Abstract
Alveolar macrophages (AM) are the most abundant antigen-presenting cells in the lungs, and they play a critical role in regulating pulmonary immune responses to inhaled pathogens and to allergens. However, compared with macrophages in other body sites, AM have an unusual phenotype that, in many respects, resembles the phenotype of dendritic cells (DC). Therefore, to more fully define the unique nature of AM, we compared the phenotype and function of AM with the phenotype and function of resident peritoneal lavage-derived macrophages (PLM). We found striking phenotypic differences between AM and PLM, particularly with regard to CD11c expression, and we also observed that AM had a significantly better antigen-presenting capability than PLM. Therefore, we investigated the role of the local airway environment in generation of the unusual phenotype of AM. We carried out cell transfer experiments to compare macrophage differentiation in the airways with that in the peritoneal cavity. We observed significant upregulation of CD11c expression on bone marrow macrophages and peritoneal macrophages when they were adoptively transferred into the airways. In contrast, CD11c expression was not upregulated after cell transfer into the peritoneal cavity, whereas CD11b expression was significantly increased. In vitro, culture of bone marrow-adherent cells with surfactant protein D (SP-D) or granulocyte/macrophage colony-stimulating factor (GM-CSF) induced significant upregulation of CD11c expression, and in vivo GM-CSF concentrations were significantly higher in bronchoalveolar than in peritoneal lavage fluid. Finally, GM-CSF−/− mice failed to develop CD11c+ AM, but CD11c+ AM were present in SP-D−/− mice. However, macrophages from GM-CSF−/− bone marrow could upregulate CD11c expression when transferred to the airways of wild-type mice. These results suggest that the airway environment promotes development of macrophages with unique DC-like characteristics and that this unusual phenotype is determined, to a large degree, by locally high concentrations of GM-CSF and, possibly, SP-D.
Keywords: dendritic cell, surfactant, granulocyte/macrophage colony-stimulating factor, CD11c
alveolar macrophages (AM) are the most abundant antigen-presenting cells in the airways and alveolar spaces, where they play a critical role in regulating immune responses and inflammation within the lung (15, 21, 27, 30, 34, 35, 42). AM perform a number of important functions, including phagocytosis of particulate matter, secretion of cytokines and enzymes, and control of microbes. They are the first cells to contact many inhaled antigens, including infectious agents, allergens, and small particulate debris, and, therefore, play a key role in initiating and perpetuating local pulmonary immune responses. Most studies indicate that AM are ineffective at initiating immune responses relative to other antigen-presenting cells in the lung and that reduced antigen presentation abilities may serve to limit deleterious inflammatory responses within the lungs (43). For example, previous studies have shown that AM are relatively ineffective at stimulating naïve T cell responses (29, 31, 46). However, other studies have shown that AM can stimulate responses by previously activated effector T cells (48).
Under steady-state conditions, the AM population in the lungs turns over very slowly, with a reported estimated half-life of 30–60 days (4, 8) to a turnover rate of 40% in 1 yr in naïve mice (32). AM can be derived primarily from blood monocytes, although a significant percentage of AM are probably also generated from a self-renewing population of progenitor cells within the lung parenchyma (3, 5, 40, 44). The growth and survival of AM within the lungs depend, in part, on locally produced cytokines such as macrophage colony-stimulating factor (CSF), and the respiratory epithelium is also a rich source of granulocyte/macrophage CSF (GM-CSF) (11, 23, 47).
AM also exhibit unique properties, including unusual phenotypic features, compared with other tissue macrophages (2, 28, 43, 50). For example, previous studies have found that AM express high levels of CD11c, a molecule that is not expressed by other macrophages and is generally only expressed by dendritic cells (DC) and some natural killer cells and activated T cell subsets (20, 22, 33, 51). Moreover, AM have been found to express high levels of DEC-205, which also is not expressed by other macrophages and is generally only expressed by certain subpopulations of DC (2, 6, 7). We reported recently that AM recovered from the lungs of normal mice by bronchoalveolar lavage (BAL) had an unusual phenotype compared with typical tissue macrophages (6). Others have also noted this unique phenotype of AM in mice (19, 52). DC are classically characterized by CD11c expression, high major histocompatability complex (MHC) class II expression, and variable expression of costimulatory markers such as CD40, CD80, and CD86. Macrophages have been historically classified as expressing CD11b and MHC class II. Interestingly, AM have been noted previously as negative for CD11b (unless activated), although CD11b expression is uniformly high on all other macrophage populations that have been studied.
The unique features of AM could reflect the unusual environment in which they are found. For example, AM are exposed to high oxygen tension and are also bathed in high concentrations of GM-CSF and surfactant proteins, especially surfactant protein (SP)-A and SP-D, both of which have immunomodulatory properties (9, 10, 13, 53). We therefore wondered to what degree the unique phenotype of AM was determined by local factors in the lung environment and whether macrophages from sites besides the lungs could be induced to assume an AM-like phenotype when placed in the lung environment. This question is important, because if local factors were found to alter macrophage phenotype, then perhaps the AM phenotype is not predetermined but is, instead, a product of the environment in which the macrophages are found.
To address this question, we first more fully compared and contrasted the phenotype and function of AM with the phenotype and function of another relatively pure macrophage population, peritoneal lavage-derived macrophages (PLM). Next, we conducted a series of adoptive transfer experiments to assess the effects of the lung environment on the phenotype of other (nonlung) macrophages and to determine the degree to which the transferred cells could be induced to assume an AM-like phenotype. For these studies, we focused primarily on changes in CD11c expression, since expression of this molecule exhibited the most consistent differences between AM and other macrophage populations. In addition, we investigated the role that key soluble factors in the lung microenvironment could play in regulating the AM phenotype. The results of these studies indicated that the lung environment was a strong stimulus for induction of the AM phenotype in macrophages from nonpulmonary sources and that GM-CSF appeared to be the most critical factor responsible for induction of the AM phenotype.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free, 6- to 8-wk-old, female C57BL/6, ICR, and BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) or Harlan Laboratories (Indianapolis, IN). C57BL/6 mice transgenic for expression of the green fluorescent protein (GFP) gene under control of the ubiquitin promoter (UBI-GFP/BL6 mice) were kindly provided by Dr. Phillipa Marrack (National Jewish Medical Center) (38). Mice were housed in sterile microisolater cages in the laboratory animal resources facility at Colorado State University, with sterile water and food provided ad libitum. All research involving animals was conducted in accordance with guidelines and animal protocols approved by the Animal Care and Use Committee at Colorado State University.
Collection of BAL cells.
