Abstract
Carcinoembryonic cell adhesion molecule 6 (CEACAM6) is a glycosylated, glycosylphosphatidylinositol (GPI)-anchored protein expressed in epithelial cells of various human tissues. It binds gram-negative bacteria and is overexpressed in cancers, where it is antiapoptotic and promotes metastases. To characterize CEACAM6 expression in developing lung, we cultured human fetal lung epithelial cells and examined responses to differentiation-promoting hormones, adenovirus expressing thyroid transcription factor-1 (TTF-1), and silencing of TTF-1 with small inhibitory RNA. Glucocorticoid and cAMP had additive stimulatory effects on CEACAM6 content, and combined treatment maximally increased transcription rate, mRNA, and protein ∼10-fold. Knockdown of TTF-1 reduced hormone induction of CEACAM6 by 80%, and expression of recombinant TTF-1 increased CEACAM6 in a dose-dependent fashion. CEACAM6 content of lung tissue increased during the third trimester and postnatally. By immunostaining, CEACAM6 was present in fetal type II cells, but not mesenchymal cells, and localized to both the plasma membrane and within surfactant-containing lamellar bodies. CEACAM6 was secreted from cultured type II cells and was present in both surfactant and supernatant fractions of infant tracheal aspirates. In functional studies, CEACAM6 reduced inhibition of surfactant surface properties by proteins in vitro and blocked apoptosis of electroporated cultured cells. We conclude that CEACAM6 in fetal lung epithelial cells is developmentally and hormonally regulated and a target protein for TTF-1. Because CEACAM6 acts as an antiapoptotic factor and stabilizes surfactant function, in addition to a putative role in innate defense against bacteria, we propose that it is a multifunctional alveolar protein.
Keywords: alveolar type II cells; thyroid transcription factor-1; glucocorticoid; adenosine 3′,5′-cyclic monophosphate; apoptosis; surfactant
the igcam superfamily is a diverse group of adhesive receptor glycoproteins that contain an amino-terminal N domain, which is homologous to the Ig variable domain, and different numbers of domains homologous to the Ig constant domain. In the human, the carcinoembryonic antigen (CEA) gene family is an IgCAM subset consisting of 29 related genes and pseudogenes clustered at q13.2 on chromosome 19 (22). CEA proteins function as intercellular homophilic and heterophilic adhesion molecules and have signaling properties.
Carcinoembryonic cell adhesion molecule (CEACAM)6 (also called NCA, NCA-50/90, and CD66c) and CEACAM5 (originally called CEA and also designated CD66e) share ∼90% homology of the N domain but differ in the number of IgC2-like domains (A and B domains). Both proteins contain a glycosylphosphatidylinositol (GPI) membrane anchor (28) and are targeted to lipid rafts in apical membranes of polarized epithelial cells (32). CEACAM5/6 bind a variety of gram-negative bacteria and mediate internalization/phagocytosis, participating in innate immune defense in the intestine (10). The two genes are not found in rodents, and they may represent pathogen-host coevolution, providing different protein structures with selective bacterial binding properties.
Expression of CEACAM5/6 is deregulated and overexpressed in cancers of colorectal epithelium, with surface levels inversely correlated with both the degree of colonocyte differentiation (26) and positive clinical outcome (27). The two CEACAMs are also expressed in a high proportion of tumor cell lines derived from breast, ovary, pancreas, prostate, and lung (8). It has been proposed that CEACAM5/6 overproduction has a causative role in tumorigenesis, acting via an imbalance of cell surface adhesion molecules that disrupts differentiation, inhibits apoptosis, and promotes both tumor formation and metastases (26, 39).
Two earlier studies identified both CEACAM5 and CEACAM6 immunoreactivity in normal adult lung, with CEACAM5 localized to type II cells and CEACAM6 present in both alveolar and airway epithelium (43, 44). At present, there is no published information regarding regulated expression or function of these proteins in the lung epithelium. In a recent study with human fetal lung we identified CEACAM6 as one of the genes upregulated during hormone-induced differentiation of type II cells in vitro (46). In addition, we found in preliminary experiments that CEACAM6 was also upregulated in undifferentiated lung epithelial cells by expression of recombinant (r) thyroid transcription factor-1 (TTF-1, product of Nkx2.1), which is critical for differentiation of type II cells and expression of surfactant-associated proteins (31). Mature type II cells produce pulmonary surfactant, a phospholipid-protein mixture that is essential for normal respiration by virtue of its ability to reduce surface tension and prevent collapse of air spaces. Type II cells also produce proteins that participate in innate defense against microorganisms and modulate fluid and ion flux between the alveolus and the epithelium, and they proliferate during fetal lung development and after lung injury to generate type I cells for gas exchange (16).
Studies with human fetal lung explants and isolated epithelial cells have been useful in determining biochemical, morphological, and functional changes in response to glucocorticoid plus cAMP treatment, which is carried out with serum-free, fully defined culture conditions using plastic substratum (2, 11, 19, 21). Hormone-treated fetal epithelial cells differentiate to closely resemble mature adult type II cells with regard to synthesis of surfactant components, formation and regulated secretion of surfactant-containing lamellar bodies, and establishment of a cellular monolayer with high transepithelial resistance. Here, we used cultured human lung cells to characterize hormonal and TTF-1 regulation of CEACAM6 expression, describe type II cell-specific synthesis and secretion, and provide evidence for functions of CEACAM6 in the lung alveolus.
MATERIALS AND METHODS
Materials.
Cell culture media, antibiotics, fetal calf serum, annexin V and TOTO-3 dye were obtained from Invitrogen (Carlsbad, CA). Restriction enzymes, modifying enzymes, and other molecular biology reagents were purchased from Promega (Madison, WI) and New England Biolabs (Beverly, MA). Complete Protease Inhibitor cocktail tablets were obtained from Roche Applied Sciences (Indianapolis, IN). Dexamethasone, 8-bromoadenosine 3′,5′-cyclic monophosphate (8-BrcAMP), and all other chemicals were obtained from either Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). Enhanced chemiluminescence reagent was purchased from Dupont-NEN (Bradford, MA).
Antibodies.
Rabbit polyclonal anti-sheep SP-B was purchased from Chemicon International (Temecula, CA), and mouse monoclonal anti-β-actin and anti-GAPDH were obtained from Abcam (Cambridge, UK). Goat anti-human SP-A was from US Biological (Swampscott, MA). Anti-TTF-1 polyclonal antibody (amino acids 1-190, SC) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Cytokeratin (pan) antibody was from Zymed Laboratories (South San Francisco, CA). CEACAM6 (CEA/CEACAM6) monoclonal antibodies were obtained from Novus Biologicals (Littleton, CO) (43), US Biological (clone 5E506), and Biogenex (clone BY114, San Ramon, CA).
Lung cell isolation and culture and tracheal aspirates.
Human fetal lung of 11- to 22-wk-gestation abortuses was obtained from Advanced Bioscience Resources (Alameda, CA) and/or the Birth Defects Laboratory of the University of Washington (Seattle, WA); specimens of postnatal lung were obtained from postmortem tissue. Lung epithelial cells were isolated and cultured as previously described under Institutional Review Board (IRB)-approved protocols of the Children's Hospital of Philadelphia (21). Briefly, after overnight culture as explants (20) the tissue was digested with trypsin, collagenase, and DNase, and fibroblasts were removed by differential adherence. Nonadherent cells were plated on 60-mm plastic culture dishes in Waymouth medium containing 10% fetal calf serum. After overnight culture (day 1), we cultured attached cells an additional 3–9 days in 1 ml of serum-free Waymouth medium alone (control) or with dexamethasone (10 nM) plus 8-BrcAMP (0.1 mM) and 3-isobutyl-1-methylxanthine (0.1 mM), a combination that is referred to as DCI, or with dexamethasone or 8-BrcAMP/3-isobutyl-1-methylxanthine separately. These concentrations maximally induce surfactant components in human lung explant cultures (20). Epithelial cell purity by this method is 86 ± 2% by cytokeratin staining, with fibroblasts as the primary contaminating cell type (19, 21).
Type II cells were isolated from adult human lungs as described by Fang et al. (15). Purity for five preparations was 94 ± 1% by staining for SP-B and SP-C, with fibroblasts as the major contaminating cell type.
Tracheal aspirate samples were collected from premature infants under an IRB-approved protocol. Large-aggregate surfactant was prepared by centrifugation at 27,000 g for 60 min.
Secretion studies.
After 4-day culture in DCI fetal lung cells received fresh medium, and both medium and cells were collected at subsequent time points. CEACAM6 content in cells and medium (concentrated by Amicon Centricon-10 filtration) was determined by Western blot analysis as described below. Percent secretion in each culture dish was calculated as density units in medium divided by units in medium + units in cells. A secretagogue mixture added to some cells contained TPA (10 nM) plus terbutaline (10 μM) plus Ca2+ ionophore A-23187 (100 nM). In some experiments cells were cultured in DCI for 24 h, with addition of mannosamine (10 mM) for the next 24–48 h, at which time medium and cells were harvested.
