Abstract
We investigated the regulatory role of 14-kDa secretory group V phospholipase A2 (gVPLA2) in the development of acute lung injury (ALI) and neutrophilic inflammation (NI) caused by intratracheal administration of LPS. Experiments were conducted in gVPLA2 knockout (pla2g5−/−) mice, which lack the gene, and gVPLA2 wild-type littermate control (pla2g5+/+) mice. Indices of pulmonary injury were evaluated 24 h after intratracheal administration of LPS. Expression of gVPLA2 in microsections of airways and mRNA content in lung homogenates were increased substantially in pla2g5+/+ mice after LPS-administered compared with saline-treated pla2g5+/+ mice. By contrast, expression of gVPLA2 was neither localized in LPS- nor saline-treated pla2g5−/− mice. LPS also caused 1) reduced transthoracic static compliance, 2) lung edema, 3) neutrophilic infiltration, and 4) increased neutrophil myeloperoxidase activity in pla2g5+/+ mice. These events were attenuated in pla2g5−/− mice exposed to LPS or in pla2g5+/+ mice receiving MCL-3G1, a neutralizing MAb directed against gVPLA2, before LPS administration. Our data demonstrate that gVPLA2 is an inducible protein in pla2g5+/+ mice but not in pla2g5−/− mice within 24 h after LPS treatment. Specific inhibition of gVPLA2 with MCL-3G1 or gene-targeted mice lacking gVPLA2 blocks ALI and attenuates NI caused by LPS.
Keywords: airway inflammation, chemotaxis, pulmonary compliance
secretory phospholipase a2s (PLA2) are a family of lipolytic enzymes that catalyze the cleavage of fatty acids from the sn-2 position of phospholipids leading to the generation of free fatty acids and lysophospholipids (6, 9, 20, 27, 33, 36, 38). There are at least 12 isoforms of secreted PLA2 in mice (27, 33, 36, 38); all of these isoforms are expressed in humans. Among the secreted PLA2s, gIIaPLA2, gVPLA2, and gXPLA2 have been implicated in a variety of inflammatory lung diseases, e.g., acute lung injury (3, 10, 21, 40), acute respiratory distress syndrome (11, 37), sepsis (1, 41), and asthma (12, 24, 30). However, the relative contributions of secretory PLA2s in these diseases remain unclear, as disparate findings have been demonstrated (3, 12, 24, 27, 30, 37, 40, 41).
Increased concentrations of gIIaPLA2 are detected in sepsis (1, 41), rheumatoid arthritis (34), and ARDS (11, 37). However, LY-31592ONA/S-5920 ({[3-(aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy} acetate), an inhibitor of 14-kDa secretory PLA2s, did not improve survival in patients with severe sepsis (41), even though LY-31592ONA/S-5920 blocked oleic acid-induced acute lung injury (ALI) in rabbits (10). Previous investigations have reported that snake venom sPLA2 causes ALI in rats (31, 35) and that this enzyme hydrolyzes phospholipids in lung surfactant (5).
Secretory gVPLA2 is a 14-kDa protein and structurally similar to gIIaPLA2 (6, 20, 27, 33, 38). However, gVPLA2 has ∼50-fold the hydrolytic capacity for outer cell surface phosphatidylcholine-rich plasma membrane compared with gIIaPLA2 in cell-free systems (13, 15). gVPLA2 is a proinflammatory enzyme that has diverse biological effects including airway inflammation (24), airway hyperresponsiveness (24), cell adhesion (25, 39), transcellular communication (25, 39), and generation of lipid mediators (4, 16, 17, 23, 25, 32, 39).
Secretory gVPLA2, which is not expressed in eosinophils (23, 25, 26, 39), is contained in neutrophils, epithelial cells, airway smooth muscle, T cells, and macrophages (7, 14, 20, 24–27, 33, 36). There is no known receptor for gVPLA2 (4, 20, 23–27, 33); gVPLA2 binds to the outer cell membrane due to its high affinity to its interfacial binding site located at Trp-31 (13, 16, 17, 23–25). Mutation of Trp-31 to alanine reduces the hydrolytic activity of gVPLA2 (13, 16, 17, 23–25). Internalization of gVPLA2 by binding to heparan sulfate proteoglycan further hydrolyzes the nuclear membrane adding more arachidonic metabolites to the external membrane hydrolysis products, which have been taken up by the cell (16).
Deletion of the gene encoding gVPLA2 attenuates eosinophilic infiltration and airway hyperresponsiveness in allergic mice (24). However, there have been no studies on the effects of gVPLA2 in ALI and neutrophilic inflammation (NI). In this study, we investigated the effect of LPS-induced gVPLA2 on ALI and NI using pla2g5−/− knockout (KO) and pla2g5+/+ littermate control mice. We found 1) that gVPLA2 synthesis is induced by LPS administration and 2) an association between gVPLA2 activity and the severity of ALI as measured by change in pulmonary compliance, neutrophilic infiltration, and neutrophil activation. Our data are the first demonstration that 14-kDa secretory gVPLA2 is an intracellular messenger protein in regulating LPS-induced vascular leak in mice in vivo.
MATERIALS AND METHODS
Animals
Homozygous gVPLA2 KO (pla2g5−/−) and littermate control (pla2g5+/+) were derived from C57/BL6 mice as previously described (4, 32). C57BL/6 mice, aged 10–12 wk old, were maintained on standard laboratory chow ad libitum. pla2g5−/− were bred in the University of Chicago Animal Facility. Briefly, mice heterozygous for disruption of the gene encoding group V sPLA2 (pla2g5) were crossed to a C57/BL6 background. Mating pairs of pla2g5−/− and pla2g5+/+ mice were derived from the litters of C57/BL6 heterozygous breeding pairs. Experimental protocols conformed to the principles outlined by the Animal Welfare and the National Health services guidelines for the care and use of animal in biomedical research and were approved by the University of Chicago IACUC Review Board.
