Abstract
Organic dust exposure in agricultural environments results in an inflammatory response that attenuates over time, but repetitive exposures can result in chronic respiratory disease. Animal models to study these mechanisms are limited. This study investigated the effects of single vs. repetitive dust-induced airway inflammation in mice by intranasal exposure method. Mice were exposed to swine facility dust extract (DE) or saline once and once daily for 1 and 2 wk. Dust exposure resulted in increased bronchoalveolar lavage fluid neutrophils and macrophages after single and repetitive exposures. Lavage fluid TNFα, IL-6, keratinocyte chemoattractant, and macrophage inflammatory protein-2 were significantly increased after single and repetitive dust exposures, but were dampened in 2-wk dust-exposed mice compared with single exposure. Dust exposure induced PKCα and -ɛ activation in isolated tracheal epithelial cells but were dampened with repetitive exposures. Ex vivo stimulation of alveolar macrophages from 2-wk animals demonstrated reduced cytokine responsiveness and phagocytic ability. Significant lung pathology occurred with development of mixed mononuclear cellular aggregates (T and B lymphocytes, phagocytes) after repetitive dust exposure, a novel observation. Airway hyperresponsiveness to methacholine occurred after single dust exposure but resolved after 2 wk. Collectively, intranasal exposure to DE results in significant lung inflammatory and pathological responses marked by a modulated innate immune response to single and repetitive dust exposures that is associated with PKC activity.
Keywords: antigen presenting cell, phagocytosis, cytokines, aggregate, airway hyperresponsiveness
agricultural workers, particularly swine farmers, exhibit a high prevalence of airway disease including chronic bronchitis, chronic obstructive pulmonary disease, and exacerbation of asthma, which is thought to be due, in part, to chronic organic dust exposure (37). Initial exposure to an organic dust environment induces an intense airway inflammatory response marked by fevers, bronchial hyperresponsiveness, and increases in bronchoalveolar lavage (BAL) fluid neutrophils, macrophages, and proinflammatory mediators including TNFα, IL-6, and IL-8 (CXCL8) (11, 41). Repetitively exposed swine farmers also experience an increase in inflammatory cells and cytokines, but this response is diminished compared with acutely exposed naive subjects (28), suggestive of an adaptation response (37). However, despite an apparent adaptation response, persons repetitively exposed to organic dust experience a high prevalence of chronic respiratory disease and a progressive loss of lung function (34).
Animal models to study the mechanisms that regulate the immune inflammatory response to single vs. repetitive organic dust exposure may be relevant for a better understanding of the respiratory symptoms of organic dust-induced diseases and the well-recognized adaptation-like response. We have previously found, in vitro, that single exposure to this dust induces significant secretion of proinflammatory mediators (TNFα, IL-6, IL-8) in human epithelial cells and monocytes/macrophages, and that this response is, in part, regulated through PKC activity, particularly the PKCα and PKCɛ isoforms (31, 32). In addition, repeat dust exposure results in an adaptation/tolerance response in human monocytes (31), and repetitive dust exposure impairs the differentiation and function of macrophages derived from monocytes (30). However, the mechanisms underlying this apparent modulated inflammatory response to single vs. repetitive dust exposures in vivo are not clear.
Animal models to investigate these observations are limited. One potential model is placing rodents in hanging cages within the swine barn. This attractive but difficult model has demonstrated airway hyperresponsiveness (AHR) after 1 day of exposure and an AHR adaptation-like response after 1 mo. However, mechanistic studies would be limited as this model fails to demonstrate consistent increases in lung lavage inflammatory cytokines, and lung parenchymal changes, even after 1 mo, are subtle (8, 9). An alternative model proposed in this study is intranasal inhalation of the organic dust. Intranasal administration of other agents, including, but not limited to, ultrafine particles, biomass particles, bacterial components, microbial superantigens, and allergens, are becoming increasingly utilized to investigate mechanisms of exaggerated airway inflammation in vivo (10, 15, 22, 24, 26).
In this study, we hypothesized that single vs. repetitive organic dust extract (DE) from swine confinement facilities intranasally delivered would result in significant airway and lung parenchymal inflammation in mice, and that these inflammatory responses would parallel activation of PKCα and PKCɛ. We also hypothesized that the alveolar macrophage (aMφ) would be impaired in mice repetitively exposed to the dust. To test these hypotheses, we compared lavage fluid inflammatory cells and cytokines, PKC isoform activity in isolated tracheal epithelial cells, ex vivo alveolar macrophage responses, lung pathology, and airway hyperresponsiveness to single and repetitive dust exposure in mice.
METHODS
Swine facility organic DE.
Organic dust was obtained from settled surface dust from modern swine confinement animal feeding operation facilities, housing ∼500–700 animals. For all experimental studies, the dust was placed into solution and sterile filtered by a standard published procedure (32). Briefly, 1 g of the dust was placed in 10 ml of Hanks’ balanced salt solution without calcium (Biofluids). The mixture was vortexed and allowed to stand at room temperature for 1 h. The mixture was centrifuged for 10 min, and the supernatant was recovered and centrifuged again. The final supernatant was filter (0.22 μm) sterilized. Complete analysis of this dust has been previously published (30). The analysis of the dust before placing into extract form revealed trace metals (B, Mg, Ti, Mn, Fe, Co, Ni, Cu, Rb, Mo, Zn) and predominance of gram-positive bacteria colonies (98%; Staphylococcus, Bacillus, Streptomycetes, and Enterococcus species) with the remainder being gram-negative bacteria. Gas chromatography-tandem mass spectrometry analysis revealed high muramic acid (predominant biomarker of gram-positive bacteria but to a lesser extent gram-negative bacteria) and a slight increase in 3-hydroxy fatty acids (markers of LPS; Ref. 30). The LPS equivalent concentration in 12.5% DE is 0.6 μg/ml (range: 0.325 μg/ml-0.875 μg/ml) as determined by the Limulus amebocyte lysate gel clot assay (Cambrex, Walkersville, MD).
Animal model and exposure.
Male C57BL/6 mice (6–8 wk old) obtained from Charles River (Wilmington, MA) were housed in group cages and fed commercial rodent chow and water ad libitum for a 1-wk acclimation. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Omaha Veterans Affairs Medical Center and the University of Nebraska Medical Center.