Recombinant adenovirus construction.
The recombinant sense construct for TTF-1 (Ad12A2) was generated as described previously (31) with the Adeno-X Expression System per the manufacturer's instructions (BD Biosciences, Clontech, Palo Alto, CA). The construct was generated under the cytomegalovirus (CMV) promoter in replication-deficient adenovirus H5.010 to facilitate high expression levels and was subcloned into a pShuttle plasmid to generate an expression cassette. Purified adenovirus was used to infect fetal lung epithelial cells. Control adenovirus (AdGFP) was constructed, containing pEGFP coding sequence under a CMV promoter, and was used in initial experiments to determine infection efficiency at various viral titers. For transduction studies, cells were exposed to either AdGFP or Ad12A2 at titers of 0.2–7 plaque-forming units (PFU)/cell and residual virus was removed by medium change after 24 h.
Small inhibitory RNA silencing of TTF-1 and CEACAM6.
Silencing small inhibitory RNA oligonucleotides (siRNA) for TTF-1 and CEACAM6 were synthesized and purified by Qiagen-Xeragon (Germantown, MD). Oligonucleotides A and D for TTF-1 each reduced TTF-1 protein in fetal lung cells by ≥50% (31). For CEACAM6, sequences are as follows: forward: r(5′-GUC CUG CUC ACA GCC UCA CUU CUA A); reverse: r(5′-UUA GAA GUG AGG CUG UGA GCA GGA C). Inhibition in the presence of siRNA was ≥90%. Scrambled (control) oligonucleotides had no homology with relevant human genes by BLAST analysis; the sequences were forward: r(5′-GUC CUC GGA CAC UCC UUC ACU CUA A); reverse: r(5′-UUA GAG UGA AGG AGU GUC CGA GGA C). Cells were treated with siRNA oligonucleotides (200 nM) by nucleofection according to the manufacturer's protocol with epithelial cell-specific buffer (AMAXA, Gaithersburg, MD). Cell viability after electroporation was ∼50%.
Real-time RT-PCR.
Total RNA was prepared from lung cells with RNA STAT-60 (Tel-Test, Friendswood, TX) and treated with RQ1 RNase-free DNase. Integrity and purity were determined with an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA). Real-time PCR reactions were performed in the Real-Time PCR Facility of the Stokes Research Institute at Children's Hospital of Philadelphia as previously described (31). Specific primers and probe sequences are available on request.
Transcription rate.
Nuclei were isolated from cultured cells, and nuclear run-on reactions were performed by a slight modification of the method of Hildebrandt and Neufer (24). The nascent RNA transcripts were extracted with RNA STAT-60 (Tel-Test), and real-time PCR was performed (in triplicate) as described above. CEACAM6 transcription rate data were expressed relative to the transcription rate of GAPDH.
Whole cell extract preparation.
Whole cell extracts were prepared from fetal lung cells by standard procedures. In brief, cells were washed once with PBS and lysed by sonication in ice-cold buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 1 mM EDTA, 2 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 μg/ml leupeptin, 5 μg/ml apoprotein, and 5 μg/ml antipain. The lysates were centrifuged at 10,000 g for 10 min at 4°C, and supernatants were used for Western blot analysis. Protein concentrations were determined with a Bio-Rad protein assay reagent kit and a microplate assay protocol (Bio-Rad Laboratories, Hercules, CA).
Western blot analysis.
Western blot analyses for TTF-1, SP-B, CEACAM6, β-actin, and GAPDH were performed as described previously (21). Cell extracts were run on NuPAGE Bis-Tris gels with MES SDS Running Buffer per the manufacturer's protocol (Invitrogen). Proteins were transferred to Duralose membrane (Stratagene, La Jolla, CA) and probed with primary antibodies [mouse anti-CEACAM5+6 (1:1,000), Novus; rabbit anti-TTF-1 (1:1,000), Santa Cruz; rabbit anti-sheep SP-B (1:2,000), Chemicon]. In some experiments, horseradish peroxidase-conjugated secondary antibodies were added, and detected with enhanced chemiluminescence (Dupont-NEN). Alternatively, infrared-detectable secondary antibodies [goat anti-mouse IgG-Alexa 680-tagged (Molecular Probes, Eugene, OR) or goat anti-rabbit IgG-IRDye800 (Rockland)] were used, and the Odyssey Imaging system (Licor Biosciences, Lincoln, NE) was used for detection and quantitation.
Immunostaining.
Cells cultured on cover glass (Fisher Scientific) were fixed in 1% paraformaldehyde in PBS and permeabilized with 0.3% Triton X-100. Explants of fetal lung (15 wk gestation), cultured 5 days with or without DCI, were fixed in 1% paraformaldehyde, cryoprotected with sucrose, and imbedded for frozen sectioning. We carried out immunostaining for CEACAM6 by methodology previously described (21), using anti-human CEACAM6 [clone BY114 (1:100), Biogenex; clone 5E506 (1:200), US Biological; or clone 9A6 (1:100), Santa Cruz] plus Cy3-conjugated or Alexa 488-tagged secondary antibodies (1:200; Molecular Probes). In some experiments nuclei were stained with DAPI.
Apoptosis assays.
Freshly isolated cells were electroporated (AMAXA) with anti-CEACAM or scrambled siRNA, cultured overnight on coverslips in the presence of serum, and then cultured 24 or 72 h in serum-free medium plus DCI. After washing, cells were incubated with annexin V (conjugated to either Alexa 488 or 594) according to the manufacturer's recommendations (Invitrogen) or with the DNA-binding dye TOTO 3 (Invitrogen) as described previously (35). Cells were examined by confocal microscopy (TE300 Nikon coupled Radiance 2000 imaging system, Carl Zeiss). Cells were assayed for caspase-3 activity (50 μg cell protein) with Ac-DEVD-AFC (fluorigenic substrate VII, Calbiochem, La Jolla, CA) as substrate.
Pulsating bubble surfactometry.
We used surfactometry to evaluate the influence of CEACAM6 on surfactant function in the presence of inhibitory proteins. Freshly isolated fetal lung epithelial cells were electroporated (AMAXA) with either CEACAM6-specific or scrambled oligonucleotide. Conditioned media were collected on day 5 and concentrated. Commercially available surfactant (Infasurf, Forest Pharmaceuticals, St. Louis, MO) was diluted to 1.5 mg phospholipid/ml with fresh Waymouth medium or with conditioned medium and incubated for 15 min at 37°C. Surface tension was then assessed in a pulsating bubble surfactometer (Electronectics, Buffalo, NY) as previously described (7).
RESULTS
CEACAM6 is developmentally regulated and transcriptionally induced by glucocorticoid and cAMP.
Using microarray analysis, we determined the expression and hormonal responsiveness of CEACAM family members in fetal lung cells (Table 1). In control fetal epithelial cells, cultured 3 days in the absence of serum and hormones, mRNAs for CEACAMs 1, 4, 5, and 6 were present at significantly detectable levels, whereas there was no significant signal for CEACAMs 3, 7, and 8. Expression of CEACAMs 1 and 4 was not affected by exposure to DCI, CEACAM5 was modestly induced (2-fold), and CEACAM6 was induced 10-fold. In type II cells from adult lung, CEACAMs 1, 5, and 6 were expressed at higher levels than for fetal cells. These findings indicate that CEACAM6 is the most highly expressed and regulated member of the CEACAM family of genes in fetal lung epithelial cells and suggest developmental regulation.
Table 1.
Selected CEACAM family members are expressed and induced by DCI in human lung type II cells
|
Fetal Type II Cells |
Adult Type II Cells Signal Intensity | ||
|---|---|---|---|
| Signal Intensity | DCI Induction, fold vs. control | ||
| CEACAM1 | 31±4 | 0.7 | 154±21 |
| CEACAM3 | ND | ND | |
| CEACAM4 | 56±8 | 0.9 | ND |
| CEACAM5 | 100±33 | 2.0 | 1,020±312 |
| CEACAM6 | 863±203 | 10.1 | 3,288±272 |
| CEACAM7 | ND | ND | |
| CEACAM8 | ND | ND | |
Intensity data (in fluorimetric units) are means ± SE; ND, expression below level of detection by microarray (designated “absent”). RNA was analyzed by microarray using Affymetrix U133A chips (46). Four of seven carcinoembryonic cell adhesion molecule (CEACAM) family members (1, 4, 5, 6) were expressed in both control and dexamethasone + 8-bromoadenosine 3′,5′-cyclic monophosphate (8-BrcAMP) + IBMX (DCI)-treated fetal cells (cultured 72 h), but only CEACAMs 5 and 6 were induced by DCI (2-fold and 10-fold, respectively; n = 5, P < 0.003). Freshly isolated adult cells from 5 experiments expressed CEACAMs 1, 5, and 6 at higher levels than for fetal cells.
