Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by a fibrotic thrombus persisting and obliterating the lumen of pulmonary arteries; its pathogenesis remains poorly defined. This study investigates a potential contribution for progenitor cell types in the development of vascular obliteration and remodeling in CTEPH patients. Endarterectomized tissue from patients undergoing pulmonary thromboendarterectomy was collected and examined for the structure and cellular composition. Our data show an organized fibrin network structure in unresolved thromboemboli and intimal remodeling in vascular wall tissues, characterized by smooth muscle α-actin (SM-αA)-positive cell proliferation in proximal regions (adjacent to thromboemboli) and neoangiogenesis/recanalization in distal regions (downstream from thromboemboli). Cells that are positively stained with CD34 and fetal liver kinase-1 (Flk-1) (CD34+Flk-1+) were identified in both the proximal and distal vascular tissues; a subpopulation of CD34+Flk-1+CD133+ cells were further identified by immunostaining. Triple-positive cells are indicative of a population of putative endothelial progenitor cells or potential colony-forming units of endothelial cells. In addition, inflammatory cells (CD45+) and collagen-secreting cells (procollagen-1+) were detected in the proximal vascular wall. Some of the CD34+ cells in CTEPH cells isolated from proximal regions were also positive for SM-αA. Our data indicate that putative progenitor cell types are present in the neointima of occluded vessels of CTEPH patients. It is possible that the microenvironment provided by thromboemboli may promote these putative progenitor cells to differentiate and enhance intimal remodeling.
Keywords: pulmonary embolism, endarterectomy, vascular remodeling
chronic thromboembolic pulmonary hypertension (CTEPH) is resultant of a single or recurrent pulmonary thromboemboli arising from sites of deep venous thrombosis that are not resolved by conventional anticoagulant therapy. Although its actual prevalence remains uncertain, CTEPH is estimated to occur in up to 2–4% patients surviving acute pulmonary embolisms (3, 25, 30, 31). The mechanism for the poor resolution of the initial thromboembolism in CTEPH patients remains to be elucidated. The persistent thrombus occludes the central pulmonary artery preventing blood flow to distal lung regions. The pathophysiological changes in pulmonary arteries of CTEPH patients lead to elevated pulmonary vascular resistance, increased pulmonary arterial pressure (20), and ultimately to progressive right heart failure (26). Pulmonary thromboendarterectomy (PTE) is currently the treatment of choice for CTEPH patients with unresolved thromboemboli (16, 19, 27).
Very few studies have investigated the molecular determinants of CTEPH. Lang and colleagues (22), while finding that an inherent endothelial cell-mediated fibrinolytic imbalance was not a generalized phenomenon in CTEPH, discovered that the prevalence of type 1 plasminogen activator inhibitor (PAI-1) expression in pulmonary thromboemboli was increased and may be responsible for vascular thrombus stabilization. It has also been shown that the levels of plasma factor VIII [detected by von Willebrand Factor (vWF)] are elevated in CTEPH (5). However, the pathogenic mechanisms for the development of CTEPH are still unclear.
Recently, a role of progenitor cells in various cardiopulmonary and vascular diseases has been a focus of research. Progenitor cells have been identified and characterized in atherosclerotic vessels (13, 23, 36) in normal lung and lung cancer tissues (17, 21). Resident and nonresident progenitor cells have both been shown to contribute to the repair and regeneration of chronic lung diseases (11, 15). Alvarez et al. (1) showed that pulmonary resident microvascular endothelial progenitor cells (EPCs) (CD31+, CD144+, and CD45−) have vasculogenic activity and the potential to form de novo vessels (1). In chronic hypoxia-induced pulmonary hypertension, circulating mesenchymal precursors of a monocyte/macrophage lineage (fibrocytes) were recruited, and these cells contributed directly and substantially to the pulmonary artery adventitial remodeling (8, 9, 34). Hayashida et al. (12) also showed bone marrow-derived cells mobilized to the hypertensive pulmonary arteries and contributed to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Most recently, Asosingh et al. (2) provided evidence showing that mobilization of high levels of proliferative bone marrow-derived proangiogenic precursors (CD34+CD133+) is a characteristic of idiopathic pulmonary arterial hypertension (IPAH) and may participate in the pulmonary vascular remodeling process. With unique access to endarterectomized tissues from CTEPH patients who have undergone PTE surgery, our initial study was designed to examine the structural changes in these PTE tissues and test the hypothesis that progenitor cell types such as early/late outgrowth EPCs or colony-forming units of endothelial cells (CFU-ECs) and circulating mesenchymal cells (fibrocytes) are present in PTE tissues and may contribute to the development and progression of CTEPH.
MATERIALS AND METHODS
Tissue collection and histological preparation.