BAL cells were obtained by BAL, as previously described (6). Cells from the three lavages for each mouse were pooled, centrifuged at 1,200 rpm for 5 min at 4°C, counted, and then resuspended in complete medium [cMEM: MEM supplemented with 10% heat-inactivated FBS, 2 mmol/l l-glutamine, 1% nonessential amino acids, 50 IU/ml penicillin, and 50 mg/ml streptomycin (all from Life Technologies, Carlsbad, CA) and 7.5% bicarbonate] or fluorescein-activated cell sorter (FACS) buffer (PBS with 2% FBS and 0.05% sodium azide) before analysis.
Collection of peritoneal lavage cells.
PLM were collected from the peritoneal cavity of naïve mice by peritoneal lavage. Briefly, ∼8–10 ml of ice-cold HBSS were injected into the peritoneal cavity. The abdominal cavity was massaged to dislodge loosely adherent peritoneal cells, and the injected fluid was slowly withdrawn using a syringe and a no. 22 needle. The procedure was repeated twice for each mouse, and the cells obtained from each mouse were pooled, quantitated, centrifuged, and resuspended in cMEM or FACS buffer before analysis.
Flow cytometry and analysis of AM and PLM.
Cell populations in the BAL and peritoneal lavage fluid were assessed by immunostaining and flow cytometry. Directly conjugated antibodies used for these analyses were purchased from Cedarlane Laboratories (Westbury, NY), Serotec (Raleigh, NC), Pharmingen (San Diego, CA), and eBiosciences (San Diego, CA). The following antibodies in various combinations were used for flow cytometric analysis: anti-CD11c [allophycocyanin (APC) or phycoerythrin (PE); clone N418], anti-CD11b (PE-Cy5, APC-Cy7, or Pacific Blue; clone M1/70), anti-GR-1 (pEY7; clone RB6-8C5), anti-Ly6G (FITC; clone 1A8), anti-B220 (APC-Cy7; clone RA3-6B2), anti-CD8 (FITC or PE; clone 53-6.7), anti-CD4 (APC; clone RM4-5), anti-CD3 (APC-Cy7; clone 145-2C11), anti-I-A/I-E (MHC class II, biotin, or PE; clone M5/114.15.2), anti-CD86 (PE; clone GL1), anti-DEC-205 (biotin; clone NLDC-145), and anti-CD40 (pEY5; clone 1C-10). Preparation and staining of cells for flow cytometry have been previously described (6). Flow cytometry was carried out on a Cyan MLE or Cyan ADP flow cytometer (DakoCytomation/Beckman Coulter, Fort Collins, CO). Approximately 25,000 events (BAL samples) and 100,000 events (peritoneal lavage samples) were analyzed for each sample. Isotype control antibodies were included initially to ensure specificity of staining. Data were analyzed using Summit software (DakoCytomation). The percentage of each cell population was determined, then total cell numbers for each population were calculated from the total number of viable cells collected. Experimental and control groups consisted of three to four animals each.
Assessment of macropinocytosis by labeled dextran uptake.
Cells from BAL and peritoneal lavage were collected as described above and placed in culture. After 1–2 h in culture, the nonadherent cells were removed, and the remaining cells were incubated with 100 μg/ml Alexa 488-conjugated dextran (Invitrogen, Carlsbad, CA) for 1 h at 37°C. Then the labeled dextran was washed off, and the cells were detached by vigorous pipetting in PBS. The detached cells were immunostained in FACS buffer for determination of dextran uptake and phenotype. In some experiments, cells were pretreated overnight with 10 μg/ml Salmonella minnesota R595 LPS (InVivogen, San Diego, CA) and then incubated with labeled dextran and analyzed by flow cytometry.
Assessment of cross-priming by AM and PLM.
AM and PLM were collected from C57BL/6 mice as described above. Cells (1 × 105 per well) were cultured for 24 or 48 h in cMEM in triplicate wells of 24-well plates to reduce the immunosuppression that occurs when AM are used in these assays immediately ex vivo. The cells were then incubated for 2 h with 100, 1, or 0.1 μg/ml filtered ovalbumin (Sigma-Aldrich, St. Louis, MO). After the cells were washed, B3.17 CD8+ T cell hybridoma cells (5 × 105 per well) specific for H-2Kb/SIINFEKL (a kind gift of Nibla Shastri, University of California, Berkley) were added to the AM or PLM, and the combined cells were cultured overnight. Negative controls included AM and PLM with or without B3.17 cells. For positive controls, AM or PLM were pulsed with 1 μM SIINFEKL peptide and then B3.17 cells were added. After overnight culture with B3.17 cells, supernatants were harvested and assayed for IL-2 concentration by mouse IL-2 ELISA according to the manufacturer's directions (eBiosciences). The mean IL-2 concentration was determined for triplicate wells and plotted.
Assessment of mixed lymphocyte reaction.
AM and PLM were harvested from ICR mice as described above and placed in triplicate wells of 96-well plates at the indicated cell concentrations in complete medium without cytokines and cultured for 1, 24, or 48 h before addition of responder T cells to assess the effects of different duration of preincubation on presentation capabilities. At the indicated time points, allogeneic T cells were prepared from the spleens of C57BL/6 mice and added to 96-well plates in cMEM at 5 × 105 per well. In some experiments, AM or PLM were activated before addition of spleen cells by incubation overnight in 10 μg/ml LPS. Proliferation was assessed after 72 h in culture by overnight incubation with 0.5 μCi of [3H]thymidine (Amersham, Piscataway, NJ). Controls included spleen cells cultured alone or AM or PLM incubated alone and pulsed with [3H]thymidine. Thymidine-pulsed cells were harvested using an automated cell harvester (Wallac-Microbeta, Wellesley, MA), and thymidine incorporation was assessed using an automated beta counter (Wallac-Microbeta).
Adoptive transfer of labeled bone marrow or peritoneal lavage cells.
Bone marrow cells were obtained from GFP transgenic mice as described previously (6). PLM were obtained from GFP+ donor mice as described above. The cells were adjusted to a final concentration of 1–2 × 108/ml in HBSS and stored on ice before adoptive transfer. For intratracheal transfer of cells, mice were anesthetized by intraperitoneal injection of 200 μl of a 2.5% solution of Avertin (Sigma, St. Louis, MO). After the mice were anesthetized, a steel gavage tube was passed by palpation through the larynx and into the trachea, and 50 μl of the cell suspension were injected intratracheally into each anesthetized mouse as described previously (7). For adoptive transfer into the peritoneal cavity, bone marrow cells (typically 2 × 107 total cells) were injected intraperitoneally.