In prior experiments, optimal DE concentration was determined by randomly assigning mice to a treatment group: no handling, PBS control (Invitrogen, Carlsbad, CA), or concentrations of 1%, 5%, 12.5%, 19%, and 25% DE once daily for 2 wk. In subsequent time course studies shown here, the optimal concentration of 12.5% DE or saline control was utilized. Mice assigned to an inhalation group received saline control or DE once daily for 1 day, 1 wk, and 2 wk with weekends excluded. To conduct a semiquantitative evaluation of the dose-response inflammatory changes observed after 2 wk of once-daily dust exposure, concentrations of 1%, 5%, and 12.5% or saline (control) were utilized. The procedure for nasal inhalation was based on an established model using cigarette smoke extract (13, 25). Briefly, mice were anesthetized by isoflurane inhalation before inhaling into the nasal cavity 50 μl of saline or DE. No mice exhibited respiratory distress. All mice were weighed weekly throughout the instillation time course. No weights of any group of mice were significantly different from another group.
BAL.
Five hours postexposure, animals were euthanized by intraperitoneal injection of 50 mg/kg body wt of pentobarbital sodium (Nembutal; Abbott Labs, Chicago, IL). The trachea was exposed, and a cannula was inserted just below the larynx. The proximal end of the trachea was held around the cannula with forceps while 1 ml of sterile PBS was instilled into the lungs and recovered by aspiration a total of three times. The BAL fluid was centrifuged at 250 g to collect cells. The supernatant from the first milliliter of BAL fluid recovered was frozen at −80°C to assay for chemokines/cytokines. Cells from all 3 ml were resuspended, pooled, and spun onto slides with a Cytopro cytocentrifuge (Wescor, Logan, UT) and stained with DiffQuik (Dade Behring, Newark, DE). Counts of the cells determined the differential ratio of cell types in 200 cells per slide per mouse.
Lung collection and semiquantitative evaluation of inflammation.
After whole lung lavage, lungs were harvested from each treatment group and cinched via the trachea to the cannula. Once removed from the thoracic cavity, the lungs were slowly inflated with 0.8 ml of 10% formalin (Sigma, St. Louis, MO). To obtain uniform lung inflation during fixation, the lungs were hung under a pressure of 15 cmH2O for 24 h while submerged in 10% formalin. Subsequently, by routine histology processing, the fixed lung tissue was embedded in paraffin. Sections (4–5 μM) were cut and either stained with hematoxylin and eosin (H&E) or utilized later for immunohistochemistry. Two H&E-stained slides of the lung from each animal were reviewed and semiquantitatively assessed for the degree of inflammation as well as the distribution of the inflammation. Each slide was entirely reviewed at scanning magnifications (×2, ×4, and ×10 objectives; Nikon Eclipse model E600 microscope). The histopathological scores were determined by a reviewer (pathologist) blinded to the treatment conditions. The scoring system utilized a standardized set of photos representing the spectrum of inflammatory changes for each parameter. The parameters included: 1) alveolar compartment inflammation, 2) bronchiolar compartment inflammation, and 3) intrapulmonary mononuclear cellular aggregates. Each parameter was independently assigned a value from 0 to 3 (the greater the score, the greater the inflammatory changes in the lung). This semiquantitative evaluation included assessment of all lung tissue present on each slide.
Cytokine assays.
Murine TNFα, IL-6, keratinocyte chemoattractant (KC; CXCL1), and macrophage inflammatory protein-2 (MIP-2; CXCL2) concentrations in BAL fluid supernatant were determined according to the manufacturer's instructions using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
PKC activity in isolated epithelial cells and whole lung.
To extend our in vitro bronchial epithelial cell observations that implicate PKCα and PKCɛ in modulating dust-induced inflammation (46), PKCα and PKCɛ activity were determined in whole lung tissue and isolated epithelial cells from murine trachea. These tissues were chosen because epithelial cells can be isolated from the trachea and provide insight into bronchial and alveolar epithelial cell responses, whereas whole lung tissue represents a multicellular organ response. Epithelial cells were isolated from tracheae after whole lung lavage by exposing the lumen of the trachea and gently scraping off the epithelial cells by sterile cell lifter (Fisher Scientific) as previously described (14). The whole lung and the epithelial cell lysate were placed in 50 mM Tris·HCl (pH 7.4) lysis buffer with protease inhibitors as previously described (47) and then immediately flash-frozen in liquid nitrogen and stored at −80°C until assayed for PKC activity.
PKCα and PKCɛ activity was determined as previously described (14, 44). Briefly, epithelial cell lysates and whole lungs were sonicated and centrifuged, the supernatant was removed (cytosolic fraction), and the pellet was resuspended in cell lysis buffer containing 0.01% Triton X-100 and sonicated again (particulate fraction). To measure PKC isoform activity specifically, 24 μg/ml PMA, 30 mM dithiothreitol, 150 μM ATP, 45 mM Mg-acetate, PKCα or PKCɛ isoform-specific substrate peptide (Calbiochem, San Diego, CA), and 10 μCi/ml [γ-32P]ATP were mixed together to form an activation. Chilled cell lysate (cytosolic or particulate) samples were added to the activation mix and incubated. This mixture was then spotted onto P-81 phosphocellulose papers (Whatman, Clinton, NJ) to halt incubation, and papers were subsequently washed with phosphoric acid (75 mM) for 5 min, washed once in 100% ethanol, dried, and counted in nonaqueous scintillant (National Diagnostics, Atlanta, GA). PKC activity was expressed in relation to the total amount of cellular protein assayed as picomoles of phosphate incorporated per minutes per milligram. PKC activity is reported as fold-increase from baseline: dust-induced kinase activity divided by media-alone kinase activity.
Ex vivo alveolar macrophage studies.
To determine if alveolar macrophages (aMφ) in the 2-wk repetitive dust-treated mouse group were modulated compared with the saline control group, aMφ for each group were pooled due to limited aMφ numbers: eight mice exposed to saline for 2 wk and eight mice exposed to DE (12.5%) for 2 wk. After BAL, equal numbers of aMφ were allowed to adhere for 18 h at 37°C and 5% CO2 incubator in complete RPMI, which consisted of l-glutamine-RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated FBS (Biofluids, Rockville, MD), 2-mercaptoethanol (5 × 10−5 M), 50 μg/ml of streptomycin (Invitrogen), and 80 U/ml amphotericin B (Invitrogen). Adherent aMφ were then collected, counted, and assured for viability by trypan blue exclusion method. For ex vivo cytokine responsiveness studies, 2 × 105 cells/ml were stimulated with DE (1%) for 5 h, and cell-free supernatants were subsequently harvested and stored at −20°C until assayed for cytokine secretion by sandwich ELISA, as described above. Cytokine secretion is reported as concentration (in picograms per milliliter) per 2 × 105 viable cells, as determined on completion of the experimental protocol by means of the trypan blue exclusion method.