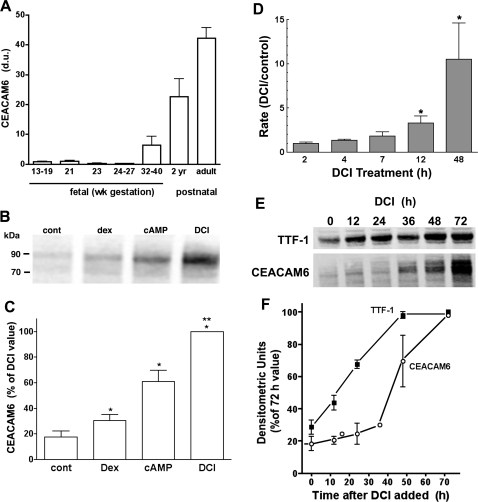
We further examined the developmental pattern for CEACAM6 by Western blot analysis of lung tissue. As shown in Fig. 1A, protein content was low to undetectable between 13 and 27 wk of fetal life and expression was progressively increased in third-trimester and postnatal specimens. This pattern is consistent with developmental expression in parallel with type II cell differentiation.
Fig. 1.
Developmental and hormonal regulation of carcinoembryonic cell adhesion molecule (CEACAM)6. A: developmental increase in CEACAM6. Western blots were performed on specimens of fetal and postnatal lung. Relative densitometric units (du), normalized to GAPDH, are shown as means ± SE with n = 3–7/group except 2 yr (n = 2). B–F: fetal lung epithelial cells were cultured in the absence (control) or presence of dexamethasone (Dex), cAMP+IBMX (cAMP), or both agents (DCI). B: Western blot from representative experiment. C: summary protein data from 3 experiments (means ± SE). *P < 0.05 vs. control, **P < 0.05 vs. cAMP and Dex alone. D: transcription rate for CEACAM6 mRNA. Nuclei were isolated after exposure of cells to DCI or diluent (control) for 2–48 h. Data are normalized to GAPDH mRNA and expressed as means ± SE for fold difference relative to control cells. *P < 0.05 vs. control. E: representative Western blot for time course of CEACAM6 and thyroid transcription factor (TTF)-1 induction. F: densitometric quantification of Western blots. An increase in CEACAM6 was delayed >24 h, consistent with TTF-1 dependence for CEACAM induction (n = 3).
To characterize the induction process, we first examined the response of CEACAM6 to individual hormone treatments. On Western blot analysis of cell sonicates, the major CEACAM6 immunoreactive band was detected at ∼90 kDa, with a minor band at ∼70 kDa (Fig. 1B). CEACAM6 protein content increased approximately fivefold with 4-day exposure to combined hormone treatment (DCI), with lesser and additive responses to each agent alone (Fig. 1C).
Increased CEACAM6 mRNA content could reflect higher transcription rate and/or changes in transcript stability. To address the former mechanism, we performed nuclear run-on experiments for CEACAM6 gene transcription. After a lag of 2–4 h, the transcription rate increased in a linear fashion on DCI exposure and was 10.6 ± 4.1-fold greater than control at 48 h (Fig. 1D). Increased transcription preceded the increase in both cellular CEACAM6 mRNA content, which was not significantly increased until 12–16 h (data not shown), and CEACAM6 protein (Fig. 1, E and F). In the same experiments the transcription rate for SP-B, which is synergistically induced by glucocorticoid plus cAMP (19), was maximal by ∼12 h (data not shown). The delayed time course of increased CEACAM6 transcription rate suggests that induction might be a secondary rather than a primary response to hormones.
Because of the low level of CEACAM6 expression in untreated cells, it was not possible to directly compare mRNA stability with and without hormone treatment. Instead, we determined content of CEACAM6 mRNA, by real-time PCR, with time after removal of DCI from treated cells, anticipating a slower decay rate initially if hormones were stabilizing transcript. In three experiments there was a log-linear decay of CEACAM6 mRNA, with a calculated half-life of 15.3 ± 4.0 h and r ≥ 0.94. This finding is consistent with a primary effect of hormones on CEACAM6 transcription rate.
CEACAM6 is a TTF-1 target gene.
We previously observed (31) that expression of CEACAM6 mRNA and protein in control lung cells was increased by exposure to adenovirus (Ad12A2) expressing rTTF-1, a key, inducible transcription factor in type II cell differentiation. Additional experiments were performed to further characterize the role of TTF-1 in CEACAM6 expression. As shown in Fig. 1, E and F, TTF-1 content was significantly increased at 12 h (or less) of hormone exposure, whereas CEACAM6 increased only after 24 h. This temporal relationship is consistent with a role for TTF-1 in regulating CEACAM6 gene expression.
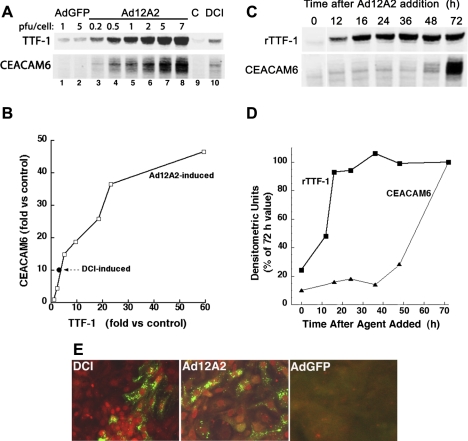
We next examined responses to rTTF-1 achieved by transduction of cells with Ad12A2. In a representative dose-response study with Western blot analysis, cellular CEACAM6 content increased with increasing amount of Ad12A2 (Fig. 2A, lanes 3–8) compared with nontransduced cells (control, lane 9) and cells exposed to adenovirus expressing rGFP (lanes 1 and 2). At higher doses of Ad12A2, content of both TTF-1 (lanes 3–8) and CEACAM6 (lanes 5–8) were greater than for DCI-treated cells (lane 10). There was an approximately linear relationship between induction of CEACAM6 and rTTF-1 over a 25-fold range, with an apparent plateau response for CEACAM6 at the highest dose of virus (Fig. 2B). In nine experiments, CEACAM6 protein was induced 74.3 ± 25.3-fold vs. control by Ad12A2 (at 6 PFU/ml) and 12.9 ± 3.2 fold vs. control by DCI (P < 0.05, Ad12A2 vs. DCI); in the same experiments, TTF-1 content increased 47.2 ± 12.2-fold and 5.0 ± 1.5-fold by Ad12A2 and DCI, respectively (P < 0.01). In time-course studies, rTTF-1 content was maximally increased by ∼18-h exposure to Ad12A2, whereas CEACAM6 increased only after 36-h exposure of cells to virus (Fig. 2, C and D). The delayed increase in CEACAM6 relative to rTTF-1 content is consistent with involvement of TTF-1-induced regulatory factor(s) in the induction mechanism.
Fig. 2.
Induction of CEACAM6 by expression of recombinant (r)TTF-1. Human fetal lung epithelial cells were transduced by exposure to Ad12A2 overnight and then cultured in control medium (Waymouth). A: representative Western blot for dose response. Exposure to Ad12A2 resulted in dose-dependent increases in rTTF-1 and CEACAM6 (lanes 3–8) compared with both AdGFP-treated cells (lanes 1 and 2) and control (C) cells (lane 9), with both proteins reaching levels manyfold greater than achieved with DCI (lane 10). PFU, plaque-forming units. B: densitometric quantification of Western blot data. Fold induction for DCI (•) is shown for comparison. C: representative Western for time course. Expression of rTTF-1 protein (Ad12A2 at 6 PFU/cell) was maximal by ∼16 h after transduction, whereas CEACAM6 protein increased markedly only after 36 h. D: densitometric quantification of Western blot data. E: immunofluorescent staining for TTF-1 and CEACAM6 in cultured cells. The intensity of nuclear TTF-1 staining (red) was variable for cells transduced with Ad12A2 (4 PFU/cell, center) or treated with DCI (left). CEACAM6 staining (green) was primarily punctate with both treatments, and more cells were CEACAM6 positive after Ad12A2 transduction compared with DCI treatment. Staining in AdGFP-treated control cells (right) was at low background level.
The response to rTTF-1 is further illustrated by immunostaining. Compared with low background staining in control cells (not shown), exposure to Ad12A2 (Fig. 2E, center) resulted in positive CEACAM6 staining in more cells than seen with DCI treatment (Fig. 2E, left). In a separate experiment with cell counting, 27% of TTF-1-positive cells stained for CEACAM6 in virus-treated cells compared with 14% for DCI-treated cells (data not shown). Thus it is likely that the higher expression of CEACAM6 with rTTF-1 vs. DCI (Fig. 2B) reflects both more cells expressing CEACAM6 and a higher level of CEACAM6 per cell.