RT-PCR
An ∼2-mm tail piece was cut from each weaning. DNA was isolated, and genotyping was performed using PCR. Mice with the required genotype, gVPLA2 littermate control (pla2g5+/+) and gVPLA2 KO (pla2g5−/−), were kept until immunologically mature. Primers used for gVPLA2 were forward, 5′-GATGCACGACCGTTGTTATG-3′, reverse, 5′-TAATCTAATGGAAGAGCCTCAGGT-3′.
Determination of gVPLA2 expression
mRNA expression for gVPLA2.
Total RNA was isolated using TRIzol reagent (Life Technologies, Rockville, MD). cDNA was synthesized using 0.5 μg of total RNA in a 20-μl reverse transcriptase reaction using iScript cDNA synthesis kit (Bio-Rad Laboratories; Hercules, CA). The primers for gVPLA2 were sense 5′-GTAACCCGTTGAACCCCATT-3′, antisense 5′-GGTCTAGAGTCAGTCACACTTGGGCG-3′.
Immunohistochemistry.
Lungs were fixed by inflation with 10% paraformaldehyde solution at a pressure of 20 cmH2O and embedded in paraffin. Briefly, lung tissues, ∼5- to 6-μm-thick sections, were subsequently cut and processed as previously described (24). Endogenous peroxidase activity and nonspecific antibody binding were prevented by incubating the tissue section for 15 min in 0.5% hydrogen peroxide solution, methanol (vol/vol), and normal goat serum (1/5 dilution). Slides containing lung tissues were incubated for 1 h in primary antibody (MCL-3G1) or IgG1 antibody (negative control) diluted in normal saline solution (26). Antibody binding was localized with a biotinylated secondary antibody, avidin-conjugated HRP, and diaminobenzidine chromogenic substrate (Vector Laboratories, Burlingame, CA). Stained airway microsections prepared from treated pla2g5+/+ or pla2g5−/− mice were examined for the presence of gVPLA2 using light microscopy.
Murine Model of ALI
Preparation of LPS.
Purified LPS from Escherichia coli (Sigma-Aldrich, St. Louis, MO) was dissolved in 10 ml of endotoxin-free saline. The mixed solution was placed in a 37°C shaking water bath for 30 min and was subjected to sonication for 2 min in an ultrasonic water bath to obtain a homogenous solution. The LPS stock solution was aliquoted to the desired volume and stored in −20°C. The final dose of LPS used in this study was 10 mg/kg per intratracheal administration.
Preparation of animals.
gVPLA2 wild-type littermate control (pla2g5+/+) and KO (pla2g5−/−) mice were anesthetized with ketamine-xylazine (100 mg/kg:20 mg/kg) (24) and were instilled through a catheter inserted into the trachea with either sterile saline solution or 10 mg/kg LPS. The dose of LPS was chosen to be consistent with previous studies wherein the greatest neutrophil emigration in the lungs was achieved. Animals were studied 24 h after administration of LPS.
Generation of Pressure-Volume Curves (Transthoracic Static Compliance)
Mice weighing 25–32 g were anesthetized with intraperitoneal ketamine-xylazine (100 mg/kg:20 mg/kg). The animals were placed in a supine position, and a tracheostomy was performed using an 18-gauge needle secured with a 3–0 suture. The intubated animal was connected to a computer-controlled small animal ventilator (Flexivent; Scireq, Vancouver, Canada) that delivered a tidal volume of 6 ml/kg at a frequency of 120 breaths/min (24); the mice were allowed to breathe 100% oxygen for 5 min. The ventilator was stopped, and the tube connected to the trachea was clamped to ambient air. Lungs were inflated with ≥30 cmH2O transthoracic pressure, and volume was gradually decreased in 0.1-ml increments. The static lung compliance was determined by recording the lung pressure change associated with each volume change in the system when the trachea was occluded to ambient air (zero flow). Two or more full inflation-deflation cycles were determined for each mouse to achieve a volume history, and the pressure-volume characteristics of the lungs were generated.
Determination of Wet:Dry Weight Ratio (Lung Fluid Accumulation)
Animals treated as above were killed, and lungs were immediately excised, blotted dry, and weighed. Lung dry weights were recorded after treatment in an oven for 7 days at 65°C. The lung wet:dry weight ratio was determined by dividing the wet weight by the dry weight. The data were normalized to wild-type (WT) control where 1 = 0 represents no fluid accumulation in the lung.
Total and Differential Inflammatory Cell Counts
NI was assessed 24 h after LPS treatment in 1) pla2g5+/+ mice alone, no MCL-3G1, 2) pla2g5+/+ mice + MCL-3G1 MAb, or 3) pla2g5−/− mice alone. The trachea was exposed and cannulated with an 18-gauge needle; bronchoalveolar lavage (BAL) was performed by injecting 0.8 ml of PBS into the lung and gently aspirating the fluid. The procedure was performed 3× to recover a total volume of 2–3 ml. The BAL fluid samples were centrifuged to obtain the cell pellet until analysis. Cytoslides were prepared and stained with Diff-Quick (Dade Diagnostics, Deerfield, IL), and the total and differential cells counts were determined using a hemocytometer (24).