The phagocytic ability of the aMφ from the 2-wk dust-treated mouse group and saline control group was assessed by flow cytometry utilizing previously published methods (1, 30). Briefly, Saccharomyces cerevisiae zymosan A BioParticles (Molecular Probes, Eugene, OR) conjugated to FITC were opsonized with opsonizing reagent (IgG) for 45 min. After being washed, aMφ at 5 × 104 cells/ml were incubated with FITC-labeled zymosan (Molecular Probes) at 5 × 106 particles/ml (1:10 ratio) for 0 and 60 min in the presence of 10% murine serum. Cells were fixed with 1% paraformaldehyde and analyzed on the same day of particle exposure by flow cytometry. Flow cytometric analyses were performed with the FACSCalibur dual-laser cytometer (Becton-Dickinson, Lincoln Park, NJ). Particle uptake was identified as a rightward shift in fluorescence on histogram analysis, and phagocytic ability was determined by assessing the average mean fluorescence intensity (MFI) from the proportion of cells in the zymosan-exposed population at 60 min compared with cells exposed for 0 min (expressed as fold change in MFI).
Immunohistochemistry.
Formalin-fixed, paraffin-embedded sections of 4- to 5-μm-thick tissue were deparaffinized through two exchanges of xylene and rehydrated using a graded series of alcohol washes (100%, 95%, 80%, 50% ethanol) and rinsed twice in PBS. Antigen unmasking was performed using the heat-induced epitope retrieval method (42). Slides were immersed in preheated antigen retrieval solution (DIVA Decloaker solution; Biocare Medical, Concord, CA) and steamed for 30 min at 95°C in a vegetable steamer. After being cooled, slides were rinsed with TBST washing buffer (Tris-based with sodium chloride and Tween 20). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 15 min. After being washed, slides were blocked for 30 min in a humidity chamber in 10% normal goat serum or 10% normal rabbit serum (Vector, Burlingame, CA) before application of primary antibodies. Slides were incubated with the following primary antibodies for 1 h in a humidity chamber: rabbit anti-CD3 (Pan-T cell marker, dilution 1:300; Dako, Carpinteria, CA), rat anti-CD45R/B220 (Pan-B cell marker, clone RA3-6B2, dilution 1:200; BD Pharmingen, San Jose, CA), anti-Mac-3 (mononuclear phagocytes, clone M3/84, dilution 1:50; BD Pharmingen).
After being washed, slides were incubated with the appropriate biotinylated goat-anti-rabbit IgG (dilution 1:500) or biotinylated anti-rat IgG mouse-absorbed (dilution 1:500) secondary antibody in a humidity chamber. Biotinylated antibodies were purchased from Vector. After 1 h, slides were rinsed, and primary antibody binding was detected using the avidin-biotin-immunoperoxidase method (Vectastain Elite ABC ready-to-use kit, Vector). Chromogen substrate (IMMPACT DAB, Vector) developer was used, and slides were counterstained with 1% Meyer's hematoxylin. Slides were dehydrated through a series of ethanols and fixed with xylene.
Invasive pulmonary function measurement.
The standard model to measure respiratory mechanics is by the linear first-order compartment model. This model provides dynamic resistance such that increased resistance values signal constriction of the lungs. In these studies, 3 h following intranasal instillation of DE or saline, mice were anesthetized with a ketamine (166 mg/kg) and xylazine (8 mg/kg) cocktail, tracheostomized with a steel 18-guage cannula, and mechanically ventilated at a rate of 160 breaths/min and tidal volume of 0.15 ml, using a computerized small animal ventilator (Finepoint; Buxco Electronics, Wilmington, NC) as previously described (27). Mice were allowed to stabilize on the ventilator for 5 min before measurements commenced. Once stabilized, dose responsiveness to aerosolized methacholine (1.5–48.0 mg/ml) was obtained and reported as total lung resistance.
Statistical analysis.
Data are presented as means ± SE. Statistics were performed using a two-tailed, non-paired t-test, one-way ANOVA, and nonparametric Mann-Whitney U-test to determine significant changes among treatment groups using SPSS (16.0) software.
RESULTS
Dust-induced cellular inflammation.
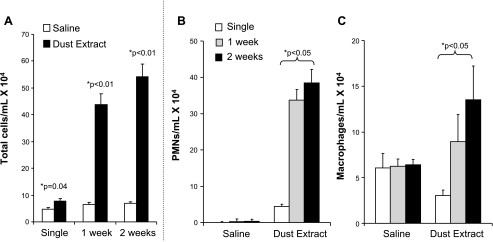
A single exposure to intranasal inhalation of swine confinement facility organic DE induced a significant increase in total leukocyte counts in lavage fluid compared with saline control 5 h after exposure (Fig. 1A, P = 0.04; n = 8 mice/treatment group). There was a further increase in lavage fluid total cellularity after once-daily DE instillations for 1 and 2 wk compared with respective time-matched saline control (Fig. 1A). The increase in lavage cellularity was predominately due to expansion of neutrophils, but also macrophages (Fig. 1, B and C). These data demonstrate that an increase in inflammatory cells occurs immediately after single DE and that there is a progressive increase in inflammatory cells, predominately neutrophils, but to a lesser degree macrophages, over time with repetitive DE.
Fig. 1.
Lung lavage fluid mean concentration of total cells (A) and neutrophils (B) and macrophages (C) after single and repetitive intranasal inhalation of dust extract (DE; 12.5%) and saline in mice. Error bars are SE (n = 8 mice/group). *P < 0.05 is statistically significant of DE vs. saline-treated mice.
Dust-induced cytokine and chemokine response.