PTE tissues were obtained directly from operating room and preserved in cold PBS for transport to the laboratory for preparation. All protocols were approved by the Institutional Review Board of University of California, San Diego. The PTE tissues were separated to three segments in the laboratory for the histological study and isolation of cells: 1) the piece of tissues cut from regions surrounding the occlusion (referred to as proximal vascular wall tissue in this study); 2) the piece of tissues cut from regions distal to the occlusion (referred to as distal vascular tissue in this study); and 3) the piece of tissues cut from the occluding coagulant material (referred to as coagulant tissue in this study). Tissues were embedded and frozen in optimal cutting temperature compound (Sakura Tissue Tek). Ten-micrometer frozen sections were cut in a cryostat machine and collected onto glass slides. Standard hematoxylin and eosin (HE) staining was performed on the slides. PTE samples obtained from a total of six patients undergoing PTE were examined in this study.
Cell isolation and culture.
The PTE tissues from each region were cut into approximately 1- to 2-mm2 squares under a Nikon SMZ-2T microscope and incubated in HBSS containing 2.5 mg/ml collagenase (Worthington Biochemical), 0.5 mg/ml elastase (Sigma), and 1.0 mg/ml BSA (Sigma) for 55 min at 37°C to create a cell suspension. After the incubation, the cells were washed in DMEM containing 20% FBS. Cells were then transferred into smooth muscle growth medium (SMGM; Lonza) containing smooth muscle basal medium (SMBM; Lonza), 5% FBS, 5 μg/ml insulin, 0.5 ng/ml human epidermal growth factor, 2 ng/ml human fibroblast growth factor, gentamicin sulphate, and amphotericin B. The cells were incubated at 5% CO2, 100% humidity, and 37°C. Confluent cells were passaged with 0.05% trypsin-EDTA (Cambrex) and used for experiments as primary cells and at passages 2-6. Human pulmonary artery smooth muscle cells (PASMCs) grown in SMGM and human pulmonary artery endothelial cells (PAECs; Lonza) grown in endothelial growth media (EGM; Lonza) were used as controls. EGM is composed of endothelial basal medium (EBM), 2% FBS, 0.5 ng/ml human epidermal growth factor, 2 ng/ml human fibroblast growth factor, and 5 μg/ml insulin.
Immunofluorescence and immunohistochemistry.
Frozen tissue sections (prepared from coagulant tissues, proximal vascular wall tissues, and distal vascular tissues) and coverslips with isolated cells were thawed and fixed in 4% paraformaldehyde/PBS. Sections were blocked in PBS (with 2% BSA, 0.1% Triton X-100, and 2% normal serum) before incubation with primary antibodies. Antibodies include smooth muscle α-actin (SM-αA), vWF, CD34, CD45, Flk-1, procollagen-1, and CD133 purchased from Sigma, Santa Cruz Biotechnology, and Abcam. Sections were then washed and incubated with fluorophore-conjugated secondary antibodies diluted in blocking solution. Nuclei were counterstained in 4′,6′-diamidino-2-phenylindole (DAPI; 5 μM) solution before sections were mounted in antifade mounting solution. For immunohistochemistry (IHC), sections were incubated in 1% H2O2 before blocking, and biotin-labeled secondary antibodies were used. The signals were detected using ABC Staining Systems (Santa Cruz Biotechnology). Fluorescence images were taken from a DeltaVision RT Deconvolution Microscope System. IHC pictures were taken from an Olympus microscope. Controls consisted of cells or slices incubated in secondary antibody alone and were carried out for all secondary antibodies used. An average of 3–6 slices/fixed cell coverslips per patient were stained and analyzed.
RT-PCR.
Total RNA was extracted from primary cultured CTEPH cells or PTE tissues using TRIzol (Invitrogen) method and treated with DNase I (Invitrogen) before being reverse-transcribed to cDNA with SuperScript II reverse transcriptase (Invitrogen). PCR was performed by a DNA thermal cycler (MyCycler; Bio-Rad Laboratories) using Platinum Blue PCR SuperMix (Invitrogen). PCR products were separated by gel electrophoresis (1.2% agarose gel), and amplified cDNA bands were visualized by GelStar nucleic acid stain (Lonza). GAPDH was used as an internal control for semiquantification. PCR was carried out for a minimum of six different samples. The net intensity values of cDNA bands (from electrophoretically separated PCR products) measured by a Kodak electrophoresis documentation system were normalized to the net intensity values of the GAPDH signals; the ratios are expressed as arbitrary units for quantitative comparison. The sense and antisense primers were specifically designed from the coding regions of CD34, Flk-1, CD133, CD45, and SM-αA. The primers are listed in Table 1. The fidelity and specificity of the sense and antisense oligonucleotides were examined using Basic Local Alignment Search Tool (BLAST) program.
Table 1.