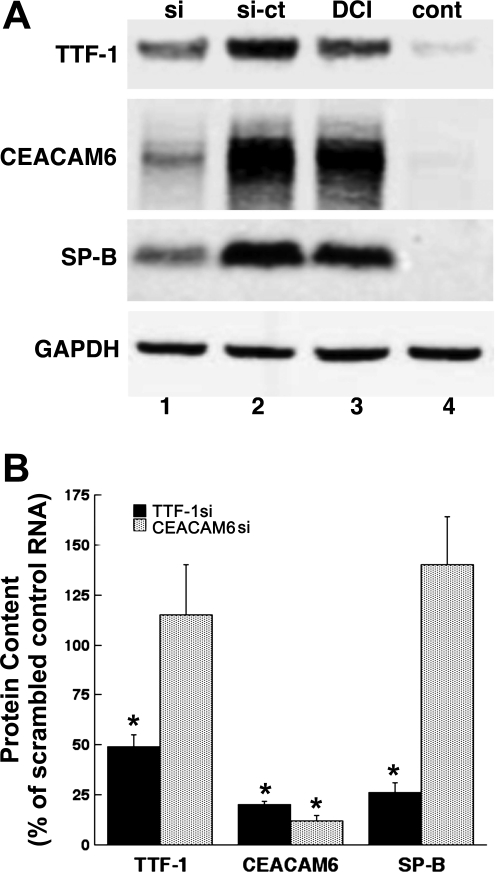
Other experiments were performed with siRNA to knock down TTF-1 expression in DCI-treated cells, predicting that induction of CEACAM6 would be blocked. Treatment with anti-TTF-1 siRNA, compared with scrambled control oligonucleotide, markedly reduced Western signals (Fig. 3A) for both CEACAM6 (compare lanes 1 and 2) and SP-B (compare lanes 1 and 2), a known TTF-1 target gene. Quantitative results for silencing of TTF-1 are shown in Fig. 3B. TTF-1 content was reduced to 46% of control, and CEACAM6 and SP-B levels were decreased to 20% and 26%, respectively, of control. For comparison, siRNA directed at CEACAM6 reduced CEACAM6 protein content to 12% of control, indicating effective silencing by the si oligonucleotides, but did not significantly affect content of TTF-1 and SP-B. This finding indicates, as expected, that induction of CEACAM6 is not required for differentiation of the fetal type II cell phenotype. Collectively, the findings with rTTF-1 and silencing establish a role for TTF-1 in regulating expression of CEACAM6.
Fig. 3.
Effect of TTF-1 and CEACAM6 knockdown. Freshly isolated epithelial cells were electroporated with small inhibitory RNA (siRNA) oligonucleotide (si) or scrambled control oligonucleotide (si-ct) and then cultured for 72 h. A: Western blot in representative experiment of TTF-1 knockdown. DCI alone (lane 3) increased TTF-1, CEACAM6, and SP-B compared with control (lane 4, Waymouth medium). Addition of TTF-1 siRNA oligos in the presence of DCI (lane 1) reduced levels of TTF-1, CEACAM6, and SP-B compared with the scrambled control oligo (lane 2). B: densitometric data comparing knockdown of TTF-1 vs. knockdown of CEACAM6. Knockdown of TTF-1 (filled bars) significantly reduced content of TTF-1 (46 ± 6% of control), CEACAM6 (20 ± 2% of control), and SP-B (26 ± 5% of control) in 4 experiments (all *P < 0.01). In contrast, knockdown of CEACAM6 (n = 3, gray bars) reduced content of CEACAM6 (12 ± 3% of control, *P < 0.01) but did not affect either TTF-1 or SP-B.
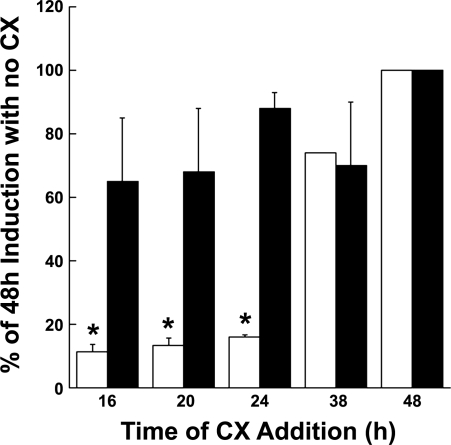
TTF-1 regulation of CEACAM6 expression could represent either a direct response, mediated via binding to promoter elements, or an indirect effect that requires other TTF-1- or DCI-inducible factors. We therefore examined the requirement for continuing protein synthesis in DCI induction of CEACAM6. Figure 4 shows the time course for induction of CEACAM6 mRNA in the presence of cycloheximide, an inhibitor of protein synthesis, added at various times after exposure to hormones. Cycloheximide added as late as 24 h after hormones completely blocked induction of CEACAM6 transcript, suggesting a requirement for de novo synthesis of protein(s) during this time interval. However, the presence of cycloheximide at 16–36 h after addition of hormones had no effect on induction of TTF-1 protein, which occurs rapidly on hormone exposure (Fig. 1E). This finding suggests that hormonally regulated factor(s) in addition to TTF-1 are involved in hormonal induction of CEACAM6. In view of the ability of rTTF-1 to induce CEACAM6 (Fig. 2), these putative regulatory factors are likely induced or activated by TTF-1.
Fig. 4.
DCI induction of CEACAM6 mRNA requires de novo protein synthesis. Epithelial cells were cultured in control medium for 3 days (to deplete endogenous TTF-1), DCI was added, and at various times thereafter (16–38 h) cycloheximide (CX, 1 μg/ml) was added to some dishes for the remainder of the 48-h culture. In response to DCI, TTF-1 protein (filled bars) increased maximally within 16 h (4-fold vs. control) and was not affected by cycloheximide. Induction of CEACAM6 mRNA (13-fold at 48 h, open bars) was sensitive to inhibition of protein synthesis for up to 24 h after addition of hormones. Data are means + SE of 3–4 replicates in 2 experiments expressed as % of 48-h value (100%). *P < 0.01 vs. 48-h value.
CEACAM6 is induced in type II but not mesenchymal cells.
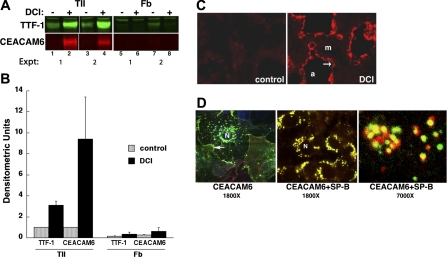
The fetal lung cell cultures used in this study contain ∼14% nonepithelial cells, which are primarily fibroblasts (21). Accordingly, we investigated expression of CEACAM6 by lung cell type. Fibroblasts were isolated at >95% purity from lung tissue in parallel with epithelial cell isolation. By Western blot analysis (Fig. 5A), CEACAM6 was barely detectable in control cultures of both epithelial cells (lanes 1 and 3) and lung fibroblasts (lanes 5 and 7). After exposure to DCI, strong CEACAM6 staining was observed for epithelial cells (Fig. 5A, lanes 2 and 4) but not for fibroblasts (lanes 6 and 8). Similarly, TTF-1 content increased with DCI treatment of epithelial cells but not fibroblasts (Fig. 5A, top). In quantitative analysis of the Western blots, TTF-1 and CEACAM6 were induced three- and ninefold, respectively, in epithelial cells, with no significant responses in fibroblasts (Fig. 5B).
Fig. 5.
Induction of CEACAM6 is cell type specific. A: representative Western blot of enriched epithelial cells and fibroblasts from the same lung cultured with or without DCI for 4 days. Both TTF-1 and CEACAM6 expression were markedly increased in type II epithelial cells (TII) but not in fibroblasts (Fb). The weak TTF-1 band observed in lane 7 is likely due to contaminating epithelial cells. Left portion of image is adapted from Kolla et al. (31). B: densitometric analysis of Western blots. Data are means and ranges from 2 experiments and demonstrate DCI induction of CEACAM6 in epithelial cells but not in fibroblasts. C: fluorescence immunostaining of CEACAM6 induction in explant cultures (5 days) of human fetal lung (19 wk of gestation). CEACAM6 staining was markedly greater in DCI-treated (right) vs. control (left) explants and was detected only in the epithelial cell layer (arrow) lining the air spaces (a), not in the mesenchymal (m) region. D: localization of CEACAM6 in DCI-treated cells by confocal microscopy. Left: cells were immunostained with monoclonal anti-CEACAM6 and anti-mouse Alexa 488-tagged secondary antibody (green). CEACAM6 was localized to large perinuclear vesicles (lamellar bodies) and also to plasma membrane (arrow). Center and right: cells were stained sequentially with anti-CEACAM6 antibody (as above) and then rabbit polyclonal anti-SP-B (Chemicon), with anti-rabbit-Cy3 tagged secondary antibody (red). CEACAM6 colocalized with SP-B (yellow color) to large perinuclear vesicles characteristic of lamellar body staining by SP-B in these cells. At higher power (right), colocalization of CEACAM6 (green) and SP-B (red) was apparent in most vesicles. N, nucleus.