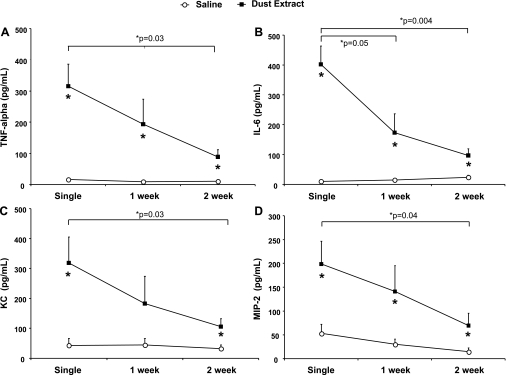
To characterize the immune inflammatory response with single vs. repetitive intranasal DE, BAL fluid was assayed for the presence of neutrophil attractant CXC chemokines KC (CXCL1) and MIP-2 (CXCL2) and the cytokines TNFα and IL-6. There was a significant increase in TNFα, IL-6, KC, and MIP-2 5 h after single DE compared with saline control (Fig. 2, A–D; n = 8 mice/treatment group; P < 0.05). Compared with saline control, TNFα, IL-6, KC, and MIP-2 levels in the lavage fluid remained significantly elevated after 2 wk (P < 0.05) of once-daily DE. However, there was a significant reduction of these cytokines/chemokines in the murine lavage fluid after 2 wk of DE compared with a single dust exposure (P < 0.05; n = 8 mice/treatment group). These findings demonstrate a consistent robust inflammatory response following single DE, and although diminished, a persistently elevated lavage inflammatory mediator response with repetitive DE.
Fig. 2.
Lung lavage fluid mean concentration of TNFα (A), IL-6 (B), KC (C; CXCL1), and MIP-2 (D; CXCL2) 5 h after single and repetitive intranasal inhalation of DE (12.5%) and saline. Error bars are SE (n = 8 mice/group). *P < 0.05 is statistically significant of DE vs. saline-treated mice.
Organic dust-induced PKCα and PKCɛ activity.
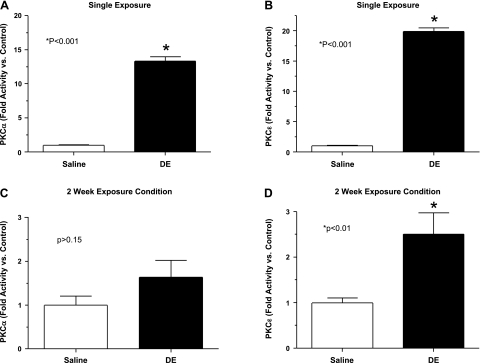
Our previous work has demonstrated a significant role for PKC, specifically PKCα and PKCɛ isoforms in swine facility organic dust-induced inflammation in cultured bronchial epithelial cells (T. A. Wyatt, R. E. Slager, A. J. Heires, J. M. DeVasure, S. G. Von Essen, J. A. Poole, and D. J. Romberger, unpublished observations, and Ref. 32). In these current studies, we sought to determine whether DE in a murine model also modulated epithelial PKCα and PKCɛ activity. PKCα and PKCɛ activity was assayed in whole lung and isolated epithelial cells from trachea after single DE and 2 wk of once-daily DE, 5-h postintranasal exposure to DE, or saline as described in methods. As shown in Fig. 3, A and B, there was a robust activation of PKCα (13.3 ± 0.6-fold increase) and PKCɛ (19.9 ± 0.6-fold increase) in the isolated epithelial cells after single DE compared with control (P < 0.05; n = 6 mice/treatment group). Compared with control, after 2 wk of DE, PKCɛ activation remained significantly elevated (2.5 ± 0.5-fold increase; P < 0.01; Fig. 3D), but PKCα activity was not significantly elevated (1.6 ± 0.4-fold increase; P > 0.15; Fig. 3C). However, activation of PKCα and PKCɛ was significantly dampened after 2 wk of DE compared with single DE (P < 0.05).
Fig. 3.
Isolated tracheal epithelial cell PKC activity in saline and DE (12.5%)-treated mice. PKCα (A) and PKCɛ (B) activity demonstrated 5 h after single inhalation of DE (12.5%) and saline in mice. PKCα (C) and PKCɛ (D) activity after 2 wk of once-daily DE exposure, 5 h after last dose. Error bars are SE (n = 6 mice minimum/group). *P < 0.05 is statistically significant of DE vs. saline-treated mice.
In the whole lung tissues (a multicellular organ response), there was no change in PKCα (1.0 ± 0.08-fold increase) and PKCɛ (1.0 ± 0.04-fold increase) after single DE compared with saline control (n = 6 mice/group). After 2 wk, there was a small but statistically significant increase in whole lung tissue PKCα (1.4 ± 0.1-fold increase, P = 0.03, n = 6 mice/group) in the DE mice compared with saline control, but no significant change in PKCɛ (1.4 ± 0.2-fold increase, P > 0.05, n = 6 mice/group).
These results in the isolated epithelial cells from the trachea are used to model lung epithelial cell responses and are consistent with prior in vitro findings in cultured bronchial epithelial cells (32). Moreover, these data paralleled cytokine/chemokine data in lavage fluid and suggest an important role for PKCα and PKCɛ in modulating dust-induced inflammation.
Organic dust modulates alveolar macrophage function.
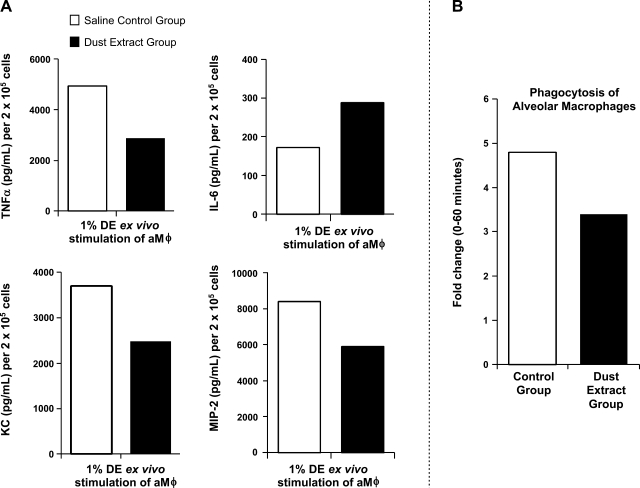
Previous in vitro studies have found that repetitive organic DE exposure significantly impairs the function of macrophages derived from monocytes (30). In these studies, aMφ from the lavage fluid of the repetitive (2 wk) saline control and dust-exposed mice were collected, and functional studies as described in methods were conducted. Alveolar macrophages from the dust-treated mouse group stimulated with DE (1%) for 5 h demonstrated a modulated inflammatory response compared with the saline control group (Fig. 4A). Specifically, TNFα, KC, and MIP-2 levels were diminished in the 2-wk dust-exposed mouse group, but IL-6 levels were increased compared with the control group (pooled aMφ, n = 8 mice/group). The phagocytic ability of the aMφ from the dust-exposed mouse group was also reduced compared with the control group. These data suggest that repetitive dust exposure affects the function of the aMφ in the lung lavage fluid, consistent with an impaired response.