Oligonucleotide sequences of the primers used for RT-PCR
| Standard Name (acc. no.)* | Size, bp | Predicted Sense/Antisense | Location, nt | Gene, Chromosome |
|---|---|---|---|---|
| CD34 (M81104) | 195 | 5′-GCAAGCCACCAGAGCTATTC-3′/ 5′-GGTCCCAGGTCCTGAGCTAT-3′ | 1022–1216 | 1q32 |
| Flk-1 (AF035121) | 386 | 5′-CAGTGGTATGGTTCTTGCCTCA-3′/ 5′-CTACTTCCTGCTGGTGGAAAGA-3′ | 4073–4458 | 4q11–q12 |
| CD133 (NM_006017) | 189 | 5′-ACCAGGTAAGAACCCGGATCAA-3′/ 5′-CAAGAATTCCGCCTCCTAGCACT-3′ | 322–985 | 4p15.32 |
| CD45 (Y00062) | 276 | 5′-CCTGCTCAGAATGGACAAGT-3′/ 5′-CAGCTAACAGGAGGTTTGGA-3′ | 3774–4049 | 1q31–q32 |
| CD11b (J03925) | 428 | 5′-CAAGCGGCAATACAAGGACA-3′/ 5′-CCAGAGACATCTGAGTCACA-3′ | 3373–3800 | 16p11.2 |
| SM-α-actin (X13839) | 308 | 5′-GCATCCATGAAACCACCTAC-3′/ 5′-GAAGCATTTGCGGTGGACAA-3′ | 890–1197 | 10q22–q24 |
| GAPDH (AF261085) | 243 | 5′-GACAACGAATTTGGCTACAGC-3′/ 5′-GATGGTACATGACAAGGTGC-3′ | 1091–1333 | 12p13.31 |
The accession numbers in GenBank for the sequence used in designing the prime. Flk-1, fetal liver kinase-1; SM-α-actin, smooth muscle α-actin.
Statistical analysis.
The composite data are expressed as means ± SE. Differences between groups were examined for statistical significance using Student's t-test or one-way ANOVA. Differences were considered to be statistically significant when P < 0.05.
RESULTS
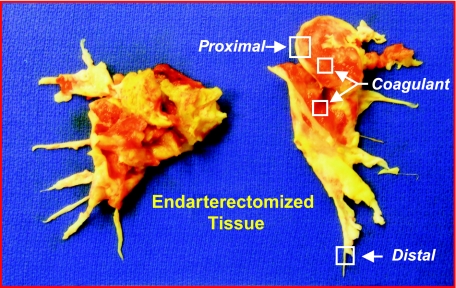
In CTEPH, the thromboembolic material is fibrotic and incorporated into the native vascular wall. During PTE, the pseudointima is identified, and a dissection plane is created, often down to the media of the vessel, to free the occluding tissues. In the PTE tissue shown in Fig. 1, the vessel wall appears white/yellowish in color with the occluding nonresolved embolic material appearing red. In this paper, we collected the occluding material (referred to as coagulant material) and the vascular wall tissue from regions adjacent (referred to as proximal vascular wall tissue) and distal (referred to as distal vascular tissue) to the occluding material (Fig. 1). These tissues were used to: 1) determine the structural changes occurring in the occluded pulmonary vascular wall; and 2) identify the presence of putative progenitor-type cells in the PTE tissues.
Fig. 1.
Endarterectomized tissues obtained from a chronic thromboembolic pulmonary hypertension (CTEPH) patient undergoing pulmonary thromboendarterectomy (PTE) surgery. The patient had bilateral disease, and the tissues show segmental extension. The areas where tissue samples were taken are labeled as defined in the text: proximal vascular wall tissue, distal vascular tissue, and occluding coagulant material (red-colored tissue).
Structure of endarterectomized tissues.
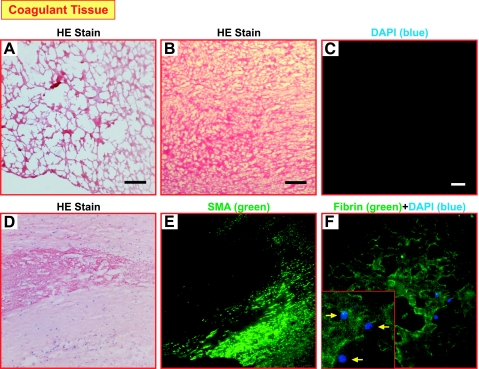
The HE-stained coagulant material is indicative of the presence of fibrous structures (Fig. 2, A and B). A lack of functional cells is suggested by the negative DAPI staining for nuclei within the central occluded region of the endarterectomized tissue (Fig. 2C). This staining suggests that the occluding coagulant material of the endarterectomized tissue is primarily composed of a fibrin network. The HE staining in Fig. 2D indicates the presence of a fibrin network, and, again, nuclei are rarely seen within this region. In an additional section taken from the same patient, the SM-αA staining in Fig. 2E demonstrates that the fibrin network in the center of the image does not contain SM-αA+ cells, but in the adjacent area there are organized layers of SM-αA+ cells. Immunostaining of fibrin in Fig. 2F indicates a network with a few cells scattered within this network, determined by DAPI nuclear counterstaining (blue).
Fig. 2.