We next examined expression of CEACAM6 in other cell types by immunostaining of fetal lung explants, which retain morphological characteristics of fetal lung in situ. Control explants had low background staining in all cells (Fig. 5C, left). After exposure of explants to DCI for 4 days, strong CEACAM6 staining occurred in epithelial cells lining primitive saccules, but not in mesenchymal cells (Fig. 5C, right). The epithelial cells represent type II cells, as indicated by presence of lamellar bodies (20) and positive staining for SPs (not shown).
We used confocal microscopy to localize expression of CEACAM6 within fetal type II cells. As shown in Fig. 5D, left, CEACAM6 was observed as punctate perinuclear immunostaining and also at the cell periphery (e.g., arrow). As illustrated in Fig. 5D, center, and at higher magnification in Fig. 5D, right, most of the punctate CEACAM6 staining colocalized with that for SP-B, a marker for lamellar bodies (47). This colocalization staining pattern is different from that observed for DC-LAMP, which is localized to the lamellar body periphery (limiting membrane), and is distinct from SP-B staining (46). This finding indicates the presence of CEACAM6 within surfactant-containing organelles of type II cells.
CEACAM6 is secreted from fetal type II cells.
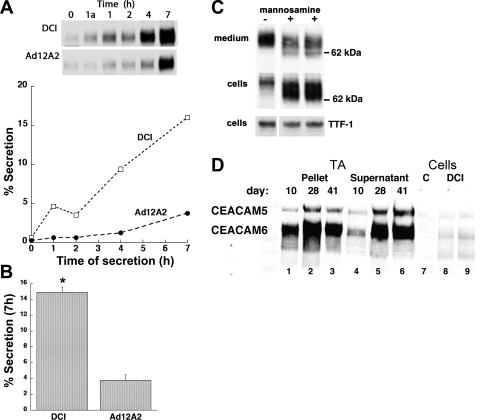
Elevated levels of CEACAM6 are found in plasma of many patients with lung cancer (3, 33); however, secretion of the protein into lung air spaces has not been examined. We determined the secretion rate of CEACAM6 from cultured fetal lung epithelial cells exposed for 4 days to either DCI or Ad12A2. As shown in the Western blot analysis of Fig. 6A, top, CEACAM6 content in the medium increased with time for both DCI- and Ad12A2-treated cells; CEACAM6 medium content was less in cells kept for 1 h at 4°C [Fig. 6A, 2nd lane (1a)] compared with cells at 37°C (3rd lane). The secretion rate (content in medium/cell+medium) was approximately linear over 7 h for both groups of cells (Fig. 6A, bottom) and was approximately fourfold higher for DCI-treated cells compared with Ad12A2-treated cells (Fig. 6B), which may reflect hormone-induced effects on intracellular protein transport, secretory mechanisms, and/or formation and exocytosis of lamellar bodies. Secretion of CEACAM6 from DCI-treated cells was similar to that for constitutive secretion of SP-A (9.1 ± 4.7% and 23.2 ± 11.2% of cell content at 4 and 7 h, respectively). Release of CEACAM6 from cells does not reflect cell lysis or leakage because medium content of cytosolic proteins at 7 h was very low (β-actin 0.3 ± 0.1%) or not detectable (GAPDH).
Fig. 6.
CEACAM6 secretion from cultured epithelial cells and in vivo. A: representative secretion experiment. Release of CEACAM6 was approximately linear for 7 h for both DCI-treated and Ad12A2-transduced (6 PFU/cell) cells. Western blot (inset) shows reduced secretion after 1 h at 4°C (1a lane) compared with 1 h at 37°C (1 lane) for both DCI-treated and Ad12A2-transduced cells. B: secretion summary. At 7 h, secretion of CEACAM6 was ∼5-fold higher for DCI-treated cells than for Ad12A2-transduced cells (n = 3, P < 0.05). C: effect of mannosamine on secretion. Cells were cultured 72 h in DCI with or without mannosamine (10 mM) added for the last 48 h to inhibit mannose-related glycosylation. Less CEACAM6 was released into the medium for mannosamine-treated cells (2nd and 3rd lanes) compared with control (1st lane) and conversely treated cells had higher CEACAM6 content. TTF-1 expression (bottom) was similar for cells with or without mannosamine. In 3 experiments the recovery ratio (+mannosamine/−mannosamine) in the medium was 0.48 ± 0.09 (P < 0.01 for n = 5). D: CEACAM6 in tracheal aspirate (TA) fluid from a newborn infant. By Western blot analysis with antibody recognizing both CEACAM5 and CEACAM6, both proteins were detected in both the large-aggregate surfactant pellet and supernatant from a ventilated premature infant, with samples obtained on different postnatal days as shown in lanes 1–6. CEACAM6 and CEACAM5 bands migrated identically to those in cultured cells (lanes 7–9). Results are representative of 3 infants with serial sampling of tracheal aspirates.
In the secretion experiments, the large-aggregate surfactant fraction from pooled media contained ∼2.1% of the total media CEACAM6, which is 30-fold more than the expected contribution from media contamination of the pelleted surfactant, and CEACAM6 was ∼0.3% of the total protein (n = 2). Thus a fraction of CEACAM6 in conditioned medium is lipid associated, which could occur either before lamellar body release or after release. In preliminary experiments we found no increase in CEACAM6 release from cultured cells in the presence of secretagogues (TPA + terbutaline + calcium ionophore A-23187).
To explore the role of glycosylation and the GPI anchor of CEACAM6 in secretion, cells were treated with mannosamine, which competes for mannose and blocks glycosylation and GPI anchor formation (34). By Western blot analysis, most of the CEACAM6 in mannosamine-treated cells (Fig. 6C, middle) migrated faster than for control cells (2nd and 3rd lanes vs. 1st lane), consistent with reduced glycosylation and molecular mass. All CEACAM6 in control medium (Fig. 6C, top) migrated at 90 kDa, whereas lower-molecular-mass forms were observed in mannosamine-treated medium (Fig. 6C, middle). In contrast, mannosamine treatment did not affect TTF-1 content or apparent molecular size (Fig. 6C, bottom). In the presence of mannosamine there was increased content of cellular CEACAM6, and less CEACAM6 was recovered in the medium (2nd and 3rd lanes compared with 1st lane). In three experiments, mannosamine treatment decreased CEACAM6 secretion rate by 52 ± 9% (P < 0.01), and total cellular content increased, reflecting the cellular retention. For comparison we examined secretion of SP-A, a non-GPI-anchored, constitutively secreted glycoprotein. Neither secretion rate (33.6 ± 7.1% in 7 h) nor apparent molecular size on Western blot analysis was affected by mannosamine treatment of cells (data not shown). The observed responses to mannosamine suggest a role for the glycosyl groups and/or the GPI anchor in CEACAM metabolism.
To examine secretion in vivo, we assessed CEACAM6 in tracheal aspirates from intubated premature infants. Tracheal aspiration uses saline instillation and suctioning and collects epithelial lining fluid from upper airways; tracheal aspirate fluid has been used to obtain surfactant for analysis (7). As shown in the representative Western blot of Fig. 6D, we found both CEACAM5 and CEACAM6 (lanes 1–6) in three aspirate samples from an infant at 10–41 days after birth; the migration patterns and ratio of CEACAM5 to CEACAM6 signal were comparable to those observed for cultured fetal lung cells (Fig. 6D, lanes 8 and 9). CEACAM5 and CEACAM6 were present in both the large-aggregate surfactant pellet (Fig. 6D, lanes 1–3) and the supernatant fraction (lanes 4–6) of each tracheal aspirate. In analysis of 40 tracheal aspirate samples, 41 ± 4% (mean ± SE) of total aspirate CEACAM6 was associated with the surfactant pellet. These findings indicate that CEACAM6 is present in airways of human infants with lung disease, presumably secreted in part from alveolar type II cells and associated with pulmonary surfactant.
CEACAM6 is a multifunctional lung protein.