Effects of SP-A or SP-D on CD11c expression by bone marrow cells.
Bone marrow cells were collected from femurs of healthy C57BL/6 mice as described above. Cells were plated at a density of 2 × 106/ml in 24-well plates (Costar, Corning, NY) in cMEM. Nonadherent cells were removed as described above. Purified human SP-A and recombinant SP-D (49) were added at the indicated concentrations in medium containing 1.8 mM calcium (cMEM) to triplicate wells of adherent bone marrow cells. Other wells were incubated with 1 μg/ml LPS or recombinant GM-CSF (PeproTech, Rocky Hill, NJ). After 5 days in culture, cells were detached, via incubation with 5 mM EDTA in PBS at 4°C for 10 min followed by vigorous pipetting. Cells were then immunostained and analyzed by flow cytometry.
SP-D−/− and GM-CSF−/− mice.
SP-D−/− and GM-CSF−/− mice were obtained from Jackson Laboratories and housed as described above. Airway and peritoneal lavage cells were recovered and stained for flow cytometry as described above. For short-term transfer experiments involving GM-CSF−/− bone marrow cells, 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) was used to label transferred cells. Briefly, bone marrow and peritoneal lavage cells were labeled by incubation in 10 μM CFSE solution for 15 min at room temperature, washed twice, and resuspended in PBS, and cell viability was assessed using trypan blue exclusion. After the CFSE-labeling procedure, cell viability was routinely >90% (data not shown). The labeled cells were washed in PBS immediately before adoptive transfer.
Statistical analyses.
Statistical differences between two treatment groups were determined using Student's t-test, whereas differences between three or more treatment groups were determined using ANOVA followed by Tukey's multiple-means comparison test. Statistical analyses were done using GraphPad Prism software (San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
Unique phenotype of AM resembles that of immature DC.
To compare the phenotype of AM and PLM, we assessed the expression of two markers commonly expressed on other populations of DC (CD11c and DEC-205) and two markers expressed on other macrophage populations in the body (CD11b and F4/80). Other cell surface determinants, including Ly6-G, B220, CD4, CD8, MHC class II, and CD86, were assessed. The population of large cells with high-forward-scatter and side-scatter characteristics were studied, inasmuch as these cells were considered to represent AM and PLM (18, 52). AM were obtained from normal BALB/c, as well as ICR, C57BL/6, and 129 Sv/Ev mice. In our studies, AM from these different strains of mice had a similar phenotype (data not shown), with virtually all cells coexpressing high levels of CD11c and DEC-205 (Fig. 1, Table 1). In addition, a small percentage (typically <5%) of cells were positive for CD11b and CD11c and expressed only very low levels of F4/80, which we interpreted as representing true airway myeloid DC. The identity of this subpopulation of cells as DC was also confirmed by cell sorting and cytology experiments (data not shown). In contrast, PLM were uniformly negative for expression of CD11c and DEC-205, whereas they expressed high levels of CD11b, and most also expressed high levels of F4/80 (Fig. 1, Table 1). Granulocytes (Ly6-G+/CD11b+) were rarely observed in BAL samples, whereas they were more numerous in peritoneal lavage samples. Most of the CD11c+/CD11b− AM did not express CD86, whereas CD86+ cells were more numerous among PLM (Table 1). Similar findings regarding the phenotype of AM have been reported previously (22). Taken together, these results further suggest that the phenotype of AM was consistent with that of immature DC, whereas the phenotype of PLM was consistent with that of previous reports of typical macrophages.
Fig. 1.
Phenotypic comparison of alveolar macrophages (AM) and peritoneal lavage-derived macrophages (PLM). Cells were collected by bronchoalveolar lavage (BAL) or lavage of the peritoneal cavity of naïve BALB/c mice. Airway and peritoneal cells were immunostained with antibodies [anti-CD11c-allophycocyanin (APC), anti-CD11b-phycoerythrin (PE)/Cy5, anti-major histocompatability complex class II (MHC II)-PE, anti-GR-1-PE/Cy7, and anti-DEC-205-biotin], incubated with streptavidin-Alexa 488, and analyzed by multiparameter 5-color flow cytometry. AM coexpressed CD11c and DEC-205 but did not express CD11b, and ∼30% were MHC class II positive. PLM were CD11b positive and CD11c and DEC-205 negative. Similar results were obtained in numerous repeated experiments using lavage cells obtained from several different strains of mice, including C57BL/6, 129, and ICR.
Table 1.
Phenotype of murine airway DC and peritoneal macrophages
| Determinant | Airway DC Macrophages | Peritoneal Macrophages |
|---|---|---|
| CD11c | +++ | − |
| CD11b | − | +++ |
| F4/80 | + | +++ |
| DEC-205 | ++ | − |
| MHC class II | + | ++ |
| B220 | + | − |
| Gr-1 | − | + |
| CD4 | − | − |
| CD8 | − | − |
| CD86 | ± | + |
Dendritic cells (DC) and peritoneal macrophages freshly isolated from airways and peritoneum, respectively, of uninfected mice were analyzed by flow cytometry. +, ++, and +++, Number and intensity of staining; −, marker observed in <2% of cells; ±, marker observed in 2-5% of cells.
AM exhibit high levels of spontaneous macropinocytosis.
Next, we compared the functional properties of AM and PLM. Immature DC typically exhibit high levels of spontaneous macropinocytosis, a property that is not exhibited by mature tissue macrophages (37). We incubated freshly isolated AM and PLM in vitro with labeled dextran and assessed macropinocytosis by flow cytometry. Labeled dextran was readily engulfed by freshly isolated AM, whereas dextran uptake was significantly reduced in freshly isolated PLM (Fig. 2A). For example, >90% of AM were positive for uptake of labeled dextran, whereas only 10–15% of PLM exhibited dextran uptake. Activation and maturation of DC by inflammatory stimuli such as LPS are known to induce a rapid reduction in macropinocytosis (37). Therefore, AM and PLM were activated by LPS for 24 h in vitro, and labeled dextran uptake was assessed. After exposure to LPS, macropinocytosis in AM was rapidly downmodulated: it decreased from 90% dextran-positive cells before LPS treatment to 30% dextran-positive cells after LPS treatment (Fig. 2B). In contrast, the already low level of dextran uptake by PLM was relatively unaffected by exposure to LPS (Fig. 2B). These results illustrate an important functional difference between AM and PLM.
Fig. 2.