Fibrous networks are present in the coagulant tissue/material. Hematoxylin and eosin (HE) staining (A and B) of representative tissue sections shows a fibrous network-like structure in the occluding coagulant tissue. 4′,6′-Diamidino-2-phenylindole (DAPI) staining for cellular nuclei is shown in C. Scale bars reflect 150 μm (A–C). The HE staining (D) and smooth muscle (SM) α-actin (SMA) staining (E) show the lack of nuclei and SMA+ cells in the central coagulant region. A fibrin network (green) with a few cells (indicated by arrows in inset) scattered within this network, determined by DAPI nuclear counterstaining (blue; F).
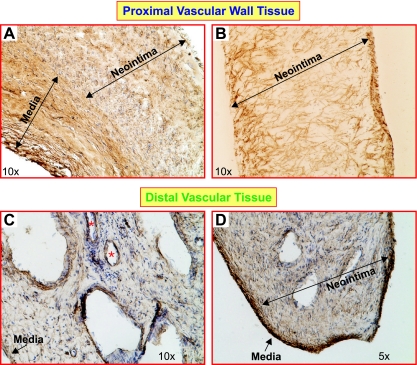
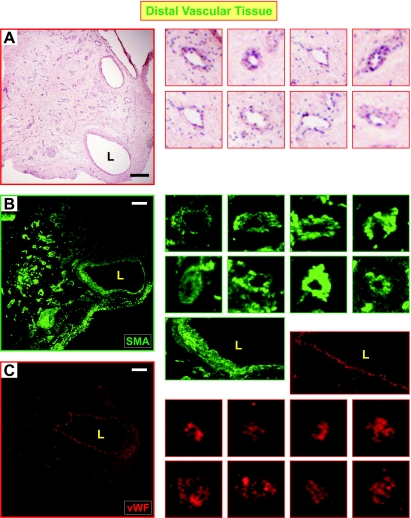
IHC revealed different structural characteristics in proximal and distal vascular tissues. As expected, part of the medial layer of the vascular wall removed during surgery can be clearly seen, in which smooth muscle cells are compact and aligned (indicated on Fig. 3, A and D). The neointimal layer of proximal vascular wall tissue is composed of a large amount of SM-αA+ cells. These SM-αA+ cells are highly disorganized (Fig. 3, A and B), indicating an abnormal distribution of smooth muscle cells or myofibroblasts in the intimal layer of the pulmonary arterial wall that is directly attached or adjacent to the thromboemboli. Compared with the proximal tissue, the neointimal layer of distal vascular tissue has less SM-αA+ cells but contains multiple-channel structures (Fig. 3, C and D, and Fig. 4A). Most importantly, the channels are surrounded by smooth muscle cells (SM-αA+; Fig. 4, A and B) and endothelial cells (vWF+; Fig. 4C), indicating that neovascularization or neoangiogenesis occurs in the pulmonary vessel segments downstream from the attached thromboemboli. Whether the outgrowth of SM-αA+ cells and recanalization are related to the intimal thickening of the occluded pulmonary arteries in CTEPH patients remain unknown and need further studies.
Fig. 3.
The structure and cellular composition of the occluded proximal pulmonary arteries and the vessels distal to the occlusion. Immunohistochemistry shows that a large amount of highly disorganized SM α-actin-positive (SM-αA+) cells (brown) in the proximal vascular wall (A and B). Tissue sections for A and B were prepared from 2 different patients. In the distal vascular tissue, the lumen was separated by the recanalizing fibrotic thrombus, and multiple channels (*) were observed in patients with CTEPH (C and D). Several medial layers of occluded vessels are shown in the proximal vascular tissue (A; labeled with “Media”) with strong SM-αA staining. The medial layer of the pulmonary vessel distal to the occlusion was clearly distinguished from neointima as a compact SM-αA+ cell layer (D). Medial and neointimal layers are indicated with arrows.
Fig. 4.
The recanalized regions in distal PTE tissues are surrounded by SM and endothelial cells. A: HE staining of a distal vascular tissue showed the lumen (L) and multiple-channel structure (as shown in images on the right), which was positive for SM-αA (B) and von Willebrand Factor (vWF; C) as shown by immunofluorescent staining. The images on the right are magnified channels from the panels on the left highlighting SM and endothelial cell staining in the distal pulmonary vessels. The 2 large images on the right in B and C are magnified areas around the lumen with SM-αA (green) and vWF (red) staining. The SM-αA and vWF panels are images from different sections obtained from the same region of the endarterectomized tissue; they are not overlaid images. Scale bar reflects 100 μm.
Identification of putative EPCs and mesenchymal precursor cells in endarterectomized tissues.