Fig. 4.
Ex vivo studies of alveolar macrophages (aMφ) from 2-wk saline control and DE (12.5%)-treated mouse groups (pooled aMφ; n = 8 mice/group). A: TNFα, IL-6, KC, and MIP-2 secretion in culture supernatant 5 h after stimulation with 1% DE. B: phagocytosis of IgG-opsonized Saccharomyces cerevisiae zymosan bioparticles with fold change in mean fluorescence intensity (proportion of cells in zymosan-exposed population at 60 min compared with cells exposed for 0 min).
Dust-induced lung inflammation progresses with repetitive exposures.
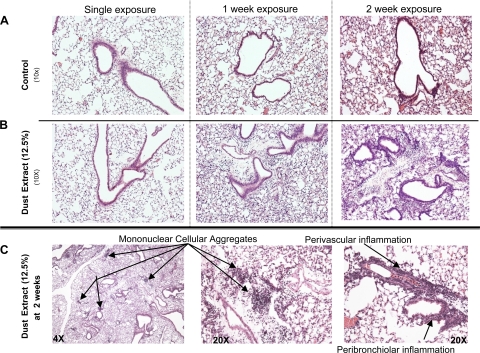
To determine the effect on the lung parenchyma following dust exposure, formalin-fixed, paraffin-embedded whole lungs were sectioned and stained with H&E. The lung histology was notable for increased inflammatory cells within the bronchiolar and alveolar compartments with increasing duration of dust exposure compared with saline control [Fig. 5A depicts saline control and Fig. 5B depicts 12.5% DE histology changes after single and repetitive dust exposures (1 and 2 wk)]. Predominately perivascular and peribronchiolar mixed mononuclear cellular aggregates were consistently observed after 2 wk of daily intranasal dust exposure (Fig. 5C) and were not observed in saline-exposed mice. Microscopic review of the lung tissue demonstrated varying degrees of alveolar and bronchiolar inflammation as well as a spectrum of mononuclear cell aggregates in the dust-exposed mice. Although there were varying degrees of inflammation, no alveolar destructive process (emphysema) was identified in any of the animals.
Fig. 5.
Histology of lung inflammation following intranasal inhalation of saline and DE (12.5%). A (saline control-treated mice) and B (DE-treated mice): H&E-stained lung sections after single and repetitive exposures shown at ×10 magnification. C: 2-wk DE-treated murine lung sections with arrows indicating mononuclear cellular aggregates (×4 and ×20 magnification) and bronchiolar and perivascular inflammation (×20). A representative 4- to 5-μm-thick section of 1 of 4 mice/treatment group is shown. All lung specimens were inflated to 15 cmH2O during fixation to avoid atelectasis artifact.
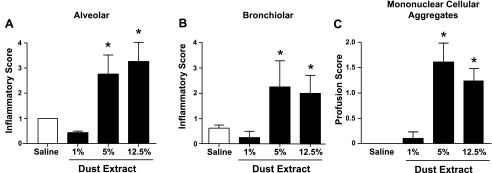
To semiquantitatively assess the range of dust-induced histopathological changes, six mice per group were challenged with saline or DE (1%, 5%, 12.5%) once daily for 2 wk, and the pathology inflammatory scores were determined by a reviewer (pathologist) blinded to the treatment conditions as described in methods. There was a concentration-dependent effect in the semiquantitatively graded distribution of lung alveolar inflammation (Fig. 6A), bronchiolar inflammation (Fig. 6B), and mononuclear cellular aggregates (Fig. 6C). Lung inflammation was not observed with 1% DE but was significantly observed at the 5% and 12.5% DE concentration. These observations demonstrate significant, semiquantifiable lung pathology occurring with repetitive dust exposures.
Fig. 6.
Semiquantitative inflammatory score of lungs after intranasal inhalation of saline and DE (1%, 5%, and 12.5%) after 2 wk of daily DE intranasal inhalation exposure. Semiquantitative distribution of lung alveolar inflammation (A), bronchiolar inflammation (B), and mononuclear cellular aggregates (C) in mice (n = 6 mice/group). Error bars are SE. *P < 0.05 is statistically significant of DE vs. saline-treated mice.
Heterogeneity of dust-induced mononuclear cellular aggregates.
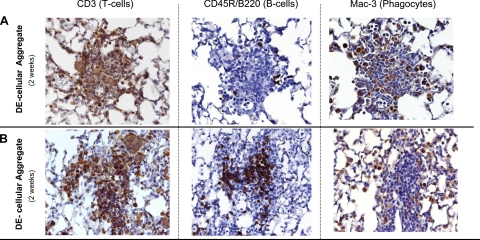
To determine the composition of these organic dust-induced mononuclear cellular aggregates observed after 2 wk of daily DE exposure, formalin-fixed, paraffin-embedded slides were analyzed for CD3 (pan-T cell marker), CD45R/B220 (pan-B cell murine marker), and Mac-3 (phagocytes) by immunohistochemistry as outlined in methods. As shown in Fig. 7, mononuclear cellular aggregates were heterogenous, representing a mixture of T lymphocytes, B lymphocytes, and phagocytes. The majority of aggregates contained T lymphocytes and phagocytes with a smaller proportion of B lymphocytes (Fig. 7A). However, other mononuclear cellular aggregates observed were composed of primarily T and B lymphocytes with a smaller fraction of phagocytes (Fig. 7B). These data demonstrate that the novel mononuclear cellular aggregates observed with repetitive dust exposure are heterogeneous, predominately characterized by T lymphocytes and macrophages, and, to a lesser extent, B lymphocytes.
Fig. 7.
Heterogeneous mononuclear cellular aggregates observed in mice after 2 wk of once-daily intranasal inhalation with DE (12.5%). A and B depict a different, but representative (2 of 4 mice), serial 4- to 5-μm-thick lung section of a focal mononuclear aggregate stained with anti-CD3 antibody for T lymphocytes, anti-murine CD45R/B220 antibody for B lymphocytes, and anti-Mac-3 antibody for phagocytes.
Dust-induced AHR.