BAL cells were harvested, and the number of migrated neutrophils was determined by flow cytometric analysis. Briefly, cells were washed with cold FACS buffer (PBS with 1% BSA and 0.1% NaN3) and centrifuged at 400 g for 10 min. The pellets were washed with FACS buffer once before adding 4 μl of Gr-1 (clone RB6-8C5; EBioscience, San Diego, CA), a MAb that has been used to identify monocytes and peripheral blood PMNs. Thirty minutes later, the cells were washed 3× with FACS buffer and incubated with FITC-conjugated goat anti-mouse Ig for 30 min at 4°C. Treated cells were fixed in 1% paraformaldehyde solution until analysis (22).
Determination of Total Lung MPO Content
The activity of neutrophil MPO (22) was determined from homogenized lungs of treated animals. MPO activity was proportional to the content of MPO in PMNs obtained from BAL fluid. Briefly, 50 μl of lung homogenates was added to 100 μl of HBSS + 10% FBS buffer and 100 μl of developing solution (8 ml 100 nM NaH2PO4, pH 5.5, 1,000 μl 10% hexadecyltrimethyammonium bromide, 3 μl 30% hydrogen peroxide, 1,000 μl 10% o-dianisidine dihydrochloride). The reaction mixture was terminated by addition of 50 μl of sulfuric acid; absorbance was measured at 405 nm in a microplate reader (Thermomax; Molecular Devices, Menlo Park, CA). A standard curve was generated on each sample plate, and linear regression curve was generated.
Data Analysis
All values were expressed as means ± SE. The Student's t-test was used for comparison between two groups. When more than two groups were compared, differences among the groups were determined by one-way ANOVA. The differences between groups were considered significant if P < 0.05.
RESULTS
Expression of Secretory gVPLA2
We first examined the expression of gVPLA2 in airway microsections obtained from gVPLA2 wild-type littermate control (pla2g5+/+) mice 24 h after LPS administration. Representative photomicrographs (n = 6) demonstrated that LPS caused upregulation of gVPLA2 expression as determined by immunohistochemical staining (Fig. 1). Intracellular gVPLA2 was detected in abundant quantities in microsections of pla2g5+/+ airways receiving LPS (Fig. 1A). gVPLA2 was weakly expressed in saline-treated pla2g5+/+ airways or parenchyma (Fig. 1B) compared with LPS-treated pla2g5+/+ mice. Expression of gVPLA2, as indicated by dark brown color, was evident in peripheral airways and lung parenchyma (Fig. 1A). IgG1, an isotype-matched, irrelevant antibody, was used as negative control (Fig. 1, A and B).
Fig. 1.
Localization of group V phospholipase A2 (gVPLA2) expression caused by intratracheal administration of LPS or sterile saline in murine airways. gVPLA2 littermate control (pla2g5+/+) or gVPLA2 knockout (pla2g5−/−) mice were treated with LPS or saline control for 24 h, and gVPLA2 expression was determined by immunohistochemical analysis. Microsections of airways and lung parenchyma were stained with either MAb directed against gVPLA2 (A–D, left) or isotype-match control, IgG1 (A–D, right). Representative photomicrographs from 6 airway microsections of pla2g5+/+ mice were shown that gave similar results. Original magnification, ×100 pixel. Ab, antibody.
To confirm further the specificity of MAb directed against gVPLA2, airway microsections from saline- and LPS-treated gVPLA2-KO mice (pla2g5−/−) were stained with MCL-3G1 MAb or IgG1 antibody. Expression of gVPLA2 was neither localized for pla2g5−/− mice receiving saline nor LPS (Fig. 1, C and D). Negative control (IgG1 stained) is shown in Fig. 1, C and D.
We next determined the mRNA expression for gVPLA2 in lung tissues obtained from these same animals by RT-PCR analysis (32). In pla2g5+/+ mice, mRNA expression for gVPLA2 was upregulated after LPS exposure compared with saline-exposed mice (Fig. 2). All data were normalized per 18S housekeeping gene and expressed as the ratio of gVPLA2 mRNA/18S. mRNA expression for gVPLA2 was 0.97 ± 0.36-fold/18S for pla2g5+/+ mice after LPS administration compared with baseline control expression of 0.48 ± 0.20-fold/18S for saline-treated pla2g5+/+ mice (P < 0.05). By contrast, mRNA expression for gVPLA2 was 0.006 ± 0.002-fold/18S for pla2g5−/− mice after LPS treatment (P <0.001 vs. LPS-treated pla2g5+/+ mice) and 0.005 ± 0.002-fold/18S for pla2g5−/− mice having saline treatment (P <0.001 vs. LPS-treated pla2g5+/+ mice).
Fig. 2.
Effect of LPS on mRNA expression for gVPLA2. mRNA expression for gVPLA2 caused by LPS in wild-type (WT) littermate control (pla2g5+/+) or gVPLA2 knockout (pla2g5−/−) mice was measured in lung homogenates by RT-PCR as described in materials and methods. The upregulation of mRNA was normalized as the ratio of mRNA/18S compared with vehicle-stimulated control airways. RT-PCRs were performed in triplicate. The data represent the means ± SE of 6 samples; *P < 0.05 and **P < 0.001 by Student's t-test.