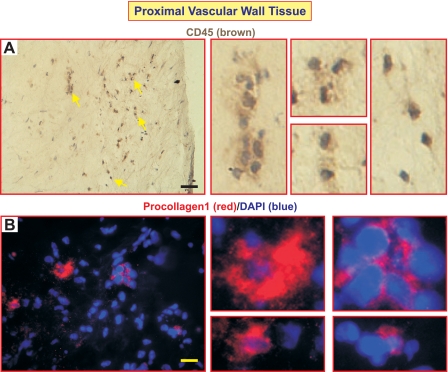
We performed IHC and immunofluorescent staining using cell markers typically indicative of mesenchymal precursor cells (8) or EPCs to determine their presence in the endarterectomized tissues and their potential contribution to the structural changes and vascular remodeling we observed in the PTE tissue sections. CD45 and procollagen-1 are two accepted markers for circulating mesenchymal cells of the monocyte/macrophage lineages called fibrocytes (8). CD45+ cells (arrows in Fig. 5A) and procollagen-1+ cells (Fig. 5B) were found in proximal vascular wall tissue but not in distal vascular tissue. In addition, we did not find cells expressing both CD45 and procollagen-1 in the proximal tissues. The presence of CD45+ cells, however, may indicate the accumulation of inflammatory cells (leukocytes) in the pulmonary vascular wall adjacent to the thromboemboli. Procollagen-1+ cells secrete ECM protein collagen, which may contribute to the establishment of a microenvironment for cell proliferation and differentiation.
Fig. 5.
CD45+ and procollagen-1+ cells were detected in the proximal vascular wall tissue. Immunohistochemistry using anti-CD45 antibody shows the accumulation of CD45+ cells (arrows in A). Immunofluorescent staining using procollagen-1 antibody identified clusters of procollagen-1+ cells. Nuclei were counterstained with DAPI (blue). The images on the right are magnified sections of the main panels highlighting either CD45+ (A) or procollagen+ cells (B). Scale bars reflect 100 μm in A and 25 μm in B.
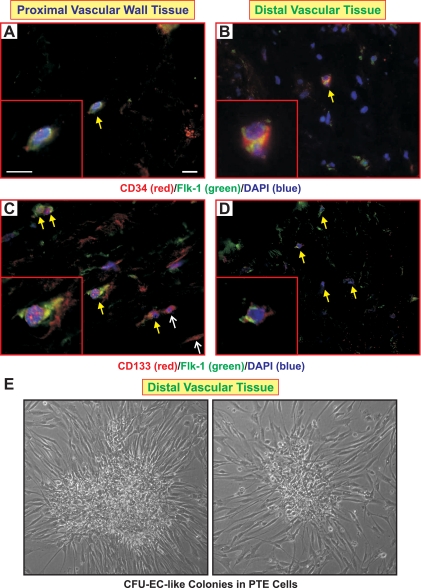
CD34 and Flk-1 (VEGFR2) when coexpressed in the same cell are typical markers for vascular wall progenitor cells (40). As shown in Fig. 6, A and B, CD34+Flk-1+ double-positive cells were identified in both the proximal and distal vascular tissues. It should be noted that we also found cells positive for either one of these markers. CD133 is characteristically a marker for hematopoietic stem cells (38). Combination of surface markers, CD34, Flk-1 and CD133, has been used in several studies to define a population of early/late outgrowth EPCs or CFU-ECs (10, 29). We found that both proximal and distal vascular walls contain CD133+Flk-1+ cells (arrows in Fig. 6, C and D) and some single-positive cells staining for either CD133 or Flk-1 (thin arrows in Fig. 6C) in proximal vascular wall. In addition, the combination of CD34/Flk-1/Tie-2 is a typical marker set for identification of vascular wall resident EPCs (VW-EPCs) (40). Staining of the tissues with CD34 and Tie-2 antibodies did not identify any double-positive CD34+Tie-2+ cells (data not shown). Therefore, our data suggest that we have identified a population of CD34, Flk-1, and CD133 expressing cells, indicative of putative EPCs, present in neointimal layers of pulmonary vessels both adjacent and downstream to thromboemboli attachment site. Our continuing investigations will strive to precisely define and characterize the properties of this population of cells. Indeed, plastic, but not glass adherent, colonies with the morphology of CFU-EC were frequently observed in primary culture and early subculture of the PTE cells (Fig. 6E) isolated from the remodeled artery wall regions as indicated in Fig. 1.
Fig. 6.
Putative endothelial progenitor cells (EPCs) were identified in the occluded vessel wall. Immunofluorescent staining using CD34 (red) and fetal liver kinase-1 (Flk-1; green) antibodies identified CD34+Flk-1+ cells in both proximal (A) and distal (B) vascular wall tissues (arrows in A and B as well as the magnified image in inset). Antibodies against hematopoietic stem cell marker, CD133 (red), and Flk-1 (green) were used to identify putative EPCs. CD133+Flk-1+ cells were found in proximal vascular wall (arrows in C and the magnified image in inset) and distal vascular wall (arrows in D and the magnified image in inset). Cells only positive for CD133 in proximal tissue are identified by the thinner arrows in C. Double-/triple-positive cells are shown at higher magnification in the insets of A–D. Nuclei were counterstained with DAPI (blue). Scale bars reflect 25 μm. E: phase-contrast images show that cultured cells prepared from distal PTE tissues can form colony-forming units of endothelial cell (CFU-EC)-like colonies on plastic coverslips.