Comparison of macropinocytosis by AM and PLM. Freshly harvested airway and peritoneal lavage cells were placed in complete medium and incubated for 1 h with 10 μg/ml Alexa 488-labeled dextran. Cells were then washed extensively in PBS, immunostained for CD11c and CD11b, and analyzed by flow cytometry. A: uptake of labeled dextran by AM and PLM after 1 h of incubation in vitro. B: uptake of labeled dextran assessed by flow cytometry after 24 h of culture of AM and PLM with or without 10 μg/ml LPS. Similar results were obtained in 1 additional experiment.
AM present antigens more efficiently than PLM.
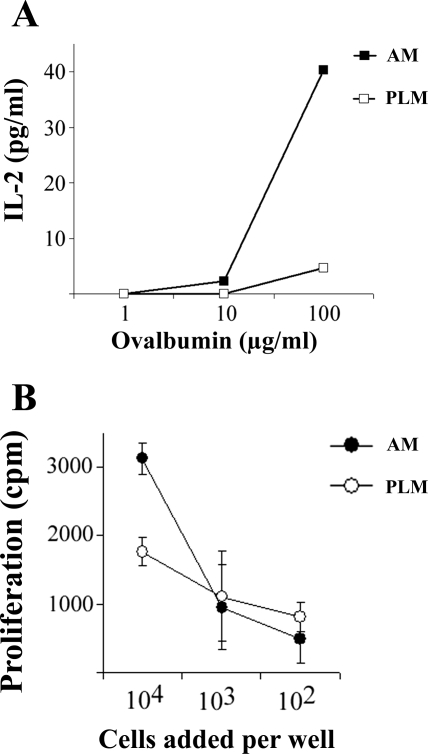
Antigen-presenting functions were assessed to compare the relative effectiveness of AM and PLM in stimulating T cell responses. The ability to process and present soluble antigens via an MHC class I-restricted pathway to CD8+ T cells (cross-priming) is a unique property of DC. Therefore, we assessed the ability of AM and PLM to cross-prime CD8+ T cell responses. For this assay, an ovalbumin peptide-specific CD8+ T cell hybridoma cell line (B3.17) was used to detect surface expression of the dominant processed SIINFEKL epitope presented by the H-2Kb molecule. We found that, after 48 h in culture, AM were able to efficiently cross-prime CD8+ T cell responses to soluble ovalbumin protein (Fig. 3A). In contrast, PLM cultured under the same conditions much less efficiently cross-primed CD8+ T cell responses.
Fig. 3.
Antigen presentation by AM and PLM as demonstrated by the ability of AM and PLM to cross-prime T cell responses and stimulate mixed leukocyte responses in vitro. A: airway and peritoneal lavage cells were collected from C57BL/6 mice and placed in triplicate wells of 96-well plates in culture at 1 × 105 cells/well. After overnight culture, medium was removed, wells were rinsed with fresh medium, and cells were incubated in medium alone or medium containing 100, 1, or 0.1 μg/ml ovalbumin. After 2 h of incubation with ovalbumin, cells were washed twice with fresh medium. CD8+ T cell hybridoma cells specific for the ovalbumin peptide SIINFEKL were added at 5 × 105 cells/well and cultured overnight. Supernatants were harvested and assayed for IL-2 concentration by ELISA, and IL-2 concentration was plotted. Values are means ± SE. Similar results were obtained in 1 additional experiment. B: AM and PLM were collected from C57BL/6 mice and placed in triplicate wells of 96-well plates in culture for 24 h, then medium was changed. After 24 h, spleen cells from ICR mice that had been depleted of adherent cells were added at 5 × 105 cells/well to AM or PLM. Cultures were incubated for 72 h and then pulsed overnight with [3H]thymidine to assess proliferation. Values are means ± SE for triplicate wells. Similar results were obtained in 1 additional experiment.
Next, the ability of AM and PLM to stimulate a mixed lymphocyte reaction (MLR) was assessed. AM and PLM were collected from ICR mice, cultured for 24 h in vitro, and then incubated for 72 h with allogeneic spleen cells (depleted of adherent antigen-presenting cells) obtained from C57BL/6 mice. When freshly isolated AM were utilized, only very weak stimulation of MLR responses was observed (data not shown). However, when AM were cultured for 48 h before addition of responder cells, significant stimulation of MLR responses by AM was observed (Fig. 3B). In contrast, PLM cultured under the identical conditions were poorer stimulators of MLR responses (Fig. 3B). The degree of T cell stimulation in the MLR assay using AM as stimulators was comparable to that in the MLR assay using cultured bone marrow-derived DC as stimulators (data not shown) and was also similar to that in the MLR assay reported by others using blood monocyte-derived DC as stimulators (37). Moreover, the T cell stimulation elicited by AM could be significantly increased if the AM were activated overnight with 10 μg/ml LPS before addition of spleen cells (data not shown). Therefore, AM were much more effective stimulators of MLR responses than PLM. Freshly isolated AM were, however, not able to cross-prime T cell responses or to stimulate MLR responses but acquired these properties after ≥24 h in culture. The recovery of functional properties by AM after in vitro culture has been noted previously (52). However, in a previous study (52), recovery of function by AM in vitro generally required the addition of exogenous GM-CSF.
Influence of the lung environment on differentiation of bone marrow cells.
The preceding experiments suggested that the lung and peritoneal environments might differ in their influence on the development of newly emigrated macrophages. Therefore, we carried out adoptive transfer experiments using bone marrow cells and PLM obtained from GFP+ syngeneic mice (38). Changes in expression of CD11c and CD11b were used to assess and compare the effects of the lung and peritoneal microenvironments, since these two surface determinants gave the clearest phenotypic distinction between normal AM and PLM.
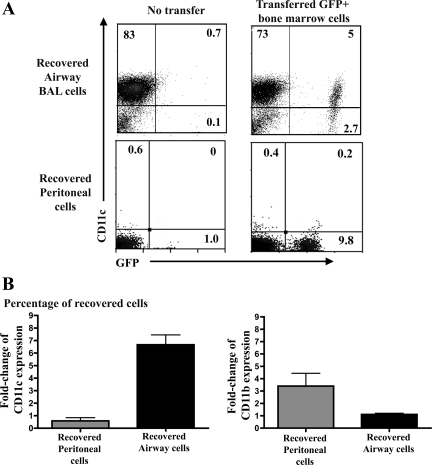
In the first cell transfer experiment, 1 × 107 GFP+ bone marrow cells were transferred to the airways of wild-type recipient mice. After 7 or 14 days, the transferred cells were recovered by lavage, and their phenotype was determined by flow cytometry. We found that the percentage of GFP+ cells expressing CD11c was increased significantly after adoptive transfer compared with the percentage of CD11c+ cells in the initially transferred bone marrow cell population (Fig. 4A). For example, the mean percentage of GFP+/CD11c+ recovered from the airway increased significantly (P < 0.05) from an average of 1.9% GFP+/CD11c+ cells before transfer to a mean of 19% GFP+/CD11c+ cells 7 days after transfer (Fig. 4B). At the same time, the percentage of CD11b+ cells in bone marrow was 25% at the time of transfer and was relatively unchanged in recovered GFP+ cells 7 days after transfer into the airways. The percentage of GFP+/CD11c+ in the airways 14 days after transfer increased significantly to an average of 20% (data not shown). These data indicated that exposure to the airway environment selectively upregulated CD11c expression by the transferred bone marrow cells but had a minimal effect on CD11b expression.
