κB activation inhibits apoptotic pathways, and, as a result, generic proteasomal inhibition in skeletal muscle would be expected to always potentiate caspase activation and caspase-mediated contractile protein degradation.
An alternative therapeutic strategy would be to employ inhibitors of either caspase or calpain activation or to administer activators of protein synthesis to septic patients. Theoretically, concomitant administration of proteasomal inhibitors may be of benefit clinically once caspase/calpain enzymes are also inhibited, since this combination therapy would block both factors (myofibrillar lattice disruption, degradation of protein lattice components) that contribute to muscle protein degradation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-80429, HL-81525, HL-63698, HL-80609, and HL-69821.
REFERENCES
- 1.Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski GS. Free radical induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol 24: 210–217, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Close RI The dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972. [DOI] [PubMed] [Google Scholar]
- 3.Divangahi M, Matecki S, Dudley RW, Tuck SA, Bao W, Radzioch D, Comtois AS, Petrof BJ. Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 169: 679–686, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290: R1589–R1597, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Hasselgren PO Role of the ubiquitin-proteasome pathway in sepsis-induced muscle catabolism. Mol Biol Rep 26: 71–76, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Kadlcikova J, Holecek M, Safranek R, Tilser I, Kessler BM. Effects of proteasome inhibitors MG132, ZL3VS and AdaAhx3L3VS on protein metabolism in septic rats. Int J Exp Pathol 85: 365–371, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, Gygi SP, Goldberg AL. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J Biol Chem 282: 17375–17386, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YP, Lecker SH, Chen Y, Waddell ID, Goldberg AL, Reid MB. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 17: 1048–1057, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med 175: 1134–1138, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Mier-Jedrzejowicz M, Brophy C, Green M. Respiratory muscle weakness during upper respiratory tract infections. Am Rev Respir Dis 138: 5–7, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Park JW, Qi WN, Cai Y, Urbaniak JR, Chen LE. Proteasome inhibitor attenuates skeletal muscle reperfusion injury by blocking the pathway of nuclear factor-kappaB activation. Plast Reconstr Surg 120: 1808–1818, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337–R344, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds TH, Krajewski KM, Larkin LM, Reid P, Halter JB, Supiano MA, Dengel DR. Effect of age on skeletal muscle proteolysis in extensor digitorum longus muscles of B6C3F1 mice. J Gerontol A Biol Sci Med Sci 57: B198–B201, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth GA, Moser B, Krenn C, Roth-Walter F, Hetz H, Richter S, Brunner M, Jensen-Jarolim E, Wolner E, Hoetzenecker K, Boltz-Nitulescu G, Ankersmit HJ. Heightened levels of circulating 20S proteasome in critically ill patients. Eur J Clin Invest 35: 399–403, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690–26697, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol 100: 1770–1777, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Supinski GS, Callahan LA. Free radical mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol 102: 2056–2063, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Supinski G, Nethery D, Stofan D, DiMarco A. Comparison of the effects of endotoxin on limb, respiratory, and cardiac muscles. J Appl Physiol 81: 1370–1378, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Svanberg E, Frost RA, Lang CH, Isgaard J, Jefferson LS, Kimball SR, Vary TC. IGF-I/IGFBP-3 binary complex modulates sepsis-induced inhibition of protein synthesis in skeletal muscle. Am J Physiol Endocrinol Metab 279: E1145–E1158, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Thorpe JA, Christian PA, Schwarze SR. Proteasome inhibition blocks caspase-8 degradation and sensitizes prostate cancer cells to death receptor-mediated apoptosis. Prostate 68: 200–209, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 64: 1348–1353, 2005. [DOI] [PubMed] [Google Scholar]
- 24.van Hees HW, Li YP, Ottenheijm CA, Jin B, Pigmans CJ, Linkels M, Dekhuijzen PN, Heunks LM. Proteasome inhibition improves diaphragm function in congestive heart failure rats. Am J Physiol Lung Cell Mol Physiol 294: L1260–L1268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wenon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29: 1325–1331, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35: 698–705, 2003. [DOI] [PubMed] [Google Scholar]