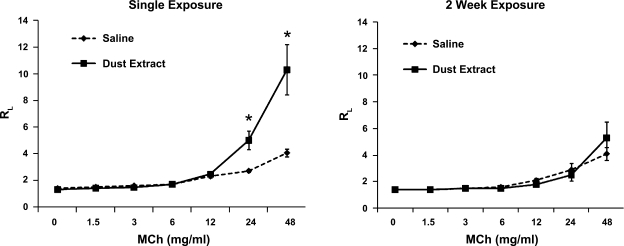
To determine if there were changes in lung function in response to single vs. repetitive intranasal DE vs. saline exposure, mice were assessed for AHR to methacholine by invasive pulmonary measurements, and there was a significant increase in AHR after single exposure to DE (12.5%) compared with saline-exposed controls (Fig. 8; 24 mg/ml and 48 mg/ml methacholine dose; P < 0.05; n = 4 mice/treatment group). However, after 2 wk, there was no change in AHR among the DE vs. saline (control)-treated mice. These results are consistent with an adapted response reported by others with swine barn air exposure (8).
Fig. 8.
Methacholine (MCh)-induced airway hyperresponsiveness following single and 2-wk repetitive intranasal inhalation of DE (12.5%) and saline in mice. Total lung resistance (RL) was directly measured using a mechanically ventilated mouse system. Data are expressed as means with standard error bars (n = 4 mice/group). *P < 0.05 is statistically significant.
DISCUSSION
Organic dust is an important agriculture environmental exposure that has been implicated in increased morbidity and lung disease among repetitively exposed subjects. Exposed individuals are at increased risk for developing significant respiratory diseases, particularly chronic bronchitis and obstructive pulmonary disease (37). In this study, a murine model utilizing single and repetitive intranasal inhalation of swine facility organic DE resulted in a reproducible, activated inflammatory airway response. A one-time exposure to dust resulted in significant increases in cellular influx, airway cytokine/chemokine release, and AHR. As hypothesized, airway mediator release dampened with repetitive exposures compared with single dust exposure, but the proinflammatory mediators in the lavage fluid remained significantly elevated after repetitive dust exposure compared with control. There was also expansion of airway neutrophils and macrophages with quantifiable increases in lung parenchymal inflammation marked by dense foci (mononuclear cellular aggregates) after 2 wk of repetitive dust exposure. Furthermore, PKC isoform activation paralleled lung inflammation suggesting PKC activation is an important signaling enzyme in organic dust-induced lung inflammation.
Proinflammatory mediators in the airway are suggested to contribute to the development of respiratory disease in swine farmers (2, 37). We found that TNFα, IL-6, KC (CXCL1), and MIP-2 (CXCL2) were robustly elevated in dust-exposed mice compared with control animals after single exposure, and albeit significantly dampened, remained persistently elevated after 2 wk of exposure conditions. These cytokines/chemokines are potent inflammatory mediators responsible for inducing pyrexia, neutrophil recruitment, activation of airway epithelial cells, and direct bronchial hyperreactivity (4). Our results with the intranasal inhalation method are consistent with other murine models of environmental inhalation injury such as endotoxin and cigarette smoke whereby proinflammatory cytokine release in the lavage fluid occurs within hours after exposure and dampens over time (3, 5, 35). In comparison, the swine barn air animal model has failed to demonstrate increases in the lavage fluid cytokines evaluated (TNFα, IL-1β, and IL-6; Ref. 9). This current model produced a consistent increase in lavage fluid inflammatory mediators, mediators that are well recognized to be increased in humans with organic dust-induced airway disease. We suggest here that this model provides an exaggerated, reproducible inflammatory response to dust exposure and that this model will be informative to future investigations of potential mechanisms driving organic dust-induced airway diseases.
In subjects with organic dust-induced respiratory disease, the airway lavage fluid is marked predominately by neutrophils, but also to a lesser degree lymphocytes and macrophages (2, 37). This current animal model demonstrated enhanced neutrophils, and, to a lesser degree, macrophages following dust exposure. In contrast, the swine barn air animal model found resolution of lavage cellularity (neutrophils) with repetitive exposures instead of a progressive enhancement of cellularity over time (8, 9). Our intranasal model is entirely consistent with nebulizer inhalation injury animal models. For example, repetitive daily inhalation of endotoxin by nebulizer to mice results in an expansion of lavage neutrophils over time, not dampening (5). Although these experimental manipulations in rodents over relatively short time intervals are not indicative of a real world human exposure (years of exposure), artificial animal models are important investigative tools for future mechanistic studies, and limitations are acknowledged. In addition, organic dust samples from various geographical locations may also contribute to variability in inflammatory outcomes.
There are several potential explanations for the increase or persistence of lavage neutrophils. First, it has been previously recognized that various agricultural organic DE directly exhibit chemotactic activity in vitro (6, 38, 39), and unpublished data from our laboratory also demonstrate that this swine facility dust directly results in neutrophil chemotaxis (data not shown). The mechanisms of these findings are not fully understood, but porcine IL-8 (45) and a leukotriene B-like component (6) (both neutrophil chemoattractants) have been directly detected in low levels in organic DE. Second, swine facility organic DE results in increased ICAM expression on epithelial cells (23), which would increase neutrophil adherence and thus their predominance in the airway. Indeed, we have pilot data demonstrating that organic dust directly enhances neutrophil adherence to epithelial cells (data not shown). Finally, albeit dampened, MIP-2 and KC are still significantly elevated after 2 wk of daily dust exposure compared with control, and, therefore, their role in neutrophil chemoattraction persists. Collectively, we suggest that the in vivo organic dust-induced airway neutrophil response is not completely dependent on the classic neutrophil chemoattractants, but may be a direct result of the dust itself.
Another potential explanation for the adaptation-like and/or modulated inflammatory response observed here may be due to the role of aMφ. Monocytes can be recruited to sites of inflammation, and depending on which maturation and differentiation factors are present in the airway milieu, differentiate into dendritic cell (DC) phenotypes or macrophages (17, 36). We have previously found that the presence of organic dust alters monocytes’ ability to differentiate into macrophages in cell culture as evident by impaired cytokine responsiveness and phagocytic ability (30). In this study, due to a limited number of lavage aMφ, aMφ were pooled from repetitively exposed (2-wk) saline control and DE mouse groups and studied for cytokine responsiveness and phagocytosis. Ex vivo stimulation of aMφ with DE resulted in a modulated immune response marked by reduced TNFα, MIP-2, and KC, but IL-6 secretion was increased. These findings are interpreted as consistent with the “tolerant” or adaptation response. Reduction in TNFα is considered the universal characteristic of tolerance, whereas others have found either suppression or augmentation of IL-6 (43). The aMφ phagocytic ability was also reduced in the 2-wk dust-treated group compared with the control group. We speculate that a reduction in aMφ function could impair clearing of the dust and/or neutrophils from the airway, and, as a result, contribute to the persistent inflammatory response observed.