Fig. 4.
Effects of airway vs. peritoneal environment on phenotype of bone marrow cells after adoptive transfer into normal mice. Bone marrow cells were collected C57BL/6 mice transgenic for expression of the green fluorescent protein (GFP) gene under control of the ubiquitin promoter (UBI-GFP/BL6), and 1 × 107 cells were transferred into the airways of normal C57BL/6 mice (n = 3–4) by intratracheal injection or into the peritoneum (n = 3–4) by intraperitoneal injection. Airway and peritoneal cells were recovered from transferred mice 7 or 14 days (data not shown for 14 days) later by BAL or peritoneal lavage, immunostained, and analyzed by flow cytometry. A: representative plots of expression of CD11c on GFP+ airway cells in naïve C57BL/6 (top left) and adoptively transferred mice (top right), as assessed by flow cytometry. Expression of CD11c by GFP+ peritoneal cells in naïve C57BL/6 (bottom left) and adoptively transferred mice (bottom right) was assessed by flow cytometry. B: fold change in CD11c and CD11b expression of recovered GFP+ cells in peritoneum and airways of adoptively transferred mice. Percentage of CD11c+ cells was significantly increased in bone marrow cells recovered from airways of transferred mice compared with pretransfer bone marrow (P < 0.05, by t-test). Percentage of CD11b+ cells was significantly increased in bone marrow transferred peritoneum compared with untransferred cells (P < 0.01).
Influence of the peritoneal environment on differentiation of bone marrow cells.
Similar adoptive transfer experiments were done to determine whether the peritoneal environment had similar effects on CD11c and CD11b expression by adoptively transferred GFP+ bone marrow cells. Bone marrow cells (1 × 107 per mouse) were transferred into the peritoneal cavity and then recovered after 7 or 14 days by peritoneal lavage. In contrast to transfer into the airways, transfer of bone marrow cells into the peritoneal cavity did not produce significant upregulation of CD11c expression (Fig. 4A). For example, the mean percentage of CD11c+/GFP+ cells in bone marrow was 1.9% before transfer and 1.7% after 7 days in the peritoneal cavity (Fig. 4B). In contrast, the percentage of CD11b+ cells was significantly increased (P < 0.01) after transfer into the peritoneal cavity: CD11b+ cells increased from 25% before transfer to an average of 65% 7 days after transfer. Thus the environment of the peritoneal cavity promoted the upregulation of CD11b, rather than CD11c, expression on transferred bone marrow cells.
Influence of the lung environment on CD11c expression by transferred PLM.
Next, experiments were conducted to determine whether the lung environment could promote the upregulation of CD11c expression by PLM, which normally expressed very low levels of CD11c. PLM were harvested from GFP transgenic mice by peritioneal lavage, and 5 × 106 GFP+ cells were injected into the airways of each recipient mouse. Airway lavage samples were collected 7 and 14 days later, and the phenotype of the GFP+ cells was analyzed by flow cytometry. We observed a significant (P < 0.01) increase in the percentage of CD11c+/GFP+ cells recovered from the lungs after 7 days in the airways compared with CD11c expression by the PLM cells freshly collected from the peritoneal cavity (Fig. 5A). For example, the percentage of CD11c+ PLM cells was typically ≤2% after the initial harvest from the peritoneum but increased to an average of 24% 1 wk after transfer into the airways (P < 0.01; Fig. 5B). The percentage of CD11b+/GFP+ cells also significantly increased (P < 0.01) after transfer into the airways: CD11b+ cells increased from 45% before transfer to 70% after transfer. These results indicate that even relatively well-differentiated macrophages, such as PLM, were capable of further differentiation to more closely resemble AM when placed in the lung environment.
Fig. 5.
Effects of airway environment on phenotype of PLM after adoptive transfer into airways of normal mice. Peritoneal lavage cells were collected from UBI-GFP/BL6 mice, and 5 × 106 cells were transferred into airways of normal C57BL/6 mice (n = 3–4) by intratracheal injection. Airway cells were recovered from transferred mice 7 days later by BAL, immunostained, and analyzed by flow cytometry. A: expression of CD11c (left) and CD11b (right) by airway and peritoneal cells from naive C57BL/6 mice (top) and GFP+ peritoneal cell transferred mice (bottom) as assessed by flow cytometry. B: fold change of CD11c and CD11b expression in retrieved, posttransfer GFP+ PLM compared with percent expression of these markers before transfer.
Effects of depletion of CD11c+ cells from bone marrow or peritoneal lavage cells before airway transfer.
It was possible that the increased percentage of CD11c+ cells observed in the airways after adoptive transfer of unsorted bone marrow or peritoneal cells represented selective survival or expansion of the originally transferred CD11c+ cells, rather than acquisition of CD11c expression by CD11c− transferred cells. To address this possibility, CD11c+ cells were depleted from GFP+ bone marrow or peritoneal lavage cells via magnetic cell sorting before transfer into the lungs of recipient mice (data not shown). In mice receiving CD11c-depleted GFP+ bone marrow cells, an average of 13% of the transferred cells became CD11c+ 1 wk after transfer. Interestingly, ∼60% of the transferred peritoneal cells became positive for CD11c expression 1 wk after transfer, suggesting that the peritoneum may contain more cells with the potential to upregulate expression of CD11c when placed in the lung environment (P = 0.025). These findings suggest that the increase in CD11c expression by bone marrow and peritoneal cells placed in the lung environment was due to de novo upregulation of CD11c expression, rather than selective survival or expansion of the original CD11c+ cells that were transferred into the airways.
Influence of in vitro culture in GM-CSF on phenotype of airway and peritoneal lavage cells.