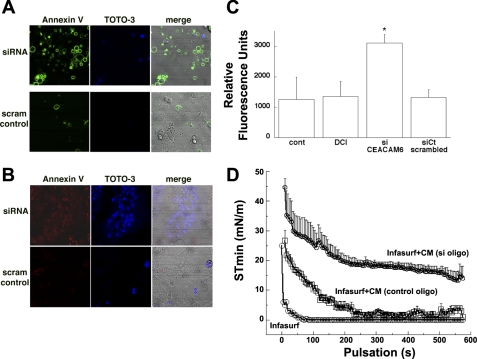
The ability of CEACAM6 to bind selected bacteria, including human-specific respiratory microorganisms, has been well characterized both in vitro and in studies with nonpulmonary epithelial primary cells and cell lines (10, 25, 45). Because CEACAM6 is secreted from type II cells and is present in epithelial lining fluid of the lung, it likely contributes to innate defense against microorganisms in lung alveoli. In addition, CEACAM6 is upregulated in metastatic tumors, and overexpression in cancer cell lines inhibits apoptosis (12–14, 26, 39, 41). We hypothesized that CEACAM6 inhibits apoptosis in type II cells, which serve as progenitors of alveolar type I cells during fetal development and proliferate in response to injury after birth (29). To test this possibility, we first performed experiments to examine apoptotic markers in DCI-treated cells with and without siRNA silencing of CEACAM6 expression (as illustrated in Fig. 3). As shown in the representative immunofluorescence images of Fig. 7A, many of the cells electroporated with CEACAM6-specific siRNA demonstrate peripheral annexin V-Alexa 488 staining (top left) compared with cells exposed to control RNA (bottom left) at 24 h after treatment (results were similar at 18 h). siRNA treatment reduced cellular content of CEACAM6 by 75% and 85% at 18 h and 24 h of DCI exposure, respectively. TOTO-3 staining, which detects cells with altered membrane permeability, was also greater in siRNA-treated cells (Fig. 7A, top center) compared with control cells (bottom center). At this time point after treatment, more cells were positive for annexin V than for TOTO-3 (overlay images in Fig. 7A, right). In studies at 72 h after electroporation, more cells with CEACAM6 knockdown were positive for TOTO-3 staining (Fig. 7B, center), and annexin V-Alexa 594 staining was more diffuse over cells (Fig. 7B, left).
Fig. 7.
CEACAM6 effects on apoptosis and surfactant function. A and B: annexin V and TOTO-3 staining at 24 h (A) and 72 h (B) by confocal microscopy. Freshly isolated epithelial cells were electroporated with anti-CEACAM6 oligonucleotides (siRNA, top) or scrambled control oligonucleotides (scram control, bottom). Cells treated with siRNA (top left, A) showed more annexin V (conjugated to Alexa 488) binding to the plasma membrane compared with cells treated with scrambled control oligos (bottom left). TOTO-3 stained few cells at 24 h (center), indicating good membrane integrity. At 72 h increased annexin V binding (conjugated to Alexa 594; B, left) occurred with knockdown of CEACAM6 similar to the response at 24 h (A). In addition, there was increased nuclear TOTO-3 staining, consistent with more cell death at this later time interval. The cellular density was similar for both groups as shown in the differential interference contrast images (right). Images are representative of 5 fields in 3 experiments. C: CEACAM6 knockdown increases caspase-3 activity. Freshly isolated cells were electroporated with anti-CEACAM6 si oligonucleotide, scrambled control oligonucleotide, or no oligonucleotide [control (cont) and DCI] and cultured 18–24 h in the absence (cont) or presence (all other groups) of DCI. Caspase-3 activity was elevated ∼3-fold in cells with knockdown of CEACAM6 compared with si control oligonucleotide value (n = 4; *P < 0.05 vs. cont). D: CEACAM6 protects surfactant from protein inhibition. Freshly isolated cells were electroporated with either CEACAM6-specific siRNA (80% knockdown by Western blot) or scrambled control oligonucleotide and cultured 4 days in the presence of DCI. Conditioned media (CM) were concentrated to a similar total protein level (range 0.2–0.6 mg/ml) in each experiment. Infasurf was diluted to 1.5 mg phospholipid/ml with fresh Waymouth medium (Infasurf alone control) or with conditioned medium, and surface tension was assessed in a pulsating bubble surfactometer. Infasurf alone rapidly achieved a low minimum surface tension (∼0 mN/m). In conditioned medium with low content of CEACAM6 (si oligo), the presence of exogenous protein increased equilibrium surface tension (time 0), delayed the time to minimum surface tension (∼550 s), and increased minimum surface tension (∼14 mN/m). In the presence of CEACAM6-replete conditioned medium (control oligo), a low minimum surface tension (∼2 mN/m) was achieved by ∼250 s.
Second, we measured caspase-3 activity in cells with/without silencing of CEACAM6 expression. As shown in Fig. 7C, caspase activity was increased 2.5-fold in type II cells treated with siRNA compared with cells receiving control RNA or no RNA (electroporated control and DCI-treated cells). The results from both staining and caspase assay indicate that expression of CEACAM6 inhibits apoptosis of cultured fetal type II cells in the face of electroportion as an apoptotic stimulus (40). Of interest, caspase-3 activity was relatively low in electroporated control cells, which contain low levels of CEACAM6. We propose that hormone-induced differentiation of fetal epithelial cells induces a proapoptotic change in the cells that is repressed by increased levels of CEACAM6. Previous microarray data indicated that expression of a number of pro- and antiapoptotic genes changed after exposure to DCI (46).
Because of our finding that CEACAM6 was associated with surfactant both intracellularly (lamellar bodies, Fig. 5D) and in epithelial lining fluid in vivo (Fig. 6D), we investigated potential effects of CEACAM6 on surfactant function. In initial studies we tested for effects of CEACAM6 on surface active properties of surfactant in the presence of inhibitory exogenous proteins. A commercial natural surfactant (Infasurf) was incubated with conditioned medium from fetal type II cells treated with either CEACAM6 siRNA or scrambled oligonucleotide (control). Conditioned medium from CEACAM6-silenced cells (Fig. 7D) was inhibitory for development of a low minimum surface tension in a pulsating bubble surfactometer; the minimum surface tension achieved was ∼15 mN/m compared with ∼0 for surfactant incubated with fresh culture medium (Infasurf). By contrast, medium without knockdown of CEACAM6 (control oligo) was much less inhibitory, achieving a minimum surface tension <2 mN/m after 4-min pulsation.
DISCUSSION
In this study we report the first characterization of CEACAM6 in human lung epithelial cells and provide evidence for unique regulation and lung-specific functions. We initially identified CEACAM6 as a protein of interest based on upregulation of its gene expression during hormone-induced fetal type II cell differentiation. In our model of differentiation, using microarray analysis, a subset of genes (7.1% of expressed genes) are induced >1.5-fold by hormone treatment; CEACAM6 was notable for a relatively high level of induction (∼10-fold) (46). In this culture system, undifferentiated epithelial cells of fetal lung develop morphological and biochemical characteristics of mature type II cells after exposure to glucocorticoid and cAMP, agents that modulate fetal lung maturation in vivo (21). Functional differentiation responses include synthesis of various surfactant components, secretion of active surfactant, and formation of a tight epithelial monolayer with regulated ion and fluid transport. Accordingly, the in vitro culture model represents precocious type II cell differentiation mimicking the process that occurs in vivo during the third trimester of human gestation. It is likely, therefore, that the properties of CEACAM6 regulation and function that we describe also occur in vivo.
Maximal expression of CEACAM6 in fetal lung cells required the presence of both dexamethasone and cAMP agents, with a response pattern similar to that for induction of TTF-1 (21). In this regard, hormonal regulation of CEACAM6 resembles that for many of the upregulated genes during in vitro type II cell differentiation (21). In addition, for those studied, hormonally induced genes of fetal lung are developmentally regulated and expressed in mature, adult type II cells as we found for CEACAM6 (4). Both glucocorticoids and cAMP are known to promote tissue differentiation; in vivo, lung corticoid content increases with development, and PGE2 is a possible stimulus for increased cAMP in fetal lung cells (1, 5, 6).
When hormones were added 72 h after cell isolation, the time course for CEACAM6 induction was relatively slow, with a considerable lag time. Delayed induction suggests that CEACAM6 is a product of differentiation rather than a signaling molecule involved in the differentiation process. In vivo, the content of CEACAM6 in colonic epithelial cells increases as the cells migrate and differentiate (22); however, the mechanism of induction in colonocytes has not been examined. For CEACAM6 of fetal lung cells, hormone treatment increased the rate of transcription comparable to the level of increased mRNA. Similarly, transcriptional regulation, rather than enhanced transcript stability, occurs for most other target genes in fetal lung cells that have been examined (4).
Using both gain- and loss-of-function approaches we have established that CEACAM6 is a target gene for TTF-1. This transcription factor, which is encoded by TITF1 (formerly Nkx2.1), is a homeodomain-containing phosphoprotein that is expressed in thyroid, lung, and areas of the brain. TTF-1 is required for normal structural development of the mouse lung (30) and regulates expression of a limited number of genes in developing type II cells (9, 31, 50). Factors that repress TTF-1 would be expected to decrease TTF-1 target genes such as CEACAM6, and we have found that this response occurs for transforming growth factor (TGF)-β (36). TTF-1 is decreased in type II cells after bleomycin lung injury in rats, correlating temporally and spatially with decreased SP-B (42). Our findings for regulation of CEACAM6 by TTF-1 in cultured cells support the possibility that CEACAM6 content of human lung cells in vivo is also influenced by TTF-1 both during development and in response to injury or disease.