Transthoracic Static Compliance: Pressure-Volume Curves
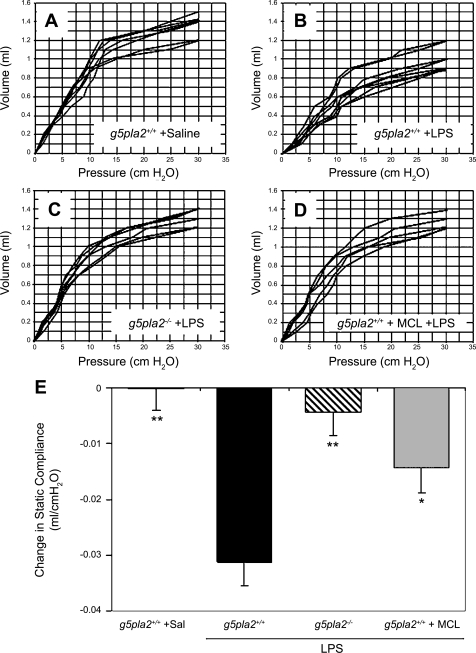
We next measured the transthoracic static compliance, an index of lung edema caused by ALI. Pressure-volume (P-V) curves were generated in 1) saline-treated pla2g5+/+ mice (n = 7 mice), 2) LPS-treated pla2g5+/+ mice (n = 8 mice), 3) LPS-treated pla2g5−/− mice (n = 6 mice), and 4) MCL-3G1 plus LPS-treated pla2g5+/+ mice (n = 5 mice) in vivo. Lung volume in experimental animals was measured beginning at ≥30 cmH2O, which corresponded to total lung capacity (Fig. 3, A–D).
Fig. 3.
Effects of neutralizing MAb against gVPLA2 in wild-type littermate control (pla2g5+/+) mice, or gVPLA2 knockout (pla2g5−/−) mice reduced transthoracic static compliance caused by intratracheal administration of LPS. gVPLA2 littermate control (pla2g5+/+) mice were treated with saline (A) or 10 mg/kg LPS (B), and pressure-volume (P-V) curves were generated after 24 h. In other studies, LPS was instilled intratracheally into gVPLA2 knockout (pla2g5−/−) mice before generation of P-V curves (C). Blockade of decrease in transthoracic lung compliance was measured by pretreating the wild-type (pla2g5+/+) mice with MCL-3G1 before LPS administration and P-V curve measurement (D). E: decrease in transthoracic static compliance caused by LPS treatment vs. control. Measurements are means ± SE at 10 cmH2O for each group. Pla2g5+/+ is a saline control group receiving no LPS. *P < 0.05 compared with LPS-treated pla2g5+/+ mice; **P < 0.01 compared with LPS-treated pla2g5+/+ mice.
Individual deflation P-V curves for each animal studied are shown in Fig. 3, A—D. LPS caused substantial reduction in transthoracic static compliance. Administration of intratracheal LPS into pla2g5+/+ wild-type mice 24 h before measurement caused a rightward shift of P-V curves (Fig. 3B) compared with baseline P-V curves generated from saline-treated pla2g5+/+ mice (Fig. 3A). Deletion of the gene encoding gVPLA2 (Fig. 3C) or blockade with MCL-3G1 (Fig. 3D) significantly blocked the increased lung stiffness caused by LPS. There was no difference between P-V curves generated from LPS-treated KO (pla2g5−/−) mice (Fig. 3C) vs. wild-type littermate control (pla2g5+/+) mice treated with MCL-3G1 before LPS administration (Fig. 3D), which were both comparable to baseline (no LPS) P-V curves (Fig. 3A).
For more precise analysis, composite changes in transthoracic static compliance were derived from the linear portion of the P-V curves in each group. Transthoracic static compliance was measured at 10 cmH2O (Fig. 3E). Static compliance in pla2g5+/+ mice decreased by −0.03 ± 0.004 cmH2O after LPS treatment (P < 0.01 vs. pla2g5+/+ + saline). This reduction in transthoracic static compliance caused by LPS was attenuated to 0.004 ± 0.004 cmH2O in gVPLA2-deficient mice treated with LPS (P < 0.01 vs. LPS-treated pla2g5+/+ mice) and to 0.014 ± 0.005 cmH2O for pla2g5+/+ mice pretreated with MCL-3G1 before LPS treatment (P < 0.05 vs. LPS-treated pla2g5+/+ mice not pretreated with MAb).
LPS-Increased Lung Fluid (Wet:Dry Weight Ratio)
We also determined the total lung wet:dry (W/D) weight ratio of the excised lungs from all treated groups (Fig. 4). Intratracheal instillation of LPS into pla2g5+/+ mice induced an increase in lung fluid at 24 h as reflected in the W/D weight ratio compared with saline-treated mice. The W/D weight ratio, normalized to WT control, increased from 1.0 ± 0.06 (saline-treated) to 1.32 ± 0.02 after LPS stimulation (P < 0.05). Edema formation was substantially attenuated to a ratio of 1.04 ± 0.05 when LPS was administered to gVPLA2 KO mice (P < 0.01 vs. LPS-treated pla2g5+/+ mice; P = not significant vs. saline-treated pla2g5+/+). Although less effective, MCL-3G1 MAb significantly reduced the W/D weight ratio to 1.17 ± 0.07 in LPS-treated pla2g5+/+ mice (P < 0.05 vs. LPS-treated pla2g5+/+ mice).
Fig. 4.
Effects of neutralizing MAb against gVPLA2 in wild-type littermate control (pla2g5+/+) mice or gVPLA2 knockout (pla2g5−/−) mice on increased wet:dry weight ratio 24 h after LPS administration. *P < 0.05 compared with LPS-stimulated pla2g5+/+ mice; **P < 0.01 compared with LPS-stimulated pla2g5+/+ mice. Data are normalized to WT control wherein 1 = 0 represents no lung edema.
LPS-Induced Neutrophilic Inflammation: Histological Examination
Histological examination revealed increased cellular infiltrates in lungs of mice receiving intratracheal LPS (Fig. 5A); these changes were much less pronounced in saline-treated pla2g5+/+ mice (Fig. 5B). Airway neutrophilic inflammation caused by LPS activation was substantially attenuated in gVPLA2 KO mice (pla2g5−/−; Fig. 5C) and in pla2g5+/+ mice treated with MCL-3G1, a MAb directed against gVPLA2 (Fig. 5D) before LPS treatment.