In mice challenged for 2 wk with dust, significant increases in lung parenchymal macrophages, lymphocytes, and neutrophils with distinct mononuclear cellular aggregates were found. These mononuclear cellular aggregates have not been described before in other dust-induced airway inflammatory models, and they consisted mainly of T lymphocytes and macrophages, and, to lesser extent, B lymphocytes. Similar mononuclear inflammatory cell aggregates in murine lung have also been noted in transgenic mice overexpressing proinflammatory cytokines (12, 18, 40). Specifically, in transgenic mice overexpressing IL-1β and IL-6, lymphocytic infiltrates without macrophages are observed, but in mice overexpressing TNFα, a heterogeneity of mixed mononuclear cellular aggregates is observed. It is possible that the mixed, heterogeneous cellular aggregate in our dust-induced model is secondary to the variety of the mediators released. It is also possible that increases in epithelial and endothelial cell adhesion molecules might be playing a role in formation of these cellular aggregates. It is recognized that swine facility DE induces epithelial cell adhesion of lymphocytes through ICAM expression (23). Others have found dust-induced expression of intercellular adhesion molecules in cultured monocytes (7), and enhanced endothelial cell markers (sICAM) in blood samples from swine dust-exposed humans have been shown (16).
A possible mechanism by which this organic dust exposure may modulate inflammation is through PKC activation. Exposure of cultured human airway epithelial cells to swine facility DE in vitro augments epithelial cell PKCα and PKCɛ activity resulting in epithelial cell IL-6 and IL-8/CXCL8 release (32). Studies also suggest that PKCα mediates TNFα, IL-6, and IL-8 release in cultured human epithelial cells and that PKCɛ is involved in IL-8 release, but not IL-6 and TNFα (T. A. Wyatt, R. E. Slager, A. J. Heires, J. M. DeVasure, S. G. Von Essen, J. A. Poole, and D. J. Romberger, unpublished observations). In this in vivo study, there was a robust activation of PKCα and PKCɛ after single exposure to DE in isolated epithelial cells, but after 2 wk, this response was dampened. However, while dampened, PKCɛ remained significantly activated compared with saline control after 2 wk of dust exposure. Because of their accessibility, isolated epithelial cells from the trachea were used to model bronchial and alveolar epithelial cell responses throughout the lung. It is noted that studies with whole lung tissue were less informative as total PKC activity from the entire organ is likely composed of variable states of PKC activity in the numerous cell types of the lung. Still, PKCα was slightly increased in the 2-wk dust-treated murine whole lung tissue compared with saline control. Together, we propose that PKCα and PKCɛ play a significant role in organic dust-induced inflammatory responses in the airway and may represent targets for future therapeutic strategies.
When naive individuals are exposed to swine barns, there is a marked decline in postexposure peak flows and forced expiratory volume in 1-s (FEV1) with an associated increase in respiratory symptoms after a single exposure (19, 20). In contrast, swine farmers (repetitively exposed workers) do not experience such pronounced fluctuations in airflow or respiratory symptoms after exposure (28). Our murine model was consistent with the swine barn air model (8) in that single dust exposure evoked airway AHR to methacholine challenge and that this response was attenuated after 2 wk of repetitive dust exposure. The mechanism of organic dust-induced increases in AHR is not known. Neutrophil recruitment (33) and TNFα (29) can result in AHR, but AHR can also occur independently of neutrophil recruitment or TNF in other inflammatory models (21). In this study, increased airway responsiveness to swine barn dust inhalation corresponded to increases in airway lavage TNFα, IL-6, KC, and MIP-2 secretion, but not to cellular influx.
In conclusion, single and repetitive intranasal exposure to swine animal confinement facility DE in mice resulted in a reproducible model of exaggerated airway inflammatory and pathological injury. This model will allow qualitative and quantitative measures of dust-induced inflammation that may be useful for generating hypotheses of mechanistic regulation of environment-triggered respiratory disease. One potential target includes the PKC isoform(s) PKCα and PKCɛ. Furthermore, this murine model appears to mimic the well-recognized adaptation response described in humans.
GRANTS
This study was supported by National Institutes of Health Grants K08-ES-015522-01 (J. A. Poole), 1R01-OH-008539-01 (D. J. Romberger), R01-AA-017663 (T. A. Wyatt), and 5R37-AA-008769-17 (J. H. Sisson), and a Veterans Affairs Merit Review Grant (T. A. Wyatt).
Acknowledgments
We thank Jane DeVasure, Jacqueline Pavlik, Tracy Mathisen, Angela Burrell, and Conrad Parks for assisting in the experiments. We thank Victoria Smith and Dr. Charles Kuszynski (UNMC Cell Analysis Facility) for assistance with flow cytometric measurements. We also thank Dr. Thomas Jerrells for assistance with digital microscopy images prepared for this manuscript and Lisa Chudomelka for manuscript preparation assistance. Some of the results shown here have been presented in abstract form at the 2005 Experimental Biology meeting.