Regulation of CEACAM6 expression by TTF-1 appears to be indirect. In transduction experiments, an increase in CEACAM6 transcription is delayed ∼36 h after elevated content of rTTF-1 (∼12 h). Inhibition of protein synthesis blocked hormone induction of CEACAM6 mRNA even under conditions of TTF-1 induction, consistent with a requirement for other upregulated factors. Also, we found no putative response elements for TTF-1 in the proximal 1 kb of the CEACAM6 promoter. At least two possible models for hormonal induction of CEACAM6 are considered. TTF-1 may increase CEACAM6 expression indirectly via induction of one or more unidentified factors that bind to the CEACAM6 promoter; in this model, TTF-1 could also bind to the CEACAM6 promoter but would be inactive in the absence of other TTF-1-induced regulators. A second indirect mode of action involves a TTF-1-induced regulator(s) that decreases binding activity of an inhibitor of CEACAM6 gene expression, thereby increasing CEACAM6. This seems less likely because of the observation that treatment with cycloheximide does not induce CEACAM6.
Regulation of CEACAM6 by TTF-1 has possible implications for the origin and metastatic potential of lung adenocarcinomas. A proportion of these tumors apparently arise from type II cells and continue to express TTF-1, in some cases at elevated levels. Moreover, both TTF-1 and CEACAM6 are marker genes that are specific for lung adenocarcinomas versus other types of lung cancers, and both proteins are highly expressed in some lung adenocarcinoma cell lines (8). We propose that transformation of type II cells in some lung adenocarcinomas involves mutagenic insults resulting in increased TTF-1 content, elevated and dysregulated expression of CEACAM6, and subsequent alterations in cell proliferation and adhesion properties. Accordingly, TTF-1 and CEACAM6 may be potential therapeutic targets for selected lung adenocarcinomas.
In addition to CEACAM6, fetal type II cells expressed CEACAMs 1, 4, and 5 at relatively low levels, and adult cells also express CEACAMs 1 and 5. CEACAM5 was the only other family member in fetal cells that was responsive to hormones. This pattern of CEACAM expression is similar to that in human colonocytes. Moreover, expression of both CEACAM5 and CEACAM6 is upregulated in vivo during colonocyte differentiation (26, 43). We did not study regulation of CEACAM5, which is modestly induced in fetal lung cells. CEACAM5 and CEACAM6, along with other members of the CEA subgroup, are clustered at q13.2 on chromosome 19, and the proximal promoters of the two genes are highly homologous (23). It is possible that these two CEACAMs display similar responses to hormone exposure and developmental signals and have overlapping functions in the lung.
In confocal immunofluorescence studies we found that CEACAM6 localized to intracellular vesicles as well as the plasma membrane of fetal type II cells. Colocalization with SP-B indicated that CEACAM6 was present within lamellar bodies rather than in the limiting membrane, such as occurs for alkaline phosphatase, LAMP3 (DC-LAMP, lysosome-associated membrane protein), and ABCA3 (ATP binding cassette A3), which has a putative phospholipid transport function (37, 38, 46). Other identified proteins within lamellar bodies include SP-A, SP-C, and pepsinogen C (Guttentag S, unpublished observation), which are all related to pulmonary surfactant function or synthesis (18). The function of CEACAM6 in lamellar bodies is not known; however, we speculate that it is targeted to developing lamellar bodies and interacts with the phospholipid bilayers via its GPI anchor.
CEACAM6 that is released from fetal type II cells into the media may represent both secretion of lamellar bodies and shedding of protein from the plasma membrane. We did not observe a significant increase in CEACAM6 release in the presence of secretagogues, which is likely due to the high level of release in the absence of secretagogues (i.e., a masking effect). In vivo, CEACAM6 is presumably secreted with surfactant during lamellar body exocytosis, and a portion remains surfactant associated in the alveolus. Approximately half of CEACAM6 in epithelial lining fluid of infant tracheal aspirates was found in the large-aggregate surfactant fraction, and half was present in the supernatant fraction, which contains small-aggregate (recycling) surfactant and nonsurfactant components. Supernatant CEACAM6 could represent in part protein released from the plasma membrane, which presumably lacks the GPI anchor and has a lower affinity for surfactant. Secretion was reduced in cells exposed to mannosamine, which inhibits glycosylation and GPI formation. Alternatively, the presence of CEACAM6 in both surfactant and supernatant fractions may reflect a moderate binding affinity of CEACAM6 for surfactant phospholipid and thus equilibrium distribution. Ongoing studies are addressing this question as well as content of secreted CEACAM6 related to the clinical status of infants.
We found that CEACAM6 content of fetal lung cells was a strong determinate of apoptosis. Overexpression of CEACAM5/CEACAM6 in transformed cell lines inhibits apoptosis of cells that are detached from their basement membrane (26, 39); however, to our knowledge, this is the first observation for a role of endogenous CEACAM6 in programmed death of normal, nontransformed cells. The stimulus for apoptosis in our studies is apparently electroporation and/or presence of transfected RNA. The physiological role for CEACAM6 in type II cell proliferation/apoptosis is uncertain; future studies will identify targets of CEACAM6 related to apoptosis.
To date, four different proteins have been assigned the name “pulmonary surfactant protein.” SP-B and SP-C are highly hydrophobic proteins that are packaged within lamellar bodies and remain surfactant associated in the alveolus, where they participate in formation and stability of the surfactant surface film (48). SP-A is primarily secreted by a constitutive mechanism, independent of surfactant, but associates with surfactant in the alveolus and recycles to intracellular lamellar bodies along with surfactant phospholipids. SP-A participates in lung innate host defense by virtue of its ability to bind microorganisms, enhance phagocytosis, and modulate inflammatory responses of lung epithelium. SP-D is partially associated with surfactant in the alveolus, but its only known function is innate defense similar to SP-A (49). We report the new observations that CEACAM6 localizes in part to lamellar bodies of cultured fetal type II cells and is found associated in part with large-aggregate surfactant isolated from both cells and epithelial lining fluid of infants. Because of this finding of surfactant association, we explored the possibility that CEACAM6 might have surfactant-related function(s). We found that the presence of CEACAM6 reduced the inhibitory activity of proteins on surfactant function in vitro. Surfactant activity is inhibited in the presence of excess protein, and this process is considered physiologically important under conditions of pulmonary edema with influx of plasma proteins or with sloughing of damaged epithelial cells after lung injury (51). Other possible effects of CEACAM6 on surfactant function or metabolism (e.g., recycling, adsorption of phospholipid to the surface) remain to be explored. We speculate that CEACAM6 is upregulated with lung injury or disease and that the increased content in the alveolus helps protect surfactant from inactivation by proteins.
CEACAM6 is upregulated during neutrophil activation, and ligation of CEACAM6 by antibodies promotes activation (32). On both neutrophils and epithelial cells, CEACAM5/CEACAM6 bind a variety of gram-negative bacteria, including microorganisms of the genera Escherichia, Salmonella, Neisseria, Morexella, and Haemophilus, and mediate internalization/phagocytosis (10). An additional role for CEACAMs in the inflammatory response is suggested by two in vitro studies showing that addition of CEACAM5 to cultured blood and Kupffer cells increased release of various cytokines (17). The importance of innate immune defense in the lung is well recognized (49). It is reasonable to speculate that CEACAM5/6 in the alveolar space promotes bacterial clearance and that cell-attached CEACAMs signal cellular responses on binding of microorganisms during alveolar infection. Currently there is no information related to these putative roles for CEACAMs in the lung alveolus.
In summary, we found that CEACAM6 in fetal lung epithelium is developmentally and hormonally regulated and that expression is specific for type II alveolar cells. Increased content of TTF-1 is sufficient to induce CEACAM6, but this response appears to be indirect and to involve other induced factor(s). CEACAM6 is localized to both the plasma membrane and lamellar bodies of cells and is found in surfactant isolated from airways, suggesting that CEACAM6 is a newly identified member of the surfactant-associated protein family. Because CEACAM6 acts as an antiapoptotic factor for cultured type II cells and stabilizes surfactant function in vitro, in addition to a putative role in alveolar innate defense, we propose that it is a multifunctional alveolar protein in postnatal lung. The unique regulation and multiple functions suggest an important role for CEACAM6 in vivo in type II cell proliferation and function, particularly in lung injury, infection, or tumorigenesis.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-19737 (P. L. Ballard, V. Kolla), HL-56401 (P. L. Ballard, Linda W. Gonzales), and HL-88193 (P. L. Ballard, Linda W. Gonzales).