Fig. 5.
Effects of neutralizing MAb against gVPLA2 in wild-type littermate control (pla2g5+/+) mice or gVPLA2 knockout (pla2g5−/−) mice on cell inflammation caused by LPS stimulation. Cell infiltration in littermate control (pla2g5+/+) mice + LPS (A), pla2g5+/+ mice + saline (B), pla2g5−/− mice + LPS (C), or pla2g5+/+ mice + MCL-3G1 + LPS (D) was analyzed using hematoxylin and eosin dye staining. Original magnification, ×100 pixel. Inset: ×350 pixel (original magnification from the same airway microsection). Dark blue cells with lobulated nuclei are neutrophils (see Figs. 6 and 7).
Cell Infiltration in BAL Fluid
Intratracheal instillation of LPS caused pulmonary inflammation characterized by cellular infiltrates into the alveolar space, as reflected by the increased number of neutrophils recovered in the BAL fluid (Fig. 6). NI was attenuated in gVPLA2 KO mice and MCL-3G1-treated mice; NI was not observed in saline-treated pla2g5+/+ mice. Quantification of the number of cellular inflammatory cells in BAL was assessed by differential cell counting after Diff-Quick staining (Fig. 6). LPS alone caused some NI even in pla2g5−/− mice (see discussion). However, total BAL fluid cell number was greater in LPS-treated pla2g5+/+ littermate controls than pla2g5−/− mice even after instillation with the same concentration of LPS (Fig. 6A). Baseline (pla2g5+/+ + saline) total cell count was 0.20 × 106 ± 0.05 cells and increased to 5.33 × 106 ± 1.27 cells after LPS administration (P < 0.01). Total cell number caused by LPS in gVPLA2 KO (pla2g5−/−) mice decreased to 2.73 × 106 ± 0.87 cells (P < 0.05 vs. LPS-treated pla2g5+/+ mice) and 3.17 × 106 ± 0.87 cells for wild-type pla2g5+/+ mice receiving MCL-3G1 before LPS exposure (P < 0.05 vs. LPS-treated pla2g5+/+ mice).
Fig. 6.
Effects of neutralizing MAb against gVPLA2 in wild-type littermate control (pla2g5+/+) mice or gVPLA2 knockout (pla2g5−/−) mice on total or differential cell count on BAL fluid caused by LPS administration. A: total cell count in BAL fluid. B: number of neutrophils in BAL fluid. C: number of macrophages in BAL fluid. *P < 0.05 compared with LPS-treated wild-type (pla2g5+/+) mice; **P < 0.01 compared with LPS-treated pla2g5+/+ mice.
Differential cell count demonstrated that neutrophils were the predominant cell type recovered from BAL fluid after LPS (Fig. 6B). Macrophages increased to a lesser extent, but this difference was not statistically significant (Fig. 6C). The number of neutrophils in the BAL fluid increased from 0.004 × 106 ± 0.003 cells (saline-treated) to 5.07 × 106 ± 1.25 cells after LPS administration (P < 0.01). A ≥50% reduction in neutrophil migration from basal count was observed in LPS-treated KO (pla2g5−/−) mice (2.43 × 106 ± 0.77 cells; P < 0.05 vs. LPS-treated pla2g5+/+ mice) and 2.95 × 106 ± 0.74 cells in pla2g5+/+ treated with MCL-3G1 + LPS (P < 0.05 vs. LPS-treated pla2g5+/+ mice).
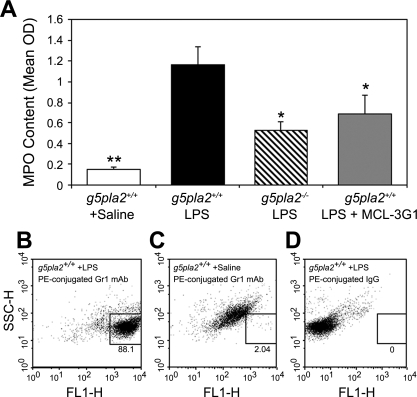
LPS-Increased MPO Activity
MPO content, which is linearly proportional to its activity (see materials and methods), was substantially increased by LPS in pla2g5+/+ mice and attenuated by specific blockade of gVPLA2 and in pla2g5−/− mice (Fig. 7A). Lung MPO concentration, expressed as optical density per 150 μg/ml protein (OD), increased to 1.17 ± 0.02 OD after LPS administration compared with 0.15 ± 0.02 OD for saline-treated pla2g5+/+ mice (Fig. 7A; P < 0.01). The concentration of MPO in pla2g5−/− mice treated with LPS was 0.52 ± 0.09 OD (P < 0.05 vs. LPS-treated pla2g5+/+ mice). Pretreatment of pla2g5+/+ (wild-type) mice with MCL-3G1 caused comparable decrease in MPO concentration after LPS as for pla2g5−/− mice.
Fig. 7.
Effects of neutralizing MAb against gVPLA2 or gVPLA2 knockout (pla2g5−/−) mice on neutrophil MPO content caused by LPS stimulation. A: MPO content of treated animals. MPO content is linearly proportional to MPO activity (see materials and methods). Data, means ± SE, are expressed as optical density per 150 μg/ml protein, measured in treated lung homogenates. *P < 0.05 compared with LPS-stimulated wild-type pla2g5+/+ mice; **P < 0.01 compared with LPS-stimulated wild-type littermate control (pla2g5+/+) mice. B–D: dot plot is all cells from BAL fluid. Cells contained in the box are solely granulocytes recovered from the BAL fluid. B: immunofluorescence staining of BAL cells from wild-type pla2g5+/+ mice treated with LPS was determined by anti-Gr1 MAb (see materials and methods). C: wild-type pla2g5+/+ treated with saline and stained with PE-conjugated anti-Gr1 MAb. D: wild-type pla2g5+/+ treated with LPS and stained with isotype-matched control (IgG1).