REFERENCES
- 1.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 117: 1396–1403, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ Small airways in COPD. N Engl J Med 350: 2635–2637, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Betsuyaku T, Hamamura I, Hata J, Takahashi H, Mitsuhashi H, Adair-Kirk TL, Senior RM, Nishimura M. Bronchiolar chemokine expression is different after single versus repeated cigarette smoke exposure. Respir Res 9: 7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol 111: S460–S475, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brass DM, Hollingsworth JW, Fessler MB, Savov JD, Maxwell AB, Whitehead GS, Burch LH, Schwartz DA. The IL-1 type 1 receptor is required for the development of LPS-induced airways disease. J Allergy Clin Immunol 120: 121–127, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck MG, Schachter EN, Fick RB, Merrill WW, Cooper JA Jr, Keirns JJ, Oliver J, Wall JH. Biologic activity of purified cotton bract extracts in man and guinea pig. Environ Health Perspect 66: 37–44, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burvall K, Palmberg L, Larsson K. Organic dust-induced activation, adhesion to substrate and expression of intercellular adhesion molecules in THP-1 monocytes. Life Sci 80: 1598–1607, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res 6: 50, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 34: 19–35, 2008. [DOI] [PubMed] [Google Scholar]
- 10.de Haar C, Kool M, Hassing I, Bol M, Lambrecht BN, Pieters R. Lung dendritic cells are stimulated by ultrafine particles and play a key role in particle adjuvant activity. J Allergy Clin Immunol 121: 1246–1254, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Deetz DC, Jagielo PJ, Quinn TJ, Thorne PS, Bleuer SA, Schwartz DA. The kinetics of grain dust-induced inflammation of the lower respiratory tract. Am J Respir Crit Care Med 155: 254–259, 1997. [DOI] [PubMed] [Google Scholar]
- 12.DiCosmo BF, Geba GP, Picarella D, Elias JA, Rankin JA, Stripp BR, Whitsett JA, Flavell RA. Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest 94: 2028–2035, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott MK, Sisson JH, West WW, Wyatt TA. Differential in vivo effects of whole cigarette smoke exposure versus cigarette smoke extract on mouse ciliated tracheal epithelium. Exp Lung Res 32: 99–118, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol 36: 452–459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp S, von Aulock S, Leendertse M, Haslinger I, Draing C, Golenbock DT, van der Poll T. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol 180: 3478–3484, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Kolbeck KG, Sardh E, Gyllenhammar H, Palmberg L, Larsson KK, Palmblad J. Modulation of plasma levels of soluble adhesion molecules and nitric oxide in healthy volunteers by exposure to swine dust. Inflammation 26: 291–296, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med 11: 653–660, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol 32: 311–318, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Larsson K, Malmberg P, Eklund A. Acute exposure to swine dust causes airway inflammation and bronchial hyperresponsiveness. Am J Ind Med 25: 57–58, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Larsson KA, Eklund AG, Hansson LO, Isaksson BM, Malmberg PO. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med 150: 973–977, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Lefort J, Singer M, Leduc D, Renesto P, Nahori MA, Huerre M, Creminon C, Chignard M, Vargaftig BB. Systemic administration of endotoxin induces bronchopulmonary hyperreactivity dissociated from TNF-alpha formation and neutrophil sequestration into the murine lungs. J Immunol 161: 474–480, 1998. [PubMed] [Google Scholar]
- 22.Marazuela EG, Rodriguez R, Fernandez-Garcia H, Garcia MS, Villalba M, Batanero E. Intranasal immunization with a dominant T-cell epitope peptide of a major allergen of olive pollen prevents mice from sensitization to the whole allergen. Mol Immunol 45: 438–445, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Mathisen T, Von Essen SG, Wyatt TA, Romberger DJ. Hog barn dust extract augments lymphocyte adhesion to human airway epithelial cells. J Appl Physiol 96: 1738–1744, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Mazzoli-Rocha F, Magalhaes CB, Malm O, Saldiva PH, Zin WA, Faffe DS. Comparative respiratory toxicity of particles produced by traffic and sugar cane burning. Environ Res 108: 35–41, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Miller LM, Foster WM, Dambach DM, Doebler D, McKinnon M, Killar L, Longphre M. A murine model of cigarette smoke-induced pulmonary inflammation using intranasally administered smoke-conditioned medium. Exp Lung Res 28: 435–455, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Muralimohan G, Rossi RJ, Guernsey LA, Thrall RS, Vella AT. Inhalation of Staphylococcus aureus enterotoxin A induces IFN-gamma and CD8 T cell-dependent airway and interstitial lung pathology in mice. J Immunol 181: 3698–3705, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldenburg PJ, Wyatt TA, Factor PH, Sisson JH. Alcohol feeding blocks methacholine-induced airway responsiveness in mice. Am J Physiol Lung Cell Mol Physiol 296: L109–L114, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmberg L, Larssson BM, Malmberg P, Larsson K. Airway responses of healthy farmers and nonfarmers to exposure in a swine confinement building. Scand J Work Environ Health 28: 256–263, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Pennings HJ, Kramer K, Bast A, Buurman WA, Wouters EF. Tumour necrosis factor-alpha induces hyperreactivity in tracheal smooth muscle of the guinea-pig in vitro. Eur Respir J 12: 45–49, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, Larsson L, Allen-Gipson D, Von Essen SG, Romberger DJ. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol 122: 375–382, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, Romberger DJ. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol 120: 366–373, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 93: 289–296, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA. Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 283: L952–L962, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz DA, Donham KJ, Olenchock SA, Popendorf WJ, Van Fossen DS, Burmeister LF, Merchant JA. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. Am J Respir Crit Care Med 151: 47–53, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Betsuyaku T, Nagai K, Fuke S, Nasuhara Y, Kaga K, Kondo S, Hamamura I, Hata J, Takahashi H, Nishimura M. Decreased airway expression of vascular endothelial growth factor in cigarette smoke-induced emphysema in mice and COPD patients. Inhal Toxicol 20: 349–359, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 172: 530–551, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 9: 185–196, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Von Essen SG, O'Neill DP, Robbins RA, Rennard SI. Neutrophil chemotaxis to extracts of grain plant components. Am J Ind Med 25: 85–88, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Von Essen SG, Robbins RA, Thompson AB, Ertl RF, Linder J, Rennard S. Mechanisms of neutrophil recruitment to the lung by grain dust exposure. Am Rev Respir Dis 138: 921–927, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Vuillemenot BR, Rodriguez JF, Hoyle GW. Lymphoid tissue and emphysema in the lungs of transgenic mice inducibly expressing tumor necrosis factor-alpha. Am J Respir Cell Mol Biol 30: 438–448, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J 10: 381–387, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Ward JM, Erexson CR, Faucette LJ, Foley JF, Dijkstra C, Cattoretti G. Immunohistochemical markers for the rodent immune system. Toxicol Pathol 34: 616–630, 2006. [DOI] [PubMed] [Google Scholar]
- 43.West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med 30: S64–S73, 2002. [PubMed] [Google Scholar]
- 44.Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 163: 1157–1166, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyatt TA, Sisson JH, Von Essen SG, Poole JA, Romberger DJ. Exposure to hog barn dust alters airway epithelial ciliary beating. Eur Respir J 31: 1249–1255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ. Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase Cɛ in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 293: L1163–L1170, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 275: L827–L835, 1998. [DOI] [PubMed] [Google Scholar]