Detection of CD34, Flk-1, and CD133 in a population of isolated PTE cells.
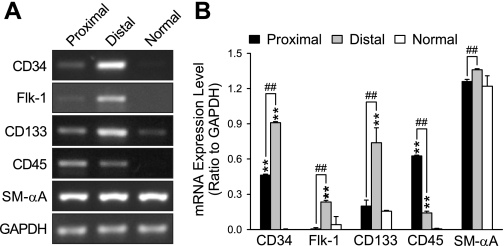
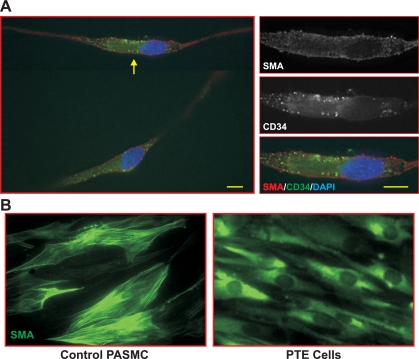
Since the numbers of marker-positive cells found in endarterectomized tissues were low, the expression of these phenotypical markers was studied in cells isolated from PTE tissues and propagated in culture. RT-PCR was performed to detect transcripts for CD34, Flk-1, CD133, and CD45 as selected typical markers used to define putative EPCs or CFU-ECs. PASMCs isolated from normal subjects were used as controls. The data (Fig. 7, A and B) show that cells from both vascular regions, proximal and distal to the thromboemboli, expressed very high levels of SM-αA. It should be noted, however, that many of the cells positive for SM-αA did not exhibit typical filament structure as in mature or contractile phenotype of vascular smooth muscle cells (Fig. 8). These data are consistent with the large number of SM-αA+ cells observed in IHC (Fig. 3, A–D). Cells from the proximal region expressed CD34, low levels of Flk-1, CD133, and a relatively high level of CD45, whereas cells from distal regions expressed high levels of CD34, Flk-1, CD133, and low levels of CD45. The data support our immunostaining results (Fig. 3A). CD34, Flk-1, and CD45 were not detected in normal PASMCs (Fig. 7, A and B). In addition, we also identified CD11b expression using RT-PCR in both proximal and distal vascular tissues, which is suggestive of the presence of monocytes (data not shown).
Fig. 7.
Putative EPCs in isolated CTEPH cells were identified by RT-PCR. Transcripts for phenotypical cell type markers CD34, Flk-1, CD133, CD45, and SM-αA were detected in isolated cells by RT-PCR. A: representative images of RT-PCR for markers from proximal and distal vascular cells and “normal” human PASMCs. GAPDH was used as an internal control. B: the PCR products, expressed as a ratio of GAPDH, are compared between cells isolated from the proximal and distal vascular tissues. Normal PASMCs were used as a control. **P < 0.01 vs. Normal; ##P < 0.01 between Proximal and Distal tissues. n = 6 samples.
Fig. 8.
Cells isolated from PTE tissues exhibit diffuse SM-αA staining. A: double immunostaining using anti-CD34 (green) and SM-αA (red) antibodies was performed on isolated CTEPH cells. CD34+SM-αA+ cells were identified from cells isolated from proximal vascular wall. Higher magnification images of the cell indicated by the arrow are shown in the panels on the right. SM-αA signals are diffused in the cytoplasm (top), whereas CD34 signals show an aggregated or clustered pattern (middle and bottom). Scale bars represent 10 μm. B: representative images showing filamentous SM-αA staining in control human PASMCs (left) and diffuse SM-αA staining in PTE cells (right).
DISCUSSION
In the present study, endarterectomized tissues were collected from CTEPH patients who underwent PTE surgery. We carefully examined the histological characteristics and cellular compositions of the unresolved thromboemboli (reddish coagulant material attached to the central pulmonary artery) and the vascular wall tissues adjacent (proximal) and downstream (distal) to the thromboemboli. The histological data are consistent with observations previously reported including intimal thickening/remodeling of both the larger and smaller pulmonary arteries (4, 26, 37). In this investigation, we report that the larger vessel intimal remodeling could involve dispersed myofibroblast and/or smooth muscle cell (SM-αA+) proliferation and migration, whereas distal intimal remodeling typically involved neovascularization or recanalization. Our data showed recanalized areas with channels surrounded by vWF+ and SM-αA+ cells suggesting that the occluded regions acquire cells with the potential to form new channels. The data indicate that these cells are putative EPCs, which are known to be involved in the de novo formation of vessels.