Neutrophils in BAL fluid were identified as likely the source of MPO as determined by flow cytometric analysis (Fig. 7, B–D). Cells contained within the box are granulocytes from BAL fluid after saline or LPS treatment as determined by Gr1, a MAb used to detect the granulocytes including neutrophils. Cells outside the box are other cells in the BAL fluid aside from granulocytes. Granulocytes constituted 88.1% of all cells in the BAL fluid after treatment with LPS. Total cell population in BAL fluid of saline-treated pla2g5+/+ mice showed insignificant numbers of granulocytes (2.04%) as determined by Gr1 MAb staining (Fig. 7C). The isotype-matched IgG (LPS-treated pla2g5+/+ + PE-IgG antibody) served as control for Gr1 MAb alone and also showed no granulocyte infiltrates in BAL fluid (Fig. 7D). Histological examination of cytoslides showed that PMNs were the predominant granulocytes increasing in number in pla2g5+/+ mice.
DISCUSSION
The objective of this investigation was to determine the role of the highly hydrolytic phospholipase, gVPLA2, in mediating ALI induced by LPS. Studies were performed to assess whether LPS causes upregulation of gVPLA2 in murine airways. Further studies were performed to determine if this upregulation of gVPLA2 was responsible for decreased transthoracic static compliance, increased W/D weight ratio, NI, and subsequent neutrophil activation caused by LPS. These physiological and inflammatory effects are commonly used to monitor ALI in animal models of ARDS (11, 21).
We and others (25, 39) previously have shown that gVPLA2 is secreted from epithelial cells to cause eosinophil activation and surface integrin adhesion of eosinophils to endothelial counterligands in vitro. Immune sensitization also was shown to upregulate gVPLA2 expression (24). Blockade of gVPLA2 by specific MAb attenuated substantially this effect in vivo and further blocked airway hyperresponsiveness to methacholine (24). In preliminary studies with neutrophils, we have demonstrated that gVPLA2 also is capable of activating neutrophil adhesion (16, 17). Thus, it is likely that activation of neutrophils caused by this protein results from transport of gVPLA2 from activated endothelium by LPS. However, neutrophils, epithelium, macrophages, T cells, or airway smooth muscle also contain gVPLA2 (7, 14, 16, 17, 19, 20, 24).
Secretory gVPLA2 has not been shown to be chemotactic, so the in vivo process for initial activation remains undefined. Because of the very small blood volume of mice, it has not been possible to measure directly the concentration of gVPLA2 after endothelial or epithelial activation in vivo or to determine which tissues participate in gVPLA2 secretion that can be demonstrated in vitro.
From these studies and others demonstrating that neutrophil cytokines and leukotriene B4 activate neutrophil adhesion (16, 17, 22) and subsequent migration, we postulated that upregulation and activation of gVPLA2 by LPS would cause vascular leak and contribute substantially to lung edema in mice. In a first series of studies, we demonstrated that gVPLA2 content of the lung parenchyma was upregulated substantially within 24 h of exposure to LPS (Fig. 1). No upregulation of gVPLA2 content was demonstrated in gVPLA2 KO mice or in isotype-matched controls. The baseline concentration of mRNA in lungs of littermate controls also was substantially upregulated 24 h after tracheal instillation of LPS (Fig. 2). These data indicate that LPS causes synthesis and upregulation of gVPLA2 content within 24 h.
Upregulation of gVPLA2 by LPS also corresponded to decreased transthoracic static compliance (Fig. 3), which corresponded to an increased W/D weight ratio (Fig. 4) in control mice. The decrease in transthoracic static compliance and increase W/D weight ratio was blocked almost completely by deletion of the gene encoding gVPLA2 (gVPLA2 KO mice) and by specific MAb directed against gVPLA2 (MCL-3G1). These data suggest a substantial role for gVPLA2 in mediation of the response leading to ALI in this murine model.
We also found that cell infiltrates (predominantly neutrophils) into the small peripheral airways and alveolar spaces caused by LPS was blocked substantially by MAb directed against gVPLA2 and were substantially attenuated in gVPLA2 KO mice (Fig. 5). gVPLA2 may serve to amplify neutrophil chemotaxis indirectly, possible by activation of smaller numbers of neutrophils elicited by LPS treatment alone (Fig. 6). A model for this process has been suggested from prior in vitro studies of neutrophil activation by gVPLA2 (16, 17). The precise sequence of events in vivo, however, has not yet been elucidated. Nonetheless, blockade of gVPLA2 by either gVPLA2 KO or neutralizing MAb against gVPLA2 substantially attenuated NI caused by LPS. Although substantial, NI was not completely blocked by these processes (Figs. 6 and 7). The absence of complete blockade of either MPO concentration or all cell migration, even in gVPLA2 KO mice, suggests that the pathogenesis of neutrophil activation is not linked solely to gVPLA2, but may also, to a lesser extent, be caused by LPS alone. Nagase and coworkers (28, 29) demonstrated increased lung elastance in mice treated with hydrochloric acid that was blocked by genetic deletion of the intracellular phospholipase gIVaPLA2, which is activated downstream by gVPLA2 in neutrophils (16, 17). In these studies, some neutrophils still were present in gIVaPLA2 KO mice, even though the indirect measurements of lung compliance were blocked fully and histological sections of lung were similar to those seen in gVPLA2 KO mice (Fig. 5). In these studies, we did not establish the downstream mechanism of gVPLA2 blockade of lung edema caused by LPS. However, it appears that substantial reduction in neutrophil secretion and NI, which is not absolute, may still be sufficient to block the formation of lung edema in ALI.