Acknowledgments
We thank P. Minoo for the murine TTF-1 plasmid construct 12A2, M. Strayer for construction of the adenoviral constructs expressing sense TTF-1, L. Lu for amplification and purification of adenovirus, and X. Fang and J. W. Lee for preparation of adult type II cells.
Present address of M. Madesh: Dept. of Biochemistry, Temple University, Philadelphia, PA.
REFERENCES
- 1.Acarregui MJ, Snyder JM, Mitchell MD, Mendelson CR. Prostaglandins regulate surfactant protein A (SP-A) gene expression in human fetal lung in vitro. Endocrinology 127: 1105–1113, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Alcorn JL, Smith ME, Smith JF, Margraf LR, Mendelson CR. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am J Respir Cell Mol Biol 17: 672–682, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Allard WJ, Neaman IE, Elting JJ, Barnett TR, Yoshimura H, Fritsche HA, Yeung KK. Nonspecific cross-reacting antigen 50/90 is elevated in patients with breast, lung, and colon cancer. Cancer Res 54: 1227–1234, 1994. [PubMed] [Google Scholar]
- 4.Ballard PL The glucocorticoid domain in the lung and mechanisms of action. In: Endocrinology of the Lung: Development and Surfactant Synthesis, edited by Mendelson CR. Totowa, NJ: Humana, 2000, p. 1–44.
- 5.Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol 173: 254–262, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Ballard PL, Gonzales LW, Williams MC, Roberts JM, Jacobs MM. Differentiation of type II cells during explant culture of human fetal lung is accelerated by endogenous prostanoids and adenosine 3′,5′-monophosphate. Endocrinology 128: 2916–2924, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Ballard PL, Merrill JD, Godinez RI, Godinez MH, Truog WE, Ballard RA. Surfactant protein profile of pulmonary surfactant in premature infants. Am J Respir Crit Care Med 168: 1123–1128, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal RD, Hansen HJ, Goldenberg DM. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res 65: 8809–8817, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bohinski RJ, Huffman JA, Whitsett JA, Lattier DL. Cis-active elements controlling lung cell-specific expression of human pulmonary surfactant protein B gene. J Biol Chem 268: 11160–11166, 1993. [PubMed] [Google Scholar]
- 10.Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med 185: 1557–1564, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, Guttentag S, Ballard PL, Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1266–L1273, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Montero CM, McIntyre BW. Acquisition of anoikis resistance in human osteosarcoma cells does not alter sensitivity to chemotherapeutic agents. BMC Cancer 5: 39, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duxbury MS, Ito H, Benoit E, Ashley SW, Whang EE. CEACAM6 is a determinant of pancreatic adenocarcinoma cellular invasiveness. Br J Cancer 91: 1384–1390, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene 23: 465–473, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fehrenbach H Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res 2: 33–46, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filella X, Molina R, Alcover J, Coca F, Zarco MA, Ballesta AM. Influence of AFP, CEA and PSA on the in vitro production of cytokines. Tumour Biol 22: 67–71, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Gerson KD, Foster CD, Zhang P, Zhang Z, Rosenblatt MM, Guttentag SH. Pepsinogen C proteolytic processing of surfactant protein B. J Biol Chem 283: 10330–10338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzales LW, Angampalli S, Guttentag SH, Beers MF, Feinstein SI, Matlapudi A, Ballard PL. Maintenance of differentiated function of the surfactant system in human fetal lung type II epithelial cells cultured on plastic. Pediatr Pathol Mol Med 20: 387–412, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales LW, Ballard PL, Ertsey R, Williams MC. Glucocorticoids and thyroid hormones stimulate biochemical and morphological differentiation of human fetal lung in organ culture. J Clin Endocrinol Metab 62: 678–691, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol 283: L940–L951, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Hammarstrom S The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 9: 67–81, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Han SU, Kwak TH, Her KH, Cho YH, Choi C, Lee HJ, Hong S, Park YS, Kim YS, Kim TA, Kim SJ. CEACAM5 and CEACAM6 are major target genes for Smad3-mediated TGF-beta signaling. Oncogene 27: 675–683, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hildebrandt AL, Neufer PD. Exercise attenuates the fasting-induced transcriptional activation of metabolic genes in skeletal muscle. Am J Physiol Endocrinol Metab 278: E1078–E1086, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Hill DJ, Virji M. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol 48: 117–129, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Ilantzis C, Jothy S, Alpert LC, Draber P, Stanners CP. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab Invest 76: 703–716, 1997. [PubMed] [Google Scholar]
- 27.Jantscheff P, Terracciano L, Lowy A, Glatz-Krieger K, Grunert F, Micheel B, Brummer J, Laffer U, Metzger U, Herrmann R, Rochlitz C. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J Clin Oncol 21: 3638–3646, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kammerer R, Popp T, Singer BB, Schlender J, Zimmermann W. Identification of allelic variants of the bovine immune regulatory molecule CEACAM1 implies a pathogen-driven evolution. Gene 339: 99–109, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kasper M, Haroske G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol Histopathol 11: 463–483, 1996. [PubMed] [Google Scholar]
- 30.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10: 60–69, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Kolla V, Gonzales LW, Gonzales J, Wang P, Angampalli S, Feinstein SI, Ballard PL. Thyroid transcription factor in differentiating type II cells: regulation, isoforms, and target genes. Am J Respir Cell Mol Biol 36: 213–225, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroki M, Abe H, Imakiirei T, Liao S, Uchida H, Yamauchi Y, Oikawa S, Kuroki M. Identification and comparison of residues critical for cell-adhesion activities of two neutrophil CD66 antigens, CEACAM6 and CEACAM8. J Leukoc Biol 70: 543–550, 2001. [PubMed] [Google Scholar]
- 33.Kuroki M, Matsushita H, Matsumoto H, Hirose Y, Senba T, Yamamoto T. Nonspecific cross-reacting antigen-50/90 (NCA-50/90) as a new tumor marker. Anticancer Res 19: 5599–5606, 1999. [PubMed] [Google Scholar]
- 34.Lisanti MP, Field MC, Caras IW, Menon AK, Rodriguez-Boulan E. Mannosamine, a novel inhibitor of glycosylphosphatidylinositol incorporation into proteins. EMBO J 10: 1969–1977, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol 170: 1079–1090, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDevitt TM, Gonzales LW, Savani RC, Ballard PL. Role of endogenous TGF-beta in glucocorticoid-induced lung type II cell differentiation. Am J Physiol Lung Cell Mol Physiol 292: L249–L257, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Meban C Fine structural localization of alkaline phosphatase in the granular pneumonocytes of hamster lung. Histochemistry 43: 367–372, 1975. [DOI] [PubMed] [Google Scholar]
- 38.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem 277: 22147–22155, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Ordonez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 60: 3419–3424, 2000. [PubMed] [Google Scholar]
- 40.Pinero J, Lopez-Baena M, Ortiz T, Cortes F. Apoptotic and necrotic cell death are both induced by electroporation in HL60 human promyeloid leukaemia cells. Apoptosis 2: 330–336, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Samara RN, Laguinge LM, Jessup JM. Carcinoembryonic antigen inhibits anoikis in colorectal carcinoma cells by interfering with TRAIL-R2 (DR5) signaling. Cancer Res 67: 4774–4782, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Savani RC, Godinez RI, Godinez MH, Wentz E, Zaman A, Cui Z, Pooler PM, Guttentag SH, Beers MF, Gonzales LW, Ballard PL. Respiratory distress after intratracheal bleomycin: selective deficiency of surfactant proteins B and C. Am J Physiol Lung Cell Mol Physiol 281: L685–L696, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Scholzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol 156: 595–605, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsutsumi Y, Onoda N, Misawa M, Kuroki M, Matsuoka Y. Immunohistochemical demonstration of nonspecific cross-reacting antigen in normal and neoplastic human tissues using a monoclonal antibody. Comparison with carcinoembryonic antigen localization. Acta Pathol Jpn 40: 85–97, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Virji M, Makepeace K, Ferguson DJ, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol 22: 941–950, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Wade KC, Guttentag SH, Gonzales LW, Maschhoff KL, Gonzales J, Kolla V, Singhal S, Ballard PL. Gene induction during differentiation of human pulmonary type II cells in vitro. Am J Respir Cell Mol Biol 34: 727–737, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver TE, Na CL, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol 13: 263–270, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 347: 2141–2148, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Wright JR Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Yan C, Sever Z, Whitsett JA. Upstream enhancer activity in the human surfactant protein B gene is mediated by thyroid transcription factor 1. J Biol Chem 270: 24852–24857, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Zuo YY, Veldhuizen RA, Neumann AW, Petersen NO, Possmayer F. Current perspectives in pulmonary surfactant—inhibition, enhancement and evaluation. Biochim Biophys Acta 1778: 1947–1977, 2008. [DOI] [PubMed] [Google Scholar]