The preceding experiments indicated that the lung environment was uniquely effective in promoting the development of cells with a DC-like phenotype. Even relatively well-differentiated cells, such as PLM, could be induced to assume a more DC-like phenotype when placed in the lung environment. Therefore, we asked the following question: To what degree does the local environment influence the development of this cell phenotype? To address this question, we first examined the role of key cytokines that influence DC and macrophage development on the phenotype of AM and PLM. For example, development of DC is critically dependent on exposure to GM-CSF, whereas development of macrophages is heavily influenced by exposure to CSF-1 (12). We found significant concentrations of GM-CSF in BAL fluid from naïve mice, whereas GM-CSF could not be detected in peritoneal fluid (Fig. 6A). This finding is consistent with the previously demonstrated ability of lung epithelial cells to produce large amounts of GM-CSF (36). In contrast, we did not detect CSF-1 protein in peritoneal lavage or BAL fluid (data not shown).
Fig. 6.
Levels of granulocyte/macrophage colony-stimulating factor (GM-CSF) in BAL and effects of surfactant protein D (SP-D) on CD11c expression by bone marrow cells. A: 1 ml of cold EDTA-PBS and 1 ml of cold HBSS were used to obtain BAL and peritoneal fluid, respectively, from 5 ICR mice. ELISA was performed using a recombinant standard and corrected for the dilution factor. No detectable levels of GM-CSF were observed in peritoneal fluid. PL, peritoneal lavage. B: bone marrow cells from C57BL/6 mice were plated in triplicate wells of 24-well plates at 2 × 106 cells/well, then nonadherent cells were removed after 2 h. Remaining adherent cells were cultured in medium alone or medium with recombinant SP-D (20 μg/ml), SP-A (20 μg/ml), GM-CSF (3% conditioned medium), or LPS (1 μg/ml) for 5 days. After 5 days, cells were harvested and immunostained for expression of CD11c and other determinants. Values are means ± SE in triplicate wells. CD11c expression was significantly increased in wells treated with SP-D compared with other treated cells or control cells (P < 0.001, by ANOVA and Tukey's multiple-means comparison test). Similar results were obtained in 1 additional experiment.
Next, we examined the effects of in vitro exposure to high concentrations GM-CSF or CSF-1 on the phenotype of cultured AM or PLM. Culture of PLM in GM-CSF failed to generate cells with a DC phenotype in vitro (data not shown). For example, the percentage of CD11c+ cells in PLM cultures remained very low (<2%), despite 1 wk of culture in 100 ng/ml GM-CSF. Moreover, culture of AM in GM-CSF or CSF-1 also failed to significantly alter their original phenotype (data not shown). These results indicated that mature AM and PLM in culture were relatively unresponsive to the cytokines GM-CSF and CSF-1. This finding suggested that perhaps factors in addition to GM-CSF in the lungs might be necessary to induce the characteristic AM phenotype.
Influence of surfactant proteins on in vitro differentiation of bone marrow cells.
We next conducted in vitro experiments to determine whether surfactant proteins, which are expressed at high levels in the small airways and alveoli, might play a role in regulating the unique AM phenotype. We cultured bone marrow-adherent cells with recombinant SP-A or SP-D for 5 days and determined the phenotype of the cultured cells by flow cytometry. We found that culture of bone marrow-adherent cells in the presence of SP-D resulted in significant (P < 0.001) upregulation of CD11c expression. Culture in recombinant GM-CSF also triggered significant upregulation of CD11c expression, whereas exposure to LPS did not upregulate CD11c expression (Fig. 6B). In contrast, culture with SP-A did not induce upregulation of CD11c expression by bone marrow cells. These findings suggest that SP-D may be a critical factor in regulating the unique phenotype of AM.
Analysis of AM from SP-D−/− and GM-CSF−/− mice suggests a critical role for GM-CSF in regulation of the CD11chi AM phenotype.
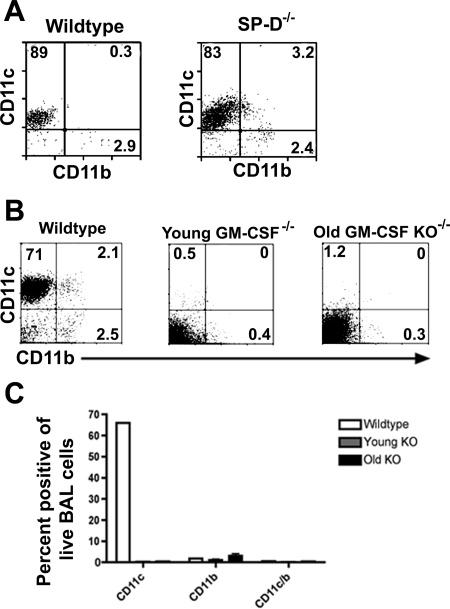
The preceding results indicated that GM-CSF and SP-D were capable of upregulating CD11c expression by cultured bone marrow cells. Therefore, we conducted studies with SP-D−/− and GM-CSF−/− mice to directly assess the role of these two factors in the development and differentiation of AM. First, we found that AM recovered from the lungs of SP-D−/− mice maintained a phenotype very similar to that of wild-type mice (Fig. 7A). In addition, the phenotype of PLM from SP-D−/− mice (SP-D is known to be produced in the peritoneal cavity) was also normal (data not shown).
Fig. 7.
Phenotype of airway cells in SP-D−/− and GM-CSF−/− mice. A: representative plot of phenotypic markers of BAL cells from SP-D−/− and wild-type C57BL/6 mice assessed by flow cytometry. Phenotypic profile of BAL cells did not differ between SP-D−/− and wild-type mice. B: representative plots of flow cytometric data from wild-type C57BL/6 mice vs. young (2-mo-old) and older (4-mo-old) GM-CSF−/− [GM-CSF knockout (KO)] mice. C: CD11c and CD11b expression of a wild-type B6 mouse and multiple young and older GM-CSF−/− mice.
However, the phenotype of AM from GM-CSF−/− mice was markedly altered compared with that of wild-type mice. The most striking difference was the nearly complete absence of CD11c expression by AM from GM-CSF−/− mice (Fig. 7B), consistent with results that have been reported previously (1, 26, 33, 39, 41, 45). The PLM of the GM-CSF−/− mice also showed a decrease in the number of CD11c+ cells, but the percentage of CD11b+ cells was not significantly different from that in wild-type mice. These results therefore suggest that locally produced GM-CSF in the airways played a critical role in generating the CD11chi phenotype of AM in vivo.