Based on these histological characteristics of the occluded vessel wall, we hypothesized that progenitor cells may be trapped or have migrated to the thromboemboli. Indeed the stabilized thromboemboli may provide a unique “niche” for differentiation of these cells in patients with CTEPH. CD34+Flk-1+ cells, which may be putative EPCs, were present in the occluded vessel wall in addition to cells positive for CD45 or procollagen-1 in the vascular wall adjacent to thromboemboli. The SM-αA+ cells present in the neointima of occluded vessels may be migrated smooth muscle cells from medial layer or fibroblasts from the adventitial layer (33). Fibroblasts have been recently shown to have proangiogenic properties regulating vasa vasorum neovascularization that occurs in pulmonary artery adventitia during periods of chronic hypoxia (6). Detection of CD45+ (leukocyte common antigen) cells may indicate infiltration of inflammatory cells such as leukocytes or monocytes into the vascular wall (18). The accumulation of leukocytes/monocytes in this region could release proinflammatory cytokine macrophage chemoattractant protein-1 (MCP-1; Ref. 18) and interleukins 1 and 6 (14), which can potentially act as chemoattractants for the migration of fibroblasts or smooth muscle cells. Procollagen-1+ cells are collagen-secreting, providing an ECM, which may contribute to the establishment of a microenvironment for SM-αA+ cell proliferation. It is possible that at least some of the SM-αA+ cells we found in endarterectomized tissues are transdifferentiated from “injured ECs” in the intimal layer. This small percentage of ECs may originate from bone marrow-derived progenitor cells or resident vascular wall progenitor cells, which can incorporate into endothelial monolayer and differentiate into ECs (8, 9). These observations support the possibility that bone marrow-derived outgrowth EPCs may be present in the endarterectomized tissue and that they may contribute to the neointimal formation in CTEPH patients. Furthermore, the resident vascular wall progenitor cells described by Zengin and colleagues (40) could also contribute to the vascular remodeling observed in these patients (40). Future studies will be necessary to determine the presence of functional progenitor cells in the endarterectomized tissues. Another potential source of SM-αA+ cells are differentiated mesenchymal progenitor cells, positive for markers such as CD105, CD73, and CD90 (7).
The presence of CD34+Flk-1+ cells may explain the neoangiogenesis or recanalization in distal vascular tissue. There are several possible origins for such putative vascular progenitor cells: bone marrow-derived EPCs (BM-EPCs; Ref. 32), VW-EPCs (40), early/late outgrowth EPCs (28), CFU-ECs, or resident microvascular EPCs (1). BM-EPCs are a heterogeneous group of cells that are most frequently characterized by the expression of surface markers, such as CD34, Flk-1, and CD133 in addition to their clonogenic potential (10). VW-EPCs are CD34+Flk-1+Tie-2+ and found in a distinct zone of human adult vascular wall, which is localized between smooth muscle and adventitial layers (40). Our immunostaining data showed the cells we found in endarterectomized tissues to be CD34+Flk-1+CD133+ but not to express Tie-2. Peichev and colleagues (29) have demonstrated CD34+Flk-1+CD133+ cells to be functional EPCs. We believe that it is likely that the cells identified in our study represent putative EPCs, potentially CFU-ECs or resident intima-derived EPCs. The high frequency at which the CFU-EC colony morphology (Fig. 6E) is observed in our low-passage cultures is supportive of this. It must be noted that, due to the low quantities of cells derived from the available PTE tissues, the appropriate functional studies on the CD34+Flk-1+CD133+ cells to support their identity has not been carried out as part of the current study. We are performing a complex new series of experiments that will include a comparison the PTE-retrieved cells with respective positive controls for the potential sources and function of these cells.
It is noteworthy that, despite a comparable number of CD34+Flk-1+CD133+ cells being found in the proximal and distal PTE regions (Fig. 6C), recanalization was rarely observed in proximal PTE tissue. This indicates that at least two distinct microenvironments exist in the occluded vascular wall region; each region could provide a specific environment that differentially regulates the fate of these progenitor type cells. The presence of inflammatory cells, collagen-secreting cells, and secreted ECM proteins (such as fibrin, collagen, and elastin; Ref. 4) in the proximal region may be favorable for proliferation and differentiation of progenitor cells and myofibroblasts. It has been shown that, in setting of tissue ischemia or endothelial damage, EPCs mobilized from the bone marrow into the circulation home to sites of injury and incorporate into foci of neovascularization (1, 39). Although this promises therapeutic benefit in patients with pulmonary hypertension, we provide evidence that in CTEPH the very unique microenvironment, while recruiting progenitor cells for revascularization of the tissues, potentially harbors a pathogenic role in supporting vascular remodeling. Interestingly, recent reports show that PAECs isolated from patients with IPAH exhibit an unusual hyperproliferative and dysfunctional angiogenic potential (24, 35). It is possible that the PAECs in the intimal layer of distal PTE tissues may have a similar dysfunctional potential and thus contribute to angiogenesis.