It is important to consider some further limitations of our findings. These studies are performed in a pathogenetic mouse model, and data cannot be extrapolated to therapeutics in the human situation. We also administered blocking antibody in each study 30 min before LPS administration. It is not clear whether post hoc administration of gVPLA2 MAb would block edema formation. The purpose of this paper was to determine the possible pathogenetic role of gVPLA2 as an effector of ALI. Studies examining the potential of gVPLA2 in preventing incipient or already existent ALI were not performed, as the focus of this study was pathogenesis, not treatment. Such determinations will require administration of gVPLA2 MAb in wild-type mice over multiple time intervals after LPS administration. We believe it is likely that once substantial lung edema develops, reversal will not be possible. Nonetheless, these studies outline some potential pathogenetic mechanisms for the formation of lung edema through a newly identified messenger protein, gVPLA2. Further studies are also required to identify the predominant source of gVPLA2, which is induced (Figs. 1 and 2) and upregulated within 24 h in the lung parenchyma following administration of LPS.
We conclude that gVPLA2 mediates ALI and NI caused by LPS. Blockade of this enzyme or genetic deletion (KO) preventing synthesis of gVPLA2 blocks substantially the NI and increased lung elastance caused by LPS. The potential role of gVPLA2 in the prevention or treatment of ALI and NI in animal models and in humans remains to be assessed.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-85779.
Acknowledgments
We thank Dr. Jonathan P. Arm, Harvard Medical School and Brigham and Women's Hospital, Boston, MA, for providing the gVPLA2 littermate control (pla2g5+/+) and gVPLA2 knockout (pla2g5−/−) mice. We thank Terry Li, University of Chicago Immunohistochemistry Core Facility, for technical assistance.
REFERENCES
- 1.Abraham E, Naum C, Bandi V, Gervich D, Lowry SF, Wunderink R, Schein RM, Macias W, Skerjanec S, Dmitrienko A, Farid N, Forgue ST, Jiang F. Efficacy and safety of LY315920Na/S-5920, a selective inhibitor of 14-kDa group IIA secretory phospholipase A2, in patients with suspected sepsis and organ failure. Crit Care Med 31: 718–728, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Alba-Loureiro TC, Marlins EF, Miyasaka CK, Lopes LR, Landgraf RG, Jancar S, Curi R, Sannomiya P. Evidence that arachidonic acid derived from neutrophils and prostaglandin E2 are associated with the induction of acute lung injury inflammation by LPS of Escherichia coli. Inflamm Res 53: 658–663, 2204. [DOI] [PubMed] [Google Scholar]
- 3.Attalah HL, Wu Y, Alaoui-El-Azher M, Thouron F, Koumanov K, Wolf C, Brochard L, Harf A, Delclaux C, Touqui L. Induction of type-IIA secretory phospholipase A2 in animal models of acute lung injury. Eur Respir J 21: 1040–1045, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Balestrieri B, Hsu VW, Gilbert H, Leslie CC, Han WK, Bonventre JV, Arm JP. Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J Biol Chem 281: 6691–6698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabot S, Koumanov K, Lambeau G, Gelb MH, Balloy V, Chignard M, Whitsett JA, Touqui L. Inhibitory effects of surfactant protein A on surfactant phospholipid hydrolysis by secreted phospholipases A2. J Immunol 171: 995–1000, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Engle SJ, Seilhamer JJ, Tischfield JA. Cloning and characterization of novel rat and mouse low molecular weight Ca2+-dependent phospholipase A2s containing 16 cysteines. J Biol Chem 269: 23018–23024, 1994. [PubMed] [Google Scholar]
- 7.Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, Reithmeier R, Lindsay TF, Lichtenberger C, Reinisch W, Lambeau G, Arm J, Tischfield J, Gelb MH, Rubin BB. Group IV, V and X phospholipases A2s in human neutrophils. J Biol Chem 277: 5061–5073, 2002. [DOI] [PubMed] [Google Scholar]
- 8.De Luca D, Baroni S, Vento G, Piastra M, Pietrini D, Romitelli F, Capoluongo E, Romagnoli C, Conti G, Zecca E. Secretory phospholipase A2 and neonatal respiratory distress: pilot study on broncho-alveolar lavage. Intensive Care Med 34: 1858–1864, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Dennis EA The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci 22: 1–2, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Furue S, Kuwabara K, Mikawa K, Nishina K, Shiga M, Maekawa N, Ueno M, Chikazawa Y, Ono T, Hori Y, Matsukawa A, Yoshinaga M, Obara H. Crucial role of group IIA phospholipase A2 in oleic acid-induced acute lung injury in rabbits. Am J Respir Crit Care Med 160: 1292–1302, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Grigoriu RG, Balan R, Rusu V. Animal model of acute respiratory distress syndrome. Rev Med Chir Soc Med Nat Iasi 112: 174–182, 2008. [PubMed] [Google Scholar]
- 12.Henderson WR Jr, Chi EY, Bollinger JG, Tien Y, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GKS, Nevalainen T, Rudensky AY, Gelb MH. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med 204: 865–877, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han SK, Kim KP, Koduri R, Bittova L, Muñoz NM, Leff AR, Wilton DC, Gelb MH, Cho W. Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2. J Biol Chem 274: 11881–11888, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Ho IC, Arm JP, Bingham CO, Choi A, Austen KF, Glimcher LH. A novel group of phospholipase A2s preferentially expressed in type 2 helper T cells. J Biol Chem 276: 18321–18326, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Kim KP, Han SK, Hong M, Cho W. The molecular basis of phosphatidylcholine preference of human group-V phospholipase A2. Biochem J 348: 643–647, 2000. [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KP, Rafter JD, Bittova L, Han SK, Snitko Y, Muñoz NM, Leff AR, Cho W. Mechanism of human group V phospholipase A2-induced leukotriene biosynthesis in human neutrophils. A potential role of heparan sulfate binding in PLA2 internalization and degradation. J Biol Chem 276: 11126–11134, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Kim KP, Han SK, Muñoz NM, Zhu X, Sano H, Leff AR, Cho W. Group V phospholipase A2 induces leukotriene biosynthesis in human neutrophils through the activation of group IVA phospholipase A2. J Biol Chem 277: 36479–36488, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Leff AR, Muñoz NM. Future treatment to lessen exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 4: 659–666, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Lindbom J, Ljungman AG, Lindahl M, Tagesson C. Increased gene expression of novel cytosolic and secretory phospholipase A2 types in human airway epithelial cells induced by tumor necrosis factor-alpha and IFN-gamma. J Interferon Cytokine Res 22: 947–955, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Masuda S, Murakami M, Ishikawa Y, Ishii T, Kudo I. Diverse cellular localizations of secretory phospholipase A2 enzymes in several human tissues. Biochim Biophys Acta 1736: 200–210, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meliton AY, Muñoz NM, Lambertino A, Boetticher E, Learoyd J, Zhu X, Leff AR. Phosphodiesterase 4 inhibition of β2-integrin adhesion caused by leukotriene B4 and TNFα in human neutrophils. Eur Respir J 28: 920–928, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz NM, Kim YJ, Meliton AY, Kim KP, Han SK, Boetticher E, O'Leary E, Myou S, Zhu X, Bonventre JV, Leff AR, Cho W. Human group V phospholipase A2 induces group IVA phospholipase A2-independent cysteinyl leukotriene synthesis in human eosinophils. J Biol Chem 278: 38813–38820, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz NM, Meliton AY, Arm JP, Bonventre JV, Cho W, Leff AR. Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. J Immunol 179: 4800–4807, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz NM, Meliton AY, Lambertino A, Boetticher E, Learoyd J, Sultan F, Zhu X, Cho W, Leff AR. Transcellular secretion of group V phospholipase A2 from epithelium induces β2-integrin-mediated adhesion and synthesis of leukotriene C4 in eosinophils. J Immunol 177: 574–582, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz NM, Kim KP, Han SK, Boetticher E, Sperling AI, Sano H, Zhu X, Cho W, Leff AR. Characterization of monoclonal antibodies specific for 14 kDa human group V secretory phospholipase A2 (hVPLA2). Hybridoma 19: 171–176, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Murakami M, Kudo I. Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv Immunol 77: 163–194, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Nagase T, Uozumi N, Aoki-Nagase T, Terakawi K, Ishii S, Tomita T, Yamamoto H, Hashizume K, Ouchi Y, Shimizu T. A potent inhibitor of cytosolic phospholipase A2, arachidonyl trifluoromethyl ketone, attenuates LPS-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 284: L720–L726, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, Shimizu T. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nat Immunol 1: 42–46, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto K, Kim JS, Rubin BK. Secretory phospholipases A2 stimulate mucus secretion, induce airway inflammation, and produce secretory hyperresponsiveness to neutrophil elastase in ferret trachea. Am J Physiol Lung Cell Mol Physiol 292: L62–L67, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Proctor LM, Strachan AJ, Woodruff TM, Mahadevan IB, Williams HM, Shiels IA, Taylor SM. Complement inhibitors selectively attenuate injury following administration of cobra venom factor to rats. Int Immunopharmacol 6: 1224–1232, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, Arm JP. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J Biol Chem 279: 16488–16494, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761: 1246–1259, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Seilhamer JJ, Pruzanski W, Vadas P, Plant S, Miller JA, Kloss J, Johnson LK. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem 264: 5335–5338, 1989. [PubMed] [Google Scholar]
- 35.Teixeira CFP, Landucci ECT, Antunes E, Chacur M, Cury Y. Inflammatory effect of snake venom myotoxic phospholipases A2. Toxicon 42: 947–962, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Tischfield JA A reassessment of the low molecular weight phospholipase A2 gene family in mammals. J Biol Chem 272: 17247–17250, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Touqui L, Wu YZ. Interaction of secreted phospholipase A2 and pulmonary surfactant and its pathophysiological relevance in acute respiratory distress syndrome. Acta Pharmacol Sin 24: 1292–1296, 2003. [PubMed] [Google Scholar]
- 38.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim Biophys Acta 1488: 59–70, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Wijewickrama GT, Kim JH, Kim YJ, Abraham A, Oh Y, Ananthanarayanan B, Kwatia M, Ackerman SJ, Cho W. Systematic evaluation of transcellular activities of secretory phospholipases A2. High activity of group V phospholipases A2 to induce eicosanoid biosynthesis in neighboring inflammatory cells. J Biol Chem 281: 10935–10944, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Singer M, Thouron F, Alaoui-El-Azher M, Touqui L. Effect of surfactant on pulmonary expression of type IIA PLA2 in an animal model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 282: L743–L750, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Zeiher BG, Steingrub J, Laterre PF, Dmitrienko A, Fukiishi Y, Abraham E. LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit Care Med 33: 1741–1748, 2005. [DOI] [PubMed] [Google Scholar]