To test whether local airway GM-CSF expression was necessary for generation of the CD11chi AM phenotype, we labeled bone marrow cells from GM-CSF−/− mice with CSFE and then transferred these cells into the airways of wild-type (GM-CSF+/+) mice. In the bone marrow of GM-CSF−/− mice, 3.3% of the cells were CD11c+ at the time of initial transfer. The labeled cells were then transferred into the airways of wild-type mice, and the airway cells were recovered and examined 7 days later (Fig. 8). We found that virtually 100% of the transferred CSFE+ cells from GM-CSF−/− mice had become CD11c+ and ∼20% of the transferred cells also upregulated CD11b expression. These results provide strong evidence that, in vivo, GM-CSF expression by the airways was sufficient and necessary for generation of the CD11chi AM phenotype.
Fig. 8.
Transfer of GM-CSF−/− bone marrow cells into airways of naïve mice. GM-CSF−/− bone marrow cells from 2- mo-old mice were labeled with 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CSFE) and placed into airways of normal C57BL6 mice. After 7 days, BAL cells were collected and analyzed by flow cytometry for surface phenotype. A: representative plot of CD11c+-CSFE+ and CD11b+-CSFE+ BAL cells. B: fold change in expression of CD11c and CD11b compared with pretransferred GM-CSF−/− cells.
DISCUSSION
The findings reported here indicate that the microenvironment of the lungs exerts a critical influence on the development of certain unique features of AM. The different environments of the lung and the peritoneal cavity appeared to play a major role in directing the differentiation of two populations of macrophages with very different phenotypic and functional properties. Clearly, AM and typical DC share a number of key phenotypic determinants (Fig. 1, Table 1) (20, 22, 33, 51). The unusual phenotype of AM is not restricted to AM from mice. For example, we have also observed that normal human AM express high levels of CD11c, as do AM recovered from dogs (unpublished data).
Functionally, the AM in this report resembled classical DC in their ability to present antigens (Fig. 2). For example, AM that were cultured for 24–48 h presented antigens much more effectively than PLM. The relative inability of freshly isolated AM to present antigens effectively may be explained in part by the effects of surfactant proteins, which have a number of important immunomodulatory properties (53). Thus it is likely that, during in vitro culture, the surfactant proteins were metabolized by AM (14). Previous studies demonstrated that SP-A suppressed the ability of bone marrow-derived DC to mature into effective antigen-presenting cells (9, 25). Surfactant proteins are also known to tonically suppress the functions of macrophages within the lungs (17, 25). For example, SP-D has also been shown to preferentially bind to immature, and not mature, DC (10). Therefore, the relative inability of AM to present antigens within the airways of the lung is likely a consequence of their environment and not an inherent property of the cells themselves.
To what degree is the unusual AM phenotype a product of the local lung and airway environment? Results of adoptive transfer experiments (Figs. 4 and 5) suggest that the lung environment plays a critical role in promoting the development of macrophages with DC-like features. Bone marrow cells placed in the airways were able to upregulate CD11c expression. However, an alternative interpretation to the adoptive transfer experiments is that the increase in CD11c+ GFP+ double-positive cells observed in the airway after transfer of GFP+ cells could have been due to phagocytosis of the transferred cells by resident AM. We believe this explanation is unlikely, however, for the following reasons. 1) Cytological examination of airway lavage cells by immunofluorescence microscopy 1 wk after transfer did not reveal evidence of GFP+ cells within resident AM (data not shown). 2) In a recent publication, we did not observe recipient AM acquiring GFP+ staining after transfer of GFP+ thymocytes into wild-type mice (25). Thus we believe our results are most consistent with upregulation of CD11c expression directly by the transferred cells within the airways.
In vitro experiments with SP-D (Fig. 6) suggested that SP-D might play a role in upregulating CD11c expression by AM. However, in the SP-D−/− mice, we did not observe the expected decrease in CD11c expression. One explanation for the discordance between the in vivo and the in vitro results may be related to differences in the structure of the SP-D molecule. In vitro, multimerization of SP-D may occur; in vivo, however, SP-D is primarily found as a dodecamer (14). Multimeric SP-D may, in turn, be more stimulatory for AM and, therefore, result in greater upregulation of CD11c expression than the native dodecameric form of SP-D. Alternatively, the failure to detect a decrease in CD11c expression by AM in SP-D−/− mice may reflect dominant effects of GM-CSF expression or, possibly, upregulation of GM-CSF production in SP-D−/− mice. In contrast, the peritoneal cavity, which had undetectable levels of GM-CSF expression, did not promote the development of AM-like macrophages, as was observed in the lungs. Instead, the peritoneal environment favored the development of more typical, CD11c-negative tissue macrophages. Finally, although AM from GM-CSF−/− mice failed to express CD11c+ cells in the airways, bone marrow cells derived from these mice were capable of upregulating CD11c expression after transfer into the high-GM-CSF environment of the airways of wild-type mice (Figs. 7 and 8). Thus we propose that AM, whether recruited from the bloodstream or generated by proliferation of endogenous progenitors, are stimulated by GM-CSF and other factors in the small airways to develop into macrophages with many DC-like features.
The fact that AM share many features with DC has important implications for understanding regulation of lung immunity and pulmonary disease pathogenesis, particularly with regard to antigen presentation. If AM possess the functional capabilities of DC, then it is apparent that the lung must tonically regulate and suppress antigen presentation by AM to avoid triggering harmful inflammatory responses in the lungs. For example, surfactant proteins are thought to play a key role in this process, as evidenced by the dual abilities of surfactant proteins to suppress and activate AM, which we recently described (17). Moreover, it is also known that lymphocytes in the lungs of mice lacking SP-D are spontaneously activated (16).
Thus the unusual phenotype of AM appears to represent a response to unique environmental signals provided by the lung and airways. These findings also raise additional questions. Do monocytes that enter the lung during inflammation or infection develop into typical AM, or do they develop, instead, into more classical DC? How quickly do newly emigrated cells in the lung develop the AM phenotype, and how do different disease states alter this phenotype? Can the unique features of macrophages in other body sites be used to predict the environmental influences to which they may be exposed? Finally, as has been noted recently, these findings also suggest that the distinction between macrophages and DC is not always clear, particularly in the lung (24).
GRANTS
This research was supported by National Institute on Aging Grant U01 AI-056487-01 and a grant from the Colorado State University College Research Council.
Acknowledgments
The authors acknowledge the assistance of Dr. Lance U'ren and Shayna Warner.
Present address of C. M. Bosio: Immunity to Pulmonary Pathogens Section, Laboratory of Intracellular Parasites, Rocky Mountain Laboratories, NIAID, NIH, Hamilton, MT 59840.