In summary, our study shows the presence of putative EPCs, CD34+Flk-1+CD133+ cells, in the PTE tissue from patients with CTEPH. Further studies are necessary to fully determine 1) the precise identity and function of the progenitor cells identified in this study, and 2) the factors that stimulate the recruitment of progenitor cells in the regions of the pulmonary arteries in close proximity to the occluded area.
GRANTS
This project is supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL-066012, HL-066012, and HL-054043 to J. X.-J. Yuan). A. L. Firth is supported by a postdoctoral fellowship from the California Institute for Regenerative Medicine (CIRM).
Acknowledgments
We thank Dr. Nigel K. Woolf for providing the cryostat and microscope for the histological studies.
REFERENCES
- 1.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Asosingh KA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, Anand-Apte B, Yoder MC, Tuder RM, Erzurum SC. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 172: 615–627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becattini CA, Pesavento R, Silingardi M, Poggio R, Taliani MR, Ageno W. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 130: 172–175, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Blauwet LA, Tazelaar HD, McGregor CG. Surgical pathology of pulmonary thromboendarterectomy: a study of 54 cases from 1990 to 2001. Hum Pathol 34: 1290–1298, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bonderman DT, Jakowitsch J, Weltermann A, Adlbrecht C, Schneider B, Kneussl M, Rubin LJ, Kyrle PA, Klepetko W, Maurer G, Lang IM. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost 90: 372–376, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Davie NJ, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol 168: 1793–1807, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici ML, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Stenmark KR. Circulating mononuclear cells with a dual, macrophage-fibroblast phenotype contribute robustly to hypoxia-induced pulmonary adventitial remodeling. Chest 128: 583S–584S, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich EB, Scharlau J, Nickenig G, Werner N. CD34−/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res 98: e20–e25, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, Strieter RM. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol 176: 1916–1927, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hayashida KF, Miyake Y, Kawada H, Ando K, Ogawa S, Fukuda K. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest 127: 1793–1798, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hu YZ, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113: 1258–1265, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 151: 1628–1631, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 556: 249–252, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson S, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: experience and lessons learned in 1500 cases. Ann Thorac Surg 76: 1457–1462, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Kim C, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H, Okada O, Tanabe N, Tanaka Y, Terai M, Takiguchi Y, Masuda M, Nakajima N, Hiroshima K, Inadera H, Matsushima K, Kuriyama T. Plasma monocyte chemoattractant protein-1 and pulmonary vascular resistance in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 164: 319–324, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kramm T, Mayer E, Dahm M, Guth S, Menzel T, Pitton M, Oelert H. Long-term results after thromboendarterectomy for chronic pulmonary embolism. Eur J Cardiothorac Surg 15: 579–583, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Kunieda T, Nakanishi N, Satoh T, Kyotani S, Okano Y, Nagaya N. Prognoses of primary pulmonary hypertension and chronic major vessel thromboembolic pulmonary hypertension determined from cumulative survival curves. Intern Med 38: 543–546, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Lama V, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, Peters-Golden M, Pinsky DJ, Martinez FJ, Thannickal VJ. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117: 989–996, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang I, Marsh JJ, Olman MA, Moser KM, Loskutoff DJ, Schleef RR. Expression of type 1 plasminogen activator inhibitor in chronic pulmonary thromboemboli. Circulation 89: 2715–2721, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Margariti A, Zeng L, Xu Q. Stem cells, vascular smooth muscle cells and atherosclerosis. Histol Histopathol 21: 979–985, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Masri F, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548–L554, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, Dainelli A, Giuntini C. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after pulmonary embolism. Medicine (Baltimore) 85: 253–262, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 103: 685–692, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Moser KM, Braunwald NS. Successful surgical intervention in severe chronic thromboembolic pulmonary hypertension. Chest 64: 29–35, 1973. [DOI] [PubMed] [Google Scholar]
- 28.Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, Kobayashi A, Yamaguchi T, Abe M, Amagasa T, Morita I. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res 314: 430–440, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95: 952–958, 2000. [PubMed] [Google Scholar]
- 30.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 350: 2257–2264, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro A, Lindmarker P, Johnsson H, Juhlin-Dannfelt A, Jorfeldt L. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 99: 1325–1330, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood 92: 362–367, 1998. [PubMed] [Google Scholar]
- 33.Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol 286: C416–C425, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Stenmark K, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology 21: 134–145, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Stevens T, Gillespie MN. The hyperproliferative endothelial cell phenotype in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L546–L547, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Torsney E, Mandal K, Halliday A, Jahangiri M, Xu Q. Characterisation of progenitor cells in human atherosclerotic vessels. Atherosclerosis 191: 259–264, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Yi E, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension: a morphometric and immunohistochemical study. Am J Respir Crit Care Med 162: 1577–1586, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Yin A, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90: 5002–50012, 1997. [PubMed] [Google Scholar]
- 39.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res 78: 413–421, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133: 1543–1551, 2006. [DOI] [PubMed] [Google Scholar]