REFERENCES
- 1.Berclaz PY, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU.1, regulates alveolar macrophage Fcγ R-mediated phagocytosis and the IL-18/IFN-γ-mediated molecular connection between innate and adaptive immunity in the lung. Blood 100: 4193–4200, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bilyk N, Holt PG. The surface phenotypic characterization of lung macrophages in C3H/HeJ mice. Immunology 74: 645–651, 1991. [PMC free article] [PubMed] [Google Scholar]
- 3.Bitterman PB, Saltzman LE, Adelberg S, Ferrans VJ, Crystal RG. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest 74: 460–469, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blusse van Oud Alblas A, Mattie H, van Furth R. A quantitative evaluation of pulmonary macrophage kinetics. Cell Tissue Kinet 16: 211–219, 1983. [PubMed] [Google Scholar]
- 5.Blusse van Oud Alblas A, van der Linden-Schrever B, Van Furth R. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intra-alveolar administration of aerosolized heat-killed BCG. Am Rev Respir Dis 128: 276–281, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol 175: 6792–6801, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bosio CM, Goodyear AW, Dow SW. Early interaction of Yersinia pestis with APCs in the lung. J Immunol 175: 6750–6756, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Bowden DH, Adamson IY. Role of monocytes and interstitial cells in the generation of alveolar macrophages. I. Kinetic studies of normal mice. Lab Invest 42: 511–517, 1980. [PubMed] [Google Scholar]
- 9.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol 284: L232–L241, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Brinker KG, Martin E, Borron P, Mostaghel E, Doyle C, Harding CV, Wright JR. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol 281: L1453–L1463, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Chen BD, Mueller M, Chou TH. Role of granulocyte/macrophage colony-stimulating factor in the regulation of murine alveolar macrophage proliferation and differentiation. J Immunol 141: 139–144, 1988. [PubMed] [Google Scholar]
- 12.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol 18: 39–48, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Crouch EC Surfactant protein-D and pulmonary host defense. Respir Res 1: 93–108, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Q, Wright JR. Degradation of surfactant protein D by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 274: L97–L105, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol 60: 353–369, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Fisher JH, Larson J, Cool C, Dow SW. Lymphocyte activation in the lungs of SP-D null mice. Am J Respir Cell Mol Biol 27: 24–33, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115: 13–23, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Garn H Specific aspects of flow cytometric analysis of cells from the lung. Exp Toxicol Pathol 57 Suppl 2: 21–24, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Juarrero M, Orme IM. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect Immun 69: 1127–1133, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol 171: 3128–3135, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Gordon SB, Read RC. Macrophage defences against respiratory tract infections. Br Med Bull 61: 45–61, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Grundy M, Sentman CL. GFP transgenic mice show dynamics of lung macrophages. Exp Cell Res 310: 409–416, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Higgins DM, Sanchez-Campillo J, Rosas-Taraco AG, Higgins JR, Lee EJ, Orme IM, Gonzalez-Juarrero M. Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J Immunol 180: 4892–4900, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hume DA Macrophages as APC and the dendritic cell myth. J Immunol 181: 5829–5835, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Janssen WJ, McPhillips KA, Dickinson MG, Linderman DJ, Morimoto K, Xiao YQ, Oldham KM, Vandivier RW, Henson PM, Gardai SJ. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRPα. Am J Respir Crit Care Med 178: 158–167, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis 193: 205–213, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lambrecht BN Alveolar macrophage in the driver's seat. Immunity 24: 366–368, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 70: 163–170, 2001. [PubMed] [Google Scholar]
- 29.Lipscomb MF, Lyons CR, Nunez G, Ball EJ, Stastny P, Vial W, Lem V, Weissler J, Miller LM. Human alveolar macrophages: HLA-DR-positive macrophages that are poor stimulators of a primary mixed leukocyte reaction. J Immunol 136: 497–504, 1986. [PubMed] [Google Scholar]
- 30.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J 7: 1678–1689, 1994. [PubMed] [Google Scholar]
- 31.Lyons CR, Ball EJ, Toews GB, Weissler JC, Stastny P, Lipscomb MF. Inability of human alveolar macrophages to stimulate resting T cells correlates with decreased antigen-specific T cell-macrophage binding. J Immunol 137: 1173–1180, 1986. [PubMed] [Google Scholar]
- 32.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 35: 227–235, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Paine R, Morris SB, Jin H, Wilcoxen SE, Phare SM, Moore BB, Coffey MJ, Toews GB. Impaired functional activity of alveolar macrophages from GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol 281: L1210–L1218, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Arellano JL, Alcazar-Montero MC, Jimenez-Lopez A. Alveolar macrophage: origin, kinetics and relationship with cells of the alveolo-interstitial region. Allergol Immunopathol (Madr) 18: 175–183, 1990. [PubMed] [Google Scholar]
- 35.Peters-Golden M The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 31: 3–7, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Reed JA, Whitsett JA. Granulocyte-macrophage colony-stimulating factor and pulmonary surfactant homeostasis. Proc Assoc Am Physicians 110: 321–332, 1998. [PubMed] [Google Scholar]
- 37.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182: 389–400, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell Immunol 214: 110–122, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 15: 557–567, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava M, Jung S, Wilhelm J, Fink L, Buhling F, Welte T, Bohle RM, Seeger W, Lohmeyer J, Maus UA. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol 175: 1884–1893, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 91: 5592–5596, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thepen T, Kraal G, Holt PG. The role of alveolar macrophages in regulation of lung inflammation. Ann NY Acad Sci 725: 200–206, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 170: 499–509, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 192: 1016–1018, 1976. [DOI] [PubMed] [Google Scholar]
- 45.Thomassen MJ, Barna BP, Malur AG, Bonfield TL, Farver CF, Malur A, Dalrymple H, Kavuru MS, Febbraio M. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res 48: 2762–2768, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Toews GB, Vial WC, Dunn MM, Guzzetta P, Nunez G, Stastny P, Lipscomb MF. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J Immunol 132: 181–186, 1984. [PubMed] [Google Scholar]
- 47.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol 64: 775–802, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Upham JW, Strickland DH, Robinson BW, Holt PG. Selective inhibition of T cell proliferation but not expression of effector function by human alveolar macrophages. Thorax 52: 786–795, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 169: 3978–3986, 2002. [DOI] [PubMed] [Google Scholar]
- 50.van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods 288: 111–121, 2004. [DOI] [PubMed] [Google Scholar]
- 51.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 201: 981–991, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, Holt PG, Stumbles PA. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol 175: 1609–1618, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Wright JR Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68, 2005. [DOI] [PubMed] [Google Scholar]